Abstract
Objective:
To evaluate the relationship of abdominal muscle lean and adipose tissue volumes with prediabetes and diabetes.
Research Design and Methods:
We measured abdominal muscle composition in 3,170 participants in the Coronary Artery Risk Development in Young Adults (CARDIA) Study who underwent computed tomography (CT) at year 25 (ages 43–55 years) of follow-up. Multinomial regression analyses was used to evaluate the associations of CT measured intermuscular adipose tissue (IMAT) lean muscle (lean), and visceral adipose tissue (VAT) volumes with diabetes ever during CARDIA, newly detected prediabetes, prior history of prediabetes, and normal glucose tolerance. Models were adjusted for potential confounding factors age, sex, race, center, height, history of smoking, hypertension, hyperlipidemia and cardiorespiratory fitness.
Results:
Higher IMAT, lean, and VAT volumes were all separately associated with a higher prevalence of prediabetes and diabetes. Inclusion of VAT in models with both IMAT and lean volume attenuated the association of IMAT with both prediabetes and diabetes, but higher lean volume retained its association with prediabetes and diabetes. People in the highest IMAT quartile coupled with VAT in its lower 3 quartiles had higher prevalence of diabetes, but not prediabetes, than those with both IMAT and VAT in their respective lower three quartiles. Adjusting for cardiorespiratory fitness did not substantially change the findings.
Conclusion:
Higher IMAT was associated with higher prevalence of diabetes even after adjustment for VAT. However, further study is warranted to sort out the complicated relationship of abdominal muscle and adipose tissues.
INTRODUCTION
Muscle tissue is the largest insulin sensitive organ. It accounts for the majority of glucose uptake, playing a crucial role in maintaining systemic glucose homeostasis1. Limited attention has been paid to the association between muscle volume and muscle composition (adipose tissue and lean muscle volumes) and the prevalence of type 2 diabetes mellitus (T2DM). The majority of studies have focused on the relationship of T2DM with adipose tissue (AT)2. Numerous studies have documented that high body mass index (BMI), and measures of total intra-abdominal AT, and specifically visceral AT (VAT), are associated with a higher risk of T2DM3,4.
Another ectopic AT depot is intermuscular adipose tissue (IMAT) that is present between the muscle fiber bundles resulting in increased muscle volume, which is in contrast with volume from lean skeletal muscle tissue (lean). Increased IMAT is associated with higher fasting glucose and insulin, and greater prevalence of T2DM5–9. One nationally representative US study assessed the ratio of total skeletal muscle mass to total body weight, as measured by bioelectrical impedance, and found that a higher ratio of muscle to body weight was associated with increased insulin sensitivity and lower risk of pre-diabetes or diabetes after adjusting for BMI and waist circumference10. This study may have been limited by the use of dual energy X-ray absorptiometry which fails to discriminate between muscle and IMAT and could lead to overestimation of effective muscle mass in conditions in which there is lipid infiltration such as obesity and aging11,12.
Small clinical trials have demonstrated that resistance training in lean individuals or resistance training combined with weight loss in obese participants is associated with improved insulin sensitivity and improved glucose tolerance13,14. A recent Coronary Artery Risk Development in Young Adults (CARDIA) report found that higher cardiorespiratory fitness based on treadmill performance was associated with lower risk for developing the composite outcome prediabetes/diabetes, even when adjusting for time-dependent BMI15. Another CARDIA study found that treadmill performance was inversely associated with total body fat assessed by dual energy X-ray absorptiometry, and the inverse association of fitness with fat mass was much stronger than the association of fitness with lean mass16.
We therefore sought to further examine the associations of muscle composition with prevalent prediabetes and diabetes, using data from the multicenter community-based CARDIA cohort for whom biochemical and clinical information about prediabetes and diabetes and physical fitness data was available. Participants enrolled in the CARDIA study had non-contrast enhanced abdominal computed-tomography (CT) imaging performed during study year 25, which provided measurements of lean, IMAT, and total muscle volumes for abdominal muscle groups including the psoas, paraspinous, lateral oblique and rectus muscles. Our objective was to investigate the cross-sectional associations of total muscle, lean, and IMAT volumes with diabetes and prediabetes at year 25. We hypothesized that muscle with higher IMAT volume was positively associated with both prediabetes and diabetes. These associations were hypothesized to be independent of physical fitness and VAT.
METHODS
Study Sample
The CARDIA Study was initiated in 1985 with recruitment of 5,115 participants aged 18 to 30 years at field centers located in Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA 17. Black and white adults were recruited from population-based samples approximately balanced within center by sex, age, race, and education. After the baseline exam, participants were examined in follow-up visits at 2, 5, 7, 10, 15, 20, 25, and 30 years, with at least 71% of survivors attending an in-person clinic examination at each examination. All participants provided written informed consent, and institutional review boards from each field center and the coordinating center approved the study annually.
Study Measures
Except year 20 treadmill testing and cumulative (through year 25) diabetes and hypertension variables, data were drawn from CARDIA year 25. Socio-demographic characteristics including sex, race, and education were obtained using standard questionnaires and confirmed at years 0 and 2. Age was self-reported. Height was measured to the nearest 0.5cm with the participant standing erect, back against a vertical mounted centimeter ruler without shoes. Weight was measured in light clothing to the nearest 0.5kg. Cigarette smoking status at year 25 was classified as never, former and current and self-report of high blood cholesterol were collected through interviewer-administered questionnaires. Cumulative history of hypertension ever during CARDIA was based on seated resting systolic and diastolic blood pressure (≥140/90 mmHg) or taking antihypertensive medication.
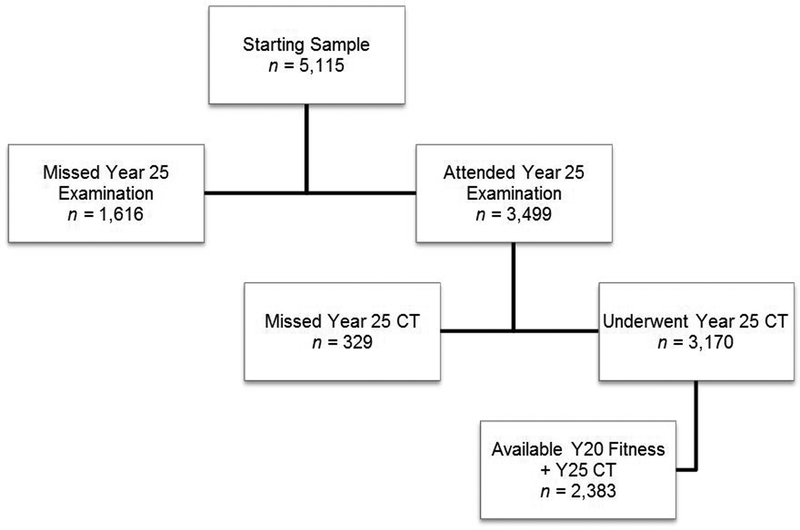
All 3499 year 25 participants were asked to participate in an abdominal CT study in year 25. Of these, 3170 participants had assessment of adipose tissue distribution in the visceral and subcutaneous compartments19,20 and measurement of the skeletal muscle size21. VAT was assessed at the L4–L5 level as previously reported19–21. Abdominal muscle composition (lean, IMAT, and total) was measured from CT images covering the lower abdomen obtained without oral or intravenous contrast agent. The MIPAV (Medical Image Processing, Analysis, and Visualization, http://mipav.cit.nih.gov/index.php) software with a custom plugin was used to perform quantitative measurements of 4 paired muscle groups: psoas, paraspinous, lateral oblique and rectus abdominis. Contiguous 1–1.25 mm slices constituting 10 mm vertically were loaded into the MIPAV viewer and the axial, coronal and sagittal reformats were used to select the center of the lumbar disk space at L3–L4 (to avoid artifacts produced by the pelvic bones encountered in some individuals at the lower L4–L5 level). Each muscle boundary was manually traced. Tissues within muscle with attenuation between −190 and −30 Hounsfield units (HU) were defined as adipose tissue, and −29 to 160 HU as lean tissue. Because findings reported here for the average across all 8 sites (left and right side for 4 muscles) were very similar to those for individual muscles (data not shown), the average across the 8 sites was used.
Analysis reliability of CT measures was assessed through blinded intra- and inter-reader re-reads of 158 scan pairs (~5%). Overall (intra- and inter-reader) technical error in re-analysis of 158 pairs of scans was 6.6% for CAC, 6.0% for VAT, and 7.7% for psoas muscle total volume with correlations for re-reads >0.95 in each measure.
In year 20, 2870 participants underwent the CARDIA cardiorespiratory fitness test, which was designed to assess the maximal, symptom-limited performance by using a modified Balke protocol18 including up to nine 2-minute stages (≤ 18 minutes total) of progressively increasing difficulty, with speed increasing from 3.0 mph in stage 1 to 5.6 mph in stage 9 and grade increasing from 2% in stage 1 to 25% in stage 9. The first 6 stages generally could be performed by walking, whereas the final 3 stages required jogging and running. Estimated metabolic equivalent tasks (METs) ranged from 4.1 in stage 1 to 19.1 in stage 9. Total seconds of treadmill exercise were recorded for each participant.
Main Outcome Measures: diabetes and prediabetes.
Individuals were asked to bring medication bottles to clinic and self-reported diabetes medications at every examination, and fasting glucose was measured at years 0, 7, 10, 15, 20 and 25. OGTT was measured at years 10, 20, and 25 and HbA1c was measured at years 20 and 25. Individuals were classified as having diabetes if at any clinic examination during the CARDIA study they had a fasting blood glucose ≥ 126mg/dl; a 2 hour post OGTT of ≥ 200mg/dl; a HbA1c ≥ 6.5% (48 mmol/mol), or a history of taking medications to treat diabetes. Individuals were classified as having prediabetes if they were not classified as having diabetes and had a fasting blood glucose between 100 mg/dl and 125mg/dl or a blood glucose 2 hours post OGTT between 140 mg/dl and 200mg/dl. Duration of diabetes was assessed as the number of years before year 25 that diabetes was first diagnosed in CARDIA. Prediabetes was much less consistently observed across examinations. The measure reflecting duration was current when prediabetes existed at year 25 or past when prediabetes was found at any previous examination, but was not noted at year 25.
Statistical Methods
We described the sample according to sex-specific quartiles of both lean muscle and IMAT volume and described the same variables according to the 4-level outcome variable included diabetes defined by testing or clinical diagnosis cumulatively through year 25, prediabetes at year 25, prediabetes before, but not at year 25, and never either diabetes nor prediabetes. Correlations among the muscle and visceral fat variables were examined. The muscle and visceral fat means were computed across diabetes of different durations. We conducted cross-sectional analysis and developed separate multinomial regression models for each independent variables. Covariates included age, sex, race, center, height, history of smoking, hypertension, and hyperlipidemia.
Odds ratios (OR) and 95% confidence intervals (CI) along with standard errors (SE) were presented. In complementary analyses, a p-value for trend treating the predictor variable as continuous was obtained. Given the complex physiological and statistical relationship between IMAT and VAT, we also focused on people who were in the highest sex-specific quartile of IMAT but a lower sex-specific quartile of VAT, that is, grouping quartiles 1–3 vs quartile 4 for each of IMAT and VAT, and crossing these groups to get 4 categories. Because those in quartiles 1–3 of both IMAT and VAT tended to be thinner, to improve comparison of diabetes probabilities according to fatness we omitted the thinnest people who were in quartile 1 of both IMAT and VAT. Within each of the 4 categories we computed mean IMAT and VAT. In multinomial logistic regression with the 4 categories as the independent variables, we adjusted for covariates and predicted probabilities of prediabetes and diabetes, setting all other predictors constant at their means (age, sex, race, center, height, history of smoking, hypertension and hyperlipidemia and fitness) by back-transforming the logit. Possible sex interaction was examined by adding the product of sex times each of lean, IMAT, and VAT volumes as continuous variables.
Missing covariate data in year 25 for smoking, height, BMI and self-reported high cholesterol reduced sample sizes to 3109, not considering the Y20 treadmill test and to 2343 in the subset of participants who did the treadmill test. In a sensitivity analysis of the people who had the year 25 abdominal fat and muscle measures and who completed the treadmill test at year 20, further adjustment was made for duration on the treadmill test. The full datasets not considering year 20 treadmill duration of 3170 people vs 3109 after removing those with missing covariates yielded similar findings (data not shown). The full data set considering year 20 treadmill duration was also little affected by loss of 40 people for missing covariates. Among the 3170 people with muscle and VAT measures at year 25, the people who completed the year 20 treadmill test were a little less likely to be African-American or to smoke or be hypertensive, obese, or self-report history of high cholesterol.
All analyses were performed using SAS version 9.4 or STATA 13. Statistical significance was defined at P < 0.05.
Results
Table 1 shows participant characteristics according to quartiles of both abdominal muscle lean and IMAT volume. Lean cutpoints were higher for male compared to female participants, but IMAT cutpoints were similar between the sexes. Age was inversely associated with lean volume and directly associated with IMAT. Treadmill duration was similar across lean quartiles, but duration was lower with higher IMAT. IMAT, lean, and total tissue volumes were each directly associated with lean and fat volumes. IMAT to total volume ratio, a measure of muscle composition which is equal to 1 – lean to total volume ratio, was similar across lean quartiles, but increased dramatically with higher IMAT quartile. VAT, BMI, and height increased across both lean and fat quartiles. Percentage of white participants decreased with higher lean quartile. Hypertension, high cholesterol, and current smoking were each more prevalent as IMAT increased. The associations of BMI, VAT, treadmill duration, diabetes duration, and hypertension appeared to be stronger with IMAT than with lean.
Table 1.
Muscle lean sex-specific quartile (left side) and IMAT sex-specific quartiles (right side) (all variables from CARDIA year 25 except treadmill).
| Muscle lean Q1 | Muscle lean Q2 | Muscle lean Q3 | Muscle lean Q4 | Muscle fat Q1 | Muscle fat Q2 | Muscle fat Q3 | Muscle fat Q4 | |
|---|---|---|---|---|---|---|---|---|
| N=782 | N=773 | N=779 | N=775 | N=782 | N=782 | N=778 | N=767 | |
| Male cutpoints | 12.0018–19.844 | 19.8445–21.939 | 21.9394–24.2463 | 24.2506–33.9118 | 0.2055–1.2976 | 1.3001–1.9126 | 1.9127–2.9684 | 2.9786–15.891 |
| Female cutpoints | 8.6629–13.1099 | 13.1116–14.5306 | 14.5308–16.1186 | 16.1325–26.6218 | 0.2081–1.2466 | 1.2466–1.8972 | 1.9016–2.9258 | 2.9279–14.7455 |
| Age (y) | 50.9±3.48a | 50.4±3.62 | 50.1±3.65 | 49.3±3.6 | 49.3±3.63 | 50.2±3.57 | 50.5±3.6 | 50.6±3.61 |
| Height (cm) | 167.9±9.7 | 170±8.92 | 170.8±9.29 | 172.5±9.28 | 169.1±8.83 | 170.3±9.31 | 170.6±9.85 | 171.3±9.63 |
| BMI (kg/m2) | 26.5±5.9 | 29±6.1 | 30.9±6.7 | 34.6±7.1 | 24.9±4.1 | 27.9±4.7 | 31±5.2 | 37.3±7.4 |
| Diabetes duration (y) | 0.7±3.1 | 0.5±2.15 | 0.8±2.93 | 1.2±3.99 | 0.4±2.32 | 0.7±3.08 | 0.7±2.87 | 1.4±3.93 |
| Lean (cc) | 14.67±3.26 | 16.94±3.53 | 18.66±3.85 | 21.67±4.61 | 16.96±4.37 | 17.73±4.5 | 18.26±4.71 | 18.98±4.65 |
| IMAT (cc) | 1.92±1.39 | 2.19±1.6 | 2.46±1.7 | 2.83±1.65 | 0.93±0.25 | 1.57±0.19 | 2.39±0.3 | 4.56±1.71 |
| Total (cc) | 16.67±3.74 | 19.22±3.97 | 21.23±4.31 | 24.64±4.98 | 17.91±4.41 | 19.35±4.54 | 20.73±4.77 | 23.8±5.06 |
| IMAT/Total (%) | 11.3±6.51 | 11.2±6.56 | 11.4±6.59 | 11.5±5.96 | 5.4±1.8 | 8.5±2.02 | 12.1±2.9 | 19.5±6.29 |
| VAT (cc) | 110.9±66.1 | 124.7±67.9 | 136.3±76.7 | 154.8±76.4 | 75.1±41.7 | 109.5±49 | 146.4±59.9 | 196.8±76.9 |
| Treadmill durationb (sec) | 437±150.6 | 440±158.6 | 433±168.6 | 393±158.1 | 494±155.9 | 454±151.7 | 409±145.7 | 332±140.5 |
| White race (%) | 68.4 (535/782) | 58.9 (455/773) | 50.6 (394/779) | 32 (248/775) | 54 (422/781) | 53.3 (417/782) | 53.8 (419/779) | 48.8 (374/767) |
| Female (%) | 56.9 (445/782) | 56.5 (437/773) | 56.5 (440/779) | 56.3 (436/775) | 56.7 (443/781) | 56.4 (441/782) | 56.6 (441/779) | 56.5 (433/767) |
| HTN (%) | 30.6 (239/782) | 34.9 (270/773) | 43.3 (337/779) | 50.2 (389/775) | 27 (211/781) | 35.2 (275/782) | 39.7 (309/779) | 57.4 (440/767) |
| High cholesterol (%) | 26.6 (208/782) | 26.4 (204/773) | 29.4 (229/779) | 30.1 (233/775) | 20.1 (938/157) | 27 (993/211) | 32.4 (1031/252) | 33.1 (1021/254) |
| Smoking status | ||||||||
| Never (%) | 59.7 (467/782) | 60.2 (465/773) | 61.5 (479/779) | 63 (488/775) | 67.4 (526/781) | 59 (461/782) | 61 (475/779) | 57 (437/767) |
| Former (%) | 21.7 (170/782) | 24.1 (186/773) | 21.7 (169/779) | 19.4 (150/775) | 17.2 (134/781) | 24 (188/782) | 22.3 (174/779) | 23.3 (179/767) |
| Current (%) | 18.5 (145/782) | 15.8 (122/773) | 16.8 (131/779) | 17.7 (137/775) | 15.5 (121/781) | 17 (133/782) | 16.7 (130/779) | 19.7 (151/767) |
Mean±Std or % (n/N). Aside from use of sex specific quartiles, data are unadjusted.
Treadmill test at year 20: Lean quartiles N = 589, 596, 603, 555, respectively; IMAT quartiles N = 626, 608, 576, 533, respectively
Definitions: IMAT, intermuscular adipose tissue; Lean, lean muscle volume; Total, total muscle volume; VAT, visceral adipose tissue; HTN, hypertension cumulative through Y25
Age, race, and sex adjusted Pearson partial correlation coefficients for the association of abdominal muscle composition with adiposity measures and cardiorespiratory fitness are shown in Supplemental Table 1. BMI and VAT had correlation coefficients over 0.6 with IMAT volume, total muscle volume, and IMAT to total muscle ratio. BMI and VAT correlations with lean volume were 0.3–0.4. IMAT was tightly correlated with IMAT to total muscle ratio (r=0.95), for which reason findings involving the ratio or the corresponding lean to total volume ratio are effectively the same as for IMAT. Further findings for the ratio are not presented. In the subset of individuals with available treadmill testing (n=2,383), higher treadmill duration was moderately associated with lower IMAT and IMAT to total muscle ratio, and, to a lesser extent, total muscle volume.
Table 2 presents participant characteristics according to prediabetes and diabetes status. Participants who developed diabetes during CARDIA were older, had lower treadmill time, higher IMAT, lean, and total muscle volumes, higher VAT, and higher BMI than normoglycemic participants while those with prediabetes were intermediate between normoglycemic and diabetic participants. Participants with prediabetes that was detected at CARDIA year 25 had higher IMAT, lean and total muscle volumes, VAT and BMI than participants with prediabetes only before Y25. Interestingly, lean volume was slightly lower in those with diabetes than in those with prediabetes at year 25. Approximately two-thirds of the diabetics were black and 55% were female while the prediabetes groups were more likely to be white and male. Three-quarters of diabetics had hypertension and also hypercholesterolemia and current smoking were higher in those with diabetes compared to those with either prediabetes or normoglycemic individuals.
Table 2.
Participant characteristics across glycemia categories
| Normoglycemia at all attended visits | Prediabetes before Y25, but not at Y25 | Prediabetes at Y25 | Diabetes at any visit | |
|---|---|---|---|---|
| N=1470 | N=474 | N=729 | N=436 | |
| Age (y) | 49.8±3.68a | 50.1±3.53 | 50.5±3.55 | 50.7±3.6 |
| Height (cm) | 168.9±9.21 | 171.8±9.11 | 172.4±9.63 | 170±9.4 |
| Treadmill duration (sec)b | 442±159.4 | 446±158.9 | 438±155.3 | 324±133.4 |
| Diabetes duration (y) | 0±0 | 0±0 | 0±0 | 5.7±6.45 |
| IMAT (cc) | 1.98±1.29 | 2.21±1.46 | 2.65±1.67 | 3.27±2.17 |
| Lean (cc) | 16.75±4.22 | 18.6±4.51 | 19.34±4.76 | 19.18±4.66 |
| Total (cc) | 18.79±4.59 | 20.89±4.85 | 22.11±5.22 | 22.67±5.46 |
| IMAT/Total (%) | 10.5±5.82 | 10.6±6.03 | 11.9±6.47 | 14.1±7.6 |
| VAT (cc) | 106.8±59.9 | 125.5±64.1 | 155.9±73.6 | 181.6±86.6 |
| BMI (kg/m2) | 28.6±6.6 | 29.2±6.3 | 31.3±6.6 | 35±8 |
| White race (%) | 55.2 (812/1470) | 54 (256/474) | 56.8 (414/729) | 34.4 (150/436) |
| Female (%) | 67.1 (987/1470) | 43.9 (208/474) | 44.3 (323/729) | 55.1 (240/436) |
| HTN (%) | 28.4 (417/1470) | 36.9 (175/474) | 42.9 (313/729) | 75.7 (330/436) |
| High cholesterol (%) | 21.4 (1785/315) | 25.7 (596/122) | 28.5 (937/208) | 52.5 (665/229) |
| Smoking status | ||||
| Never (%) | 62.6 (920/1470) | 63.7 (302/474) | 57.8 (421/729) | 58.7 (256/436) |
| Former (%) | 21.8 (321/1470) | 18.4 (87/474) | 24.4 (178/729) | 20.4 (89/436) |
| Current (%) | 15.6 (229/1470) | 17.9 (85/474) | 17.8 (130/729) | 20.9 (91/436) |
Mean±Std or % (n/N). Aside from use of sex specific quartiles, data are unadjusted.
Treadmill test at year 20, N =1125, 369, 544, 305, respectively
Definitions: IMAT, intermuscular adipose tissue; Lean, lean muscle volume; Total, total muscle volume; VAT, visceral adipose tissue; HTN, hypertension cumulative through Y25
In those with a diagnosis of diabetes ever during CARDIA, diabetes was first detected at year 25 in 39%, and duration was 5 or 10 years in 55% of the participants (Supplemental Table 2). Diabetes duration was unrelated to any of the muscle or adiposity measures.
Multinomial logistic regression models evaluating the associations of muscle composition and VAT with prediabetes and diabetes are shown in Table 3. A minimally adjusted model including age, race, and sex (Model 1) and another model adding risk factors (Model 2) are presented. We subsequently added all primary predictor variables, IMAT volume, lean volume, and VAT, to model 2 simultaneously. As shown, IMAT was significantly associated with current prediabetes (detected at Y25), prior history of prediabetes, and diabetes in both models 1 and 2. A 1-SD higher IMAT was associated with 23% higher occurrence of previous history of prediabetes, 60% higher risk of newly detected prediabetes, and 91% higher occurrence of diabetes after adjusting for model 2 covariates. Although not significantly associated with previous history of prediabetes, a 1-SD increment in abdominal lean volume was associated with 1.5-fold higher risk of newly detected prediabetes and 1.72-fold higher risk of diabetes in model 2. Higher VAT was apparently more strongly associated with the outcome variable than were either of the muscle-related variables. Inclusion of both lean volume and IMAT in model 2 slightly attenuated findings compared to model 2. Adjustment for VAT led to greater attenuation compared to model 2, with IMAT losing significance, but lean volume retaining statistical significance. VAT remained strongly associated with both prior prediabetes and diabetes [OR(95% CI) 1.7 (1.49, 1.95) and 2.29 (1.94, 2.7), respectively], but VAT was no longer associated with previously detected prediabetes in the full model. Adding terms for sex interaction did not significantly improve any model (p>0.15 in all models; data not shown). All findings were slightly attenuated in Supplemental Table 3 by adding treadmill duration at year 20 to the model (and therefore restricting the sample analyzed to N = 2,343).
Table 3.
Association of abdominal muscle composition and VAT with diabetes and prediabetes
| Model | Past Prediabetes (before but not at Y25) |
Current Prediabetes (Y25) | Diabetes (through Y25) |
|
|---|---|---|---|---|
| IMAT | 1 | 1.27(1.11, 1.45)a | 1.71 (1.54, 1.90) | 2.16 (1.93, 2.42) |
| 2 | 1.23 (1.08, 1.41) | 1.60 (1.43, 1.78) | 1.91 (1.69, 2.15) | |
| + Lean | 1.20 (1.05, 1.38) | 1.48 (1.33, 1.79) | 1.77 (1.56, 1.99) | |
| + Lean & VAT | 1.10 (0.94, 1.28) | 1.08 (0.95, 1.23) | 1.14 (0.99, 1.32) | |
| Lean | 1 | 1.10 (0.98, 1.23) | 1.53 (1.38, 1.69) | 1.77 (1.58, 1.98) |
| 2 | 1.09 (0.96, 1.23) | 1.50 (1.35, 1.67) | 1.72 (1.52, 1.96) | |
| + IMAT | 1.05 (0.93, 1.19) | 1.40 (1.26, 1.56) | 1.57 (1.38, 1.79) | |
| + IMAT & VAT | 1.03 (0.91, 1.17) | 1.29 (1.16, 1.44) | 1.35 (1.18, 1.55) | |
| VAT | 1 | 1.26 (1.12, 1.43) | 2.02 (1.82, 2.25) | 3.17 (2.80, 3.60) |
| 2 | 1.22 (1.07, 1.39) | 1.92 (1.72, 2.14) | 2.70 (2.36, 3.09) | |
| + IMAT & Lean | 1.14 (0.96, 1.34) | 1.70 (1.49, 1.95) | 2.29 (1.94, 2.70) |
standardized odds ratio(95% CI)
Model 1: age, race, sex; model 2: model 1 + center, height, smoking, hypertension, and high cholesterol
Definitions: IMAT, intermuscular adipose tissue; VAT, visceral adipose tissue; Lean, lean muscle volume
Given the high correlation between IMAT and VAT, the fully adjusted but non-interactive models in Table 3 may be misleading. These variables were examined in more detail in Table 4. To understand any independent IMAT association with diabetes prevalence, we focused on the group (N=302) with IMAT in Q4 but VAT in Q1–3 compared to the group with both IMAT and VAT in Q1–3 (N=1009). Mean VAT was comparable between the groups (137.0 and 120.0 cc, respectively), but IMAT was very different (4.1 vs 2.0 cc, respectively.) We performed a logistic regression model adjusted for covariates as in Table 3, model 2, with the 4 IMAT and VAT categories shown in Table 4 as the predictors of interest. There was no multiplicative interaction in diabetes prevalence. VAT is clearly the stronger predictor of diabetes, but the back-transformed estimated probabilities of having diabetes at or before year 25 among those with VAT in Q1–3 were 0.133 in IMAT Q4 vs 0.090 in IMAT Q1–3 (p=0.008). Corresponding differences for current or previously detected prediabetes were not significant in these models (data not shown).
Table 4.
Unadjusted IMAT and VAT volumes, and adjusted probability of having diabetes according to the crossing of IMAT with VAT in sex specific quartiles 1–3 (Q1–3) vs quartile 4 (Q4). The 495 people in quartile 1 for both IMAT and VAT were much thinner (mean±std IMAT 0.87±0.26 cc and VAT 50.43±19.61 cc; 4.6% had diabetes) and were omitted to improve comparison of diabetes probabilities according to fatness.
| IMAT Q1–3 | IMAT Q4 | |||
|---|---|---|---|---|
| IMAT Values | ||||
| N | Mean (SD) | N | Mean | |
| VAT Q1–3 | 1009 | 1.97 (0.46) | 302 | 4.09 (1.25) |
| VAT Q4 | 301 | 2.28 (0.45) | 471 | 4.97 (1.96) |
| VAT Values | ||||
| VAT Q1–3 | 1009 | 120.02 (31.46) | 302 | 136.98 (30.05) |
| VAT Q4 | 301 | 214.29 (46.6) | 471 | 240.42 (66.18) |
| Diabetes probability in Multinomial Logistic Model 2 (see definitions in Table 3) | ||||
| VAT Q1–3 | 1009 | 0.090 | 302 | 0.133 |
| VAT Q4 | 301 | 0.171 | 471 | 0.236 |
Discussion
Our findings suggest that: 1) higher abdominal IMAT volume is associated with higher risk for prediabetes or diabetes; 2) higher lean volume is also associated with higher prevalence of current prediabetes and diabetes, a finding that persisted with adjustment for IMAT volume; and, 3) the association of IMAT, which is highly correlated with muscle composition, with prediabetes and diabetes is tightly associated with VAT volume. Associations of higher IMAT, lean tissue, and VAT with prediabetes and diabetes were independent of concomitant risk factors and cardiorespiratory fitness. Though adjusting models for VAT attenuated the associations of IMAT and lean volumes with prediabetes and diabetes, those in the highest sex-specific quartile of IMAT had higher prevalence of diabetes even when VAT was not in its highest sex-specific quartile.
Our findings that IMAT and VAT are associated with prediabetes and diabetes are perhaps not surprising in that a number of clinical and observational epidemiologic studies suggest that ectopic adipose deposition is associated with insulin resistance and prevalent and incident diabetes2–6,9,10. In 2011, Srikanthan et al. examined the relationship of total skeletal muscle mass to total body weight as measured by bioelectrical impedance with prediabetes and diabetes using the National Health and Nutrition Examination Survey III (1988–1994) finding that a higher ratio of muscle, ostensibly lean muscle tissue, to body weight was associated with increased insulin sensitivity and a lower risk of prediabetes or diabetes after adjusting for BMI and waist circumference10. Bioelectrical impedance has limitations that include the potential to overestimate the muscle mass content in the setting of obesity and the inability of bioelectrical impedance to differentiate the specific contributions of the lean tissue and IMAT components of the total muscle11,12. However, studies using CT to specifically assess the role of muscle quality or IMAT have shown that lower attenuation in core or peripheral muscles consistent with higher fat infiltration is associated with insulin resistance, diabetes, and other cardiometabolic abnormalities as well6,7,22,23. Using CT, the present study shows that higher IMAT volume and higher lean volume (the major constituent of total muscle volume) independently contribute to risk for prevalent prediabetes and diabetes.
In our correlational analysis, aerobic physical fitness as assessed by total treadmill time 5 years before the abdominal CT scan was inversely associated with IMAT. Though higher aerobic fitness was moderately associated with lower IMAT, it was also, to a lesser degree, associated with lower total muscle volume. Including treadmill time in multivariable models did not explain associations between muscle composition nor VAT with prediabetes or diabetes. Prior studies have suggested that interventions such as aerobic exercise, resistance training, or dietary weight loss (especially multifaceted interventions including exercise and diet) may improve insulin sensitivity and even reduce incidence of diabetes via increase in muscle mass, specifically lean muscle tissue9,24. Although our study does not directly address these interventions, our findings suggest that it would be difficult to distinguish between metabolic improvements attributable to lean muscle gain from that contributed by loss of either IMAT or VAT in obese individuals. Thus, the benefit of exercise on diabetes incidence may be partially mediated by reductions in IMAT secondary to fitness. Indeed, any dietary or training intervention that seeks to reduce excess body fat may be expected to reduce VAT in proportion to overall weight loss and, in turn, insulin sensitivity may be expected to improve due to its inverse correlation with VAT25.
The finding that lean volume is positively related to both prediabetes and diabetes seems paradoxical but is consistent with the previous CARDIA finding that treadmill duration is only weakly related to DXA-measured lean mass16. In Zhu et al, the relation of lean mass was positively related to treadmill duration only in the lowest quartile of fat mass, while the relation was inverse in the highest quartile of fat mass16. Higher muscle volume may reflect adaptation to obesity, such as lower muscle density (ie, more lipid within adipocytes of the muscle) and the need to support body weight. We speculate that the quality of lean tissue that results in response to dynamic or isometric activity is higher than the quality of lean tissue that results solely to support a large mass of adipose tissue. Greater amount of IMAT has been associated with low quality lean tissue, inflammation, and insulin resistance9. In this view, IMAT may therefore be regarded as one measure of muscle quality, concordant with speculation by Larsen et al26.
Strengths of this study include the large, well characterized community-based sample of white and black individuals, novel and comprehensive measures of muscle mass using CT scan, and the wide array of demographic, lifestyle and chronic disease measures to assess confounding and effect modification, including cardiorespiratory fitness. However, we must also acknowledge limitations to our study. First the cross-sectional design cannot prove causality as discussed above. Second, we evaluated abdominal muscle composition and therefore our findings may not be applicable to peripheral muscles.
In conclusion, we found that higher abdominal IMAT volume was associated with higher prevalence of current prediabetes and diabetes. Abdominal IMAT is strongly associated with VAT and, as such, associations cannot be interpreted without accounting for the larger VAT depot. We also report the novel observation that lean muscle volume is positively associated with diabetes and prediabetes after adjusting for both IMAT and VAT. This observation requires further study, but it seems likely that lean muscle quality suffers as ectopic adipose depots enlarge, as well as being influenced by other metabolic factors, including inflammation and insulin resistance.
Supplementary Material
Figure 1–
Enrollment and follow up of the study participants
Acknowledgments
The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201800005I & HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I), the Intramural Research Program of the National Institute on Aging (NIA), and an intra-agency agreement between NIA and NHLBI (AG0005). This manuscript has been reviewed by CARDIA for scientific content. The Young Adult Longitudinal Trends in Antioxidants Study (YALTA) is supported by the National Heart, Lung, and Blood Institute, National Institutes of Health (R01 HL 53560). CNB and BWJHP are supported through an NWO-VICI grant (number 91811602). CT scan acquisition and analyses were supported in part by R01 HL098445 (J. Jeffrey Carr). Dr. Granados was supported by the Institutional T32 grant 5T32DK071212 during fellowship training.
Footnotes
The authors have no conflicts of interest. All authors have contributed to the manuscript in significant ways and have reviewed and agreed upon the manuscript content.
References
- 1.Carnagarin R, Dharmarajan AM, Dass CR. Molecular aspects of glucose homeostasis in skeletal muscle - A focus on the molecular mechanisms of insulin resistance. Mol Cell Endocrinol. 2015;417:52–62. doi: 10.1016/j.mce.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care. 2000;23(4):465–471. [DOI] [PubMed] [Google Scholar]
- 3.Astrup A, Finer N. Redefining type 2 diabetes: “diabesity” or “obesity dependent diabetes mellitus”? Obes Rev. 2000;1(2):57–59. [DOI] [PubMed] [Google Scholar]
- 4.N IJ, T AT, A CR, et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308(11):1150–1159. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boettcher M, Machann J, Stefan N, et al. Intermuscular adipose tissue (IMAT): association with other adipose tissue compartments and insulin sensitivity. J Magn Reson Imaging. 2009;29(6):1340–1345. doi: 10.1002/jmri.21754. [DOI] [PubMed] [Google Scholar]
- 6.Miljkovic-Gacic I, Gordon CL, Goodpaster BH, et al. Adipose tissue infiltration in skeletal muscle: age patterns and association with diabetes among men of African ancestry. Am J Clin Nutr. 2008;87(6):1590–1595. doi: 10.1093/ajcn/87.6.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Therkelsen KE, Pedley A, Speliotes EK, et al. Intramuscular Fat and Associations With Metabolic Risk Factors in the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2013;33(4):863–870. doi: 10.1161/ATVBAHA.112.301009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miljkovic I, Zmuda JM. Epidemiology of myosteatosis. Curr Opin Clin Nutr Metab Care. 2010;13(3):260–264. doi: 10.1097/MCO.0b013e328337d826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Addison O, Marcus RL, Lastayo PC, Ryan AS. Intermuscular fat: A review of the consequences and causes. Int J Endocrinol. 2014;2014:34–36. doi: 10.1155/2014/309570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the Third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2011;96(9):2898–2903. doi: 10.1210/jc.2011-0435. [DOI] [PubMed] [Google Scholar]
- 11.Volgyi E, Tylavsky FA, Lyytikainen A, Suominen H, Alen M, Cheng S. Assessing body composition with DXA and bioimpedance: effects of obesity, physical activity, and age. Obesity (Silver Spring). 2008;16(3):700–705. doi: 10.1038/oby.2007.94. [DOI] [PubMed] [Google Scholar]
- 12.Deurenberg P Limitations of the bioelectrical impedance method for the assessment of body fat in severe obesity. Am J Clin Nutr. 1996;64(3 Suppl):449S–452S. doi: 10.1093/ajcn/64.3.449S. [DOI] [PubMed] [Google Scholar]
- 13.Ishii T, Yamakita T, Sato T, Tanaka S, Fujii S. Resistance training improves insulin sensitivity in NIDDM subjects without altering maximal oxygen uptake. Diabetes Care. 1998;21(8):1353–1355. [DOI] [PubMed] [Google Scholar]
- 14.Dunstan DW, Daly RM, Owen N, et al. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care. 2002;25(10):1729–1736. [DOI] [PubMed] [Google Scholar]
- 15.Chow LS, Odegaard AO, Bosch TA, et al. Twenty year fitness trends in young adults and incidence of prediabetes and diabetes: the CARDIA study. Diabetologia. 2016;59(8):1659–1665. doi: 10.1007/s00125-016-3969-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu N, Jacobs DRJ, Sidney S, et al. Fat mass modifies the association of fat-free mass with symptom-limited treadmill duration in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr. 2011;94(2):385–391. doi: 10.3945/ajcn.110.008995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. [DOI] [PubMed] [Google Scholar]
- 18.Sidney S, Haskell WL, Crow R, et al. Symptom-limited graded treadmill exercise testing in young adults in the CARDIA study. Med Sci Sports Exerc. 1992;24(2):177–183. [PubMed] [Google Scholar]
- 19.VanWagner LB, Wilcox JE, Colangelo LA, et al. Abstract 52: Associations of Nonalcoholic Fatty Liver Disease with Subclinical Myocardial Dysfunction: The CARDIA Study. Circ. 2014;129(Suppl 1):A52–A52. http://circ.ahajournals.org/content/129/Suppl_1/A52.abstract. [Google Scholar]
- 20.Mueller NT, Pereira MA, Demerath EW, et al. Earlier menarche is associated with fatty liver and abdominal ectopic fat in midlife, independent of young adult BMI: The CARDIA study. Obesity. 2015;23(2). doi: 10.1002/oby.20950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terry JG, Shay CM, Schreiner PJ, et al. Intermuscular Adipose Tissue and Subclinical Coronary Artery Calcification in Midlife: The CARDIA Study (Coronary Artery Risk Development in Young Adults). Arterioscler Thromb Vasc Biol. 2017;37(12):2370–2378. doi: 10.1161/ATVBAHA.117.309633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miljkovic I, Cauley JA, Wang PY, et al. Abdominal myosteatosis is independently associated with hyperinsulinemia and insulin resistance among older men without diabetes. Obesity (Silver Spring). 2013;21(10):2118–2125. doi: 10.1002/oby.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71(4):885–892. [DOI] [PubMed] [Google Scholar]
- 24.Aguiar EJ, Morgan PJ, Collins CE, Plotnikoff RC, Callister R. Efficacy of interventions that include diet, aerobic and resistance training components for type 2 diabetes prevention: a systematic review with meta-analysis. Int J Behav Nutr Phys Act. 2014;11:2. doi: 10.1186/1479-5868-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tchernof A, Despres J-P. Pathophysiology of Human Visceral Obesity: An Update. Physiol Rev. 2013;93(1):359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 26.Larsen BA, Wassel CL, Kritchevsky SB, Strotmeyer ES, Criqui MH, Kanaya AM, Fried LF, Schwartz AV, Harris TB, Ix JH; Health ABC Study. Association of Muscle Mass, Area, and Strength With Incident Diabetes in Older Adults: The Health ABC Study. J Clin Endocrinol Metab. 2016. April;101(4):1847–55. doi: 10.1210/jc.2015-3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.