Abstract
Background:
Dementia in Parkinson’s disease (PD) is common and disabling. Identification of modifiable risk factors for it is essential. Vascular risk factors may be associated with cognitive decline in early PD. Biomarkers that serve as surrogates of the long-term effect of vascular risk factors on PD are needed. To that end, we aimed to quantitate white matter hyperintensities in early PD, measure associations with vascular risk factors, and examine relationships between white matter hyperintensities and longitudinal cognition.
Methods:
Participants in the Parkinson Progression Markers Initiative study (141 PD patients, 63 healthy controls) with adequate baseline structural brain MRIs were included. Hypertension and diabetes history, and body mass index, were combined to create a vascular risk score. White matter hyperintensities were quantitated via automated methods. Cognition was assessed annually with a comprehensive test battery.
Results:
In the PD group, vascular risk score was associated with white matter hyperintensities for total brain (β=0.210;p=0.021), total white matter (β=0.214;p=0.013), frontal (β=0.220;p=0.002) and temporal (β=0.212;p=0.002) regions. Annual rate of change in global cognition was greater in those with higher vascular risk score (β=−0.040;p=0.007) and greater white matter hyperintensities (β=−0.029;p=0.049). Higher temporal white matter hyperintensity burden was associated with great decline over time in verbal memory (β=−0.034;p=0.031).
Conclusion:
In early PD, modifiable vascular risk factors are associated with white matter hyperintensities on brain MRI. Temporal white matter hyperintensity burden predicts decline in verbal memory. White matter hyperintensities may serve as a surrogate marker for the effect of vascular risk factors on cognitive abilities in PD.
Keywords: Cognitive disorders, Parkinson’s disease, Cerebrovascular diseases
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder associated with numerous motor and non-motor manifestations. Many symptomatic therapies are available, but treatments for some of the more common and disabling manifestations, including cognitive impairment and dementia[1], are limited. Several predictors of cognitive decline in PD have been identified[2-5], Unfortunately, none of these are modifiable. In the non-PD population, modifiable vascular risk factors (VRFs) such as diabetes, hypertension, and obesity have been associated with cognitive decline[6]. In general, modifiable risk factors may account for one-third of all-cause dementia[7], making them of great potential relevance in PD. Increasing evidence suggests that VRFs are associated with cognitive impairment in patients with PD of several years duration[8,9] and even in early disease stages[10,11].
Generally, cognitive decline associated with VRFs is thought to be the direct consequence of cerebrovascular disease. The latter can manifest clinically with transient ischemic attack or stroke, but more often is asymptomatic while leading to various changes on structural MRI including cortical infarcts, lacunes, and white matter hyperintensities(WMH). The converse is also true: WMH most commonly reflect white matter tissue ischemia pathologically[12]. The presence of WMH predicts increased risk of dementia in older adults[13], and greater rate of cognitive decline in Alzheimer’s disease [12,14], WMH are thus being examined as surrogate end-points for the effect of modifying VRFs in non-PD populations[15].
Interventions designed to modify VRFs in PD are likely to have the greatest impact in early disease, such as untreated PD without dementia. At this disease stage, any longitudinal changes in cognition are small in magnitude, and the impact of any intervention would require long trials with large sample sizes. Thus, biomarkers that can serve as surrogates of the effect of VRFs on cognitive trajectory in PD are needed. Towards addressing this critical gap, we aimed to quantitate WMH in early PD, to measure associations with VRFs, and to examine the relationship between WMH and longitudinal changes in cognition. Based on the literature in non-PD populations, we hypothesized there would be an association between modifiable VRFs and WMH, and that WMH would independently predict change in cognition in early PD.
Methods
Table 1 defines abbreviations used.
Table 1.
List of Abbreviations
| Abbreviation | Definition |
|---|---|
| BMI | Body mass index |
| BP | Blood pressure |
| GDS-15 | 15-item Geriatric Depression Scale |
| HC | Healthy controls |
| HVLT | Hopkins Verbal Learning Test-Revised |
| JOLO | Benton Judgment-of-Line-Orientation |
| LNS | Letter-Number Sequencing (LNS) |
| MCI | Mild cognitive impairment |
| MDS-UPDRS | Movement Disorders Society Unified Parkinson’s Disease Rating Scale |
| MoCA | Montreal Cognitive Assessment |
| mFRS | modified Framingham Risk Score |
| PD | Parkinson’s Disease |
| PPMI | Parkinson Progression Markers Initiative |
| SDMT | Symbol digit modality test |
| VRFs | Vascular risk factors |
| VRS | Vascular risk score |
| WM | White matter |
| WMH | White matter hyperintensities |
| WMHS | White matter hyperintensity score |
Study Participants
We utilized data from the PPMI study. This dataset represents a recent, multicenter cohort of well-characterized newly diagnosed and untreated (at baseline) PD patients and HC. Study aims, methodology, and details of study assessments are available on the PPMI website(http://www.ppmi-info.org/study-design).
At enrollment, PD patients were required to have (i)asymmetric resting tremor or bradykinesia, or two of bradykinesia, resting tremor and rigidity (ii)been diagnosed ≤2 years of study enrollment, (iii)dopamine transporter deficit on SPECT imaging, be (iv)untreated for PD and (v)dementia-free based on the site investigator’s clinical assessment. HC were required to also have a MoCA score of >26.
Exclusion criteria for PD and HC groups relevant to these analyses included anticoagulant use, medical conditions precluding study participation at the investigator’s discretion, and previously obtained MRI scan showing clinically significant abnormality. In addition, HC participants could not have a first-degree relative with PD.
Among the 423 PD participants and 196 HC in PPMI, 220 underwent MRI with FLAIR and T1-weighted MRI sequences. Of those, 9 were excluded due to poor image acquisition quality (e.g.poor contrast, significant field inhomogeneity, or significant motion), and 7 were excluded based on image processing quality control (e.g.poor WMH lesion and segmentation correspondence). The final sample for the baseline analysis included 141 PD and 63 HC participants. 134 PD and 61 HC underwent at least one follow-up cognitive assessment and were included in longitudinal analysis.
The study was approved by institutional review boards at PPMI sites, and written informed consent was obtained. Data used in this analysis were downloaded 31October, 2016.
Assessments
PPMI cohort participants undergo a range of assessments as detailed at http://www.ppmi-info.org/study-design. Baseline variables relevant to the present analyses include demographics, BMI, vital signs, PD disease duration, MDS-UPDRS as a measure of motor disease severity, and GDS-15 score as a measure of depression. Measures of cognitive function included baseline and annual scores on the neuropsychological test battery, and mild cognitive impairment (MCI) or dementia categorization (see supplement for test battery and cognitive categorization criteria)(4,16-21).
Vascular Risk Factor Ascertainment
During the PPMI screening visit, all medical conditions reported by the patient are recorded in the medical conditions log. Self-reported diabetes and hypertension were ascertained from the log. Use of anti-hypertensive medications was similarly ascertained from medication logs. The individual abstracting data from the logs (C.D.S) was blinded to MRI findings.
A VRS was generated based on the modified Framingham Risk Score (mFRS)[22]. The mFRS accounts for age, sex, self-reported hypertension and diabetes, BMI, measured BP (accounting for BP treatment), and smoking history. In PPMI, smoking history was not ascertained, so the mFRS was further modified by excluding smoking history i.e.VRS=mFRS excluding smoking history. This score thus accounts for the 2 main VRFs, diabetes and hypertension, that have been associated with cognitive dysfunction in early PD cross-sectionally[9,10]. This score has several advantages. It applies weights to demographics and acquired risk factors, while accounting for anti-hypertensive treatment. Importantly, unlike other VRF scores, mFRS does not rely on laboratory assessment of lipids. The mFRS has been validated and its association with cardiovascular disease is as strong as the traditional FRS score which includes laboratory measurements[22].
White Matter Hyperintensity Quantification
T1-weighted images were first preprocessed for correction of intensity inhomogeneities and extraction of brain tissue. A registration based multi-atlas segmentation method[23] was applied to parcellate the brain into anatomical regions of interest. WMH were segmented by applying a supervised learning-based multimodal segmentation method, WMLS[24] on T1-weighted and FLAIR images. Minimum WMH volume was set to 25mm3, to ensure ischemic origin of the detected hyperintense area. Regional WMH volumes were calculated in frontal, temporal, parietal, and occipital regions. To visualize the spatial extent of WMH accumulation, individual lesion segmentation maps of each subject were non-linearly aligned to a common template and lesion frequency maps were computed. All WMH calculations were performed using an automated process, blinded to clinical history (including VRFs). Quality control assessments were also performed in a blinded approach.
Statistical Analysis
Visual inspection of WMH variables revealed that data were not normally distributed. Therefore, a root-cube transformation was applied.
A detailed description of the statistical analysis approach is included with the supplement. Briefly, descriptive statistics were used to summarize basic demographics and parametric and non-parametric tests were applied as appropriate to compare groups and examine for associations among variables.
For cross-sectional analyses pertaining to baseline cognitive function, logistic regression models were used to examine the relationship between VRS and cognitive categorization. Linear regression models were used to examine the relationship between VRS or WMHS and cognitive test score . For the multivariable models, age at testing, sex, years of education and disease duration (PD group) were included covariates.
For longitudinal analyses linear mixed-effects models were used to examine the relationship between VRS or WMHS, and continuous measures of cognition. Age at testing, sex, years of education, baseline cognitive test score and disease duration (PD group) were included covariates. Generalized estimating equations examined the relationship of VRS, WMHS and occurrence of MCI on follow-up.
Statistical significance was set at p<0.05. Analyses were conducted with Stata-13(Stata Corp,Tx).
Results
Modifiable Vascular Risk Factors and WMH in the PD vs Healthy Control Group
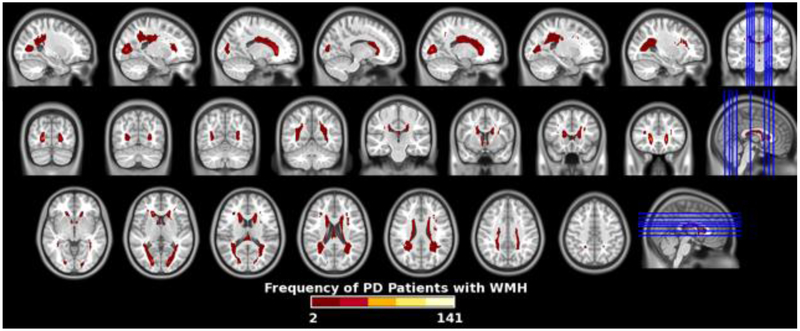
Table 2 shows baseline demographics, VRFs, and mean values of WMHS (root-transformed) in the PD and HC groups. There were no significant differences in demographics or VRFs. In both groups, regional WMHS were highest in frontal regions. There were no significant differences in global or regional WMHS in the PD vs. HC groups. Frequency maps of segmented WMH for the PD group are shown in figure 1.
Table 2.
Baseline demographics, modifiable vascular risk factor prevalence, and white matter hyperintensity scores (root-transformed) in PD and HC group. VRS=Vascular Risk Score. WM=White Matter.
| PD group n=141 |
HC group n=63 |
p-value | |
|---|---|---|---|
| Demographic Characteristics | |||
| Mean age in years (SD) | 61.23 (9.86) | 61.4 (12.42) | 0.915 |
| Mean age at diagnosis in years (SD) | 59.70 (13.31) | n/a | n/a |
| Mean disease duration in years (SD) | 0.57 (0.49) | n/a | n/a |
| Sex (M: F) (n, %) | 92 (65.24):49 (34.75) | 36 (57.14):27 (42.86) | 0.269 |
| Non-Hispanic:Hispanic | 138 (97.87):2 (1.42) | 63 (100%):0 | 0.477 |
| Race (self-reported; white: non-white or not reported) | 134 (95.04):7 (4.96) | 62 (98.41):1 (1.59) | 0.496 |
| Mean years of education (SD) | 15.88 (2.89) | 16.63 (2.71) | 0.079 |
| Vascular Risk Factors | |||
| Diabetes, self-reported Y: N (n, %) | 9 (6.4):132 (93.62) | 3 (4.76):60 (85.24) | 0.649 |
| Hypertension, self-reported Y: N (n, %) | 40 (28.37):101 (71.63) | 17 (26.98):46 (73.02) | 0.839 |
| Mean body mass index (kg/m2) (SD) | 27.16 (4.65) | 26.66 (4.62) | 0.476 |
| Mean VRS (SD) | 12.21 (4.60) | 12.02 (5.13) | 0.786 |
| White Matter Hyperintensity Scores (root-transformed) | |||
| Mean Total brain (SD, range) | 10.18 (5.06; 0-33.67) | 11.12 (6.51; 0-32.20) | 0.962 |
| Mean Total WM (SD, range) | 10.67 (5.26; 0-34.17) | 10.71 (6.36; 0-31.79) | 0.984 |
| Mean Frontal WM (SD, range) | 7.40 (7.05; 0-24.5) | 7.21 (4.19; 0-17.90) | 0.502 |
| Mean Temporal WM (SD, range) | 3.81 (4.06; 0-22.81 | 4.43 (2.57; 0-24.24) | 0.826 |
| Mean Parietal WM (SD, range) | 3.91 (3.60; 0-20.93) | 4.65 (4.91 ;0- 22.82) | 0.688 |
| Mean Occipital WM (SD, range) | 6.12 (3.39; 0-14.48 | 6.34 (3.81;0-15.93) | 0.939 |
Figure 1.
Frequency map of white matter hyperintensities sin Parkinson’s disease patients quantitated from FLAIR MRI volumes.
Baseline Associations Between Modifiable Vascular Risk Factors, WMH, and Cognitive Function
Baseline associations between VRS and demographic, clinical, and WMHS are shown in table 3. In both groups, VRS was significantly associated with age. VRS was higher in males than females in the PD group (median VRS=13 vs. 11 respectively, p=0.007) and the HC group (median VRS=14 vs. 11 respectively, p=0.031). VRS was associated with MDS-UPDRS subscores in the HC but not the PD group. There were no associations in either group between VRS and GDS-15.
Table 3.
Univariable associations in the PD group and HC group between vascular risk score (VRS) and demographics, motor and non-motor features, and white matter hyperintensity scores (root-transformed). MDS-UPDRS=Movement Disorders Society Unified Parkinson’s Disease Rating Scale. GDS-15=15-item Geriatric Depression Scale. Results shown are from linear regression models.
| PD (n=141) | HC (n=63) | |||
|---|---|---|---|---|
| Variable | β (95% CI) | p- value |
β (95% CI) | p-value |
| Demographics and Clinical Measures | ||||
| Age | 1.60 (1.36, 1.84) | <0.001 | 1.97 (1.61, 2.33) | <0.001 |
| Education | −0.015 (−0.120, 0.089) | 0.773 | −0.019 (−0.154, 0.116) | 0.783 |
| MDS-UPDRS part I | −0.027 (−0.085, 0.030) | 0.353 | 0.015 (−0.040, 0.070) | 0.584 |
| MDS-UPDRS part II | 0.019 (−0.149, 0.187) | 0.172 | 0.068 (0.017, 0.118) | 0.009 |
| MDS-UPDRS part III (motor subscale) | 0.213 (−0.094, 0.521) | 0.157 | 0.227 (0.089, 0.365) | 0.002 |
| GDS-15 | −0.036 (−0.115, 0.043) | 0.367 | 0.051; (−0.032, 0.133) | 0.225 |
| MoCA | −0.066 (−0.144, 0.011) | 0.092 | −0.034 (−0.091,0.0214) | 0.222 |
| HLVT delayed | −0.165 (−0.241, −0.089) | <0.001 | −0.082 (−0.200; 0.037) | 0.174 |
| HLVT recognition | −0.071 (−0.112, −0.030) | 0.001 | −0.040 (−0.079, −0.0002) | 0.049 |
| JOLO | −0.054 (−0.201, 0.093) | 0.469 | 0.054 (−0.065, 0.172) | 0.370 |
| LNS | −0.164 (−0.251, −0.076) | <0.001 | −0.140 (−0.250, −0.0296) | 0.014 |
| Semantic fluency | −0.930 (−1.32, −0.544) | <0.001 | −1.10 (−1.73, −0.464) | 0.001 |
| Verbal fluency | −0.057 (−0.242, 0.128) | 0.543 | −0.208 (−0.406, −0.010) | 0.040 |
| SDMT | −0.667 (−0.983, −0.350) | <0.001 | −1.48 (−1.92, −1.03) | <0.001 |
| White Matter Hyperintensity scores | ||||
| Total WM | 0.214 (0.045, 0.383) | 0.013 | 0.292 (−0.016, 0.60) | 0.063 |
| Frontal WM | 0.229 (0.088, 0.369) | 0.002 | 0.178 (−0.026, 0.382) | 0.085 |
| Temporal WM | 0.212 (0.078, 0.345) | 0.002 | 0.306 (0.035, 0.578) | 0.027 |
| Parietal WM | 0.076 (−0.046, 0.198) | 0.218 | 0.228 (−0.009, 0.466) | 0.059 |
| Occipital WM | 0.037 (−0.087, 0.160) | 0.559 | 0.038 (−0.151; 0.228) | 0.688 |
In the PD group, VRS was associated with total brain and white matter, frontal, and temporal WMHS. Only temporal WMHS associated with VRS in the HC group(table 3).
Adjusting for covariates, in the HC group, higher VRS was associated with lower JOLO scores (supplementary table 1). In the PD group, there were no associations between VRS and cognitive test scores (table 4). In the PD group, VRS was not significantly different in those with vs. without MCI (mean VRS 13.9 vs. 12.0, p=0.13 respectively).
Table 4.
In the PD group, association between (a) presence of MCI at baseline and (i) VRS score (ii) WMH (root transformed) in the PD group and (results are from multivariable logistic regression models that include age at baseline, sex, education, and disease duration as covariates) (b) individual cognitive test scores and (i) VRS score (ii) WMH (root transformed) (results are from multivariable linear regression models that include age at baseline, sex, education, and disease duration as covariates). 95%CI=95% Confidence Interval. HVLT=Hopkins Verbal Learning Task. JOLO=Judgement of Line Orientation. LNS=Letter number sequencing. MCI=mild cognitive impairment. MoCA=Montreal Cognitive Assessment. SDMT=Symbol Digit Modality. VRS=Vascular Risk Score. WM=white matter. WMH=White Matter Hyperintensity.
| (a) |
Cognitive Variable |
VRS (OR; 95%CI; p-value) |
WMH Measure (OR; 95%CI; p-value) | |||||
| Total Brain | Global WM | Frontal WM | Temporal WM | Parietal WM | Occipital WM | |||
| MCI | 1 10; 1.0, 1.25; 0.144 | 1.05; 1.0, 1.17; 0.404 | 1.05; 1.0, 1.19; 0.372 | 1 03, 0.90, 1 19; 0.633 | 1 09; 0.95, 1.24; 0.217 | 1 06; 0.91, 1.24; 0.425 | 1 08; 0.91, 1.27; 0.379 | |
| (b) |
Cognitive Variable |
VRS (β; 95%CI; adjusted R2; p- value) |
WMH Measure (β; 95%CI; adjusted R2; p-value) | |||||
| Total Brain | Global WM | Frontal WM | Temporal WM | Parietal WM | Occipital WM | |||
| MoCA | 0.014; −0.110, 0.1388; 0.823 | 0.054; −0.021, 0129; 0.034; 0.154) | 0.050; −0.029. 0.129; 0.031; 0212) | 0.088: −.005, 0.181; 0.025; 0.065) | −0.033; −0.133; 0.066; 0.023; 0.508) | −0.002: −0.110, 0.107; 0.019; 0.977) | 0.056; −0.049, 0.161; 0.028; 0.293) | |
|
HLVT delayed |
−0.121; −0.243, 0.001; 0.051 |
−0.079; −0.153, −0.004; 0.111; 0.038) |
−0.083; −0.160, −0.005; 0.111; 0.038) | −0.081; −0.174, 0.012; 0.102; 0.089) | −0.124; −0.222, −0.026; 0.155; 0.013) | −0.111; −0.218, −0.004; 0.142; 0.043) | −0.046; −0.151, 0.059; 0.120; 0.388) | |
|
HLVT recognition |
−0.0256; −0.0910, 0.040; 0.439 | −0.052; −0.091, −0.013; 0.155; 0.009) | −0.056; −0.097, −0.015; 0.157; 0.008) | −0.059; −0.107, −0.010; 0.115; 0.019) | −0.112; −0.161, −0.063; 0.227; <0.001) | −0.080; −0.135, −0.024; 0.130; 0.006) | −0.018, −0.−74, 0.038; 0.080; 0.523) | |
| JOLO | −0.090; −0.308, 0.129; 0.420 | −0.030; −0.164, 0.103; 0.131; 0.655) | −0.036; −0176, 0.104; 0.132; 0.614) | −0.058; −0.225, 0.109, 0.133; 0.495) | −0.138; −0.314, 0.038, 0.145; 0124) | 0.007, −0.186, 0.199, 0.130; 0.947) | −0.010; −0.198, 0.177; 0.130; 0.913) | |
| LNS | −0.022, −0.154, 0.110; 0.744 | 0.001, −0.079, 0.081; 0.187; 0.977) | −0.006; −0.091, 0.078; 0.187; 0.882) | 0.018. −0.083, 0.118; 0.188, 0.726) | −0.079; −0.185, 0.026, 0.200; 0.140) | −0.037; −0.153, 0.079; 0.189; 0.528) | −0.090, −0.201, 0.022; 0.202; 0.115) | |
|
Semantic fluency |
−0.256; −0.845, 0.332; 0.390 | −0.060; −0.419, 0.299; 0.211; 0.740) | −0.044; −0.421, 0.334; 0210, 0.820) | −0108; −0.557, 0.341, 0.211; 0.635) | −0.052; −0.529, 0.424; 0.210; 0.828) | −0.117; −0.635, 0.401; 0.211; 0.655) | −0.149; −0.652, 0.353; 0.212; 0.558) | |
|
Verbal fluency |
0.207; −0.080, 0.495; 0.157 | 0.036; −0.140, 0.212, 0.046, 0.685) | 0.037; −0.148, 0.222; 0.046; 0.695) | 0.121; −0.098, 0.340, 0.054; 0.278) | −0.092; −0.325, 0.141, 0.049; 0.437) | −0.099 –0.353, 0.155; 0.049, 0.441) | −0.146, −0.392, 0.100; 0.055; 0.242) | |
| SDMT | 0.318, −0.141, 0.776; 0.173 | 0.063, −0.218, 0.344; 0.259, 0.659) | 0.079, −0.217, 0.374, 0.260; 0.600) | 0.204, −0.146, 0.554, 0.265; 0.251) | −0.180; −0.552, 0.192, 0.263, 0.340) | −0.157; −0.562, 0.248; 0.261; 0.445) | 0.077; −0.317, 0.471; 0.259, 0.700) | |
data missing for 1 participant who didn’t complete baseline cognitive testing
Adjusting for covariates, there were no significant associations between WMHS in any brain region and cognitive measures in the HC group(supplementary table 1). On the other hand, in the PD group, global, temporal, and parietal WMHS were inversely associated with HVLT- delayed recall. WMHS in all brain regions except occipital were associated with HVLT-recognition(table 4). Otherwise, WMHS in any region were not associated with any other cognitive domain nor with presence of MCI.
Relationship Between Baseline Modifiable Vascular Risk Factors, WMH, and Longitudinal Changes in Cognition
Median duration of follow-up from baseline in the PD and HC groups was 731 and 761 days respectively. Cognitive categorization for the PD group at each follow-up time point is shown in supplementary table 2.
In the HC, VRS was inversely associated with annual rate of change in MoCA and JOLO (supplementary table 3). In the PD group, VRS was inversely associated with annual rate of change in MoCA score (β=−0.040, p=0.008), but not any other cognitive test score or occurrence of MCI at any point in follow-up (table 5).
Table 5.
In the PD group, results of multivariable (a) generalized estimating equations model examining the effects on probability of MCI of (i) VRS score (ii) WMH (root transformed) and (b) linear mixed effects model examining the effect on rate of change in cognitive test score over time of (i) VRS score (ii) WMH on change over time in cognitive scores in the PD group. All models include age and disease duration at baseline, baseline cognitive test score, sex, education, and disease duration as covariates. 95%CI=95% Confidence Interval. HVLT=Hopkins Verbal Learning Task. JOLO=Judgement of Line Orientation. MCI=mild cognitive impairment. LNS=Letter number sequencing. MoCA=Montreal Cognitive Assessment. SDMT=Symbol Digit Modality. VRS=Vascular Risk Score. WM=white matter. WMH=White Matter Hyperintensity.
| (a) | Cognitive Measure |
VRS score (β; 95%CI; p- value) |
WMH Measure (β; 95%CI; p-value) | |||||
|---|---|---|---|---|---|---|---|---|
| Total Brain | Global WM | Frontal WM | Temporal WM | Parietal WM | Occipital WM | |||
| MCI | −0.033; −0.069, 0.003, 0.071 | −0.004; −0.034, 0.025; 0.766 | −0.003, −0.034, 0.027; 0.823 | −0.009, −0.047, 0.028; 0.625 | −0.005, −0.041, 0.031; 0.779 | −0.005, −0.047, 0.038; 0.825 | 0.019, −0.026, 0.063; 0.413 | |
| (b) |
Cognitive Measure |
VRS score (β; 95%CI; p- value) |
WMH Measure (β; 95%CI; p-value) | |||||
| Total Brain | Global WM | Frontal WM | Temporal WM | Parietal WM | Occipital WM | |||
| MoCA | −0.040; −0.069; −0.011; 0.007 | −0.027; −0.054, 0.001; 0.059 | −0.029; −0.058, −0.0001; 0.049 | −0.023; −0.058. 0.012; 0.201 | −0.035; −0.071, 0.0007; 0.054 | −0.019; −0.060, 0.021; 0.346 | −0.041; −0.080, −0.001; 0.043 | |
| HLVT delayed | −0.019; −0.044, 0.006; 0.133 | −0.017; −0.041, 0.006; 0.152 | −0.020; −0.045, 0.005; 0.114 | −0.020; −0.050, 0.009; 0.178 | −0.034; −0.065, −0.003; 0.031 | −0.022; −0.056, 0.013; 0.219 | −0.012; −0.046, 0.021; 0.473 | |
|
HLVT recognition |
−0.003; −0.017, 0.010; 0.622 | −0.002; −0.015, 0.011; 0.785 | −0.002; −0.015, 0.012; 0.783 | −0.004: −0.020. 0.012; 0.648 | 0.004; −0.013, 0.021; 0.640 | −0.004; −0.022, 0.015; 0.694 | −0.005; −0.023, 0.014; 0.636 | |
| JOLO | 0.0008. −0.019, 0.021; 0.936 | −0.017; −0.036, 0.002; 0.073 | −0.018, −0.038, 0.001; 0.067 | −0.017; −0.041. 0.006; 0.151 | −0.018, −0.042, 0.007; 0.155 | −0.025, −0.052, 0.003; 0.075 | −0.023; −0.050, 0.004; 0.089 | |
| LNS | 0.004. −0.018. 0.026; 0.737 | −0.010; −0.031; 0.010; 0.323 | −0.010, −0.031, 0.012; 0.365 | −0.009; −0.035, 0.017; 0.480 | −0.013, −0.040, 0.014; 0.346 | −0.022; −0.052, 0.008; 0.144 | −0.013; −0.043, 0.016; 0.373 | |
| Semantic | 0.033. −0.056. 0.122; 0.465 | −0.004; −0.087, 0.080; 0.930 | −0.011; −0.099, 0.076; 0.801 | 0.052; −0.053, 0.157; 0.330 | −0.049, −0.158, 0.060; 0.382 | 0.011; −0.111, 0.132; 0.863 | −0.073; −0.192, 0.046; 0.231 | |
| Verbal fluency | −0.042; −0.086, 0.003; 0.068 | −0.026; −0.068, 0.016; 0.223 | −0.030: −0.074, 0.014, 0.186 | −0.038: −0.091, 0.015; 0.160 | −0.043; −0.099, 0.012; 0.126 | −0.023; −0.084, 0.038; 0.459 | −0.003; −0.064, 0.58; 0.925 | |
| SDMT | −0.047, −0.130, 0.037; 0.270 | −0.058; −0.138, 0.021; 0.151 | −0.066; −0.149, 0.018; 0.123 | −0.056, −0.156. 0.045; 0.277 | −0.092, −0.197, 0.012, 0.082 | −0.037, −0.152, 0.079, 0.535 | −0.084, −0.198, 0.030, 0.148 | |
There were no significant associations between WMH measures and longitudinal measures of cognition in the HC group (supplementary table 3). Global WMHS was inversely associated with annual rate of change in MoCA score with borderline significance (β=−0.029; 95%CI −0.058, −0.0001; p=0.049), as was occipital WMHS (β=−0.041; 95%CI −0.080, −0.001; p=0.043). Temporal WMHS was inversely associated with annual rate of change in HVLT-delayed recall (β=−0.034; 95%CI −0.065, −0.003; p=0.031)(table 5).
Discussion
In this cohort of patients with early PD we found several relevant associations. First, using a composite measure which accounts for VRFs in a weighted manner, we show significant association between modifiable VRFs and rate of change in a measure of global cognition (MoCA). Second, there was an association between WMH and modifiable VRFs. Third, WMH burden in total white matter, frontal, and temporal regions associated with measures of verbal memory at baseline, and temporal WMH associated with measures of verbal memory longitudinally.
Interpretation of differences between the PD and HC groups warrants caution given the differences in study inclusion criteria (HCs required MoCA>26 to enter the study). With that in mind, our findings may suggest that individuals with early PD have a susceptibility to effects of VRF and WMH above what is seen in those without PD, perhaps due to the synergistic effects of the dual pathology. Our findings require replication in PD and non-PD cohorts matched on cognition at baseline.
The relationships we found between VRS and cognitive impairment/decline in the PD group are consistent with an analysis of the PPMI cohort that examined the association of individual VRFs with cognition, not accounting for anti-hypertensive treatment[10]. The largest study to examine the association between VRFs, cognition, and WMH in early PD included 1759 subjects enrolled in the ProBAND cohort[11]. Cognitive function was examined using a single measure of global cognition (MoCA) and categorized as normal, MCI or dementia. Cognitive dysfunction was associated with both VRFs and WMH burden. In contrast, we did not find association between VRS and baseline MoCA score. These cross-sectional differences may be explained by several factors. First, mean age of the ProBAND cohort was 7 years greater than the PPMI cohort. Second, only a minority of the ProBAND cohort were unmedicated, and dopaminergic therapy may mediate effects of various factors on cognitive function[25]. Third, a smaller proportion of individuals in PPMI had MCI or dementia at baseline as defined in the ProBAND study[11]. Finally, our smaller sample size may have prevented us from detecting weaker associations in our cohort..
Regarding the longitudinal changes in cognition, we found a significant inverse association between VRS and rate of change in MoCA. Importantly, to our knowledge, VRFs are the only possible modifiable risk factor for cognitive decline in PD identified to date. If our findings are confirmed in properly designed epidemiologic and interventional studies, the care of individuals with PD may come to incorporate screening and aggressive VRF modification to prevent cognitive decline. In addition, examining whether interventions (such as exercise[26]) that improve vascular cognitive impairment in other patient populations can also improve cognition in PD will be key.
Imaging changes in PD are of interest as objective measures for both disease characterization and as potential endpoints in clinical trials. In the general population, VRFs are associated with significant increases in WMH over time[27]. Thus, WMH could be a surrogate for the long-term effects of VRFs on the brain. If indeed VRFs are a modifiable risk factor for cognitive dysfunction in PD, having an objective measure of their impact as an endpoint to clinical trials could save time and resources required to follow patients longitudinally with cognitive function as an outcome. We found significant, albeit relatively weak, associations between VRS and WMH in our cohort. Other potential contributors to WMH include VRFs unmeasured in PPMI (such as smoking and arrhythmia). However another consideration is that in PD, WMH could represent pathology unrelated to ischemic small vessel pathology, including amyloid angiopathy and/or other components reflecting an underlying neurodegenerative process[28]. Additional studies are needed to determine if WMH are a robust proxy of VRFs in PD and, more importantly, that interventions to reduce WMH in PD have a meaningful clinical impact.
In the non-PD population, WMH are associated with multidomain cognitive impairment including in executive function and delayed memory recall[29]. There are limited studies examining WMH in newly diagnosed PD. Malek et al utilized a binary measure of white matter disease, reporting worse cognition in those with leukoaraiosis[25]. Two other reports examining WMH in early PD did not find associations between WMH and cognitive function after adjusting for age[30,31]. On the other hand, the relationship between VRS, WMH, and cognition has been widely studied in more advanced PD cohorts. In a study comparing PD patients with and without MCI, the MCI group had a greater prevalence of VRFs and greater WMH burden globally and in frontal, parietal, and occipital regions. WMH associated with MCI independent of VRFs[32]. WMH were associated with abnormalities in executive function, memory, and language even in those without MCI, and again even after adjusting for VRFs. Other studies examining associations between WMH and cognitive function have reported similar findings of predominant involvement of executive function and attention in PD samples of wide ranges of disease duration[33]. In contrast, our main finding was an association between WMH and impaired verbal memory.
Most studies examining VRFs and cognition in PD have looked at individual VRFs separately. In contrast, we utilized a composite VRF score, which applies weights according to the strength of association between VRFs and cardiovascular disease, accounting for age, sex, and anti-hypertensive treatment. Furthermore, we used a quantitative measure of WMH with regional subscores. These are both seen as major strengths of this study. However, several limitations to this study warrant mention. First, autonomic dysfunction occurs in PD, and it is possible that the pathophysiology and consequences of hypertension in early PD may be different from that in the Framingham cohort. However, in early PD significant dysautonomia affecting BP is unlikely. Second, VRFs were self-reported and may be under-estimated. Third, we could not include smoking in our analysis as smoking history was not obtained in PPMI. In the PD population the association between smoking and cognitive dysfunction is not clear. Some studies show worse cognition in PD patients with a history of smoking[34] whereas other studies have not found a relationship between smoking and various PD disease manifestations and WMH[32] including cognition[11]. Concerns regarding omission of this VRF in our study are possibly mitigated by the finding that individuals with PD are less likely to smoke compared to the general population[35]. Another VRF not accounted for in the VRS is hyperlipidemia. A cross sectional analysis in the PPMI cohort did not find an association between hyperlipidemia and cognition[10], but we cannot exclude an effect longitudinally. However, we otherwise view the lack of requirements of lipid measurements in the mFRS as an advantage to allowing easier applicability for in-clinic VRF assessment. Fourth, while the neuropsychological battery administered in PPMI assesses most cognitive domains, a test for language is not administered, and it does not allow categorization of cognition at the highest level of confidence (PD-MCI level II)[36]. This may have limited limited our ability to detect additional relationships between VRF, cognition, and WMH. We did not find an association between MCI and VRS or WMH but the relatively small number with MCI and the fluctuation of MCI over time in early PD requires these results be interpreted with caution. Unfortunately we were not able to examine the risk of dementia in relation to VRS or WMH in this cohort due to missing data.
The association between modifiable VRFs and cognitive decline in early PD lends cautious optimism to a potential avenue through which to prevent or delay dementia in PD. Having an objective surrogate marker of the effects of vascular disease on cognition in PD is needed to allow for efficient clinical trials. We have demonstrated that VRS and WMH are correlated in early PD and that WMH are associated with greater rate of decline in measures of verbal memory. Future work will be needed to demonstrate that interventions to treat VRFs in PD prevent increases in WMH and that this has a clinically meaningful impact on course of cognitive decline.
Supplementary Material
Acknowledgments
Funding Sources:
The MRI analysis component of this work was partially funded by grants R01 AG054409 and R01 AG014971.
Data used in the preparation of this article were obtained from the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org. PPMI – a public-private partnership – is funded by the Michael J. Fox Foundation for Parkinson’s Research and funding partners, including Abbvie, Avid, Biogen, Biolegend, Bristol-Meyers Squibb, GE Healthcare, Genetech, GSK, Lilly, Lundbeck, Merck, Meso Scale Discovery, Pfizer, Piramal, Roche, Sanofi Genzyme, Servier, Takeda, Teva, UCB, and Golub Capital.
Footnotes
Conflicts of interest: none of the authors report conflict of interest related to this work.
Reference list
- [1].Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord 2008. April 30;23(6):837–844. [DOI] [PubMed] [Google Scholar]
- [2].Aarsland D, Bronnick K, Williams-Gray C, Weintraub D, Marder K, Kulisevsky J, et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology 2010. September 21;75(12):1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Anang JB, Gagnon JF, Bertrand JA, Romenets SR, Latreille V, Panisset M, et al. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology 2014. September 30;83(14):1253–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Caspell-Garcia C, Simuni T, Tosun-Turgut D, Wu IW, Zhang Y, Nalls M, et al. Multiple modality biomarker prediction of cognitive impairment in prospectively followed de novo Parkinson disease. PLoS One 2017. May 17;12(5):e0175674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Collins LM, Williams-Gray CH. The Genetic Basis of Cognitive Impairment and Dementia in Parkinson's Disease. Front Psychiatry 2016. May 20;7:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Knopman D, Boland LL, Mosley T, Howard G, Liao D, Szklo M, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001. January 9;56(1):42–48. [DOI] [PubMed] [Google Scholar]
- [7].Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet 2017. July 19;390(10113):2673–2734. [DOI] [PubMed] [Google Scholar]
- [8].Pilotto A, Turrone R, Liepelt-Scarfone I, Bianchi M, Poli L, Borroni B, et al. Vascular Risk Factors and Cognition in Parkinson's Disease. J Alzheimers Dis 2016. February 6;51(2):563–570. [DOI] [PubMed] [Google Scholar]
- [9].Jones JD, Jacobson C, Murphy M, Price C, Okun MS, Bowers D. Influence of hypertension on neurocognitive domains in nondemented Parkinson's disease patients. Parkinsons Dis 2014;2014:507529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Doiron M, Langlois M, Dupre N, Simard M. The influence of vascular risk factors on cognitive function in early Parkinson's disease. Int J Geriatr Psychiatry 2017. May 16. [DOI] [PubMed] [Google Scholar]
- [11].Malek N, Lawton MA, Swallow DM, Grosset KA, Marrinan SL, Bajaj N, et al. Vascular disease and vascular risk factors in relation to motor features and cognition in early Parkinson's disease. Mov Disord 2016. October;31(10):1518–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Erten-Lyons D, Woltjer R, Kaye J, Mattek N, Dodge HH, Green S, et al. Neuropathologic basis of white matter hyperintensity accumulation with advanced age. Neurology 2013. September 10;81(11):977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dong C, Nabizadeh N, Caunca M, Cheung YK, Rundek T, Elkind MS, et al. Cognitive correlates of white matter lesion load and brain atrophy: the Northern Manhattan Study. Neurology 2015. August 4;85(5):441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bilello M, Doshi J, Nabavizadeh SA, Toledo JB, Erus G, Xie SX, et al. Correlating Cognitive Decline with White Matter Lesion and Brain Atrophy Magnetic Resonance Imaging Measurements in Alzheimer's Disease. J Alzheimers Dis 2015;48(4):987–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan study. Stroke 2008. October;39(10):2712–2719. [DOI] [PubMed] [Google Scholar]
- [16].Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005. April;53(4):695–699. [DOI] [PubMed] [Google Scholar]
- [17].Smith A Symbol digit modalities test: Manual. Los Angeles: Western Psychological Services; 1982. [Google Scholar]
- [18].Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol 1999. February;14(2):167–177. [PubMed] [Google Scholar]
- [19].Gladsjo JA, Schuman CC, Evans JD, Peavy GM, Miller SW, Heaton RK. Norms for letter and category fluency: demographic corrections for age, education, and ethnicity. Assessment 1999. June;6(2):147–178. [DOI] [PubMed] [Google Scholar]
- [20].Brandt J, Benedict RHB. The Hopkins Verbal Learning Test-Revised. Odessa, FL: Psychological Assessment Reources; 2001. [Google Scholar]
- [21].Benton AL, Varney NR, Hamsher KD. Visuospatial judgment. A clinical test. Arch Neurol 1978. June;35(6):364–367. [DOI] [PubMed] [Google Scholar]
- [22].D'Agostino RB S, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008. February 12;117(6):743–753. [DOI] [PubMed] [Google Scholar]
- [23].Doshi J, Erus G, Ou Y, Resnick SM, Gur RC, Gur RE, et al. MUSE: MUlti-atlas region Segmentation utilizing Ensembles of registration algorithms and parameters, and locally optimal atlas selection. Neuroimage 2016. February 15;127:186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zacharaki EI, Kanterakis S, Bryan RN, Davatzikos C. Measuring brain lesion progression with a supervised tissue classification system. Med Image Comput Comput Assist Interv 2008;11(Pt 1):620–627. [DOI] [PubMed] [Google Scholar]
- [25].Poletti M, Bonuccelli U. Acute and chronic cognitive effects of levodopa and dopamine agonists on patients with Parkinson's disease: a review. Ther Adv Psychopharmacol 2013. April;3(2):101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liu-Ambrose T, Best JR, Davis JC, Eng JJ, Lee PE, Jacova C, et al. Aerobic exercise and vascular cognitive impairment: A randomized controlled trial. Neurology 2016. November 15;87(20):2082–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gouw AA, van der Flier WM, Fazekas F, van Straaten EC, Pantoni L, Poggesi A, et al. Progression of white matter hyperintensities and incidence of new lacunes over a 3-year period: the Leukoaraiosis and Disability study. Stroke 2008. May;39(5):1414–1420. [DOI] [PubMed] [Google Scholar]
- [28].McAleese KE, Walker L, Graham S, Moya ELJ, Johnson M, Erskine D, et al. Parietal white matter lesions in Alzheimer's disease are associated with cortical neurodegenerative pathology, but not with small vessel disease. Acta Neuropathol 2017. June 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jokinen H, Kalska H, Mantyla R, Pohjasvaara T, Ylikoski R, Hietanen M, et al. Cognitive profile of subcortical ischaemic vascular disease. J Neurol Neurosurg Psychiatry 2006. January;77(1):28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dalaker TO, Larsen JP, Bergsland N, Beyer MK, Alves G, Dwyer MG, et al. Brain atrophy and white matter hyperintensities in early Parkinson's disease(a). Mov Disord 2009. November 15;24(15):2233–2241. [DOI] [PubMed] [Google Scholar]
- [31].Dalaker TO, Larsen JP, Dwyer MG, Aarsland D, Beyer MK, Alves G, et al. White matter hyperintensities do not impact cognitive function in patients with newly diagnosed Parkinson's disease. Neuroimage 2009. October 1;47(4):2083–2089. [DOI] [PubMed] [Google Scholar]
- [32].Kandiah N, Mak E, Ng A, Huang S, Au WL, Sitoh YY, et al. Cerebral white matter hyperintensity in Parkinson's disease: a major risk factor for mild cognitive impairment. Parkinsonism Relat Disord 2013. July;19(7):680–683. [DOI] [PubMed] [Google Scholar]
- [33].Vesely B, Antonini A, Rektor I. The contribution of white matter lesions to Parkinson's disease motor and gait symptoms: a critical review of the literature. J Neural Transm (Vienna) 2016. March;123(3):241–250. [DOI] [PubMed] [Google Scholar]
- [34].Weisskopf MG, Grodstein F, Ascherio A. Smoking and cognitive function in Parkinson's disease. Mov Disord 2007. April 15;22(5):660–665. [DOI] [PubMed] [Google Scholar]
- [35].Allam MF, Campbell MJ, Hofman A, Del Castillo AS, Fernandez-Crehuet Navajas R. Smoking and Parkinson's disease: systematic review of prospective studies. Mov Disord 2004. June;19(6):614–621. [DOI] [PubMed] [Google Scholar]
- [36].Litvan I, Goldman JG, Troster AI, Schmand BA, Weintraub D, Petersen RC, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord 2012. March;27(3):349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.