Abstract
The purpose of this article is to highlight some areas of research with spores of bacteria of Firmicute species in which the methodology too commonly used is not optimal and generates misleading results. As a consequence, conclusions drawn from data obtained are often flawed or not appropriate. Topics covered in the article include the following: 1) the importance of using well purified bacterial spores in studies on spore resistance, composition, killing, disinfection and germination; 2) methods for obtaining good purification of spores of various species; 3) appropriate experimental approaches to determine mechanisms of spore resistance and spore killing by a variety of agents, as well as known mechanisms of spore resistance and killing; 4) common errors made in drawing conclusions about spore killing by various agents, including failure to neutralize chemical agents before plating for viable spore enumeration, and equating correlations between changes in spore properties accompanying spore killing with causation. It is hoped that a consideration of these topics will improve the quality of spore research going forward.
Keywords: Bacillus, Spores, Bacterial spores, Disinfection, Resistance
INTRODUCTION
Notably, just a cursory analysis of publications cited in PubMed on bacterial spores from 2015 to the present indicates that of ~ 360,000 papers on bacteria, ~ 1% focus at least peripherally on spores of bacteria of Bacillales or Clostridiales species. This research covers many aspects of spores, including their formation, composition, properties, germination, killing, resistance, disinfection and roles in food spoilage and disease, and has been supported by multiple governmental agencies in the USA including the National Institutes of Health, the National Science Foundation, the Department of Agriculture, the National Aeronautics and Space Administration and a variety of Defense Department agencies. There is also significant government support for spore research in many other countries. Worldwide, the food industry has also carried out much spore research, in particular on how to detect and kill spores and validate spore killing.
Research on the spores of bacteria of Bacillales and Clostridiales species and their close relatives has been ongoing for many, many years. This work has been driven by a number of factors including: i) the elegant developmental system of sporulation, often triggered by nutrient starvation, that leads to spore formation inside a mother cell, followed by mother cell lysis releasing the free spore (Setlow and Johnson 2012; Tan and Ramamurthi 2014); ii) the rapid return to life of spores in the process of spore germination triggered by low molecular weight compounds, often nutrients, whose presence signals that the environment is suitable for cell growth (Setlow et al. 2017); iii) spores’ extreme dormancy and resistance, generally much greater than for any other bacterial form, and the novel mechanisms that establish and maintain these spore properties (Setlow 1994; Setlow 2016); iv) the use of spores of Bacillus species to monitor organismal survival in the harsh environment of outer space, and to allow development of mechanisms to insure protection of other planets from accidental contamination by microbes from earth (Nicholson et al. 2000; Horneck et al. 2012); v) the roles of spores of many species as vectors for food spoilage and food borne disease (Setlow and Johnson 2012); and vi) because of their extreme resistance properties, spores of some species are vectors in some serious human diseases including botulism caused by Clostridium botulinum, gas gangrene and food poisoning caused by Clostridium perfringens, severe and often fatal diarrhea caused by Clostridiodes difficile, and food poisoning caused by Bacillus cereus and Bacillus licheniformis (Malozzi et al. 2010; Setlow and Johnson 2012). The recent emergence of spores of Bacillus anthracis as a major potential agent for biowarfare and bioterrorism by acting as a vector for the potentially fatal human disease anthrax has also stimulated research on spores of this organism and its close relatives (Dias et al. 2010).
I first became aware of bacterial spores in the summer of 1968 when I took up a postdoctoral position in Arthur Kornberg’s laboratory at the Stanford University School of Medicine in California. Dr. Kornberg was well known at that time as the discoverer of the first DNA polymerase which was identified in and purified from Escherichia coli in his laboratory then at Washington University in St. Louis, Missouri USA. This work led to Dr. Kornberg’s award of the Nobel Prize in 1959. While his work on DNA polymerase continued after Dr. Kornberg moved to the Stanford University School of Medicine, it is less well known that from the early 1960s to 1972 about half of the Kornberg lab worked on various aspects of spores of Bacillus species. While I knew nothing about spores when I joined the Kornberg laboratory, and not much more about microbiology, I decided that I would join the spore half of the Kornberg laboratory - this was one of the two wisest decisions I ever made in my life, and 50 years later I am still working on spores. One of the first things I had to do in the Kornberg lab was to prepare spores of Bacillus megaterium, which was the spore former of choice in the laboratory at that time. In beginning to prepare these spores, it was emphasized to me that it was absolutely essential to purify the spores well so that one would have spore preparations that would give reproducible results when experiments were repeated in either the Kornberg laboratory or elsewhere.
SPORE PURIFICATION
The emphasis in the Kornberg lab on spore purity may have stemmed from a phrase often associated with Arthur Kornberg based on his work in enzymology, in particular on DNA polymerase: “Don’t waste clean thinking on dirty enzymes!”. At the time, substituting the word “spores” for “enzymes” was great advice, and it still is! Consequently, my lab has constantly strived to be sure that all spores used in our work are as pure as possible, not just microscopically pure, but free of contaminating macromolecules. This emphasis on spore purity is somewhat foreign to microbiologists who are concerned primarily with having cultures with only one organism – in other words “pure cultures”. Consequently, when isolating growing or stationary cells from such pure cultures there will be no concerns about the purity of these cells, as long as culture medium is washed away by some osmotically-stabilizing medium. However, the situation is much different when obtaining spores. In particular, a sporulating culture, even if it is well sporulated with the great majority of cell forms observed by phase contrast microscopy being bright dormant spores, the culture will contain some significant percentage, hopefully small, of a variety of other cell forms, while purified spores will not (Fig. 1A,B). These contaminating cell forms include spores still retained in the mother cell, cells that did not sporulate and perhaps even some germinated spores. In addition, the lysis of the mother cell in which the spores are formed releases large amounts of nucleic acid, cell wall material and intracellular protein into the culture, some of which can readily adhere to the dormant spores. Indeed, when crude spore preparations are examined, they are commonly seen to contain a large amount of cellular debris (Fig. 1A). Thus, to obtain truly pure spores, all these contaminants must be removed as completely as possible. Note, however, that spore purification is much, much easier if sporulation is extremely efficient, with a minimal level of unsporulated cells and spores still in sporangia. The precise conditions to get good sporulation of the many spore formers that are studied vary significantly, but for species/strains commonly used, good sporulation conditions can be found in the literature. Importantly, with cultures that have sporulated quite poorly, spore purification can be difficult and give minimal yields of highly purified spores. Unfortunately, cataloging good sporulation conditions for myriads of spore formers is beyond the scope of this article.
Figure 1A,B.
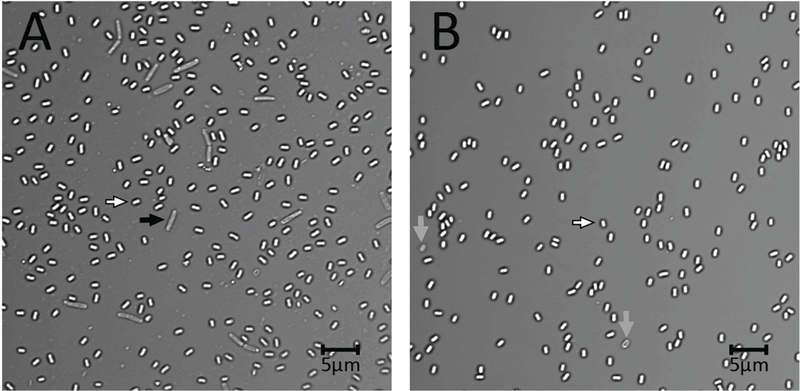
Photomicrograph of crude (A) and purified (B) B. subtilis spores. B. subtilis PS533 (Setlow and Setlow 1996) was sporulated on three 2xSG sporulation medium plates (Nicholson and Setlow 1990; Paidhungat et al. 2000) at 37°C. After 2 d spores were scraped from plates, suspended in ~ 30 ml cold water, sonicated for 3 min, centrifuged, the pellet suspended in 30 ml cold water and an aliquot photographed on an agarose-coated slide as described previously (Setlow et al. 2016; Li et al. 2017) either (A) immediately or (B) after spores were purified. For purification, the final suspension described above was sonicated for 3 min, centrifuged for 10 min at 17,000xg, suspended in 30 ml cold water with 3 min of sonication of the suspension followed by centrifugation and resuspension. The suspended spores at ~ 4°C were purified over 3 d by rounds of centrifugation, resuspension in cold water, sonication and centrifugation, ~ 3 rounds per d. In the last few d of this regimen, debris on the spore pellet surface was washed away by a gently spray of water. The scale bar in the figure is 5 microns, and white, grey and black arrows denote free dormant spores, germinated or lysed spores, and cells that did not sporulate, respectively. Note the debris in the background of panel A.
Sad to say, the “mantra” noted above of using pure spores in experiments studying spore properties is often not followed. Indeed, in much work, sporulating cultures are only centrifuged, washed with water a few times and then given a heat shock at 75–80°C for 30 min to kill contaminating sporulating or growing cells but not the highly resistant spores. Sometimes, but by no means always, the heated spore preparation is then washed with water a few more times by centrifugation. This overall procedure will certainly give spore preparations in which the only viable organisms are spores, and such preparations may appear to contain ≥ 95% dormant spores by phase contrast microscopy. However, spores “purified” in this fashion will most likely contain dead cells killed by the heat treatment as well as significant amounts of cell debris that isn’t seen easily, if at all, in light microscopy. Importantly, this contaminating material can potentially affect spore properties. For example, such contaminants can result in misleading analyses of spore composition including proteins, peptidoglycan and nucleic acids. Analyses of spore resistance can also be compromised by contaminants in spore preparations because of UV shielding by nucleic acids or protection of spores against oxidizing agents, much as organic matter protects growing cells against such agents (Russell 1999; Maillard 2011). The presence of such contaminants in spore preparations is shown easily if crude spore preparations are centrifuged in a glass centrifuge tube such that spores tend to pellet at the bottom, while debris is on the surface of the spore pellet (Fig. 2A,B), and this debris include intact cells, cell fragments and aggregated macromolecules.
Fig. 2A,B.
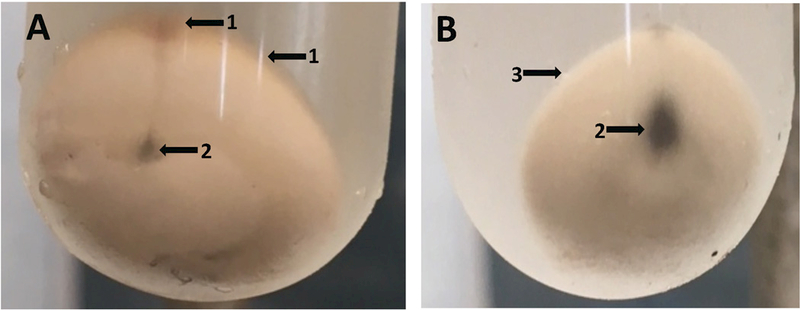
Photographs of minimally (A) and fully (B) purified B. subtilis spore pellets. Three 2xSG medium sporulation plates as described in the legend of Fig. 1 were incubated at 37°C for 2 d, at which time the spores were scraped from the plates and suspended in ~ 30 ml of cold water. The cell forms in this suspension observed by bright field microscopy were ≥ 90% free spores as seen in Fig. 1A. The initial suspension was centrifuged for 10 min at 17,000xg, washed once by resuspension in cold water with 3 min of sonication of the suspension followed by centrifugation and resuspension. In tube A, the latter suspension was centrifuged in a 30 ml glass tube for 30 min at 6700xg and the pellet was photographed. The suspended spores were then further purified over a period of 3 d as described in the legend to Fig. 1. Finally, in B the purified spore suspension was centrifuged as described above for tube A and the pellet was photographed. Arrows labeled 1 in tube A indicate debris on the surface of the spore pellet, and the arrow labeled 2 in tube B shows that the surface of the extensively purified spore pellet is free from debris. The black material indicated by arrows labeled 3 in tubes A and B is small amounts of insoluble grit in sporulation media. This can be removed from spore suspensions, by allowing it to settle out from suspensions, as the grit settles out much faster that do spores.
As a consequence of concerns about effects of impurities in spore preparations influencing spore properties, removal of the contaminating debris is crucial to obtain pure spores, such that results with these spores can be readily reproduced in other laboratories. There are a variety of methods for removal of this type of debris, ranging from incubation with a lytic enzyme such as lysozyme to digest peptidoglycan and/or nucleases to digest DNA and RNA. Sonication can also be used to break up debris to facilitate its autolysis and removal, although this is not recommended for spores that have an outermost exosporium layer (Fig. 3), which for spores of some species, albeit not all, can be damaged or even removed by sonication (Thompson et al. 2011; Escobar-Cortes et al. 2013). A simpler alternative is to wash harvested spores several times with water to remove soluble medium components, and leave the suspended spore preparation in the cold room over a period of several weeks to allow enzymatic autolysis by sporulating cell enzymes of contaminating macromolecules along with intermittent sonication (if not contraindicated) and centrifugation. (Note that if impure spore preparations are heat-treated as described above, this will inactivate autolytic enzymes in spore preparations!) In this simple method, the debris on the surface of the spore pellet will slowly disappear, and this disappearance can be hastened by flushing away surface material with a gentle spray of water. This process can be continued for several weeks until the spore pellet is uniform, and, importantly, streams away evenly when a gentle water spray is applied to the spore pellet. Overall, while this procedure sounds like a bit of an art form, it is simple to do and easy to learn, is inexpensive and gives extremely pure spores. The one proviso is that there must be good sporulation to begin with, perhaps > 50%, for this procedure to work effectively. However, with good sporulation this procedure works well for spores of Bacillus subtilis, B. megaterium, Bacillus atrophaeus and Geobacillus stearothermophilus, among others. There can, however, be problems with spores that have a very hydrophobic exterior exosporium layer, such as those of B. anthracis and its relatives such as Bacillus cereus and Bacillus thuringiensis. This hydrophobic spore surface causes these spores to clump and these clumps can trap air such that they float, making the spores difficult to pellet upon centrifugation. One way to deal with this problem is to add low levels of a mild detergent such as an antifoaming agent or Tween-20. Small amounts (≤ 0.1%) of these compounds can completely disperse spores of B. anthracis and its close relatives giving suspensions of single spores that are easily studied.
Figure 3.
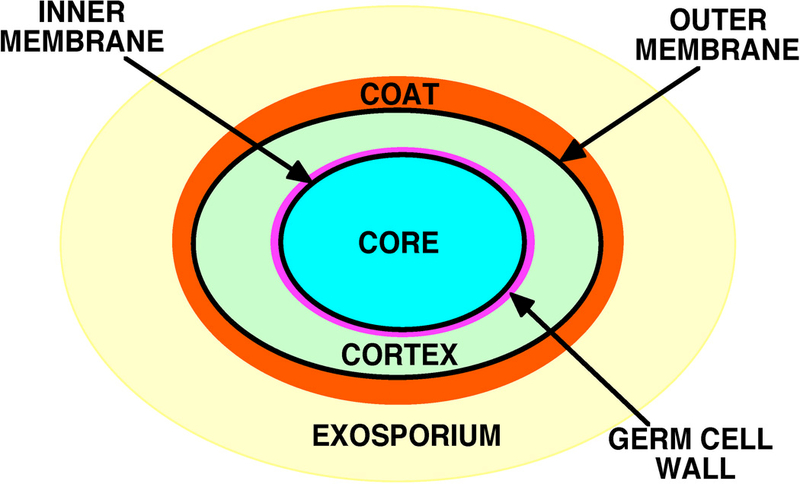
Schematic structure of a bacterial spore. Note that the exosporium is not present in spores of all species, and that spores of at least some species that lack an outermost exosporium have an outer layer termed the crust (Setlow and Johnson 2012; Tan and Ramamurthi 2014).
A final method for even further purification of moderate amounts of spores (10–30 mg dry wt) takes advantage of the extremely high wet density of the dormant spore core due to the core’s very low water content (Gerhardt and Marquis 1989). The water content of spores suspended in water is generally 25–40% of wet wt, compared to values of ~ 80% for a growing cell protoplast. In this method, spores in 200–300 μl of 20% Histodenz are layered on ~1.5–2 ml of a high-density solution such as 50% Histodenz (Sigma Chemical Company, St. Louis, MO USA) in a microcentrifuge tube and the samples are then centrifuged at top speed for 10 min at 4 or 23°C. Under these conditions, dormant spores of most species pellet while impurities float (Fig. 4). However, spores of some species will need to be layered on slightly less dense Histodenz solutions, if these spores’ core wet density is lower than that of spores that pellet with 50% Histodenz. Note that Histodenz is expensive, so centrifugation over Histodenz should be reserved for a final step in spore purification. Finally, the pelleted spores are washed with cold water to remove Histodenz, suspended in cold water at an optical density at 600 nm (OD600 nm) of 10–20 and stored at 4°C protected from light or frozen at −20°C or even −80°C. These spores are now as pure as can readily be prepared, and different labs should be able to produce pure spores with the same properties by these procedures if the same sporulation procedures are used. This should be the norm for preparing spores for further analysis, not the exception! Note that most of the discussion above has focused on spores of Bacillales. However, methods noted also give good purification of spores of Clostridiales species as well (Fimlaid et al. 2015; Donnelly et al. 2017), and using pure spores in research with Clostridiales spores is also essential.
Figure 4A,B.

Photographs of (A) crude and (B) purified B. subtilis spores centrifuged on Histodenz. Spores prepared as in the legend to Fig. 1 were suspended in ~ 30 ml of cold water, , centrifuged, and washed once with 30 ml of cold water. 1 ml of the latter suspension as well as comparable amounts of spores purified as described in the legend to Fig. 1 were pelleted. The pellets were suspended in 300 μl of 20% Histodenz, which was layered on 1.5 ml of 50% Histodenz in 2 ml Eppendorf tubes; these were centrifuged for 5 min and photographed. Arrow 1 denotes the meniscus between the 20 and 50% Histodenz, arrow 2 denotes the pelleted spores.
SPORE PROPERTIES AND MAJOR QUESTIONS ABOUT SPORE RESISTANCE AND KILLING
There are many spore properties that can be measured, including the: i) content of dipicolinic acid (DPA) (Fig. 5) present as a 1:1 chelate with divalent cations, most generally Ca2+, in the spore core (CaDPA) and comprising ~ 25% of core dry wt; ii) proteins present in various spore layers (Fig. 3) such as the exosporium, coat, membranes and core; iii) the peptidoglycan composition of the cortex and germ cell wall layers; iv) permeability of and proteins in spores’ two membranes, the outer and inner forespore membranes; v) levels of spore core small molecules in addition to CaDPA, including free nucleotides, amino acids and others; vi) spore nucleic acids, in particular the mRNA species that are found in spores (Segev et al. 2012; Bergman et al. 2006; Bettegowda et al. 2006; Keijser et al. 2007); vii) return of spores to life in the processes of germination followed by outgrowth; and viii) spore resistance to and killing by a host of known or potential sporicides, including high temperatures (wet or dry), high pressures, vacuum (in particular outer space vacuum), high fluxes of visible, UV or γ-radiation, and a host of toxic chemicals, including acids, bases, alkylating agents and oxidizing agents. Major questions that are crucial to understand the effects of any of these agents in spore killing are: 1) what are the mechanisms of spore resistance to sporicides; 2) are spores that appear killed by various sporicides truly dead, or just incapable of germinating; and 3) what are the mechanisms whereby spores are truly killed by various sporicides? Each of these major questions will be dealt with separately.
Figure 5.
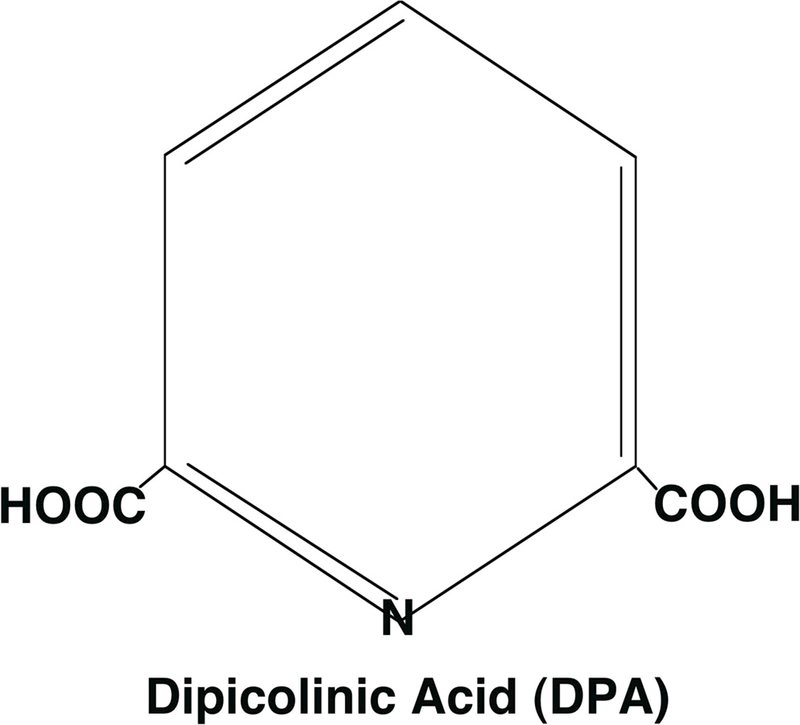
Pyridine-2,6-dicarboxylic acid, or dipicolinic acid (DPA). At the pH inside the spore core of ~ 6.5, the two carboxyl groups will be ionized and are chelated to divalent cations.
1. WHAT ARE THE MECHANISMS OF SPORE RESISTANCE TO POTENTIAL SPORICIDES?
A major area of spore research concerns spores’ extreme resistance to all manner of harsh treatments, as listed above. Importantly, as was discussed under SPORE PURIFICATION above, spore purity can have a major influence on the observed spore properties for a variety of reasons. Thus, impurities such as macromolecules released from lysing mother cells in sporulation could influence spore resistance properties by detoxifying chemical agents. This can be catalytically by an enzyme such as catalase that destroys hydrogen peroxide (note that sporulating cells often have high levels of catalase), although heat treatment of spore preparations can minimize this possibility. However, contaminating proteins can also react non-enzymatically with and detoxify chemicals such as oxidizing agents (e.g. – hypochlorite, chlorine dioxide), thus reducing these chemicals effectiveness (Russell 1999). Results obtained in different laboratories on the efficacy of such agents may then differ, if the purity of the spores used also differs between laboratories. Similarly, if spores adsorb nucleic acids, these will absorb UV radiation and again could result in decreased efficacy of this agent in spore killing. Indeed, this was shown to be the reason for anomalously high UV resistance of spores of B. anthracis (Nicholson and Galeano 2003), as with proper purification of the B. anthracis spores, their UV resistance fell significantly, and to levels observed for spores of other commonly used Bacillus species including B. subtilis. Impure spores can also often aggregate, and this plays havoc with spore enumeration by plate culture; one large aggregate behaves as a single spore on plate culture, but all individual spores in an aggregate need to be killed for the aggregate to appear dead. Spores in the center of an aggregate may also be protected against agents such as heat, radiation and chemicals. Since aggregate formation can have huge effects on measurements of killing, elimination of spore aggregates is an important part of spore purification; it is also crucial to be sure that the killing treatment used does not itself generate aggregates. To emphasize again, spore purity is of paramount importance in measurements of all spores’ resistance properties.
Work primarily with B. subtilis spores has identified five major spore resistance mechanisms (Setlow 2006, 2013, 2016; Leggett et al. 2012), including the: 1) spores’ low core water content which is important in resistance to wet heat and perhaps reactive oxygen species generated in the spore core; 2) high levels of CaDPA in the spore core which are important in spore resistance to wet heat and radiation; 3) spore DNA saturation with novel spore-specific proteins, the α/β-type small, acid-soluble spore proteins, SASP, which protect spore DNA (and thus spore viability) against wet and dry heat, radiation, high vacuum and a host of DNA damaging chemicals; 4) low permeability of spores’ inner membrane which greatly restricts access of many toxic hydrophilic compounds, in particular genotoxic chemicals, to the spore core; and 5) spore outer layers, in particular the large proteinaceous coat layer, which can restrict access of large molecules to more inner spore layers, and non-specifically react with and detoxify many reactive chemicals, in particular oxidizing agents. Determining the mechanism(s) whereby spores resist inactivation by a new sporicidal agent is easiest with B. subtilis spores, as there are isogenic mutants available that lack most coat proteins, most α/β-type SASP, or much DNA repair capacity, including missing individual DNA repair pathways (Young and Setlow 2003; Djouiai et al. 2018). There are also B. subtilis mutants that produce stable spores with elevated core water content, either because of a modified spore cortex or the complete absence of DPA (Popham et al. 1995; Magge et al. 2008). Comparable mutants are also available in a few other spore formers, such as α/β-type SASP-less C. perfringens spores and B. anthracis spores deficient in various outer layer proteins (Giorno et al. 2007; Paredes-Sabja et al. 2008; Bozue et al. 2016). However, B. subtilis is by far the easiest of the spore formers in which to carry out genetic manipulation, and many strains of this species sporulate well, and its spores are easy to purify. Importantly, studies with spores of species other than B. subtilis have indicated that spore coats and α/β-type SASP play the same roles in resistance of spores of these other species that they do in B. subtilis spores (Paredes-Sabja et al. 2008; Setlow et al. 2014; Li et al. 2017). However, whether mechanisms of the resistance of spores of all species will be similar to those of B. subtilis spores is, of course, unknown.
Other assistance in determining mechanisms of spore resistance, in particular with spores from species other than B. subtilis, are methods for chemical decoating of spores using detergents and dithiothreitol at high pHs and temperatures (Bagyan et al. 1998). Such decoating treatments, while harsh, do not eliminate spore viability and these decoated spores generally exhibit greatly increased sensitivity to a number of toxic and reactive chemicals, but with only small decreases in spore resistance to heat or high pressures. Preparation of spores at low and high temperatures can also generate spores with higher and lower core water content, respectively, and spore wet heat resistance is inversely correlated with core water content (Gerhardt and Marquis 1989; Melly et al. 2002). Spore inner membrane permeability also increases in spores made at low compared to high temperatures (Cortezzo et al. 2004, 2005). Thus, analysis of the resistance properties of spores made at different temperatures may also give insight into mechanisms of spore resistance.
2. ARE SPORES THAT APPEAR KILLED BY SPORICIDES DEAD, OR JUST INCAPABLE OF GERMINATING?
Obviously, deciding whether spores treated with a possible sporicide are really dead, or only slow to form colonies, perhaps because of a germination defect, is extremely important. If the treated spores are only defective in germination, then they are potentially alive and may be revived if they are given assistance in germination and then exert possible deleterious effects on foods or people. Indeed, there are agents that behave in this manner, one being very high pH which can inactivate a crucial enzyme(s) in spores’ outer layers that is/are essential for the completion of spore germination by degrading the large peptidoglycan cortex (Setlow et al. 2017). Thus, spores of at least some Bacillus and Clostridium species incubated at pH ≥ 13 and then brought to pH 7 and plated appear dead (Setlow et al. 2002; Paredes-Sabja et al. 2009a,b). However, they are actually not dead, and can readily be recovered if supplied with an exogenous peptidoglycan hydrolase such as lysozyme. Thus, it is crucial with a new potential sporicide to show that this agent does not simply abolish spore germination. Obviously, if spores apparently killed by this new agent can complete germination well, then this agent does not kill spores by inactivation of an essential germination protein. However, if spores treated with a putative sporicide lose the ability to germinate before or in parallel with apparent spore killing, then attempts should be made to artificially germinate the treated spores, for example with lysozyme or the almost universal spore germinant CaDPA (Setlow et al. 2017). If these agents do germinate the treated spores then spore viability should again be measured. Thankfully, spores killed by most agents lose the ability to germinate only long after spores have already been killed by other mechanisms (Young and Setlow 2003; Coleman et al. 2007, 2010; Huesca-Espitia et al. 2016; Setlow et al. 2016; Li et al. 2017).
3. WHAT ARE THE MECHANISMS OF SPORE KILLING BY SPORICIDES?
An important step in measurement of spore killing is to stop spore killing before the killing is assessed by measurement of colony forming units (CFU) on plates. This is crucial, because when spores are applied to nutrient plates for CFU determinations, spores will germinate, and germinated spores lose their resistance properties and have the lower resistance of growing cells. Thus, treatments such as high temperatures, high radiation flux, high pressure, high vacuum, and chemicals must be halted before determination of treated spore viability (Russell 1999). This is easy with most of these treatments, as radiation, pressure or vacuum sources can be turned off, spores heat-treated in water can be diluted in cold water and spores exposed to dry heat can be chilled on ice. However, for spores treated with chemicals, the chemicals must be neutralized before plating. Indeed, some chemical agents (e.g. – I2) can adsorb to spores, presumably are poorly removed by dilution and must be neutralized, generally by a reducing agent such as thiosulfate, prior to plating for CFU determination. Even an agent such as an antibiotic that does not kill dormant spores will appear to kill dormant spores if it is not neutralized and/or removed prior to plating of the treated spores (Russell 1999; Maillard 2011; Ghosh and Setlow 2018).
When all the above advice has been followed, one other concern is the precise condition of the incubation with the various agent to be used, as incubation variables such as pH, temperature and ionic strength can influence rates of spore killing. The incubation pH may be a special concern as some chemical agents can alter solution pH. Thus, it may be wise to have a buffer present in incubations to control incubation pH. Another concern is the precise spore concentration to use in incubation. Too low a spore concentration may not allow the validation of high levels of spore killing. On the other hand, too high a spore concentration might give a large amount of spore shielding against radiation, or may rapidly detoxify some chemical agents. Generally, spore suspensions of 107–108 CFU/ml have proven to give reproducible results.
It is in assignment of precise mechanisms of spore killing that has generally been of most concern in recent years. Work primarily in the Setlow lab has identified five different mechanisms by which spores are killed (Setlow 2016): 1) DNA damage by UV or γ-radiation, dry heat, very high vacuum and some DNA damaging chemicals; 2) elimination or greatly slowing the ability of spores to complete germination by high pH – but as noted above, these apparently killed spores may be induced to germinate if given extra assistance and may then return to life; 3) complete rupture of spores’ permeability barriers by concentrated mineral acids causing explosive release of all CaDPA; 4) damage likely to spores’ inner membrane by a number of oxidizing agents (e.g. - hypochlorite, chlorine dioxide, ozone), such that while the killed dormant spores appear normal and germinate normally, the germinated spores rapidly lyse, as if the damaged spores’ inner membrane cannot restrain the osmotic pressure of the spore core; and 5) damage to one or more spore proteins caused by wet heat such that the killed spores germinate normally and do not lyse, but neither make ATP nor initiate metabolic activities. In order to determine which of these mechanisms are operating to kill spores there are two principles to keep in mind as follows. The First Principle is that in order for a mechanism of killing to be definitively identified, it must be shown that spore killing takes place in parallel with changes in spores’ due to the killing mechanism and not long before. For example, when Bacillus spore killing by wet heat iodine or peracetic acid plus supercritical CO2 are examined, loss of the ability to germinate takes place well after spore killing (Coleman et al. 2007, 2010; Huesca-Espitia et al. 2016; Setlow et al. 2016; Li et al. 2017). Thus, inactivation of one or more essential germination proteins plays no role in spore killing by these agents. CaDPA release by wet heat-treated spores also occurs well after spore killing, so a breakdown in spore permeability barriers is not what kills spores by this agent either. Similarly, spores surviving a wet heat treatment giving > 90% killing do not exhibit increased mutagenesis or any obvious DNA damage (Setlow 2006), thus ruling out spore DNA damage as causing the spore killing. The Second Principle, and one as important as the First Principle, is that “Correlation does not equal Causation”. Thus, even with a close kinetic association of a change in a spore property and spore killing, this does not mean that it is the change in the spore property that caused spore death, as the change in the spore property may be just a nearly instantaneous post mortem event. This is of special concern when some gross change in spore morphology is seen upon spore treatment with an agent, for example large changes in the appearance of spores’ outer layers. One agent that can cause such gross changes in spore properties is gas plasma at various temperatures, although such changes are often well after spore death (Yardimci and Setlow 2010). It is also extremely difficult with structural changes of spores to get good kinetics of both spore killing and the structural change, especially if the latter may be an accumulation of many small, perhaps individually unseen structural changes. However, there has been an unfortunate tendency in the literature to point to any large structural change during treatment with some agent as the mechanism of spore killing. Rather, it is equally, and perhaps even more likely that the structural change only exhibits a correlation with spore killing, and often with no or minimal evidence of a causal relationship between the structural change and killing.
Unfortunately, while kinetic studies, as noted above for spore wet heat treatment, can rule out mechanisms of spore killing, alone they cannot prove that a particular mechanism is how spores are killed. This requires results from studies other than kinetics. One other source for information on mechanisms of spore killing, as well as mechanisms of spore resistance as noted above, is the use of mutants in key genes important in various spore resistance properties. As noted above, such mutants are not widely available in species other than B. subtilis, but given the advantages in genetic manipulation in other Bacillus and Clostridium strains this should be feasible at least for organisms of applied interest (Minton et al. 2010; Saldanha et al. 2013; Pomerantsev et al. 2017; Joseph et al. 2018). Notably, a few studies have shown that mechanisms of spore killing by least some agents are similar in spores of all Bacillus species tested (Coleman et al. 2010; Setlow et al. 2014; Huesca-Espitia 2016; Li et al. 2017), although there is no guarantee that this will be true for all species. Mutants that have proven most useful in B. subtilis include strains lacking: i) most spore-specific α/β-type SASP that protect spore DNA from many types of damage; ii) recA, the product of which modulates much DNA repair when spores germinate and begin outgrowth; and iii) cotE, which encodes a protein essential for assembly of much of the spore’s coat layer. Use of these mutants as well as: 1) kinetic analysis of spore killing and changes in spore properties such as loss of CaDPA with spore killing to 95–99%; 2) measurement of the germination and outgrowth of spores killed 95–99%; 3) ultrastructural analysis of spores killed 95–99%; 4) measurements of dormant and germinated spore permeability to nucleic acid stains as a function of spore killing up to 99%, as live dormant spores take up minimal amounts of nucleic acid stains, and only in spores’ outer layers, while spores killed by some agents (e.g. – concentrated mineral acid) do and use of such nucleic acid stains can even distinguish between live and dead germinated spores (Setlow et al. 2016; Li et al. 2017); and 5) examination of survivors of spores killed 95–99% for mutations. Notably, auxotrophic and asporogenous mutants in B. subtilis can easily be identified by examination of colonies formed (or not formed) on appropriate media (Fairhead et al. 1993). Generation of antibiotic resistant mutants can also be examined on plates, and rifampicin resistance has been used in some studies (Setlow et al. 2014). Together, data from all these analyses should allow identification of mechanisms of spore resistance to and killing by various agents – even if specifics, such as for example the precise protein(s) inactivated by wet heat or the precise inner membrane damage caused by oxidizing agents, remain unclear. However, there will be evidence well beyond correlation that the mechanism identified is actually the cause of spore death.
In addition to the direct killing of dormant spores discussed above, there are some agents that actually trigger spore germination and then almost immediately kill the much less resistant germinated spores. The most well studied agent that does this is dodecylamine that triggers germination of all Bacillales and Clostridiales spores that have been tested (Rode and Foster 1960, 1961; Setlow et al. 2017). This amphipathic agent triggers spore germination by activating spores’ inner membrane channel for CaDPA composed of SpoVA proteins, such that CaDPA is rapidly released (Velázquez et al. 2014; Setlow et al. 2017). In Bacillales and many, but not all Clostridiales species, this CaDPA release triggers completion of spore germination. The dodecylamine then kills the germinated spores, presumably by disrupting the germinated spores’ plasma membrane. A variety of other alkylamines, as well as many cationic dyes, can also trigger germination of spores of multiple species, probably in the same way dodecylamine does, although this has not been studied. However, dodecylamine is the most effective of this class of molecules in triggering spore germination. Recently, a member of a new class of antimicrobial agents termed ceragenins with some structural similarity to dodecylamine was found to kill B. subtilis spores (Piktel et al. 2017), and recent work has shown that this agent kills spores by first germinating them and then killing the germinated spores (Ghosh and Setlow 2018). Overall then, agents such as dodecylamine and its structural homologs do kill spores, but only germinated spores not dormant spores.
CONCLUSIONS AND FINAL THOUGHTS
With all the information noted above, what should be done in spore research going forward? There are a number of important questions about spores that remain unanswered, including but certainly not limited to the following. 1) What are the specific mechanisms of spore killing by commonly used sporicides such as wet heat and oxidizing chemicals, including what is/are the specific spore molecules to which damage causes spore killing and the precise nature of the damage. 2) What is the role of the mRNAs that modern omics technology has found in spores of all species examined – are these simply mRNA species left over from the developing spore when dormancy arrived, or might these mRNAs play some role in spore resistance, germination and outgrowth? 3) What are the mechanistic details of the process of spore germination, including: i) how do germinant receptors that respond to physiological germinants transmit information to downstream germination proteins, whether they be the components of SpoVA protein CaDPA channel or the enzymes involved in the hydrolysis of spores’ peptidoglycan cortex. 4) How is the inner membrane channel for CaDPA composed of SpoVA proteins gated, and what is the structure of this channel? 5) Are mechanisms of spore killing by and resistance to various agents with B. subtilis spores the same in spores of Clostridiales? 6) Are spores truly metabolically dormant, or do they exhibit low levels of metabolic activity as reported recently (Segev et al. 2012), and what is the role of this metabolism?
In work to answer these questions it will be essential to use spores that are as pure as possible to avoid egregious errors that come from use of impure spores. One of the best examples of such an error was the proposal that the B. anthracis EA1 protein would be an excellent marker for sensitive detection of spores of this potential biological weapon, since it was thought to be associated with the spore surface. However, subsequent work showed that EA1 was a vegetative cell protein that was a contaminant in B. anthracis spore preparations that could readily be removed by spore purification, and not a protein on these spores’ outer layers (Williams and Turnbough 2004). Similarly, all experiments designed to determine mechanisms of spore killing and resistance would be well served by paying attention to spore purity and also other concepts present in this article, so that inappropriate claims do not make their way into the spore literature where they are enshrined forever.
Finally, I believe that all individuals carrying out spore research, as well as reviewing or editing manuscripts on various properties of bacterial spores, must not: “waste clean thinking on dirty spores”. Additionally, when evaluating research purporting to elucidate mechanisms of spore resistance, in particular mechanisms of spore killing, reviewers/editors should be inoculated with a healthy dose of skepticism. Do the experiments definitively prove a mechanism for spore killing, or do they merely observe that spores are killed and the killed spores are damaged, but with no proof of a causal connection between these two observations. Individuals doing or planning to do research on bacterial spore resistance and killing, also even in the spore field itself would also be well served, if before doing experiments they: i) read the literature; ii) talk to an expert in spore research; and best of all iii) read this article carefully and take the points made to heart. If the latter happens, then this article will have contributed significantly to spore research going forward and will have been worth the effort of writing it.
ACKNOWLEDGEMENTS
I am most grateful to the late Arthur Kornberg for introducing me to the wonderful world of bacterial spores, and to Barbara Setlow who urged me to write this article after listening to me gripe about methodological errors in spore research in papers I was reviewing or editing. Emily Camilleri and George Korza were also of great help in preparing Fig. 1, 2 and 4, and Wayne Nicholson and Christopher Doona made excellent suggestions that improved the manuscript. My work on spores has received generous support over the past 50 years from a variety of USA government agencies including the National Science Foundation, National Institutes of Health, Department of Agriculture, U.S. Army Research Office, Defense Threat Reduction Agency and the Defense Advanced Research Projects Agency, as well as the 3M Corporation.
Footnotes
CONFLICTS OF INTEREST
None
REFERENCES
- Bagyan I, Noback M Bron S, Paidhungat M and Setlow P (1998) Characterization of yhcN, a new forespore-specific gene of Bacillus subtilis. Gene 212, 179–188. [DOI] [PubMed] [Google Scholar]
- Bergman NH, Anderson EC, Swenson EE, Niemeyer MW, Miyoshi AD and Hanna PC (2006) Transcriptional profiling of the Bacillus anthracis lifecycle in vitro and an implied model for regulation of spore formation. J Bacteriol 188, 6092– 6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettegowda C, Huang X, Lin J, Cheong I, Kohli M, Szabo SA, Zhang X, Diaz LA Jr., Velculescu VE, Parmigiani G, Kinzler KW, Vogelstein B and Zhou S (2006) The genome and transcriptomes of the anti-tumor agent Clostridium novyi-NT. Nat Biotechnol 24, 1573–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozue JA, Welkos S and Cote C (2016) The Bacillus anthracis exosporium: what’s the big hairy deal? In The Bacterial Spore: From Molecules to Systems ed. Eichenberger P and Driks A pp. 253–268. Washington, DC: ASM Press. [DOI] [PubMed] [Google Scholar]
- Coleman WH, Chen D, Li Y-Q, Cowan AE and Setlow P (2007) How moist heat kills spores of Bacillus subtilis. J Bacteriol 189, 8458–8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman WH, Zhang P, Li Y-Q and Setlow P (2010) Mechanism of killing of spores of Bacillus cereus and Bacillus megaterium by wet heat. Lett Appl Microbiol 50, 507–514. [DOI] [PubMed] [Google Scholar]
- Cortezzo DE, Koziol-Dube K, Setlow B and Setlow P (2004) Treatment with oxidizing agents damages the inner membrane of spores of Bacillus subtilis and sensitizes the spores to subsequent stress. J Appl Microbiol 97, 838–852. [DOI] [PubMed] [Google Scholar]
- Cortezzo DE and Setlow P (2005) Analysis of factors influencing the sensitivity of spores of Bacillus subtilis to DNA damaging chemicals. J Appl Microbiol 98, 606–617. [DOI] [PubMed] [Google Scholar]
- Dias MB, Reyes-Gonzalez L, Veloso FM and Casman EA (2010) Effects of the USA PATRIOT Act and the 2002 Bioterrorism Preparedness Act on select agent research in the United States. Proc Natl Acad Sci USA 107, 9556–9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouiai B, Thwaite JE, Laws TR, Commichau FM, Setlow B, Setlow P and Moeller R (2018) Role of DNA repair and protective components in Bacillus subtilis spore resistance to inactivation by 400 nm blue light. Appl Environ Microbiol In press. [DOI] [PMC free article] [PubMed]
- Donnelly ML, Li W, Li Y-Q, Hinkel L, Setlow P and Shen A (2017) A Clostridium difficile-specific, gel-forming protein required for optimal spore germination. mBio 17, e02085–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Cortes K, Barra-Carrasco J and Paredes-Sabja D (2013) Proteases and sonication specifically remove the exosporium layer of spores of Clostridium difficile strain 630. J Microbiol Methods 93, 25–31. [DOI] [PubMed] [Google Scholar]
- Fairhead H, Setlow B and Setlow P (1993) Prevention of DNA damage in spores and in vitro by small, acid-soluble proteins from Bacillus species. J Bacteriol 175, 1367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fimlaid KA, Jensen O, Donnelly ML, Francis MB, Sorg JA and Shen A (2015) Identification of a novel lipoprotein regulator of Clostridium difficile spore germination. PLoS Pathog 11:e1005239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt P and Marquis RE (1989) Spore thermoresistance mechanisms. In Regulation of Prokaryotic Development ed. Smith I, Slepecky RA and Setlow P pp. 43–63. Washington, DC: American Society for Microbiology. [Google Scholar]
- Ghosh S and Setlow P 2018. Unpublished results
- Giorno R, Bozue J, Cote C, Wenzel T, Moody KS, Mallozzi M, Ryan M, Wang R, Zielke. R, Maddock JR, Friedlander, A, Welkos S and Driks A. (2007) Morphogenesis of the Bacillus anthracis spore. J Bacteriol 189, 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horneck G, Moeller R, Cadet J, Douki T, Mancinelli R, Nicholson WL, Panitz C, Rabbow P, Rettberg A, Spry E, Stackebrandt E, Vaishampayan P and Venkateswaran K (2012) Resistance of bacterial endospores to outer space for planetary protection purposes – experiment PROTECT of the EXPOSE-E mission. Astrobiol 12, 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huesca-Espitia L del C, Suvira. M, Rosenbeck K, Korza G, Setlow B Li W, Wang S, Li Y-Q and Setlow P. (2016) Effects of steam autoclave treatment on Geobacillus stearothermophilus spores. J Appl Microbiol 121, 1300–1311. [DOI] [PubMed] [Google Scholar]
- Joseph RC, Kim NM and Sandoval NR (2018) Recent developments of the synthetic biology toolkit for Clostridium. Front Microbiol 9, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keijser BJF, Ter Beek A, Rauwerda H, Schuren F, Montijn R, van der Spek H and Brul S (2007) Analysis of temporal gene expression during Bacillus subtilis spore germination and outgrowth. J Bacteriol 189, 3624 –3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggett M, McDonnell G, Denyer S, Setlow P and Maillard J-Y (2012) Bacterial spore structures and their protective role in biocide resistance. J Appl Microbiol 113, 485–499. [DOI] [PubMed] [Google Scholar]
- Li Q, Korza G and Setlow P (2017) Killing of spores of Bacillus species by molecular iodine. J Appl Microbiol 122, 54–64. [DOI] [PubMed] [Google Scholar]
- Magge A, Granger AC, Wahome PG, Setlow B, Vepachedu VR, Loshon CA, Peng L, Chen D, Li Y. q. and Setlow P (2008) Role of dipicolinic acid in the germination, stability and viability of spores of Bacillus subtilis. J Bacteriol 190, 4798–4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard JY (2011) Innate resistance to sporicides and potential failure to decontaminate. J Hosp Infect 77, 204–209. [DOI] [PubMed] [Google Scholar]
- Mallozzi M, Viswanathan VK and Vedantam G (2010) Spore-forming Bacilli and Clostridia in human disease. Future Microbiol 5, 1109–1123. [DOI] [PubMed] [Google Scholar]
- Melly E, Genest PC, Gilmore ME, Little S, Popham DL, Driks A and Setlow P (2002) Analysis of the properties of spores of Bacillus subtilis prepared at different temperatures. J Appl Microbiol 92, 1105–1115. [DOI] [PubMed] [Google Scholar]
- Minton NP, Ehsaan M, Humphreys CM, Little GT, Baker J, Henstra AM, Liew F, Kelly ML, Sheng L, Schwarz K and Zhang Y (2016) A roadmap for gene system development in Clostridium. Anaerobe 41, 1104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson WL and Galeano B. (2003) UV resistance of Bacillus anthracis spores revisited: validation of Bacillus subtilis spores as UV surrogates for spores of B. anthracis Sterne. Appl Environ Microbiol 69, 1327–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson WL, Munakata N, Horneck G, Melosh HJ and Setlow P (2000) Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol Mol Biol Rev 64: 548–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson WL and Setlow P (1990) Sporulation, germination and outgrowth. In Molecular Biological Methods for Bacillus ed. Harwood CR and Cutting SM pp. 391–450. Chichester, UK: John Wiley and Sons. [Google Scholar]
- Paidhungat M, Setlow B, Driks A and Setlow P (2000) Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J Bacteriol 182, 5505–5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes-Sabja D, Raju D, Torres JA and Sarker MR (2008) Role of small, acid-soluble spore proteins in the resistance of Clostridium perfringens spores to chemicals. Int J Food Microbiol 122, 335–335. [DOI] [PubMed] [Google Scholar]
- Paredes-Sabja D, Setlow P and Sarker M (2009a) The protease CspB is essential for initiation of cortex hydrolysis and DPA release during germination of spores of Clostridium perfringens. Microbiology 155, 3464–3472. [DOI] [PubMed] [Google Scholar]
- Paredes-Sabja D, Setlow P and Sarker MR (2009b) SleC is essential for cortex peptidoglycan hydrolysis during germination of spores of the pathogenic bacterium Clostridium perfringens. J Bacteriol 191, 2711–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piktel E, Pogoda K, Roman M, Niemirowicz K, Tokajuk G, Wroblewska M, Szynaka B, Kwiatek WM, Savage PB and Bucki R (2017) Sporicidal activity of ceragenin CSA-13 against Bacillus subtilis. Sci Rep 7, 44452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantsev AP, McCall RM, Chahoud M, Hepler NK, Fattah R and Leppla SH (2017) Genome engineering in Bacillus anthracis using tyrosine site-specific recombinases. PLoS One 12, e0183346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popham DL, Illades-Aguiar B and Setlow P (1995) The Bacillus subtilis dacB gene, encoding penicillin-binding protein 5*, is part of a three-gene operon required for proper spore cortex synthesis and spore core dehydration. J Bacteriol 177, 4721–4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode LJ and Foster JW (1960) The action of surfactants on bacterial spores. Arch Mikrobiol 36, 67–94. [DOI] [PubMed] [Google Scholar]
- Rode LJ and Foster JW (1961) Germination of bacterial spores with alkyl primary amines. J Bacteriol 81, 768–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AD (1999) Factors influencing the efficacy of antimicrobial agents. In Principles and Practice of Disinfection, Preservation and Sterilization 3rd edn. ed. Russell AD, Hugo WB, and Ayliffe GAJ pp. 95–123. London, UK: Blackwell Science. [Google Scholar]
- Saldanha RJ, Pemberton A, Shiflett P, Perutka J, Whitt JT, Ellington A, Lambowitz AM, Kramer R, Taylor D and Lamkin TJ (2013) Rapid targeted gene disruption in Bacillus anthracis. BMC Biotechnol 13, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev E, Smith Y and Ben-Yehuda S (2012) RNA dynamics in aging bacterial spores. Cell 148, 139–149. [DOI] [PubMed] [Google Scholar]
- Setlow B, Korza G, Blatt KMS, Fey J and Setlow P (2016) Mechanism of Bacillus subtilis spore killing by and resistance to supercritical CO2 plus peracetic acid. J Appl Microbiol 120, 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B, Loshon CA, Genest PC, Cowan AE, Setlow C and Setlow P (2002) Mechanisms of killing of spores of Bacillus subtilis by acid, alkali and ethanol. J Appl Microbiol 92, 362–375. [DOI] [PubMed] [Google Scholar]
- Setlow B, Parish S, Zhang P, Li YQ, Neely WC and Setlow P (2014) Mechanism of killing of spores of Bacillus anthracis in a high temperature gas environment. J Appl Microbiol 116, 805–14. [DOI] [PubMed] [Google Scholar]
- Setlow B and Setlow P (1996) Role of DNA repair in Bacillus subtilis spore resistance. J Bacteriol 178, 3486–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P (1994) Mechanisms which contribute to the long-term survival of spores of Bacillus species. J Appl Bacteriol 76, 49S–60S. [DOI] [PubMed] [Google Scholar]
- Setlow P (2013) Resistance of bacterial spores to chemical agents. In Principles and Practice of Disinfection, Preservation and Sterilization, 5th edn. ed. Fraise AP, Maillard J-Y, and Sattar SA pp. 121–130. Oxford, UK: Wiley-Blackwell. [Google Scholar]
- Setlow P (2016) Spore resistance properties, In The Bacterial Spore: From Molecules to Systems ed. Eichenberger P and Driks A pp. 201–158. Washington, DC: ASM Press. [Google Scholar]
- Setlow P and Johnson EA (2012) Spores and their significance. In Food Microbiology, Fundamentals and Frontiers, 4th edition ed. Doyle MP and Buchanan R pp. 45–79. Washington, DC: ASM Press. [Google Scholar]
- Setlow P, Wang S and Li YQ 2017. Germination of spores of the orders Bacillales and Clostridiales. Annu Rev Microbiol 71, 459–477. [DOI] [PubMed] [Google Scholar]
- Tan IS and Ramamurthi KS (2014) Spore formation in Bacillus subtilis. Environ Microbiol Rep 6, 212–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BM, Binkley JM and Stewart GC (2011) Current physical and SDS extraction methods do not efficiently remove exosporium proteins from Bacillus anthracis spores. J Microbiol Methods 85, 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velásquez J, Schuurman-Wolters G, Birkner JP, Abee T and Poolman B (2014) Bacillus subtilis spore protein SpoVAC functions as a mechanosensitive channel. Mol Microbiol 92, 813–823. [DOI] [PubMed] [Google Scholar]
- Williams DD and Turnbough CL Jr. (2004) Surface layer protein EA1 is not a component of Bacillus anthracis spores but is a persistent contaminant in spore preparations. J Bacteriol 186, 566–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardimci O, and Setlow P (2010) Plasma sterilization: Opportunities and microbial assessment strategies in medical device manufacturing. IEEE Trans Plasma Sci 38, 973–981. [Google Scholar]
- Young SB and Setlow P (2003) Mechanisms of killing of Bacillus subtilis spores by hypochlorite and chlorine dioxide. J Appl Microbiol 95, 54–67. [DOI] [PubMed] [Google Scholar]


