Abstract
Purpose
HPV self-sampling has previously been shown to increase cervical cancer screening among ethnic minority and immigrant women. We conducted a randomized pragmatic trial to examine the effectiveness of HPV self-sampling delivered via in person versus by US mail for medically underserved Hispanic, Haitian, and non-Hispanic Black women living in South Florida.
Methods
We randomized women ages 30–65 who had not completed Pap smear screening in the past 3 years into two groups: 1) HPV self-sampling delivered in-person (IP) by a Community Health Worker (CHW; IP+SS) or 2) HPV self-sampling delivered via US mail (SS+Mail). Our primary outcome was HPV self-sampling completion by six months post-study enrollment.
Results
We enrolled 600 women. Approximately 65% were Hispanic and 35% were Haitian or non-Hispanic Black. Nearly half (43%) had an income of less than $20,000/year and 67% were uninsured. In intent-to-treat analyses, 71.6% of participants in the SS+Mail group and 81.0% of participants in the IP+SS group completed HPV self-sampling.
Conclusion
Mailed HPV self-sampling is an effective strategy to increase cervical cancer screening among underserved immigrant and ethnic minority women.
Keywords: Cervical cancer screening, HPV, immigrant, women, self-sampling
Background
Despite significant advances in disease prevention, ethnic minority and immigrant women living in the US remain at increased risk of developing and dying from cervical cancer, largely due to lack of access to primary (HPV vaccination) and secondary (screening) opportunities [1–5]. In Miami, FL cervical cancer is a particular problem for Hispanic, Haitian, and non-Hispanic Black women [6]. For example, the rate of cervical cancer in Little Haiti, a large enclave of Haitian settlement, is 38 per 100,000, which is over four times that of the state of Florida overall (8/100,000) [7, 8]. Previously identified barriers to cervical cancer screening among Haitian and other ethnic minority women living in Miami include: language barriers; lack of access to the formal healthcare system; preference for ethnomedical providers; limited knowledge about cancer and the importance of early detection of disease; and, cultural concerns regarding modesty/limited acceptability of traditional Pap smear screening [6, 9].
These barriers necessitate identifying alternative screening strategies for underserved ethnic minority groups. One such strategy, cervical self-sampling, enables women to self-collect a cervico-vaginal specimen in a non-clinical setting of their choosing, circumventing many of the aforementioned barriers to screening uptake [10, 11]. This specimen is then tested for high-risk Human Papilloma Virus (HPV) infection, the principal cause of cervical cancer. This screening modality has consistently been shown to demonstrate similar sensitivity to physician-collected samples for HPV detection [12, 13]. Further, with the recent FDA approval of Roche cobas, a first line test for cervical cancer screening, clinical algorithms for disease prevention increasingly prioritize HPV testing over cytology, the historical gold standard for screening [14].
We previously completed a large randomized pragmatic trial (n = 601 women) to test the effectiveness of a Community Health Worker (CHW)-delivered HPV self-sampling intervention for increasing cervical cancer screening uptake among ethnic minority women within three medically underserved Miami communities: Little Haiti, Hialeah, and South Dade [15]. Our findings upheld that HPV self-sampling was superior to navigating women to low cost Pap smears at Federally Qualified Health Centers or free clinics within their community of residence (77% HPV self-sampling completion vs. 43% Pap smear completion, respectively) [16]. Given such findings, we sought to further examine the most optimal strategies for delivering HPV self-sampling to these three underserved communities, as well as others similarly characterized by health disparity and lack of access to the formal healthcare system. Outside of the US, mailed self-sampling has been shown to be a particularly efficacious method of delivering HPV self-sampling to unscreened and under screened women. Therefore, we conducted a randomized pragmatic trial of HPV self-sampling delivered via US mail (SS+ mail) versus self-sampling delivered in-person (IP+SS). To our knowledge, this trial is the first to evaluate the effectiveness of mailed self-sampling in the US. Findings will inform future dissemination of this low-cost alternative to traditional, clinic-based cervical cancer screening.
Study Design
Guided by the principles of community-based participatory research (CBPR), we conducted a randomized trial to compare the effectiveness two modes of self-sampling delivery - SS+Mail versus IP+SS – for increasing cervical cancer screening uptake among ethnic minority and immigrant women in South Florida. All study procedures were conducted in the participants’ language of preference, either English, Spanish, or Haitian Creole. The study protocol has been described in detail elsewhere [17]. Prior to implementation, it was approved by the University of Miami Institutional Review Board and registered at clinialtrials.gov (NCT02202109).
Participants and Setting
The sample included women who self-identified as Hispanic, Haitian, or non-Hispanic Black, were 30–65 years of age, lived in Little Haiti, Hialeah or unincorporated Southern Miami-Dade (South-Dade), and reported not having had a Pap smear in the previous 3 years. Women were excluded if they had a history of hysterectomy or cervical cancer, were pregnant, or had ever been enrolled in any other cervical cancer prevention/outreach-related study, including the aforementioned trial comparing CHW-delivered HPV self-sampling to patient navigation. Based on a priori power analyses (see Kobetz et al., 2016), we sought to enroll 200 women from each neighborhood (n = 600 total) [17].
Our study CHWs, who were indigenous to the three target neighborhoods and knowledgeable of cultural mores related to health promotion, recruited and screened women for eligibility at a variety of community venues including churches, flea markets, and community events, such as health fairs. Eligible and interested women were then scheduled for a one-on-one meeting with a community health educator (CHE) at their home or place of their choosing, where they completed written informed consent and a baseline interview. After baseline data collection was completed, women were randomized by the study statistician, in a 1:1 ratio, to one of two intervention arms, IP+SS or SS+Mail. Randomization was stratified by neighborhood to ensure equal distribution of study arms by neighborhood. CHWs were immediately notified of a participant’s assignment and contacted participants within a week of randomization to inform them of their assignment; for IP+SS participants, an intervention in-person visit was also scheduled by the CHW at this time. Approximately 6 months-post study enrollment, participants were scheduled for a follow-up visit with the CHE to conduct an exit interview which examined secondary outcomes (cervical cancer knowledge, having a usual source of healthcare, and health insurance status). The follow-up interview provided the basis for our secondary outcomes analyses.
Interventions
IP+SS
Women randomized to this arm received a 30-minute in-person study visit by a CHW at a mutually-determined community location. During this visit, the CHW provided brief health education about the importance of cervical cancer screening as well as verbal and visual instructions for how to appropriately self-sample, using the POI/NIH self-sampler [17]. Women were instructed to insert the swab until they meet resistance, turn the swab 5 times, withdraw the swab, and swirl the swab in liquid ThinPrep fixative 10 times before disposing of the swab. The ThinPrep vial was then sealed in a biohazard bag and returned to the CHW [17]. Participants then were provided the option to self-sample while the CHW waited, or to self-sample at a later time, and return their sample via US mail using a pre-addressed, pre-stamped envelope. Samples collected during the study visit were stored at room temperature and delivered weekly to a CLIA-approved laboratory for testing.
SS+Mail
Women in this arm were mailed a self-sampling kit which included the self-sampler and vial for storing the specimen, a pre-addressed, pre-stamped envelope for returning the vial to the CLIA-approved laboratory, and paper copies of the instructional images for how to appropriately self-sample. The kit was mailed to the participant within a week of randomization, and CHWs contacted participants by phone one week after the mailing date to confirm receipt. At this time, the CHW also provided by telephone the same brief health education that they offered in-person to women randomized to the IP + SS arm, and reviewed the visual instructions for self-sampling.
Outcomes
Our primary outcome was self-sampling completion within 6 months of enrollment. Consistent with an intent-to-treat approach, individuals who were lost to follow-up or whose samples were not received by the lab were assumed not to have completed self-sampling. Secondary outcomes included changes in 1) cervical cancer knowledge (defined as the proportion of participants answering at least 3 of 5 items about cervical cancer signs and risk factors correctly) [18]; 2) the proportion of participants reporting having health insurance; and 3) the proportion of participants reporting a usual source of care (defined as having a place to go for routine or preventive care). All follow-up data were collected 6 months after enrollment by CHEs who were blinded to study-arm assignment.
Statistical Analysis
Baseline characteristics were compared across the two study arms using chi-square tests (categorical data) and t-tests (continuous data). Crude differences in proportion of women screened by study arm (overall and stratified by neighborhood site) were tested using chi-square tests. We then modeled the association between sociodemographic variables and HPV self-sampling completion using univariate and multivariable logistic regression analyses. We included neighborhood site, age, income, insurance status, education, Pap history, marital status and citizenship status as covariates in the multivariable analysis. Race/ethnicity was not analyzed as it was highly collinear with site (e.g. all Haitians resided in Little Haiti). We report odds ratios (ORs) and corresponding 95% confidence intervals (CI) for the regression analyses. Secondary outcomes were analyzed using paired McNemar chi-square tests for the overall sample, and stratified by neighborhood site. All statistical tests were two-sided, with significance set at the p < 0.05 level. The data were analyzed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Study Flow
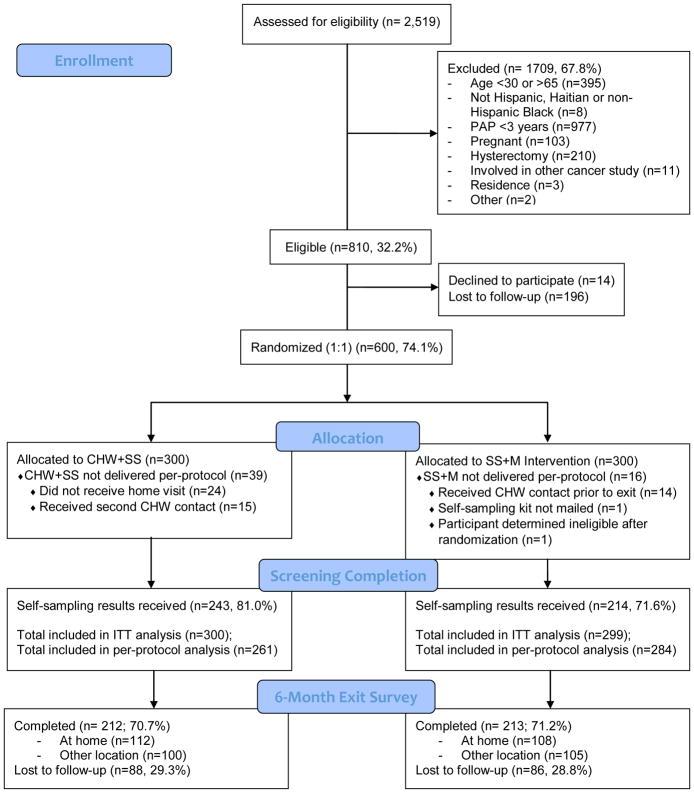
To achieve an enrolled sample size of 600, a total of 2,519 women were assessed for eligibility, of which 1709 (67.8%) were ineligible. The most common reason for ineligibility, accounting for more than half of those excluded from participating, was having had a Pap smear in the previous three years (n = 977). Among the 810 eligible women, 14 declined participation and 196 were lost to follow-up between completing the eligibility screener with the CHW and scheduling an appointment for baseline data collection with the CHE. Half of the 600 enrolled participants were allocated to IP+SS (n = 300) and half to SS+Mail (n = 300). Each arm consisted of an equal number of women from the three target neighborhoods. One woman in the SS+Mail arm was determined to be ineligible after randomization due to Pap smear history, and was thus excluded from all analyses. Six-month follow-up was greater than 70% in both study arms (70.7% in IP+SS, and 71.2% in SS+Mail) and did not significantly differ between study arms.
Study Sample
Baseline characteristics of the study sample are presented in Table 1. The mean age of participants was 45.9 years (SD = 9.2). The sample was predominantly uninsured (66.8%), low income (42.6%), without a place for routine health services (55.9%), and married or living with a significant other (53.9%). Although none of the sample had a Pap smear in the previous 3 years, 87.8% reported having had at least one previous Pap smear in their lifetime. Our sample was largely composed of recent immigrants, with only 26.9% identifying as US citizens. Consistent with our recruitment strategy, roughly one third of the sample was Haitian or non-Hispanic black (34.9%) with all other participants self-identifying as Hispanic. Of note, all participants recruited from the neighborhood of Little Haiti identified as Haitian. There were no significant differences in the distribution of baseline sociodemographic characteristics between study arms.
Table 1.
Baseline characteristics of N=599 women enrolled in a cervical self-sampling randomized controlled trial, by intervention arm
| All | CHW+SS | SS+MAIL | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
|
|
|
|
||||
| Total | 599 | 100.0 | 300 | 100.0 | 299 | 100.0 |
| Age (mean, SD) | 45.9 | 9.2 | 46.0 | 9.6 | 45.7 | 8.9 |
| Income | ||||||
| </= $20,000 | 255 | 42.6 | 130 | 43.3 | 125 | 41.8 |
| > $20,000 | 76 | 12.7 | 40 | 13.3 | 36 | 12.0 |
| Unknown/Not Reported | 268 | 44.7 | 130 | 43.3 | 138 | 46.2 |
| Race/Ethnicity | ||||||
| Hispanic | 390 | 65.1 | 197 | 65.7 | 193 | 64.5 |
| Haitian or Non-Hispanic Black | 209 | 34.9 | 103 | 34.3 | 106 | 35.5 |
| Health Insurance | ||||||
| Insured | 194 | 32.4 | 97 | 32.3 | 97 | 32.4 |
| Uninsured | 400 | 66.8 | 200 | 66.7 | 200 | 66.9 |
| Unknown/Not Reported | 5 | 0.8 | 3 | 1.0 | 2 | 0.7 |
| Has a place of care when sick | ||||||
| Yes | 328 | 54.8 | 171 | 57.0 | 157 | 52.5 |
| No | 269 | 44.9 | 128 | 42.7 | 141 | 47.2 |
| Unknown/Not Reported | 2 | 0.3 | 1 | 0.3 | 1 | 0.3 |
| Has a place of care for routine services | ||||||
| Yes | 261 | 43.6 | 139 | 46.3 | 122 | 40.8 |
| No | 335 | 55.9 | 160 | 53.3 | 175 | 58.5 |
| Unknown/Not Reported | 3 | 0.5 | 1 | 0.3 | 2 | 0.7 |
| Marital Status | ||||||
| Single, never married | 127 | 21.2 | 61 | 20.3 | 66 | 22.1 |
| Married or living with significant other | 323 | 53.9 | 165 | 55.0 | 158 | 52.8 |
| Separated, divorced or widowed | 145 | 24.2 | 73 | 24.3 | 72 | 24.1 |
| Unknown/Not Reported | 4 | 0.7 | 1 | 0.3 | 3 | 1.0 |
| Has ever had previous pap smear | ||||||
| Yes | 526 | 87.8 | 265 | 88.3 | 261 | 87.3 |
| No | 69 | 11.5 | 34 | 11.3 | 35 | 11.7 |
| Unknown/Not Reported | 4 | 0.7 | 1 | 0.3 | 3 | 1.0 |
| Educational attainment | ||||||
| Less than HS | 220 | 36.7 | 110 | 36.7 | 110 | 36.8 |
| HS or greater | 373 | 62.3 | 187 | 62.3 | 186 | 62.2 |
| Unknown/Not Reported | 6 | 1.0 | 3 | 1.0 | 3 | 1.0 |
| Current type of residence | ||||||
| Rents an apartment or home | 352 | 58.8 | 177 | 59.0 | 175 | 58.5 |
| Rents room | 22 | 3.7 | 12 | 4.0 | 10 | 3.3 |
| Pays a mortgage on a house | 80 | 13.4 | 40 | 13.3 | 40 | 13.4 |
| Owns house with no mortgage | 18 | 3.0 | 11 | 3.7 | 7 | 2.3 |
| Has other living arrangements | 120 | 20.0 | 56 | 18.7 | 64 | 21.4 |
| Unknown/Not Reported | 7 | 1.2 | 4 | 1.3 | 3 | 1.0 |
| Citizenship status | ||||||
| Permanent Resident | 231 | 38.6 | 119 | 39.7 | 112 | 37.5 |
| US Citizen | 161 | 26.9 | 78 | 26.0 | 83 | 27.8 |
| Unknown/Other | 207 | 34.6 | 103 | 34.3 | 104 | 34.8 |
No significant differences between intervention arms, at the p<0.05 level on chi-square tests (categorical variables) and t-tests (continuous variables), among participants with known values.
Self-Sampling Completion
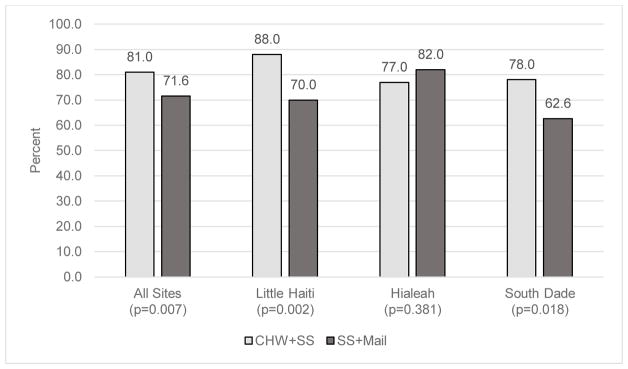
Completion of HPV self-sampling was high in both study arms, reaching 81.0% (n = 243) among IP+SS participants and 71.6% (n = 214) among SS+Mail participants (Figure 2). The overall difference in self-sampling completion by arm was statistically significant (p < 0.01). When stratified by neighborhood, self-sampling completion was higher for IP+SS participants than SS+Mail participants in Little Haiti (88.0% vs. 70.0%, p < 0.01) and South Dade (78.0% vs. 62.6%, p <0.01). Of note, within the IP+SS arm, 42 (14%) women elected to mail their specimens in rather than collect the sample the day of the in-person study visit with the CHW. Most often, these women were menstruating on the day of the in-person study visit and thus elected to self-sample at a later date.
Figure 2.
Self-sampling completion by study arm and site
Table 2 presents unadjusted and adjusted logistic regression models of HPV self-sampling completion. None of the covariates listed below were associated with HPV self-sampling completion (all ps > 0.05). Models were adjusted for study site, age, income, insurance, education, Pap smear history, marital status, and citizenship status. Additionally, the adjusted models excluded individuals with missing insurance, education, or marital status. In the full adjusted model, the odds of HPV self-sampling completion remained similar across study arms and neighborhood sites.
Table 2.
Unadjusted and adjusted odds of self-sampling completion among women enrolled in a cervical self-sampling randomized controlled trial
| No. completed | Unadjusted | Adjusted (n=584) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | ||||
|
|
|
|
|||||||
| Study arm (n=599) | |||||||||
| SS+Mail | 214 | Ref | -- | -- | -- | Ref | -- | -- | -- |
| CHW+SS | 243 | 1.69 | 1.16 | 2.48 | <0.01 | 1.81 | 1.22 | 2.69 | <0.01 |
| Site (n=599) | |||||||||
| Hialeah | 159 | Ref | -- | -- | -- | Ref | -- | -- | -- |
| Little Haiti | 158 | 0.97 | 0.60 | 1.57 | .90 | 0.92 | 0.51 | 1.66 | .79 |
| South Dade | 140 | 0.61 | 0.39 | 0.97 | .04 | 0.75 | 0.45 | 1.26 | .28 |
| Age (per year; n=599) | 1.01 | 0.99 | 1.03 | .26 | 1.01 | 0.99 | 1.04 | .30 | |
| Income (n=599) | |||||||||
| ≤ $20,000 | 184 | Ref | -- | -- | -- | Ref | -- | -- | -- |
| > $20,000 | 62 | 1.71 | 0.90 | 3.24 | .10 | 1.69 | 0.82 | 3.46 | .15 |
| Unknown/not reported | 211 | 1.43 | 0.96 | 2.13 | .08 | 1.41 | 0.88 | 2.27 | .16 |
| Insurance (n=594) | |||||||||
| Insured | 151 | Ref | -- | -- | -- | Ref | -- | -- | -- |
| Uninsured | 304 | 0.90 | 0.60 | 1.36 | .62 | 0.99 | 0.63 | 1.56 | .96 |
| Education (n=593) | |||||||||
| HS or greater | 287 | Ref | -- | -- | -- | Ref | -- | -- | -- |
| < HS | 167 | 0.94 | 0.64 | 1.40 | .77 | 1.00 | 0.64 | 1.56 | 1.00 |
| Pap History (n=595) | |||||||||
| Never had a pap | 55 | Ref | -- | -- | -- | Ref | -- | -- | -- |
| Had a pap | 400 | 0.81 | 0.44 | 1.50 | .50 | 1.00 | 0.48 | 2.05 | .99 |
| Marital Status (n=595) | |||||||||
| Single, never married | 100 | Ref | -- | -- | -- | Ref | -- | -- | -- |
| Married or living with SO | 250 | 0.93 | 0.56 | 1.52 | .76 | 0.99 | 0.58 | 1.69 | .98 |
| Separated, divorced, or widowed | 106 | 0.73 | 0.42 | 1.29 | .28 | 0.64 | 0.35 | 1.18 | .16 |
| Citizenship (n=599) | |||||||||
| US Citizen | 128 | Ref | -- | -- | -- | Ref | -- | -- | -- |
| Permanent Resident | 182 | 0.96 | 0.58 | 1.57 | .86 | 0.87 | 0.51 | 1.48 | .61 |
| Unknown/not reported | 207 | 0.63 | 0.39 | 1.03 | .06 | 0.70 | 0.39 | 1.24 | .22 |
Models exclude data from individuals with missing or unknown insurance, education, pap history, or marital status. OR = Odds Ratio; ORs statistically significant (p<0.05) in bold.
Secondary Outcomes
Participants in both study arms were more likely to report having a usual source of care at six month follow up (46.7% to 62.7% in IP+SS and 39.9% to 56.8% in SS+Mail). Among participants in the SS+Mail arm, cervical cancer knowledge significantly increased from 39.0% of participants answering at least 50% of the questions correctly at baseline to 47.9% at 6-month follow-up. Change in cervical cancer knowledge was not significant among participants in the IP+SS arm. The proportion of individuals who reported having health insurance significantly increased from 32.5% to 42.0% in the IP+SS arm, but did not change significantly in the SS+Mail arm.
Discussion
Among ethnic minority and immigrant women living in South Florida, HPV self-sampling delivered via US mail resulted in comparable cervical cancer HPV self-sampling completion as HPV self-sampling delivered in-person by a CHW. Although there was a statistically significant difference in HPV self-sampling completion between our study arms, both methods of self-sampling delivery were highly successful, yielding over 70% self-sampling completion, which is substantially higher than the observed screening rates for the target communities [6]. While formal cost analyses have yet to be undertaken, the cost of an in-person CHW self-sampling visit may outweigh the benefit of increasing HPV self-sampling completion by only 10%. These results are somewhat consistent with previous studies of mailed HPV self-sampling conducted in Europe, most of which found this method to be at least equivalent to in-person delivery [11]. However, one notable difference is that the proportion of women having completed HPV self-sampling in the European studies was substantially lower than the proportion who completed the screening in our study, ranging from 10–35% vs. 70% [11]. Many of these prior studies involved self-sampling kits mailed from clinics. Our high success rate may be attributable to our intervention having been delivered by CHWs, who are members of our target communities and knowledgeable regarding cultural norms. Our CHWs were the first point of contact for women enrolled in our study and likely alleviated any distrust and concerns participants may have had with the cervical cancer screening process. Within our mailed self-sampling arm, CHWs provided culturally-tailored health education by phone, potentially enhancing HPV self-sampling completion. This method of phone delivery of health education in combination with mailed self-sampling kits may be a potentially cost-effective strategy for screening unscreened and under screened women within these at-risk communities. Our results highlight the importance of cultural competence in the development and delivery of cancer screening interventions among these underserved populations.
Additionally, intervention outcomes differed between our three target communities. In both Little Haiti and South Dade, a significantly greater proportion of women completed in-person screening than screening delivered via mail. This finding suggests that within some communities, providing in-person HPV self-sampling may optimize screening uptake. Our CHEs noted that some women in Little Haiti and South Dade were uncomfortable going to the post office to mail their samples, as this is a government-run facility and there were concerns with immigration status. This barrier to mailed HPV self-sampling completion would not have been likely among women in Hialeah, as this area is composed of mostly Cuban immigrants who have legal status per the recently-ended wet foot, dry foot policy [19]. However, it is important to note that within both Little Haiti and South Dade, mailed HPV self-sampling yielded self-sampling completion over 60%, which is substantial, and thus the mailed approach may also be a viable, cost effective screening strategy in these communities.
In addition to HPV self-sampling completion, our overall sample reported improvements in access to care (i.e. having a usual source of care) as well as in cervical cancer knowledge across the study period. Improvements in our secondary outcomes differed slightly between study groups—while both groups experienced improvements in access to care, the IP+SS group also experienced a significant increase in proportion of participants reporting having health insurance and the SS+Mail group experienced a significant increase in cervical cancer knowledge. Although improvements in secondary outcomes were not completely consistent between study groups, taken together, these findings indicate that both forms of the self-sampling intervention have the potential to improve care above and beyond increasing HPV self-sampling completion. The increase in access to care is particularly salient, as cervical cancer screening has no impact on cervical cancer risk without timely follow-up for those with abnormal results.
Limitations
The current study has a number of limitations that should be acknowledged. Firstly, we utilized one CHW per community to deliver each intervention to community members. Thus, variation in intervention outcomes between communities may have been due, in part, to variation in intervention delivery between our CHWs. To minimize any potential differences and ensure intervention fidelity, CHWs received extensive training, including formal CHW certification, and underwent monitoring of their study activities throughout study implementation. Future work to disseminate self-sampling approaches will likely utilize several CHWs within each community, such that consistency and reliability between CHWs can be better examined. Future work must also examine how our flexible approach to self-sampler return within IP+SS arm may have affected screening uptake. We allowed women who were unable to self-sample during the in-person study visit to mail their sample in at a later date. While only a small proportion (14%) of women in this arm elected to do so, we acknowledge that allowing this option may have unnecessarily inflated completion rates for IP+SS participants.
Additionally, we implemented our interventions within Miami’s unique underserved ethnic minority and immigrant communities (Haitian and Hispanic immigrants as well as African Americans), and we acknowledge that our results may not be generalizable to other underserved ethnic minority groups. However, given our successful approach, future research should examine whether mailed HPV self-sampling is a viable screening strategy in other groups that experience cervical cancer disparities. Future qualitative research is certainly needed to further understand unique barriers to screening uptake that may exist among low-income ethnic minority women. When study participants were asked about income, a large proportion reported they did not know their income, refused to disclose their income, or selected “not applicable,” which may reflect cultural taboos and discomfort with disclosing income among our study participants. Moreover, nearly a quarter of potential study participants either declined participation or were lost-to-follow up prior to randomization, which may imply that the intervention would be less successful among the hardest-to-reach women, and that, if disseminated broadly, the intervention may have lower rates of uptake in the general population
Finally, we note that while our mailed HPV self-sampling intervention was successful in leading to self-sampling completion among a large proportion of women, we do not yet have data regarding the proportion of women who adhered to necessary follow-up after testing positive for high-risk HPV. We acknowledge that screening is not effective unless follow-up for positive results is completed. Future studies should examine not only self-sampling completion but also adherence to follow-up care to further evaluate the effectiveness of this screening intervention.
Conclusion
Developing and disseminating acceptable, efficient, and cost-effective screening is paramount to eliminating cervical cancer disparities. Our findings demonstrate that HPV self-sampling delivered via mail is a viable and effective strategy for increasing cervical cancer screening among underserved ethnic minority and immigrant women living in South Florida. Future initiatives will examine the large-scale incorporation of this screening strategy within federally-qualified healthcare centers serving unscreened and under screened women throughout the state of Florida.
Figure 1.
PARTICIPANT ENROLLMENT DIAGRAM
Acknowledgments
We wish to thank our study team who played a critical role in ensuring completion of the study. These include our Community Health Workers, Valentine Cesar (Center for Haitian Studies), Maria Azqueta (Citrus Health Inc), and Linabell Lopez (Community Health Inc.), our Health Choice Network (HCN) Project Manager, Ludmilla Paul, as well as, our data managers Carmen Linarte and Feng Miao.
Funding: This work was supported by the National Cancer Institute (1R01CA183612).
Footnotes
Conflict of Interest: The authors have no potential conflicts of interest to disclose.
Ethical approval: All procedures performed in our study were in accordance with the ethical standards of our institutional review board and with the 1964 Helsinki declaration and its later amendments.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2013 Incidence and Mortality Web-based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2016. Available at www.cdc.gov/uscs Cited January 12, 2017. [Google Scholar]
- 2.Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017 doi: 10.1002/cncr.30507. [DOI] [PubMed] [Google Scholar]
- 3.Musselwhite W, Oliveira CM, Kwaramba T, et al. Racial/Ethnic Disparities in Cervical Cancer Screening and Outcomes. Acta Cytologica. 2016;60:518–526. doi: 10.1159/000452240. [DOI] [PubMed] [Google Scholar]
- 4.Lin L, Benard VB, Greek A, Hawkins NA, Roland KB, Saraiya M. Racial and ethnic differences in human papillomavirus positivity and risk factors among low-income women in Federally Qualified Health Centers in the United States. Prev Med. 2015;81:258–61. doi: 10.1016/j.ypmed.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Downs LS, Smith JS, Scarinci I, Flowers L, Parham G. The disparity of cervical cancer in diverse populations. Gynecologic Oncology. 2008;109:S22–S30. doi: 10.1016/j.ygyno.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Seay JS, Carrasquillo O, Campos NG, McCann S, Amofah A, Pierre L, et al. Cancer Screening Utilization Among Immigrant Women in Miami, Florida. Progress in Community Health Partnerships: Research, Education, and Action. 2015;9(Suppl):11–20. doi: 10.1353/cpr.2015.0029. [DOI] [PubMed] [Google Scholar]
- 7.Florida Cancer Data System. 2014 Available at http://fcds.med.miami.edu/inc/welcome.shtml.
- 8.Centers for Disease Control and Prevention. National Program of Cancer Registries (NPCR) Available at: http://www.cdc.gov/cancer/npcr/tools.htm.
- 9.Menard J, Kobetz E, Maldonado JC, Barton B, Blanco J, Diem J. Barriers to cervical cancer screening among Haitian immigrant women in Little Haiti, Miami. J Cancer Educ. 2010;25(4):602–8. doi: 10.1007/s13187-010-0089-7. [DOI] [PubMed] [Google Scholar]
- 10.Racey CS, Withrow DR, Gesink D. Self-collected HPV testing improves participation in cervical cancer screening: A systematic review and meta-analysis. Can J Public Health. 2013;104:e159–e166. doi: 10.1007/BF03405681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verdoodt F, Jentschke M, Hillemanns P, Racey CS, Snijders PJ, Arbyn M. Reaching women who do not participate in the regular cervical cancer screening program by offering self-sampling kits: A systematic review and meta-analysis of randomized trials. Eur J Cancer. 2015 doi: 10.1016/j.ejca.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Arbyn M, Verdoodt F, Snijders PJ, Verhoef VM, Suonio E, Dillner L, et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol. 2014;15(2):172–83. doi: 10.1016/S1470-2045(13)70570-9. [DOI] [PubMed] [Google Scholar]
- 13.Belinson JL, Du H, Yang B, Wu R, Belinson SE, Qu X, et al. Improved sensitivity of vaginal self-collection and high-risk human papillomavirus testing. Int J Cancer. 2012;130(8):1855–60. doi: 10.1002/ijc.26202. [DOI] [PubMed] [Google Scholar]
- 14.Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: Interim clinical guidance. Gynecol Oncol. 2015 Jan 6; doi: 10.1016/j.ygyno.2014.12.022. pii: S0090-8258(14)01577-7. [DOI] [PubMed] [Google Scholar]
- 15.Carrasquillo O, McCann S, Amofah A, et al. Rationale and Design of the Research Project of the South Florida Center for the Reduction of Cancer Health Disparities (SUCCESS): Study Protocol for a Randomized Controlled Trial. Trials. 2014;15:299. doi: 10.1186/1745-6215-15-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carrasquillo O, Seay J, Amofah A, et al. HPV Self-Sampling for Cervical Cancer Screening among Ethnic Minority Women in South Florida: A Randomized Trial. Journal of General Internal Medicine. doi: 10.1007/s11606-018-4404-z. Manuscript currently under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobetz E, Seay J, Amofah A, et al. Mailed HPV self-sampling for cervical cancer screening among underserved minority women: study protocol for a randomized controlled trial. Trials. 2017 Jan 13;18(1):19. doi: 10.1186/s13063-016-1721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiro JA, Meissner HI, Kobrin S, Chollette V. What do women in the U.S. know about human papillomavirus (HPV) and cervical cancer? Cancer Epidemiology, Biomarkers, and Prevention. 2007;16(2) doi: 10.1158/1055-9965.EPI-06-0756. [DOI] [PubMed] [Google Scholar]
- 19.Obama, Barack. (January 12th, 2017) “Statement by the President on Cuban Immigration Policy”. The White House. Retrieved June 26th, 2017