Abstract
The glymphatic system is a glial-dependent waste clearance pathway in the brain, in place of lymphatic vessels, dedicated to drain away soluble waste proteins and metabolic products. Specifically, the glymphatic network serves as a "front end" for waste clearance, and is connected downstream to an authentic lymphatic network, associated with dura covering the brain as well as cranial nerves and large vessels at the skull exits. The anatomical and functional interconnections between these two networks are not completely understood. Several key physiological processes have been identified that control glymphatic transport function and waste clearance from brain. In this review, we aim to provide an overview and discussion of the concept behind the glymphatic system, current evidence, and controversies, while specifically focusing on the consequences of aging and evidence of its existence in human brain. Discovering novel strategies for optimizing and maintaining efficient brain waste clearance across the lifespan may in the future prove to be important for preventing cognitive decline and sustaining healthy aging.
Keywords: cerebrospinal fluid transport, brain, aging, waste solutes, pulsatility, AQP4, glymphatic, perivascular space, sleep, human
Introduction
Waste removal from the central nervous system is essential for maintaining brain homeostasis across the lifespan. Two interconnected, dynamic networks were recently uncovered, which may provide new information concerning the conundrum of how the brain manages waste removal in the absence of authentic lymphatic vessels. The glymphatic system serves as the brain’s "front end" waste drainage pathway that includes a peri-vascular network for cerebrospinal fluid (CSF) transport [1,2], which is connected to a downstream authentic lymphatic network associated with the meninges (dura), cranial nerves and large vessels exiting the skull [3–5]. The anatomical and functional components of the two systems are complex and the processes by which they physically interconnect are only partly understood.
The first pioneering studies documented that soluble amyloid beta (Aβ) protein and tau oligomers – metabolic waste products whose build up is associated with Alzheimer’s disease (AD) – was transported from the interstitial fluid (ISF) space and out of the brain via the glymphatic system [1,6]. This information was followed by another hallmark study reporting that slow wave sleep enhanced glymphatic Aβ clearance from brain when compared to wakefulness [7]. Collectively, this information was met with excitement in the neuroscience and clinical communities because maintaining efficient brain waste drainage across the lifespan – possibly by preserving normal sleep architecture - emerged as a novel therapeutic target for preventing cognitive dysfunction and decline. The idea of maximizing brain ‘waste drainage’ as a new preventive or therapeutic target for neurodegenerative disease states was further strengthened by animal studies providing evidence of declining glymphatic transport efficiency in healthy aging [8], Alzheimer’s disease models [9], traumatic brain injury [6], cerebral hemorrhage [10] and stroke [11]. Considering the novelty of the glymphatic system concept, along with the rapidly emerging literature associating key physiological processes (e.g. vascular pulsatility [12,13], and sleep [7]) with glymphatic transport function and waste solute outflow from brain, we decided it was timely to review this information cohesively. Hence the goal of this mini-review is to provide a broad overview of the current data, controversies and gaps in knowledge of the glymphatic system and waste drainage from the brain, while addressing potential consequences of aging as well as critically reviewing evidence for its existence in the human brain.
The glymphatic system concept
The glymphatic system and waste clearance process in the rodent brain was originally described as a 3-step serial process as follows: 1) CSF is continuously transported from the basal cisterns and into the subarachnoid space covering the cerebral hemispheres; and from the subarachnoid space, CSF enters the peri-arterial spaces in a bulk-flow driven manner; 2) CSF is propelled from the peri-arterial compartment into the interstitial fluid (ISF) space facilitated by aquaporin 4 (AQP4) water channels on astroglia end-feet, a process enabling CSF-ISF mixing and waste solute removal; and 3) the CSF-ISF fluid mixed with interstitial waste solutes is subsequently transported towards the peri-venous compartment of the larger central veins (Fig. 1) from where it eventually exits into lymphatic vessels and the systemic circulation [1]. The new information pertaining to the glymphatic system concept was the proposed critical role of AQP4 water channels for rapid, high volume CSF transport from the peri-vascular compartment into ISF space and waste drainage [1]. The dependency of parenchymal CSF transport on AQP4 water channels was documented by quantifying CSF solute transport and clearance capabilities in the brain of mice deficient of AQP4 water channels (AQP4−/−) in comparison to controls [1]. These experiments showed that glymphatic transport of waste solutes including Aβ was significantly reduced in AQP4−/− mice when compared to controls [1]. However, this area is currently controversial. Thus, Smith et al., reported that movement of fluorescent albumin from CSF into brain parenchyma was similar in AQP4−/− mice as well as APQ4−/− rats when compared to controls [14]. Still, data (nonpeer reviewed preprint posted on bioRxiv.org) from three independent laboratories recently refuted the negative findings of Smith et al. ([14]), replicating the dependency of glymphatic transport on AQP4 water channels in three different APQ4−/− mice models [15]. Further, another recent report documented reduced parenchymal clearance of paramagnetic contrast in the thalamus (but not caudate) of AQP4−/− rats compared to controls [16]. Even such large apparent discrepancies in results are likely to reflect differences in experimental design (e.g. anesthetics used [17,18], surgical procedures including open skull procedures and parenchymal injection techniques including volume and pressure of the administered tracers). Although the field is at an early stage of knowledge about this delicately balanced system, it is already known that all of these factors can greatly affect solute transport patterns in the brain (reviewed by Brinker et al. [19]).
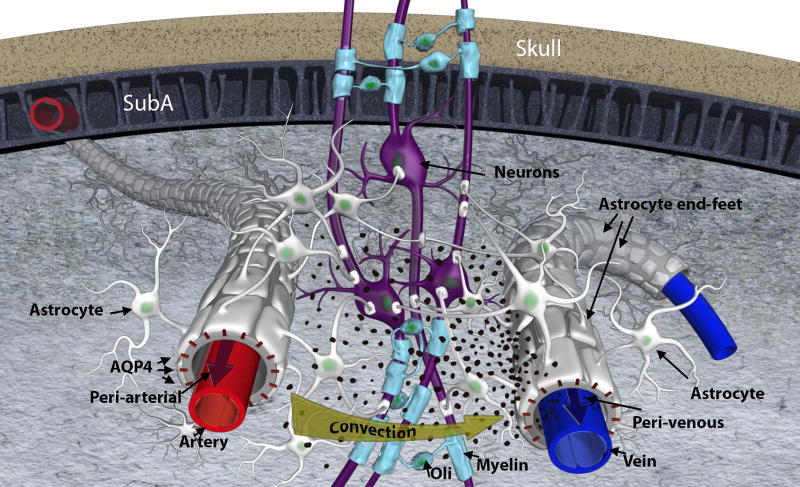
Fig 1. Glymphatic transport and waste drainage concept.
Original concept of the glymphatic transport [1], highlighting the periarterial and the perivenous space, and the astrocytic endfeet with aquaporin 4 (AQP4) water channels and forming a sheath around the blood vessels. Cerebrospinal fluid is driven by convection through the periarterial space and is propelled across the astroglia end-feet to mix with interstitial fluid and waste products. From there the waste and excess fluids are driven towards the peri-venous space, to ultimately be directed towards the lymphatic vessels and general circulation for breakdown and clearance. The black particles represent ‘waste’ particles in the interstitial fluid (e.g. amyloid-beta). SubA = subarachnoid space; Oli = oligodendrocyte; AQP4 = aquaporin 4 water channels.
Measurement of glymphatic transport
To characterize the physiological and physical processes underlying CSF and waste transport via the glymphatic system, objective and quantitative measures of its operation in live brain are required. Current strategies to track glymphatic transport in vivo, employ a wide variety of tracers (e.g. fluorescent dyes, MR paramagnetic contrast agents or radioactive ligands) administered into CSF which then act as surrogate ‘waste’ solutes which can be visualized by various imaging modalities as they pass through the brain. In most studies, the transport of CSF tracers from the peri-arterial conduits into brain tissue is referred to as glymphatic ‘influx’ while transport of the tracers out of brain tissue is referred to as glymphatic ‘clearance’ or ‘efflux’ [1,17,20,21]. In the mouse brain, glymphatic transport of small molecular weight (MW) tracers (Texas Red Dextran (TRd), MW 3000 Da) from the cisterna magna, subarachnoid space and into the ISF space of the cortex was visualized in vivo by 2-photon optical imaging [1]. These first 2-photon studies showed that the TRd transport from CSF to cortical ISF via the peri-vascular space of the penetrating arteries was fast (5–10 min); and further that the TRd molecules appeared to move freely in ISF [1]. Larger MW molecules such as FITC-dextran (MW 2000 kDa) also moved rapidly from the subarachnoid space to peri-arterial channels, but these particles did not pass into ISF due to their large size, because the gaps between the astroglial end-feet are narrow (~20–30 nm wide) [1]. Thus, when using large MW tracers to study the glymphatic system, only the peri-vascular component will be highlighted [22,23], and the critical glymphatic system components such as parenchymal influx and clearance are lost to study. For tracers that are capable of passing from the peri-vascular conduits into ISF, parenchymal influx as well as clearance of the tracers can be quantified dynamically in specific brain regions provided that the imaging method has sufficient temporal and spatial resolution [1,20,21].
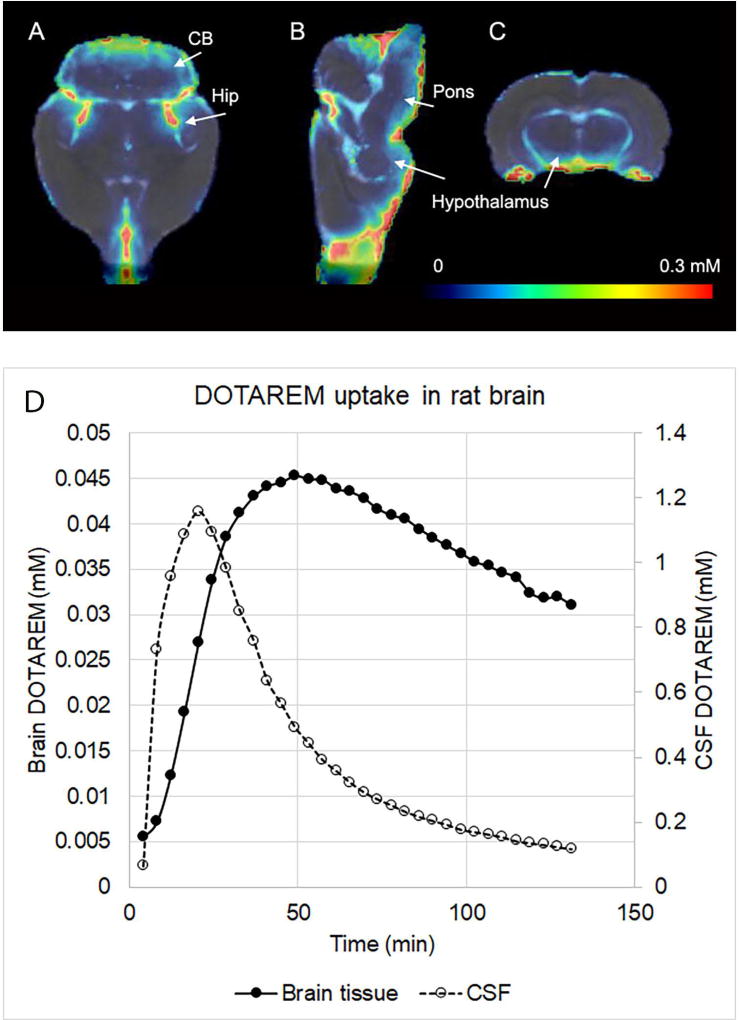
Glymphatic transport in the whole brain of rodents can be captured in vivo by dynamic contrast enhanced magnetic resonance imaging (MRI) by administering a small volume of paramagnetic contrast into CSF via the cisterna magna [20]. Fig. 2A shows population averaged (N=5) concentration maps of the macrocyclic MR contrast agent gadoterate meglumine (DOTAREM (MW 558.64 Da) in rat brain 1 hr after CSF administration via the cisterna magna (based on data from Lee et al. [24]). As shown, DOTAREM tissue uptake is evident into the cerebellum, hippocampus, hypothalamus and pons, in a concentration range of ~0.1–0.2mM (Fig. 2A–C). Fig. 2D shows the corresponding whole brain and CSF DOTAREM concentration as a function of time and also shows that the maximal retention (peak) of the contrast molecule in whole brain tissue and CSF is ~0.045mM and 1.2 mM, respectively. A similar anatomical pattern of the MR contrast agent uptake from CSF into the brain of non-human primates [10] and human brain via perivascular spaces [25] was documented recently, supporting the concept of the existence of a glymphatic system in evolutionary ‘higher’ species.
Fig. 2. Glymphatic transport measured by contrast enhanced MRI.
The population averaged (N=5) concentration maps from rat brain presented in three orthogonal planes (A–C), 1 hr after administration of the macrocyclic MR contrast agent gadoterate meglumine (DOTAREM, MW 558.64 Da) into the cisterna magna (based on [24]). The color coded DOTAREM maps are overlaid on the corresponding anatomical brain template and tissue uptake is evident into the cerebellum (CB), hippocampus (Hip), hypothalamus and pons, in a concentration range of ~0.1–0.2mM. Data based on Lee et al. 2017 [24]. CB=cerebellum; Hip=hippocampus. Color bar represents the DOTAREM concentration. D shows the corresponding whole brain and CSF DOTAREM concentrations as a function of time and it can be observed that the maximal retention of the contrast molecule in whole brain and CSF is ~0.045mM and 1.2 mM, respectively.
Glymphatic efflux pathways
While the peri-arterial influx pathways of the glymphatic system are easily captured by a variety of imaging techniques and described in great detail by several investigators [14,17,20,21,26,27], the efflux pathways are less well documented and will be reviewed in the following.
Glymphatic waste efflux pathways are most often characterized by administering solutes of interest (including Aβ and tau) into the CSF (via cisterna magna) or directly into brain parenchyma and after a pre-determined time of tracer circulation the animal will be euthanized [1,7,8]. Using this approach, the brain’s tracer loss over time (a.k.a. ‘clearance’) can be determined cross-sectionally in a series of animals exposed to incremental tracer CSF circulation times and compared across groups [1,7,8,21]. For direct visualization of glymphatic efflux pathways, the spatial distribution pattern of the tracer in the formalin-fixed brain tissue can be documented in relation to vascular and other anatomical landmarks using optical microscopy [1]. Using such ex-vivo techniques, Iliff et al. reported that tracers administered directly in the brain parenchyma accumulated along capillaries and large central veins including the caudal rhinal vein but not along arteries highlighting the peri-venous conduits as main drainage pathways of the glymphatic system [1]. It is important to compare these more recent results to previous and now classical reports of waste drainage pathways from the brain obtained with similar postmortem techniques. Thus, Cserr et al., reported that metabolically inert tracers such as dextran and horseradish peroxidase administered into the striatum of the rat brain drained along the peri-vascular spaces of arteries, arterioles (not capillaries) and veins [28]. Later, Carare et al., reported that small and large MW solutes administered into the striatum of mice were accumulating in the basement membranes of capillaries, arterioles and arteries which strongly suggested that these specific conduits acted as major waste drainage pathways [29,30]. While there appears to be agreement concerning the vascular basement membranes serving as drainage pathways [1,22,29], the controversy remains as to whether waste solutes drain against a pulse pressure gradient (i.e. upstream along arterioles and arteries) or downstream along veins (for more detail see excellent review by [31]). The discrepancies related to waste drainage and efflux pathways from the brain are likely due to differences in techniques and perhaps also influenced by post-mortem processing of the tissues. Although the fluorescent dyes administered into the tissue are aldehyde-fixable and therefore should not be erroneously redistributed during intravascular formalin perfusion fixation, any distribution pattern has to be interpreted cautiously due to artefacts which might arise during the fixation processing (e.g. shrinkage of the interstitial space [32] and collapse of vessels) and affect tracer distribution post-mortem. Clearly, in vivo imaging techniques with inherent larger field of views permitting capture of the whole brain at high temporal and spatial resolution are needed to resolve these important outstanding controversies pertaining to efflux pathways.
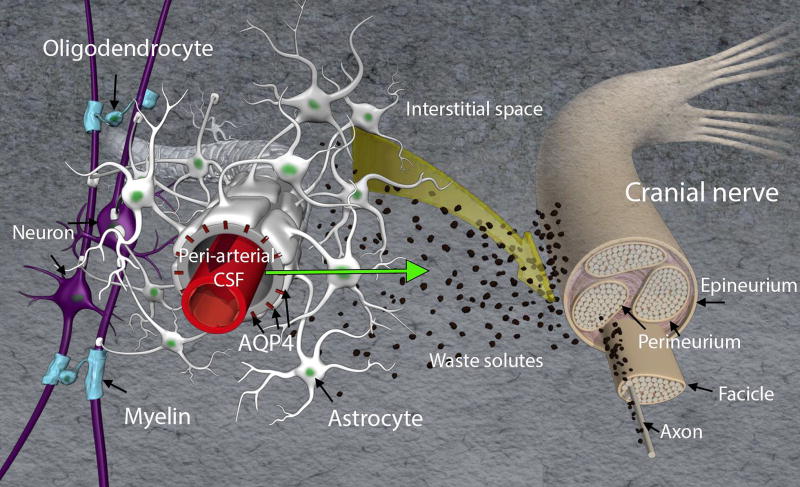
It has been known for decades that CSF communicates directly with the perineural space of cranial as well as peripheral nerves. In 1872 Quincke published seminal work on CSF transport and documented outflow pathways along intercostal nerves, lumbar and sacral nerves and cranial nerves including the olfactory nerves [33,34]. Quincke also documented that CSF drained solutes to the cervical lymph nodes [33,34]. These studies were later repeated with more elegant and modern techniques using smaller tracers showing that shortly after an injection of Indian ink into the cisterna magna of the rat, the carbon particles could be observed in the nasal mucosa and cervical lymph nodes [35]. In dynamic contrast-enhanced MRI studies, transport of the tracer out of the brain along the optical, olfactory, vagal, acoustic and hypoglossal nerves is also clearly documented in vivo following CSF administration [21,36] supporting the previous postmortem studies. Fig. 3 is a conceptual illustration of the perineural drainage pathways in relation to the glymphatic system from the point of view of a cranial peripheral nerve. It is currently unknown if interstitial waste solutes from the brain travel along the cranial nerves or inside the nerve itself (i.e. via the fascicles). It is also unknown if perineural drainage of waste solutes serves as major clearance routes after intraparenchymal administration and more systematic studies are needed.
Fig. 3. Conceptual medical illustration of brain waste clearance along cranial nerves.
The front-end of the glymphatic system is shown including peri-arterial, interstitial space and peri-arterial transport of CSF (green arrow). A cranial nerve is illustrated and waste solutes (in black) are shown to drain along the cranial nerve. Another possibility is that waste solutes with fluid enters the nerve and waste solutes are draining along fascicles and/or axons. It is currently not known if interstitial waste solutes travel along the cranial nerves or within the nerve itself (i.e. penetrating epineurium and draining along or inside fascicles). It is also unknown if perineural drainage of waste solutes serves as major clearance routes after intraparenchymal administration and more systematic studies are needed.
Another very recent study characterized CSF outflow pathways using tracers administered into CSF of Prox1-GFP mice (Prox1 is a marker of lymphatic endothelial cells and transgenic mice in which lymphatic endothelial cells express GFP are used to conveniently spot lymphatic vessels), in parallel with direct dynamic tracking of the outflow pathway from the brain using near infrared imaging [5]. They reported that the time interval from CSF tracer administration, and glymphatic circulation to detection of the tracer in LVs and cervical lymph nodes occurred fast (<10min) and was mostly active on the ventral skull surface at sites where cranial nerves and vessels exit; whereas the detection of the same tracers in the systemic circulation was delayed (>25 min) [5]. This temporal pattern strongly suggested that CSF and waste solutes predominantly drain directly into LVs along the exiting cranial nerves and not immediately into the venous circulation (e.g. dural sinuses) or into LVs along the dural sinuses as previously described [5]. The discrepancy between this and other studies in regards to the importance of meningeal LVs for brain drainage of waste and macromolecules is currently not understood.
Connections between glymphatic system and lymphatic vessels outside the brain
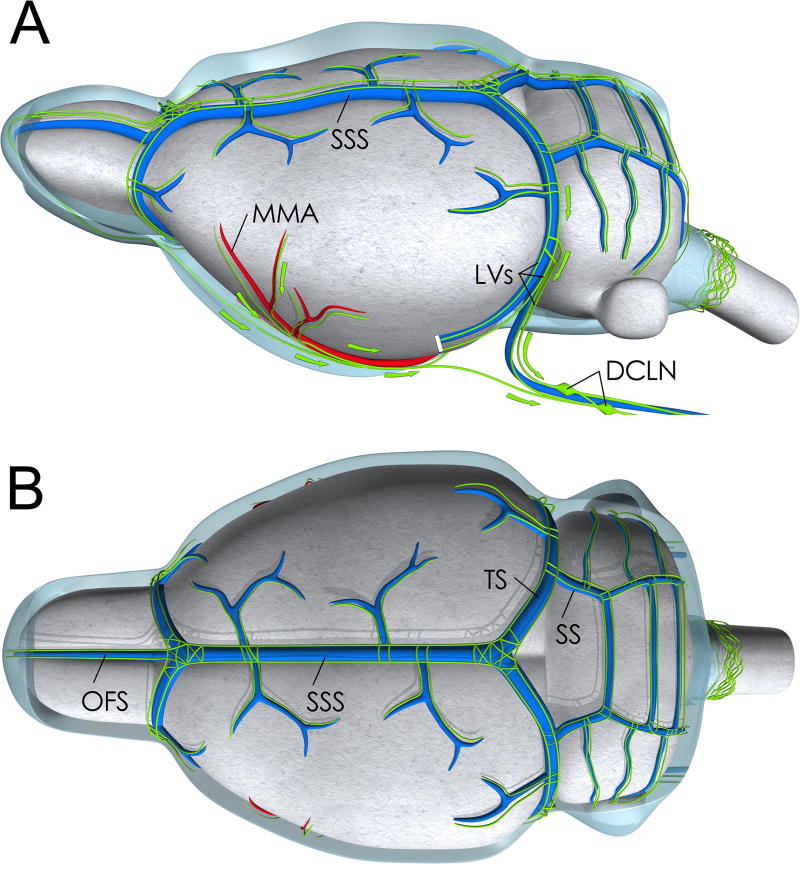
In the first introduction of the glymphatic pathway the physical merging of the peri-venous outflow from the brain parenchyma and downstream lymphatic circulation was suggested but not described in detail [1]. However, new data on the downstream lymphatic network and how it interconnects with the glymphatic pathway has recently emerged. First, authentic lymphatic vessels (LV) were confirmed at the level of the dural meninges [37] and more recently shown to be functionally capable of absorbing macromolecules from brain tissue and drain these to the deep cervical lymph nodes [3,4]. Second, lack of dural lymphatics in transgenic mouse models was shown to impede clearance of macromolecules from the brain to cervical lymph nodes [3,38]. The topography of the LV network associated with the dura has been mapped in the mouse (Fig. 4), non-human primate and human brain by means of immunohistochemistry and specific lymphatic endothelial markers [3,4,39]. In normal adult brain the topographic features are relatively consistent across species and includes LVs along the venous sinuses (e.g. superior sagittal sinus, transverse sinus, sigmoid sinus) and middle meningeal artery (Fig. 4) [3,4,39]. In the mouse brain LVs have also been demonstrated at the level of the pterygopalatine artery, rostral rhinal vein, cranial nerves at their exit points from the skull [38]. Presumably the LVs positioned at the level of the dura ensheathing these structures exit through cranial foramina along with major arteries, veins and cranial nerves as illustrated in Fig. 5 and ultimately connect to the cervical lymph nodes. Detailed topographic mapping of LV sprouting, maturation and growth during the first postnatal month was recently described in the murine CNS [38]. These and other studies show that LV growth is driven by vascular endothelial growth factor (VEGF) C; and VEGF receptor 3 signaling is required for LV maintenance in the adult murine CNS [38].
Fig. 4. Medical illustration of lymphatic vessels associated with larger vessels of the dura.
Medical illustration of the topography of the lymphatic vessel network imbedded in the dura (dura not shown) overlying the rodent brain based on work from several investigators [38,73,84]. A: Lateral view showing the dural sinuses including the superior sagittal sinus (SSS) and transverse sinus (TS). The lymphatic vessels are shown in green are running alongside the venous sinuses. Lymphatic vessels are also running alongside the middle meningeal artery (MMA), a larger dural artery originating from the external carotid artery. The lymphatic vessels (LV) drain into cervical lymph nodes including the deep cervical lymph nodes (DCLN). B: Top view of rat brain showing the topology of the lymphatic network associated with the dural sinuses and superficial veins. OFS = Olfactory sinus; SS=Sigmoid sinus.
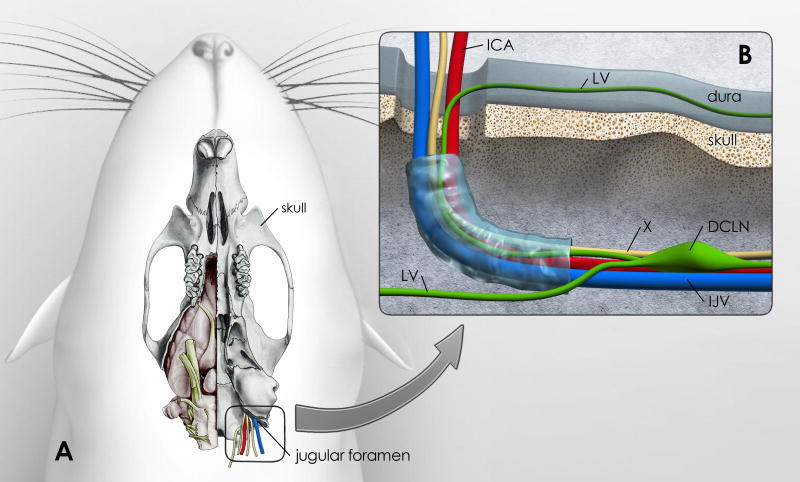
Fig. 5. Vessels, cranial nerves and lymph vessels at the site of the jugular foramen.
Fig. 5 is an illustration of how lymphatic vessels in the dura might drain to the deep cervical lymph nodes (DCLN). A: The ventral aspect of the rat skull is shown; the right side shows the brain in situ (modified from: [85]). The jugular foramen is highlighted and shows the exit of the vagal nerve (X), internal jugular vein (IJV) and internal carotid artery (ICA). B: High magnification of the area of the jugular foramen with the proposed exiting vessels and vagal nerve. Lymphatic vessels (LV) associated with dura lining the ventral surface of the skull might exit at this site into the DCLNs. This drawing is a proposed illustration of the connection between dural LVs and DCLNs.
Drivers of glymphatic transport
From the strong premise that the glymphatic system is involved in brain waste clearance and has been documented to be less efficient in the aging rodent brain [5,8], and in transgenic mouse models of AD [9] it becomes important to understand the controlling forces. As discussed, continuous CSF transport through brain parenchyma is critical for the glymphatic system’s ability to transport and drain waste solutes. Thus, any condition that affect CSF transport will also interfere with solute transport via the glymphatic system. Although the glymphatic system consists of multiple structural and functional components (Fig. 1) it is viewed as a ‘single waste processing unit’. Anatomical and physiological factors which are implicated in glymphatic pathway transport and brain waste clearance are likely to include: i) perivascular AQP4 water channels, ii) hydraulic forces associated with arterial pulsation [12,22], respiratory effort and body position [21], iii) normal CSF production and transport and iiii) state of arousal [7] (Box 1).
Aquaporin-4 (AQP4) water channels
AQP4 water channels are regulators of transcellular water flow and facilitate rapid movement of water across membranes in response to changes in tonicity [40,41]. The polarized high expression of AQP4 water channels on astroglial endfeet are viewed to be essential for efficient glymphatic influx as well as efflux [1]. Glymphatic influx often investigated using tracers administered into CSF (e.g. small molecular weight fluorescently tagged dyes or paramagnetic contrast molecules) which, do not pass through the AQP4 channels but are transported to the interstitial space via the small gaps between the astrocytic endfeet. In studies where tracers are administered directly into parenchyma it is assumed that their clearance from brain is also reflective of CSF transport via peri-vascular AQP4 channels. In other words, it is assumed that transport of the tracer of interest parallels mobility of ions and other molecules driven by the water flux via AQP4 channels. In the original study by Iliff, influx and clearance of Aβ1–40 was measured in normal and AQP4−/− mice using two different techniques [1]. At 30 min, influx of Aβ (measured via CSF administration) was reduced by 40% in AQP4−/− mice compared to controls whereas clearance (measured via striatal injection) was reduced by 20% in AQP4−/− mice compared to controls [1]. Thus, while influx as well as clearance was reduced in AQP4−/− mice supporting the concept that AQP4 channels are crucial for transport, the scales of ‘influx’ and ‘clearance’ were unbalanced for unknown reasons. Clearly, the techniques involved are imperfect and more innovative methods are needed to fully elucidate the role of AQP4 water channels on the various glymphatic transport components across the brain.
AQP4 immunohistochemistry studies of the mouse brain have shown that the expression of AQP4 is not uniform across the brain which, might reflect the regional heterogeneity observed in glymphatic transport function [20]. In mouse brain, the lowest AQP4 expression is documented in the cortex, followed by the hippocampus and the inferior colliculi; with the highest expression in cerebellum and spinal cord [42]. The effect of aging on the AQP4 water channel distribution pattern has also been investigated in mice and human brain. Kress et al., reported that the AQP4 polarization on the astroglia endfeet processes surrounding the cortical penetrating arterioles (but not the capillaries) was significantly reduced in old (18 month) mice when compared to young (2–3 month old) mice [8]. However, these data were not corroborated in recent study, reporting that AQP4 expression in membranes next to the capillary endothelial cells and arterioles was independent of age in both mice and humans [43]. The importance of AQP4 water channels have also been highlighted in rodent models of closed skull traumatic brain injury where glymphatic clearance is impaired in the area of injury due to the loss of peri-vascular AQP4 channels [6,44].
Physical forces driving glymphatic transport
There is currently debate in the literature in regards to the mix of physical forces driving solute transport in the brain. While some studies support glymphatic waste transport due to pressure-driven bulk motion of fluid referred to as ‘advection’ (independent on the solute’s MW) [1,20,45] others claim that diffusion forces (dependent on MW) govern [14,46]. Advection implies that hydraulic energy is associated with mass transport starting with the pressure head of CSF movement from the subarachnoid space into the peri-arterial space. It is highly likely that solute transport in the brain is governed by both processes (‘convection’ = ‘advection’ + diffusion) and the contribution of each may vary regionally across the brain. For example, in tissue areas with decreased AQP4 water channel expression, reduced peri-vascular polarization and sparse vascularization (e.g. white matter) diffusion forces may be more prominent. Considerable evidence in vivo supports convection driven transport in the periarterial space of the rodent brain because solute motion in this compartment appears to be independent of MW [1,20,47]. Further, Bedussi et al., injected microspheres into the CSF of rodents and documented oscillation of the particles and net positive displacement in direction parallel to blood flow at an average velocity of 17 µm/s in the peri-vascular space of leptomeningeal vessels in sync with the heart rate [22]. In additional support of rapid mass transport by convection including arterial pulse wave propagation, ligation of the internal carotid artery results in reduced glymphatic solute influx and increasing arteriolar pulsatility by dobutamine enhances glymphatic influx [12]. It is currently unclear if parenchymal waste clearance is similarly influenced by changes in pulsatility and this will need further investigation. Asgari et al., used computer simulation to model arterial pulse wave propagation and its effect on water and solute dynamics in the peri-arterial space and came to the conclusion that arterial pulsations alone are unlikely to produce pure or notable bulk flow [46]. However, Asgari et al. implemented a series of hemodynamic parameters in their modelling algorithms (e.g. arterial wall distention wave parameters) derived from both humans and rodents which might also have impacted their conclusions [46]. It has also been suggested that arterial pulsations could induce reverse flow of solutes in the peri-vascular space, however such a process which would require a valve-like mechanism which is not documented [48].
Another feature supporting pressure driven transport in CNS is related to intracranial pressure (ICP). For example, if large CSF reservoirs are open to atmospheric pressure (e.g. basal cisterns via cisternotomy), glymphatic clearance of solutes administered into brain parenchyma is significantly reduced [49]. This effect may be explained by decreased CSF transport into the peri-vascular and ISF space secondary to changes ICP. Under normal conditions ICP is a balance between CSF production and reabsorption and ICP is dependent on body position. For example, in humans ICP drops when posture is changed from supine to upright position [50] which, may also affect the pulsatile component of cerebral blood flow and therefore CSF transport [51]. In support of this, in rat studies it was shown that glymphatic transport and clearance of Aβ was decreased in the prone position (head up) when compared to lateral recumbent or supine position [21].
Pulsatility and vascular remodeling in aging brain
Arterial stiffening and reductions in compliance occurs progressively in aging and is marked by degeneration of the otherwise highly elastic lamellar architecture of the arterial wall [52,53]. Along with deposition of advanced glycation endproducts in the vessel wall, known to crosslink arterial wall proteins [54–56], and intimal and medial calcification [57], mechanical remodeling of the brain microvasculature contribute to the arterial stiffening seen in aging. Under normal conditions, the brain retains pressure pulsation throughout the capillary network [53], however, with increasing vessel stiffness due to aging, arterial pulse wave velocity and pulse pressure increases in the brain and transmits mechanical strain to even distal vessels [58,59]. Recent analysis of clinical data suggests that increased intracranial pulsatility may play a significant role in the pathophysiology of cerebral small vessel disease (SVD), which is responsible for up to 45% of dementia and 20% of all stroke occurrences worldwide [60]. Interestingly, Bedussi recently demonstrated that glymphatic bulk-flow driven transport in the hippocampus was enhanced in spontaneously hypertensive rats (SHR) which is an animal model of effects of hypertension on brain micro-vessels [45]. Specifically, in SHR rats, fluorescent tracers of different MW administered into the hippocampus spread much faster in the SHR when compared to the Wistar Kyoto (WKY) control rat. The increased ISF transport might be related to higher than normal pulse pressure and altered pulsatility in the SHR rats. Alternatively, the effect might be due to enhanced expression of AQP4 water channels in SHR rats compared to controls [61].
State of arousal
One of the most exciting studies concerning the glymphatic pathway was the report of the enhancing effect of sleep on influx and clearance of waste solutes including Aβ. Thus, in rodents, glymphatic influx was increased by 95% and Aβ was cleared 2-fold faster in the cortex during slow wave sleep (or anesthesia with ketamine/xylazine) when compared to wakefulness [7]. Because sleep is associated with improved brain waste clearance when compared to wakefulness, it is inferred that all drug-induced low arousal/sleep states will promote brain waste clearance. However, the basis for sleep-induced enhancement of glymphatic transport appears to be closely linked to central norepinephrine tone/activity. In agreement with this concept, we recently showed that rats receiving dexmedetomidine (which blocks norepinephrine release from the locus coeruleus) in combination with low-dose isoflurane exhibit a 30% higher glymphatic transport function relative to rats receiving isoflurane alone [62]. This finding may be important for clinical practice because dexmedetomidine or other drugs that reduce central norepinephrine signaling could have the added benefit of improving ‘brain waste removal’ during anesthesia and surgery. The beneficial effect of dexmedetomidine in the context of glymphatic transport might also explain why the use of dexmedetomidine decrease the incidence of postoperative delirium [63].
Does a glymphatic system exist in the human brain?
It is currently unknown if a glymphatic system for waste drainage as described in rodents [1] exists in the human brain. Clearly, fundamental anatomical and physiological differences exist between the two species which need to be considered. First, the cerebral metabolic rate of glucose utilization in young adult rat (non-anesthetized) is twice as high as that of adult human and non-human primate brain [64] which would infer that all metabolic fluxes including waste production will change in proportion to overall metabolic rate in rodent brain when compared to humans, at least in resting subjects where nearly all glucose is oxidized. Second, the much larger brain mass, greater ratio of white-to-gray matter, and surface area in human brain compared to rodent brain in regards to CSF transport and existence of a glymphatic system would also be important differences to consider. Third, from the point of view of vascular pulsatility the adult rodent has much higher resting heart rates (mice ~500 bpm; rats ~300 bpm) compared to humans (~60–70bpm) which would also theoretically drive higher overall glymphatic transport rates in the smaller species compared to humans. Nevertheless, rodent as well as human brain parenchyma is devoid of authentic lymphatic vessels, both species produce toxic metabolic waste products (e.g. Aβ, alpha-synuclein or lactate), and both species develop cognitive decline with normal aging suggesting that metabolic waste clearance in brain is an inherent vulnerability across species.
Similar to the rodent brain, evidence of a glymphatic system for solute waste removal in human brain would need to include: 1) CSF influx from subarachnoid space into peri-arterial space, 2) AQP4-dependent CSF transport from the peri-arterial space into the parenchymal ISF space, 3) peri-venous drainage of brain interstitial waste products and 4) confirmation of connections between peri-venous conduits and lymphatic circulation outside brain. In addition, one could add to this list evidence of increased brain waste clearance during sleep compared to wakefulness, given the rodent studies demonstrating the glymphatic systems’ dependency on state of arousal [7]. Peri-arterial CSF transport is dependent on normal CSF production and volume (i.e. CSF drainage via cisternotomy has been shown to impede glymphatic transport in mice [49]). The major portion of CSF is produced in the choroid plexus, and the CSF formation rate in the rat has been reported to be 300-fold higher compared to the human brain [65]. Further, CSF turnover rate in the young normal rat brain is 11 volumes/day; whereas the larger human brain turnover CSF 4 times/day [65]. In the rat brain CSF formation rate and turnover decreases with age in agreement with data demonstrating that glymphatic clearance also decreases with age [8]. In humans CSF transport and turnover is also affected by aging and by neurodegenerative disease states including iNPH. iNPH belongs to a subset of communicating hydrocephalus seen in the elderly and is defined by the clinical triad of gait disturbance, dementia, and incontinence. Ringsted et al. recently characterized CSF transport in normal human brain (examined for dural leaks) and subjects with iNPH using the MRI contrast agent ‘gadobutrol’ [25,66] which has been shown to be transported via the ‘glymphatic’ pathway in rodents [20,21,24]. In the human study, gadobutrol (0.5 ml of Gadovist® 1.0mmol/ml) was administered as a bolus injection into CSF via the lumbar intrathecal route and dynamic contrast-enhanced MRI was performed at various time intervals over a 1–2 day period [25]. Peri-arterial contrast was observed along cerebral arteries, and CSF signal enhancement peaked at 1–2 hrs in control (non-iNPH) patients and at ~5 hrs in iNPH patients following contrast administration and the influx magnitude varied regionally [25] similar to the rodent brain [20]. Parenchymal contrast uptake was slower in human brain compared to rodent brain which may relate to differences in vascular pulsatility, metabolic rate, larger brain mass, and expression pattern of AQP4.
Although AQP4 dependent CSF influx from peri-arterial to ISF has not been described in human brain, the subcellular distribution pattern of AQP4 expression in human brain is largely similar to the mouse brain [43]. In both species APQ4 channels are concentrated on the astroglia endfeet facing the endothelial cells – so-called ‘perivascular polarization’ [43]. However, perivascular AQP4 densities were reported to be 1/3 lower in human brains compared to mice and the density of APQ4 channels over parenchymal astrocytic membranes were higher in human than in mice and [43]. The differences in AQP4 distribution might infer ~30% more efficient parenchymal transport and waste drainage in the small mouse brain via a glymphatic system compared to human brain, or perhaps less dependence on the AQP4 and more dependence on other systems in larger mammalian brains. Alternatively, differences in gray-to-white matter volumes in rat versus human brain might also relate to AQP4 ‘efficiency’, because humans have a lot more white matter than grey matter compared to mice; and white matter is characterized by decreased expression and endfeet polarization of AQP4 compared to grey matter [67]. Another study demonstrated that perivascular AQP4 polarized localization was preserved in cognitively intact elderly individuals but not in tissue from age-matched individuals with AD [68]. Future studies addressing species differences in AQP4 more dynamically will be important to further address the existence of a glymphatic system in human brain.
In the recent study by Ringstad et al, [25], MR contrast in CSF was observed to penetrate along arteries as well as into brain parenchyma, however the superior sagittal venous sinus was not enhancing at any time point suggesting that dural sinuses (and arachnoid villi) are not major drainage pathways for interstitial solute waste, or that the rate of drainage in to the venous sinuses was too slow to show up as an increase in the blood signal. A recent study addressed clearance of the MR contrast agent from human entorhinal cortex [66]. MR contrast enhancement peaked at 6–9 hrs and disappeared from the entorhinal cortex of normal subjects at 24 hrs and was slower in iNPH patients [66]. However, this study did not quantify clearance rates, and specific anatomical drainage pathways were not identified. Thus there appears to be no direct documentation in human brain of waste clearance in relation to large central veins as described in rodent brain [1]. On the contrary, based on data from patients with sporadic cerebral amyloid angiopathy where Aβ is observed to accumulate in the wall of cortical cerebral arteries and arterioles as well as in leptomeningeal arteries [69–71] it has been suggested that waste clearance in human brain does not follow a glymphatic transport pattern [29,30,71] as originally described [1].
However, other waste drainage pathways have been highlighted in human brain. A recent study by de Leon et al. performed in humans used dynamic positron emission tomography (PET) to track egress of CSF from brain after IV injection of 18F-THK5117, a tracer for tau pathology [72]. By correlating the time activity curves of the 18F-THK5117 tau tracer in CSF with voxels exhibiting the same temporal profiles in extracranial areas the nasal turbinates were highlighted and provided evidence of tau CSF clearance along olfactory nerves [72]. Interestingly, the same study showed that CSF egress was less in AD patients compared to normal subjects and that ventricular CSF clearance was inversely correlated with amyloid deposition [72]. Although this study addresses CSF transport and tau clearance and support waste clearance from human brain they do not directly support the existence of a glymphatic system.
It is not fully understood how metabolic waste from brain end up in cervical lymph nodes [4,73,74]. It has been suggested that waste solutes from the ISF travel retrograde along arteries to subarachnoid space and from there perhaps reach the cervical lymph nodes via the cribriform plate and nasal lymphatics [29,75]. This would imply that waste solutes are transported against an arterial pulse propagation wave which is not supported by peri-vascular pulsatility studies in normal rodent brain [26]. Clearly new studies implementing more advanced tools and noninvasive approaches to allow tracking endogenous waste substances in ISF and subsequent drainage to the neck in live human subjects will be required.
Faster glymphatic transport and waste clearance during sleep in rodents was associated enhanced clearance of soluble Aβ from brain tissue [7]. Several new studies correlating Aβ levels in CSF and/or brain of humans with changes in sleep architecture and hours of arousal have recently emerged supporting the presence of a glymphatic system in the human brain. These studies include evidence of 1) increases in Aβ in brain after acute sleep deprivation [76], 2) increases in CSF Aβ with disruption of slow wave sleep [77], 3) increases in CSF as well as brain Aβ after 24 hrs of sleep deprivation [78], 4) correlation between self-reported sleep duration and Aβ accumulation in the brain [79] and 5) circadian variation in CSF Aβ and tau [80]. The faster glymphatic transport and waste clearance during sleep in rodents was also associated with a 40–60% increase in ISF space volume measured in the cortex [7]. Under awake conditions the ISF space volume is ~20% of total brain volume which is more restrictive to solute transport when compared to slow wave sleep where in cortex it expands to 40% inferring that more CSF will enter into the glymphatic pathway to facilitate waste drainage [7]. The mechanisms underlying the sleep-induced changes on ISF space volume are only partly understood but in animals shown to relate to central norepinephrine activity [7] and the concentration of extracellular ions [13]. Clinical MRI studies have documented diurnal changes in the brain water diffusivity [81] and changes in white matter diffusivity following acute sleep deprivation when compared to those measured after rested sleep [82], also suggesting a relationship between ISF volume and hours of arousal. However, acute changes in brain diffusivity during sleep itself as compared to that of the awake state have not yet been demonstrated in humans.
Recently it also became possible to capture the anatomical distribution pattern of meningeal LVs in the live human brain by MRI [39]. The visualization of LVs was based on the fact that the small molecular weight MR contrast molecule (Gadobutrol), leaks out of the dural blood vessels after intravenous administration and travels from the dural ISF space into adjacent LVs [39]. The ability to capture LVs in CNS using the specified MRI contrast enhancement protocol holds promise for visualizing LVs in normal subjects and subjects with neurodegenerative diseases by same technical approach. Given that clearance of MR contrast from brain parenchyma is delayed in iNPH compared to controls subjects one could hypothesize that an underlying cause of iNPH in the elderly is impaired CSF and waste drainage via the glymphatic pathway and possibly also an abnormal downstream meningeal lymphatic network. Specifically, given that animal studies have shown that 1) VEGF-C; and VEGFR-2 receptor signaling is required for LV maintenance in the adult CNS and 2) VEGF is elevated in the CSF of patients with hydrocephalus [83] it could be hypothesized that iNPH patients might develop an increased meningeal LV network which could serve as a diagnostic marker. More generally, establishing a routine method to visualize meningeal lymphatic anatomy by MRI, may have potentially wide applicability across brain disorders that may associate with as yet unidentified dysregulations in brain waste clearance.
Summary
This review summarizes recent work that has implicated the glymphatic system and meningeal lymphatic network as a tightly connected clearance system that can efficiently perform this task within the distinct environment of the brain. The glymphatic system has revived the field of CSF fluid transport and highlighted its role as a mechanism for waste drainage from the central nervous system. A large body of experimental evidence has emerged over the last few years suggesting that an efficiently functioning glymphatic system may be important for maintaining brain health across the life span. The implication of slow wave sleep for enhancing brain waste drainage including Aβ clearance has escalated research efforts focused on uncovering the different mechanisms involved in maintaining and controlling the complex system. Imaging studies visualizing CSF transport and meningeal lymphatic vessels have contributed new information adding to a beginning understanding of the drainage pathways in the live animal and human brain. However, along with novel information on glymphatic system function, unexpected and controversial findings have also emerged. Examples of unresolved issues include the physical forces driving solute transport in brain parenchyma, that is, bulk flow or passive diffusion. Other important gaps in knowledge relates to whether or not waste solutes are transported from periarteriolar to perivenular conduits before exiting the head. Further, although meningeal authentic lymphatic vessels are identified their total capacity for waste and fluid drainage is unknown; and they may serve as outlets for larger macromolecules and cells rather than small molecular weight metabolic waste solutes. Future studies with more effective functional imaging tools able to track endogenous waste molecules directly in brain parenchyma can provide further insight into the functioning of these clearance processes in human brain in normal health and disease states.
Acknowledgments
NIH R01AG048769, RF1 AG053991 and Leducq Foundation (16/CVD/05)
References
- 1.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Science translational medicine. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nedergaard M. Neuroscience. Garbage truck of the brain. Science. 2013;340:1529–1530. doi: 10.1126/science.1240514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma Q, Ineichen BV, Detmar M, Proulx ST. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat Commun. 2017;8:1434. doi: 10.1038/s41467-017-01484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, Singh I, Deane R, Nedergaard M. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:16180–16193. doi: 10.1523/JNEUROSCI.3020-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew JA, Plog BA, Ding F, Deane R, Nedergaard M. Impairment of paravascular clearance pathways in the aging brain. Annals of neurology. 2014;76:845–861. doi: 10.1002/ana.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng W, Achariyar TM, Li B, Liao Y, Mestre H, Hitomi E, Regan S, Kasper T, Peng S, Ding F, Benveniste H, Nedergaard M, Deane R. Suppression of glymphatic fluid transport in a mouse model of Alzheimer's disease. Neurobiology of disease. 2016;93:215–225. doi: 10.1016/j.nbd.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goulay R, Flament J, Gauberti M, Naveau M, Pasquet N, Gakuba C, Emery E, Hantraye P, Vivien D, Aron-Badin R, Gaberel T. Subarachnoid Hemorrhage Severely Impairs Brain Parenchymal Cerebrospinal Fluid Circulation in Nonhuman Primate. Stroke. 2017;48:2301–2305. doi: 10.1161/STROKEAHA.117.017014. [DOI] [PubMed] [Google Scholar]
- 11.Gaberel T, Gakuba C, Goulay R, Martinez De Lizarrondo S, Hanouz JL, Emery E, Touze E, Vivien D, Gauberti M. Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI: a new target for fibrinolysis? Stroke. 2014;45:3092–3096. doi: 10.1161/STROKEAHA.114.006617. [DOI] [PubMed] [Google Scholar]
- 12.Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, Deane R, Nedergaard M. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:18190–18199. doi: 10.1523/JNEUROSCI.1592-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding F, O'Donnell J, Xu Q, Kang N, Goldman N, Nedergaard M. Changes in the composition of brain interstitial ions control the sleep-wake cycle. Science. 2016;352:550–555. doi: 10.1126/science.aad4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith AJ, Yao X, Dix JA, Jin BJ, Verkman AS. Test of the 'glymphatic' hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. Elife. 2017;6 doi: 10.7554/eLife.27679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mestre H, Kress BT, Zou W, Pu T, Murlidharan G, Castellanos Rivera RM, Simon MJ, Pike MM, Plog BA, Xavier ALR, Thrane AS, Lundgaard I, Thomas JH, Xiao M, Asokan A, Iliff JJ, Nedergaard M. Aquaporin-4 dependent glymphatic solute transport in rodent brain. bioRxiv. 2017 doi: 10.7554/eLife.40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teng Ze WA, Peng Wang, Rui Wang, Wei Wang, Hongbin Han. The Effect of Aquaporin-4 Knockout on Interstitial Fluid Flow and the Structure of the Extracellular Space in the Deep Brain. doi: 10.14336/AD.2017.1115. A&D:0- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benveniste H, Lee H, Ding F, Sun Q, Al-Bizri E, Makaryus R, Probst S, Nedergaard M, Stein EA, Lu H. Anesthesia with Dexmedetomidine and Low-dose Isoflurane Increases Solute Transport via the Glymphatic Pathway in Rat Brain When Compared with High-dose Isoflurane. Anesthesiology. 2017 doi: 10.1097/ALN.0000000000001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gakuba C, Gaberel T, Goursaud S, Bourges J, Di Palma C, Quenault A, de Lizarrondo SM, Vivien D, Gauberti M. General Anesthesia Inhibits the Activity of the "Glymphatic System". Theranostics. 2018;8:710–722. doi: 10.7150/thno.19154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brinker T, Stopa E, Morrison J, Klinge P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS. 2014;11:10. doi: 10.1186/2045-8118-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, Benveniste H. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123:1299–1309. doi: 10.1172/JCI67677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee H, Xie L, Yu M, Kang H, Feng T, Deane R, Logan J, Nedergaard M, Benveniste H. The Effect of Body Posture on Brain Glymphatic Transport. J Neurosci. 2015;35:11034–11044. doi: 10.1523/JNEUROSCI.1625-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bedussi B, van der Wel NN, de Vos J, van Veen H, Siebes M, VanBavel E, Bakker EN. Paravascular channels, cisterns, and the subarachnoid space in the rat brain: A single compartment with preferential pathways. J Cereb Blood Flow Metab. 2017;37:1374–1385. doi: 10.1177/0271678X16655550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA. Evidence for a 'paravascular' fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985;326:47–63. doi: 10.1016/0006-8993(85)91383-6. [DOI] [PubMed] [Google Scholar]
- 24.Lee H, Mortensen K, Sangsgaard S, Koch P, Brunner H, Quistorff B, Nedergaard M, Benveniste H. Quantitative Gd-DOTA uptake from cerebrospinal fluid into rat brain using 3D VFA-SPGR at 9.4T. Magn Reson Med. 2017 doi: 10.1002/mrm.26779. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ringstad G, Vatnehol SAS, Eide PK. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain. 2017;140:2691–2705. doi: 10.1093/brain/awx191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bedussi B, Almasian M, de Vos J, VanBavel E, Bakker EN. Paravascular spaces at the brain surface: Low resistance pathways for cerebrospinal fluid flow. J Cereb Blood Flow Metab. 2017 doi: 10.1177/0271678X17737984. 271678X17737984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikomi F, Kawai Y, Ohhashi T. Recent advance in lymph dynamic analysis in lymphatics and lymph nodes. Ann Vasc Dis. 2012;5:258–268. doi: 10.3400/avd.ra.12.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cserr HF. Role of secretion and bulk flow of brain interstitial fluid in brain volume regulation. Ann N Y Acad Sci. 1988;529:9–20. doi: 10.1111/j.1749-6632.1988.tb51415.x. [DOI] [PubMed] [Google Scholar]
- 29.Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JA, Perry VH, Weller RO. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol. 2008;34:131–144. doi: 10.1111/j.1365-2990.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- 30.Weller RO, Subash M, Preston SD, Mazanti I, Carare RO. Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer's disease. Brain Pathol. 2008;18:253–266. doi: 10.1111/j.1750-3639.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hladky SB, Barrand MA. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS. 2014;11:26. doi: 10.1186/2045-8118-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Harreveld A, Khattab FI. Changes in extracellular space of the mouse cerebral cortex during hydroxyadipaldehyde fixation and osmium tetroxide post-fixation. J Cell Sci. 1969;4:437–453. doi: 10.1242/jcs.4.2.437. [DOI] [PubMed] [Google Scholar]
- 33.Benveniste H, Hof PR, Nedergaard M, Bechter K. Modern cerebrospinal fluid flow research and Heinrich Quincke's seminal 1872 article on the distribution of cinnabar in freely moving animals. The Journal of comparative neurology. 2015;523:1748–1755. doi: 10.1002/cne.23758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quinke H. Zur Physiologie der Cerebrospinalfluessigkeit. Arch Anat Physiol Wiss Med. 1872:153–177. [Google Scholar]
- 35.Kida S, Pantazis A, Weller RO. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol. 1993;19:480–488. doi: 10.1111/j.1365-2990.1993.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 36.Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, Benveniste H. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123:1299–1309. doi: 10.1172/JCI67677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, Zhou J, Shi Y. Scanning electron microscopy of human cerebral meningeal stomata. Annals of anatomy = Anatomischer Anzeiger : official organ of the Anatomische Gesellschaft. 1996;178:259–261. doi: 10.1016/S0940-9602(96)80059-8. [DOI] [PubMed] [Google Scholar]
- 38.Antila S, Karaman S, Nurmi H, Airavaara M, Voutilainen MH, Mathivet T, Chilov D, Li Z, Koppinen T, Park JH, Fang S, Aspelund A, Saarma M, Eichmann A, Thomas JL, Alitalo K. Development and plasticity of meningeal lymphatic vessels. J Exp Med. 2017;214:3645–3667. doi: 10.1084/jem.20170391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Absinta M, Ha SK, Nair G, Sati P, Luciano NJ, Palisoc M, Louveau A, Zaghloul KA, Pittaluga S, Kipnis J, Reich DS. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife. 2017;6 doi: 10.7554/eLife.29738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Day RE, Kitchen P, Owen DS, Bland C, Marshall L, Conner AC, Bill RM, Conner MT. Human aquaporins: regulators of transcellular water flow. Biochim Biophys Acta. 2014;1840:1492–1506. doi: 10.1016/j.bbagen.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 41.Zeuthen T. General models for water transport across leaky epithelia. Int Rev Cytol. 2002;215:285–317. doi: 10.1016/s0074-7696(02)15013-3. [DOI] [PubMed] [Google Scholar]
- 42.Hoddevik EH, Khan FH, Rahmani S, Ottersen OP, Boldt HB, Amiry-Moghaddam M. Factors determining the density of AQP4 water channel molecules at the brain-blood interface. Brain structure & function. 2017;222:1753–1766. doi: 10.1007/s00429-016-1305-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eidsvaag VA, Enger R, Hansson HA, Eide PK, Nagelhus EA. Human and mouse cortical astrocytes differ in aquaporin-4 polarization toward microvessels. Glia. 2017;65:964–973. doi: 10.1002/glia.23138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ren Z, Iliff JJ, Yang L, Yang J, Chen X, Chen MJ, Giese RN, Wang B, Shi X, Nedergaard M. 'Hit & Run' model of closed-skull traumatic brain injury (TBI) reveals complex patterns of post-traumatic AQP4 dysregulation. J Cereb Blood Flow Metab. 2013;33:834–845. doi: 10.1038/jcbfm.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bedussi B, Naessens DM, de Vos J, Olde Engberink R, Wilhelmus MM, Richard E, Ten Hove M, vanBavel E, Bakker EN. Enhanced interstitial fluid drainage in the hippocampus of spontaneously hypertensive rats. Sci Rep. 2017;7:744. doi: 10.1038/s41598-017-00861-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asgari M, de Zelicourt D, Kurtcuoglu V. Glymphatic solute transport does not require bulk flow. Sci Rep. 2016;6:38635. doi: 10.1038/srep38635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolf DA, Hesterman JY, Sullivan JM, Orcutt KD, Silva MD, Lobo M, Wellman T, Hoppin J, Verma A. Dynamic dual-isotope molecular imaging elucidates principles for optimizing intrathecal drug delivery. JCI Insight. 2016;1:e85311. doi: 10.1172/jci.insight.85311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schley D, Carare-Nnadi R, Please CP, Perry VH, Weller RO. Mechanisms to explain the reverse perivascular transport of solutes out of the brain. J Theor Biol. 2006;238:962–974. doi: 10.1016/j.jtbi.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 49.Plog BA, Dashnaw ML, Hitomi E, Peng W, Liao Y, Lou N, Deane R, Nedergaard M. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:518–526. doi: 10.1523/JNEUROSCI.3742-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andresen M, Hadi A, Petersen LG, Juhler M. Effect of postural changes on ICP in healthy and ill subjects. Acta Neurochir (Wien) 2015;157:109–113. doi: 10.1007/s00701-014-2250-2. [DOI] [PubMed] [Google Scholar]
- 51.Pickard JD, Czosnyka M. Management of raised intracranial pressure. Journal of neurology, neurosurgery, and psychiatry. 1993;56:845–858. doi: 10.1136/jnnp.56.8.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Avolio A, Chen S-G, Wang R-P, Zhang C-L, Li M-F, O'rourke M. Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation. 1983;68:50–58. doi: 10.1161/01.cir.68.1.50. [DOI] [PubMed] [Google Scholar]
- 53.O'Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension (Dallas, Tex: 1979) 2005;46:200–204. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- 54.Kass DA, Shapiro EP, Kawaguchi M, Capriotti AR, Scuteri A, deGroof RC, Lakatta EG. Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation. 2001;104:1464–1470. doi: 10.1161/hc3801.097806. [DOI] [PubMed] [Google Scholar]
- 55.McNulty M, Mahmud A, Feely J. Advanced glycation end-products and arterial stiffness in hypertension. American journal of hypertension. 2007;20:242–247. doi: 10.1016/j.amjhyper.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 56.Semba RD, Nicklett EJ, Ferrucci L. Does Accumulation of Advanced Glycation End Products Contribute to the Aging Phenotype? The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2010;65a:963–975. doi: 10.1093/gerona/glq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Atkinson J. Age-related medial elastocalcinosis in arteries: mechanisms, animal models, and physiological consequences. Journal of applied physiology (Bethesda, Md:1985) 2008;105:1643–1651. doi: 10.1152/japplphysiol.90476.2008. [DOI] [PubMed] [Google Scholar]
- 58.Tsao CW, Seshadri S, Beiser AS, Westwood AJ, DeCarli C, Au R, Himali JJ, Hamburg NM, Vita JA, Levy D, Larson MG, Benjamin EJ, Wolf PA, Vasan RS, Mitchell GF. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology. 2013;81:984–991. doi: 10.1212/WNL.0b013e3182a43e1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee HY, Oh BH. Aging and arterial stiffness. Circulation journal : official journal of the Japanese Circulation Society. 2010;74:2257–2262. doi: 10.1253/circj.cj-10-0910. [DOI] [PubMed] [Google Scholar]
- 60.Hachinski V, World Stroke O. Stroke and Potentially Preventable Dementias Proclamation: Updated World Stroke Day Proclamation. Stroke. 2015;46:3039–3040. doi: 10.1161/STROKEAHA.115.011237. [DOI] [PubMed] [Google Scholar]
- 61.Tomassoni D, Bramanti V, Amenta F. Expression of aquaporins 1 and 4 in the brain of spontaneously hypertensive rats. Brain Res. 2010;1325:155–163. doi: 10.1016/j.brainres.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 62.Benveniste H, Lee H, Ding F, Sun Q, Al-Bizri E, Makaryus R, Probst S, Nedergaard M, Stein EA, Lu H. Anesthesia with Dexmedetomidine and Low-dose Isoflurane Increases Solute Transport via the Glymphatic Pathway in Rat Brain When Compared with High-dose Isoflurane. Anesthesiology. 2017;127:976–988. doi: 10.1097/ALN.0000000000001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Su X, Meng ZT, Wu XH, Cui F, Li HL, Wang DX, Zhu X, Zhu SN, Maze M, Ma D. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;388:1893–1902. doi: 10.1016/S0140-6736(16)30580-3. [DOI] [PubMed] [Google Scholar]
- 64.Clarke DD, Sokoloff L. Regulation of cerebral metabolic rate. In: Siegel GJ, Agranoff BW, Albers RW, editors. Basic Neurochemistry: Molecular, cellular and medical aspects. Philadelphia: Lippincott-Raven; 1999. [Google Scholar]
- 65.Johanson CE, Duncan JA, 3rd, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:10. doi: 10.1186/1743-8454-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eide PK, Ringstad G. Delayed clearance of cerebrospinal fluid tracer from entorhinal cortex in idiopathic normal pressure hydrocephalus: A glymphatic magnetic resonance imaging study. J Cereb Blood Flow Metab. 2018 doi: 10.1177/0271678X18760974. 271678X18760974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stokum JA, Mehta RI, Ivanova S, Yu E, Gerzanich V, Simard JM. Heterogeneity of aquaporin-4 localization and expression after focal cerebral ischemia underlies differences in white versus grey matter swelling. Acta Neuropathol Commun. 2015;3:61. doi: 10.1186/s40478-015-0239-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zeppenfeld DM, Simon M, Haswell JD, D'Abreo D, Murchison C, Quinn JF, Grafe MR, Woltjer RL, Kaye J, Iliff JJ. Association of Perivascular Localization of Aquaporin-4 With Cognition and Alzheimer Disease in Aging Brains. JAMA Neurol. 2017;74:91–99. doi: 10.1001/jamaneurol.2016.4370. [DOI] [PubMed] [Google Scholar]
- 69.Thal DR, Ghebremedhin E, Rub U, Yamaguchi H, Del Tredici K, Braak H. Two types of sporadic cerebral amyloid angiopathy. Journal of neuropathology and experimental neurology. 2002;61:282–293. doi: 10.1093/jnen/61.3.282. [DOI] [PubMed] [Google Scholar]
- 70.Smith EE, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Curr Atheroscler Rep. 2003;5:260–266. doi: 10.1007/s11883-003-0048-4. [DOI] [PubMed] [Google Scholar]
- 71.Weller RO, Nicoll JA. Cerebral amyloid angiopathy: pathogenesis and effects on the ageing and Alzheimer brain. Neurol Res. 2003;25:611–616. doi: 10.1179/016164103101202057. [DOI] [PubMed] [Google Scholar]
- 72.de Leon MJ, Li Y, Okamura N, Tsui WH, Saint-Louis LA, Glodzik L, Osorio RS, Fortea J, Butler T, Pirraglia E, Fossati S, Kim HJ, Carare RO, Nedergaard M, Benveniste H, Rusinek H. Cerebrospinal Fluid Clearance in Alzheimer Disease Measured with Dynamic PET. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2017;58:1471–1476. doi: 10.2967/jnumed.116.187211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, Kipnis J. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest. 2017;127:3210–3219. doi: 10.1172/JCI90603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Raper D, Louveau A, Kipnis J. How Do Meningeal Lymphatic Vessels Drain the CNS? Trends in neurosciences. 2016;39:581–586. doi: 10.1016/j.tins.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carare RO, Hawkes CA, Jeffrey M, Kalaria RN, Weller RO. Review: cerebral amyloid angiopathy, prion angiopathy, CADASIL and the spectrum of protein elimination failure angiopathies (PEFA) in neurodegenerative disease with a focus on therapy. Neuropathol Appl Neurobiol. 2013;39:593–611. doi: 10.1111/nan.12042. [DOI] [PubMed] [Google Scholar]
- 76.Volkow ND, Tomasi D, Wang GJ, Telang F, Fowler JS, Logan J, Benveniste H, Kim R, Thanos PK, Ferre S. Evidence that sleep deprivation downregulates dopamine D2R in ventral striatum in the human brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:6711–6717. doi: 10.1523/JNEUROSCI.0045-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ju YS, Ooms SJ, Sutphen C, Macauley SL, Zangrilli MA, Jerome G, Fagan AM, Mignot E, Zempel JM, Claassen J, Holtzman DM. Slow wave sleep disruption increases cerebrospinal fluid amyloid-beta levels. Brain. 2017;140:2104–2111. doi: 10.1093/brain/awx148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ooms S, Overeem S, Besse K, Rikkert MO, Verbeek M, Claassen JA. Effect of 1 night of total sleep deprivation on cerebrospinal fluid beta-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol. 2014;71:971–977. doi: 10.1001/jamaneurol.2014.1173. [DOI] [PubMed] [Google Scholar]
- 79.Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, Zhou Y, Wong DF, Ferrucci L, Resnick SM. Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70:1537–1543. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ, Holtzman DM. Disruption of the sleep-wake cycle and diurnal fluctuation of beta-amyloid in mice with Alzheimer's disease pathology. Sci Transl Med. 2012;4:150ra122. doi: 10.1126/scitranslmed.3004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jiang C, Zhang L, Zou C, Long X, Liu X, Zheng H, Liao W, Diao Y. Diurnal microstructural variations in healthy adult brain revealed by diffusion tensor imaging. PloS one. 2014;9:e84822. doi: 10.1371/journal.pone.0084822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Elvsashagen T, Norbom LB, Pedersen PO, Quraishi SH, Bjornerud A, Malt UF, Groote IR, Westlye LT. Widespread changes in white matter microstructure after a day of waking and sleep deprivation. PloS one. 2015;10:e0127351. doi: 10.1371/journal.pone.0127351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shim JW, Sandlund J, Han CH, Hameed MQ, Connors S, Klagsbrun M, Madsen JR, Irwin N. VEGF, which is elevated in the CSF of patients with hydrocephalus, causes ventriculomegaly and ependymal changes in rats. Exp Neurol. 2013;247:703–709. doi: 10.1016/j.expneurol.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 84.Louveau A, Da Mesquita S, Kipnis J. Lymphatics in Neurological Disorders: A Neuro-Lympho-Vascular Component of Multiple Sclerosis and Alzheimer's Disease? Neuron. 2016;91:957–973. doi: 10.1016/j.neuron.2016.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fitzgerald MJT. Anatomy and Embryology of the Laboratory Rat. Journal of anatomy. 1987;153:256–256. [Google Scholar]