To the editor,
Avoidance of ventilator-induced lung injury (VILI), defined as the damage caused by the application of large pressures or volumes to the lung parenchyma, is one of the main objectives of contemporary ventilatory management. This specific form of injury is triggered by a variety of molecular mechanisms involving mechanosensation and mechanotransduction of physical forces, inflammatory responses, activation of intracellular signals, extracellular matrix remodeling, and dysregulation of different forms of cell death. Among these, inflammation plays a key role in the induction of early damage, but also in later repair. In spite of this knowledge, no treatment aimed to reduce VILI based on these mechanisms has been translated into the clinical practice.
The outcome of critically ill ventilated patients depends on the cause of the disease, the specific characteristics of the patient (including comorbidities), and the response to therapies and their consequences. The impact of VILI in outcome falls in this latter category. However, it is not known how previous baseline conditions and applied therapies interact.
Sex is one of the main determinants of health and disease. It has been reported that males show worse intensive care unit (ICU) outcomes [1]. In the specific field of lung injury and mechanical ventilation, females may receive lung protective ventilation less frequently, due to miscalculation of target tidal volumes [2]. Some studies have reported higher incidence of acute respiratory distress syndrome (ARDS) [3] and mortality rates [4] and worse long-term outcomes [5] in mechanically ventilated women, but others have failed to confirm these results [6, 7]. The mechanisms behind these differences have been addressed in several experimental studies (reviewed in [8]). Male and female animals have different responses in models of respiratory diseases such as chronic obstructive pulmonary disease (COPD), asthma, and fibrosis. The effects of sex hormones on inflammation and cell metabolism mediate the majority of these differences.
The role of VILI in the difference in ICU outcome between sexes has not been addressed. Most of the experimental research has been conducted in male animals, discarding their female counterparts, and no study has compared male and female animals after mechanical ventilation. To address this question, we ventilated male and female C57BL/6 mice to induce a moderate lung injury (peak pressure 15 cmH2O, PEEP 0 cmH2O, respiratory rate 100 breaths/minute, inspiratory to expiratory ratio 1:1, FiO2 0.21). After 2.5 h, animals were sacrificed and the lungs removed and fixed. There were no differences in lung injury, addressed using a semiquantitative score [9] (0.5 ± 0.5 vs 0.75 ± 0.52 for female and male animals respectively at baseline, 2.3 ± 1.2 vs 2.2 ± 1.3 for female and male animals respectively after VILI, n = 6 per condition, p < 0.001 for the effect of ventilation, p = 0.99 for the effect of sex in a two-way ANOVA). Using a more aggressive ventilatory strategy (peak pressure 20 cmH2O, PEEP 0 cmH2O, respiratory rate 50 breaths/minute, inspiratory to expiratory ratio 1:1, FiO2 0.21) yielded slightly higher scores but again without differences between sexes (2.5 ± 1.3 vs 2.7 ± 1.3 for female and male mice, n = 3 per group, p = 0.88). Similarly, there were no differences in neutrophilic infiltrates between sexes (14 ± 7 vs 11 ± 5 myeloperoxidase-positive cells per field in female and male animals respectively at baseline, 43 ± 19 and 37 ± 15 myeloperoxidase-positive cells per field in female and male animals respectively after ventilation, n = 6 per group, p = 0.001 for the effect of ventilation, p = 0.80 for the effect of sex). Figure 1 shows representative sections of these studies.
Fig. 1.

Representative histological sections stained with hematoxylin-eosin (upper row) or immunostained using an anti-myeloperoxidase (MPO) antibody (lower row) in male and female mice after injurious ventilation
In order to obtain a large-scale assessment of the putative differences between groups, RNA from lung homogenates from baseline and ventilated mice of each sex was obtained and hybridized using expression microarrays (Affymetrix Mouse Gene 2.0 ST, with a coverage above 26,500 genes). Raw data (available at GEO database, accession number GSE121550) were background-corrected and normalized to obtain expression values. These values were fitted to a linear model including sex and ventilation as factors, and F statistics computed with Bayesian moderation of the standard errors. p values were corrected using the Benjamini-Hochberg method. These methods have been detailed elsewhere [9]. Mechanical ventilation induced significant changes in 3510 genes, irrespective of the sex. When comparing the effect of mechanical ventilation between sexes, only 30 genes showed a log-fold change higher than 1.5 (this is, a 2.8-fold difference between male and female above in the ventilator-induced change in gene expression). Irrespective of the fold change, no gene showed a significant difference between sexes reaching an adjusted p value lower than 0.05 (Fig. 2).
Fig. 2.
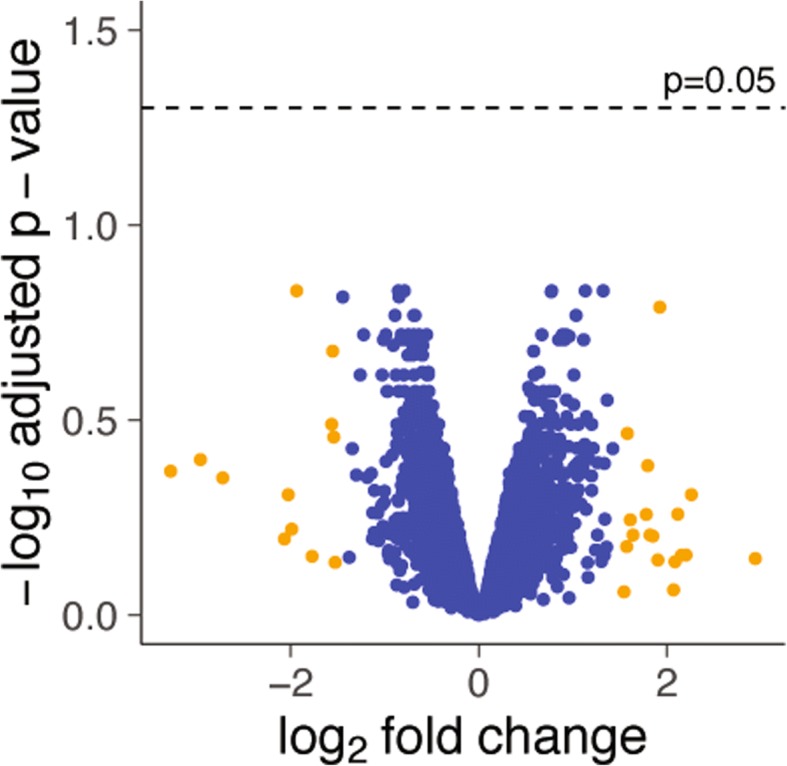
Volcano plot representing the log-fold change in gene expression with ventilation (comparing male and female mice) against the log of the statistical significance. Only 30 genes (orange dots) had a log-fold change above 1.5, and no gene reached an adjusted p value lower than 0.05
Collectively, these results suggest that there are no differences caused by sex in susceptibility to VILI. Rather than justify the use of animals of a single sex in experimental studies, these findings support the inclusion of both, as advocated by others [10]. Our data allow researchers to assume a hypothesis of no influence of sex, followed by the post hoc assessment of putative differences, if any.
Acknowledgements
Not applicable.
Funding
Supported by Centro de Investigación Biomédica en Red de Enfermedades Respiratorias (CIBERES) and Instituto de Salud Carlos III (FEDER funds, PI16/01614), Madrid, Spain. Instituto Universitario de Oncología del Principado de Asturias is supported by Fundación Bancaria Caja de Ahorros de Asturias. Instituto de Investigación Sanitaria del Principado de Asturias is supported by Fundación FINBA. I.L.-A. is the recipient of a grant from Fundación FINBA. C.H. is the recipient of a grant from Instituto de Salud Carlos III (Contratos Sara Borrell, CD16/00033). L.A.-R. is the recipient of a grant from Instituto de Salud Carlos III (Contratos Rio Hortega, CM16/00128). C.L-M. is the recipient of a grant from Asociación Española contra el Cancer (AECC).
Availability of data and materials
The rawdata from the expression microarrays generated during the current study are available in the GEO repository, accession number GSE121550 [https://www.ncbi.nlm.nih.gov/geo].
Abbreviations
- ARDS
Acute respiratory distress syndrome
- COPD
Chronic obstructive pulmonary disease
- ICU
Intensive care unit
- VILI
Ventilator-induced lung injury
Authors’ contributions
ILA and GMA designed the study. ILA, LAR, CLM, and CHF performed the experiments and acquired the data. GMA made the genomic and statistical analyses. GMA and ILA drafted the manuscript. All authors contributed to the manuscript modifications. All authors read and approved the final version.
Ethics approval
The Animal Research Ethics Committee of the Universidad de Oviedo evaluated and approved the study. All the animal experiments were performed according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Inés López-Alonso, Email: ila@lab14.org.
Laura Amado-Rodriguez, Email: lar@lab14.org.
Cecilia López-Martínez, Email: clm@lab14.org.
Covadonga Huidobro, Email: chf@lab14.org.
Guillermo M. Albaiceta, Email: gma@lab14.org
References
- 1.Mahmood K, Eldeirawi K, Wahidi MM. Association of gender with outcomes in critically ill patients. Crit Care. 2012;16:R92. doi: 10.1186/CC11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han S, Martin GS, Maloney JP, et al. Short women with severe sepsis-related acute lung injury receive lung protective ventilation less frequently: an observational cohort study. Crit Care. 2011;15:R262. doi: 10.1186/cc10524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heffernan DS, Dossett LA, Lightfoot MA, et al. Gender and acute respiratory distress syndrome in critically injured adults: a prospective study. J Trauma. 2011;71:878–883. doi: 10.1097/TA.0b013e31822c0d31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kollef MH, O’Brien JD, Silver P. The impact of gender on outcome from mechanical ventilation. Chest. 1997;111:434–441. doi: 10.1378/chest.111.2.434. [DOI] [PubMed] [Google Scholar]
- 5.Brown SM, Wilson EL, Presson AP, et al. Understanding patient outcomes after acute respiratory distress syndrome: identifying subtypes of physical, cognitive and mental health outcomes. Thorax. 2017;72:1094–1103. doi: 10.1136/thoraxjnl-2017-210337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Haddad H, Jang H, Chen W, et al. The effect of demographics and patient location on the outcome of patients with acute respiratory distress syndrome. Ann Thorac Med. 2017;12:17–24. doi: 10.4103/1817-1737.197767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epstein SK, Vuong V. Lack of influence of gender on outcomes of mechanically ventilated medical ICU patients. Chest. 1999;116:732–739. doi: 10.1378/chest.116.3.732. [DOI] [PubMed] [Google Scholar]
- 8.Card JW, Zeldin DC. Hormonal influences on lung function and response to environmental agents: lessons from animal models of respiratory disease. Proc Am Thorac Soc. 2009;6:588–595. doi: 10.1513/pats.200904-020RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.López-Alonso I, Blázquez-Prieto J, Amado-Rodríguez L, et al. Preventing loss of mechanosensation by the nuclear membranes of alveolar cells reduces lung injury in mice during mechanical ventilation. Sci Transl Med. 2018;10:eaam7598. doi: 10.1126/scitranslmed.aam7598. [DOI] [PubMed] [Google Scholar]
- 10.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The rawdata from the expression microarrays generated during the current study are available in the GEO repository, accession number GSE121550 [https://www.ncbi.nlm.nih.gov/geo].


