Abstract
Skeletal-related events (SREs) are common bone complications in multiple myeloma (MM). However, there are few real-world reports of their incidence. In this study, a database of oncology electronic health records was linked to administrative claims data. Patients identified were aged ≥18 years and newly diagnosed with MM, had ≥1 clinic visit within 1 month of diagnosis, and ≥1 year of follow-up after diagnosis. The study period was January 1, 2011 to December 31, 2016. 343 patients were included, 35% of whom had a baseline history of any SRE. During a median follow-up of 25.7 months, 34% of patients experienced SREs after diagnosis. Median time to SRE was 167 days. Among patients experiencing an SRE, 68% had an SRE within the first year. The incidence rate of SREs at 1 year following MM diagnosis for patients with baseline history was 103/100 person-years (PY) versus 16/100PY for patients without baseline history. SRE incidence rates within 3 months of initiating a line of therapy increased with subsequent lines (line 1: 81/100PY, line 2: 118/100PY, line 3: 150/100PY). Risk of SREs was similar across different anti-MM regimens, including proteasome inhibitor-based regimens. These results highlight the importance of continued surveillance and management of MM-associated bone disease.
Keywords: Skeletal-related event, Multiple myeloma, Bone disease, Proteasome inhibitor
1. Introduction
Multiple myeloma (MM), the second most prevalent hematologic malignancy in the adult United States population, is considered a disease of the elderly [1], with a median age at diagnosis of 69 years and an increasing incidence with age [2]. The incidence of MM in the United States has been found to be increasing, possibly because of earlier diagnosis or aging of the population [3].
Destructive bone lesions are one of the classic defining features of MM, which also include hypercalcemia, renal failure, and anemia (i.e., CRAB criteria [4]). It is estimated that 80–90% of patients with MM will develop bone lesions during the course of their disease [5], [6], with consequent bone destruction a devastating consequence of MM [7]. The severity of bone destruction has been associated with MM disease burden [7], [8] and prognosis [7], [9], and the presence of bone lesions increases the risk for what has been termed skeletal-related events (SREs) [9–11], which can include pathologic fractures, vertebral compression leading to spinal cord compression, and the need for radiation and surgery to treat bone lesions. SREs, in turn, have been associated with increased mortality [12], impaired quality of life [13], and higher healthcare resource utilization and costs [14] for patients with MM [11].
In clinical trials, treatment with bisphosphonates has been found to reduce the incidence of SREs compared with placebo or no treatment [15], and these agents are consequently recommended by clinical guidelines for patients with MM [[11], [16]–18]. In one seminal study of patients with MM receiving conventional chemotherapy, patients treated with pamidronate had a lower rate of SREs than patients treated with placebo (24% versus 41%; p < 0.001) [19]. In a head-to-head study of two frontline bisphosphonate therapies, the Medical Research Council Myeloma IX trial showed that compared with oral clodronic acid, treatment with intravenous zoledronic acid resulted in a significant reduction in the proportion of patients with an SRE before disease progression (27% versus 35%; p = 0.0004) and improved overall survival (median, 50.0 versus 44.5 months; p = 0.04) in patients with newly diagnosed MM [20]. Recently, denosumab, a fully human monoclonal antibody that binds RANKL, was approved in the United States for the prevention of SREs in patients with MM [21], [22]. This approval was based on results from the phase 3 482 study, which demonstrated that denosumab met the primary endpoint of noninferiority to the bisphosphonate zoledronic acid for time to first on-study SRE in patients with newly diagnosed MM and bone disease (hazard ratio [HR] = 0.98; 95% confidence interval [CI]: 0.85–1.14) [22].
Although the frequency of SREs among patients receiving conventional chemotherapy in clinical trials has been well studied, less is known about the real-world incidence of SREs, particularly in the era of novel agents. These novel agents, which include proteasome inhibitors (PIs) and immunomodulatory drugs (IMiDs), have improved efficacy outcomes for patients with MM [23]. PIs have certain anabolic bone activity [24], and there is some evidence that bortezomib can reduce the frequency of SREs [25], [26]; however, this effect requires further investigation and there are data to support the observation that SREs remain a frequent complication in the era of novel agents [10], [27].
Given the limited contemporary data on the real-world incidence of SREs in MM, we conducted this study to describe the incidence of SREs among patients with MM in a real-world setting of outpatient oncology clinics in the United States.
2. Material and methods
2.1. Data source
Oncology electronic health records (EHRs) contained in Amgen's Oncology Services Comprehensive Electronic Records (OSCER) database, generated by Flatiron Health (New York, NY, April 30, 2016), were linked to administrative claims data from the IBM-Truven Marketscan® administrative claims database and used in this study. The OSCER database contains EHRs of patients treated at over 265 outpatient oncology clinics across the United States, representing approximately 20% of oncology patients nationally. The IBM-Truven Marketscan® administrative claims database contains health insurance claims from employer-based insurance plans and covers approximately 350 private payers. Linkage of the 2 databases allows more complete insights to patients’ health status: details on oncology treatments, diagnoses, and lab values are obtained from the EHR, while data on diagnoses, treatments, and hospitalizations that occur outside of the oncology clinic setting are obtained from the commercial claims database.
2.2. Study design and population
This was a retrospective cohort study covering the time period from January 1, 2011 through December 31, 2016. Patients were included in the study population if they were 18 years or older, newly diagnosed with MM (ICD-9: 203.00; ICD-10: C90.00), had at least 1 clinic visit within 1 month of diagnosis, had at least 1 year of follow-up after MM diagnosis, had received anti-MM therapy, and had patient-level data that was successfully linked between the OSCER and MarketScan databases. The time period analyzed for a given patient required overlap in the 2 databases. When data did not overlap, time was censored at the point where follow-up was shortest. Patient diagnoses, treatment dates, and administrations were ascertained from OSCER EHRs.
2.3. Measures
The primary outcomes of interest were occurrence of and time to SRE. SREs were ascertained from diagnosis codes (Supplementary Appendix) in insurance claims from the MarketScan database and included the following: spinal cord compression, pathologic fracture, surgery to bone, and radiation to bone. Open and closed fractures of all bones were included in the analysis, including but not limited to: humerus, vertebrae, femur, tibia, fibula, ribs, and skull (Supplementary Appendix). SREs occurring within 60 days of MM diagnosis were classified as baseline SREs.
Multiple SREs that occurred within a 21-day span [28] were considered as a single SRE and ordered based on the following hierarchy: (1) spinal cord compression, (2) pathologic fracture, (3) surgery to bone, and (4) radiation to bone. This approach was used because individual SREs occurring within a limited timespan may be serially interdependent as described by Aly et al. [28]. The baseline period for comorbidities was 12 months prior to the MM diagnosis date [28]. Patients were classified as having a history of SREs if these complications occurred during the 12-month baseline period through 60 days on or after the MM diagnosis date.
2.4. Calculations
Descriptive statistics (mean/median) were used to summarize the proportion of patients with an SRE and the time to SRE. Subgroup analyses were conducted to stratify results by history of baseline SRE, anti-MM regimen type, and line of therapy. A time-to-event analysis was also conducted to evaluate the cumulative incidence of SREs and total number of SREs by type of SRE. A Cox model was used to assess the relationship between duration of treatment and the development of SREs.
3. Results
3.1. Cohort characteristics
A total of 343 patients with MM who met the study inclusion criteria were identified (Fig. 1). Of these, 187 (54.5%) were aged 65 years or older, 185 (53.9%) were male, and 241 (70.3%) were white (Table 1). Approximately one-third of patients had an estimated glomerular filtration rate of less than 60 ml/min, and 68.8% of patients presented with anemia.
Fig. 1.
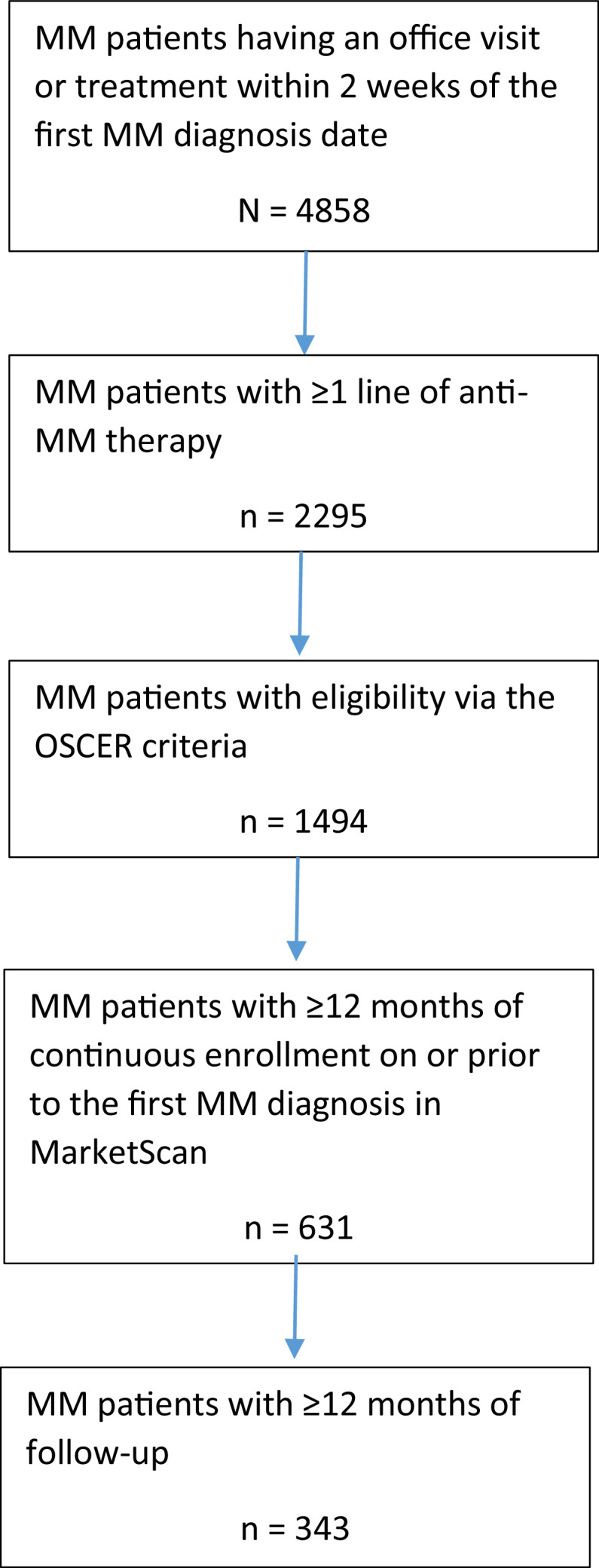
Patient attrition. MM, multiple myeloma; OSCER, oncology services comprehensive electronic records.
Table 1.
Demographic and clinical characteristics of the MM study population at baseline.
| Characteristic, n (%) | N = 343 |
|---|---|
| Age at diagnosis, years | |
| 18–39 | 3 (0.9) |
| 40–49 | 20 (5.8) |
| 50–64 | 133 (38.8) |
| 65–74 | 88 (25.7) |
| ≥75 | 99 (28.9) |
| Sex | |
| Male | 185 (53.9) |
| Female | 158 (46.1) |
| Race | |
| White | 241 (70.3) |
| Black | 52 (15.2) |
| Asian | 2 (0.6) |
| Hispanic | 1 (0.3) |
| Other | 13 (3.8) |
| Unknown | 34 (9.9) |
| ISS stage | |
| I | 57 (16.6) |
| II | 34 (9.9) |
| III | 40 (11.7) |
| Unknown | 212 (61.8) |
| ECOG PS | |
| 0 | 71 (20.7) |
| 1 | 41 (12.0) |
| 2 | 13 (3.8) |
| 3 | 3 (0.9) |
| 4 | 1 (0.3) |
| Unknown | 214 (62.4) |
| Presence of renal impairmenta | |
| Yes | 109 (31.8) |
| No | 163 (47.5) |
| Unknown | 71 (20.7) |
| Presence of hypercalcemia | |
| Yes | 29 (8.5) |
| No | 249 (72.6) |
| Unknown | 65 (19.0) |
| Presence of anemia | |
| Yes | 236 (68.8) |
| No | 78 (22.7) |
| Unknown | 29 (8.5) |
| Baseline/history of SREb | |
| Spinal cord compression | 16 (4.7) |
| Pathologic fractures | 94 (27.4) |
| Surgery to bone | 3 (0.9) |
| Radiation to bone | 29 (8.5) |
| Lines of anti-MM therapy | |
| Line 1 | 343 (100.0) |
| Line 2 | 181 (52.8) |
| Line 3 | 90 (26.2) |
| Line 4 | 42 (12.2) |
| Line 5 | 16 (4.7) |
| Line 6+ | 13 (3.8) |
Defined by creatinine clearance <60 mL/min/1.73 m2.
Patients can be in multiple SRE categories. History of an SRE was measured during the 12-month baseline period through 60 days on or after the MM diagnosis date. ECOG PS, Eastern Cooperative Oncology Group performance status; ISS, International Staging System; MM, multiple myeloma; SRE, skeletal-related event.
With respect to baseline history of SREs, 4.7% of patients had a history of spinal cord compression, 27.4% had a history of pathologic fracture, 0.9% had a history of surgery to bone, and 8.5% had a history of radiation to bone.
A total of 220 (64%) of patients received a bone-targeting agent during the follow-up period. We have previously observed that over 52% of patients with MM in the OSCER database had sporadic usage of bone-targeting agents [29].
3.2. Frequency of SREs and time to SREs in the overall population
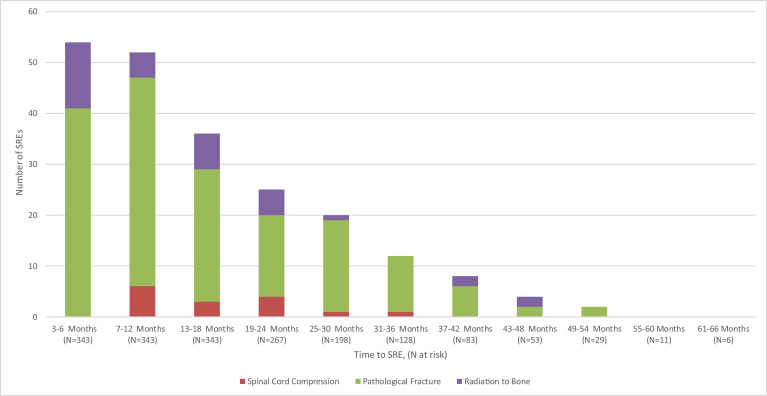
With a median follow-up time of 25.7 months after start of follow-up (i.e., 60 days after MM diagnosis), 117 patients (34.1%) experienced a subsequent SRE after start of follow-up (Table 2). The incidence rates of any SRE and specific types of SREs are shown in Table 3. The incidence rate of any SRE was 46.1 per 100 person-years (PYs) 1 year following MM diagnosis. The proportion of patients with spinal cord compression, pathologic fracture, surgery to bone, and radiation to bone at this timepoint were 1.7%, 18.1%, 0.0% (all surgery to bone events were reclassified as another type of SRE based on the previously described hierarchy for multiple SREs occurring within a 21-day span), and 5.0%, respectively. The distribution of the timing of SREs is shown in Fig. 2. The median time to first SRE was 167 days after start of follow-up. Among patients that experienced an SRE, 68% (n = 79) occurred within the first year from start of follow-up.
Table 2.
Proportion of patients with any SRE and time to first SRE.
| Characteristics | Any SRE |
||||
|---|---|---|---|---|---|
| Time to first SRE during follow-up (days) |
|||||
| Patients at risk, n | Patients with any SRE, n (%)a | Mean | Median | Standard deviation | |
| Overall | 343 | 117 (34.1) | 282.1 | 167.0 | 316.3 |
| History of SREb | |||||
| Yes | 119 | 72 (61.5) | 200.9 | 88.0 | 262.4 |
| No | 224 | 45 (38.5) | 395.9 | 305.0 | 351.7 |
| Lines of anti-MM therapyc,d | |||||
| Line 1 | 343 | 51 (14.9) | 136.0 | 80.0 | 152.5 |
| Line 2 | 181 | 20 (11.0) | 109.7 | 71.0 | 169.6 |
| Line 3 | 90 | 7 (7.8) | 33.6 | 30.0 | 13.9 |
| Line 4 | 42 | — | — | — | — |
| Line 5 | 16 | 1 (6.3) | 134.0 | 134.0 | — |
| Line 6+ | 13 | 1 (7.7) | 30.0 | 30.0 | — |
There are 15 patients with multiple SREs.
History of SRE was measured during the 12-month baseline period (prior to the MM diagnosis date) thru 60 days on or after the MM diagnosis date.
SREs had to occur during each specified anti-MM line of therapy.
Time to first SRE was calculated as the number of days between the line of therapy start date and the first SRE date for the specified line of therapy (i.e., first SRE date within specified anti-MM line of therapy − line of therapy start date +1). MM, multiple myeloma; SRE, skeletal-related event.
Table 3.
Time-to-event analysis of the cumulative incidence rate of SREs and total number of SREs by SRE type.
| Cumulative follow-up time (median follow-up: 25.7 months) | Type of SRE |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any SRE |
Spinal cord compression |
Pathologic fracture |
Radiation to bone |
|||||||||
| Patients with SREs, % | SREs, n | Incidence rate per 100 PYs | Patients with event, % | Events, n | Incidence rate per 100 PYs | Patients with event, % | Events, n | Incidence rate per 100 PYs | Patients with event, % | Events, n | Incidence rate per 100 PYs | |
| 3 months | 6.1 | 25 | 29.6 | 0 | 0 | 0 | 5.0 | 19 | 22.5 | 1.7 | 6 | 7.1 |
| 6 months | 14.9 | 77 | 45.6 | 0 | 0 | 0 | 12.0 | 61 | 36.1 | 3.8 | 16 | 9.5 |
| 9 months | 19.0 | 116 | 45.8 | 1.2 | 4 | 1.6 | 14.9 | 95 | 37.5 | 4.1 | 17 | 6.7 |
| 12 months | 23.0 | 156 | 46.1 | 1.7 | 6 | 1.8 | 18.1 | 128 | 37.9 | 5.0 | 22 | 6.5 |
| 15 months | 24.5 | 177 | 42.4 | 2.3 | 8 | 1.9 | 18.7 | 143 | 34.2 | 5.8 | 26 | 6.2 |
| 18 months | 28.0 | 204 | 41.8 | 2.6 | 9 | 1.8 | 21.6 | 163 | 33.4 | 6.7 | 32 | 6.6 |
| 21 months | 29.2 | 224 | 40.8 | 2.9 | 10 | 1.8 | 22.4 | 179 | 32.6 | 7.0 | 35 | 6.4 |
| 24 months | 30.6 | 243 | 40.4 | 3.5 | 14 | 2.3 | 22.7 | 189 | 31.4 | 7.6 | 40 | 6.7 |
| 60 months | 34.1 | 311 | 38.9 | 3.5 | 16 | 2.0 | 26.8 | 248 | 31.1 | 8.2 | 47 | 5.9 |
PYs, person-years; SRE, skeletal-related event.
All surgery to bone events were reclassified as either spinal cord compression or pathologic fracture due to all events occurring within 21 days of each other.
Fig. 2.
Time from MM diagnosis to SRE during the follow-up period. No patients had a SRE of surgery to bone during the follow-up period. SREs had to occur more than 60 days after the MM diagnosis to be considered as a follow-up SRE. Patients had to have at least 12 months of follow-up. MM, multiple myeloma; SRE, skeletal-related event.
3.3. Frequency of SREs and time to SREs by history of baseline SREs
The proportion of patients who experienced an SRE after start of follow-up was 61.5% for the subgroup of patients who had a history of any SRE (n = 119) and 38.5% for the subgroup of patients that did not have a history (n = 224) (Table 2). The incidence rate of SREs was 103.2 per 100 PYs for patients with a baseline history (n = 58) and 15.9 per 100 PYs for patients with no baseline history (n = 21) 1 year following MM diagnosis. The median time to first SRE was 88 days for patients who had a history of any SRE and 305 days for patients who did not have a history.
3.4. Frequency of SREs by line of therapy
The incidence rate of SREs in each line of therapy within 3 months of the initiation of each line increased with each subsequent line (line 1: 81.1 per 100 PYs, line 2: 117.9 per 100 PYs, line 3: 150.3 per 100 PYs). For patients with relapsed disease (initiated second- or third-line therapy), the incidence rate of SREs was highest at the beginning of each relapse (Table 4). For patients on second-line therapy, the incidence rate of SREs was 117.9 per 100 PYs at 3 months within initiation of second-line therapy and declined to 82.4 per 100 PYs at 24 months within initiation of second-line therapy. For patients on third-line therapy, the incidence rate of SREs was 150.3 per 100 PYs at 3 months within initiation of third-line therapy and declined to 116.6 per 100 PYs at 24 months within initiation of third-line therapy.
Table 4.
Incidence rate of total SREs by line of anti-MM therapy among patients initiating anti-MM therapy.
| Cumulative follow-up time (median follow-up: 25.7 months) |
Line 1 |
Line 2 |
Line 3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients with SREs, n | SREs, n | Incidence rate per 100 PYs | Patients with SREs, n | SREs, n | Incidence rate per 100 PYs | Patients with SREs, n | SREs, n | Incidence rate per 100 PYs | |
| 3 months | 41 | 59 | 81.11 | 29 | 43 | 117.88 | 17 | 28 | 150.29 |
| 12 months | 65 | 127 | 70.44 | 41 | 87 | 94.25 | 23 | 55 | 130.72 |
| 24 months | 70 | 140 | 57.49 | 43 | 94 | 82.35 | 23 | 57 | 116.55 |
MM, multiple, myeloma; PYs, person-years; SREs, skeletal-related events.
3.5. Frequency of SREs by duration of treatment
Patients receiving continuous anti-MM therapy (no break greater than 90 days) had a 46% increased risk of developing an SRE (HR: 1.46, 95% CI: 1.01–2.11) compared with patients who had a break in therapy. However, when adjusting for numerous baseline covariates, the risk of SRE between groups was 24% (HR: 1.24, 95% CI: 0.81–1.89).
3.6. Frequency of SREs by type of anti-MM regimen
Anti-MM regimens were classified into three categories: regimens that contained a PI and an IMiD (PI + IMiD) regimens that contained a PI but not an IMiD (PI without IMiD), and regimens that did not contain a PI (non-PI regimens). For patients receiving first-line therapy, the incidence rate of SREs within 1 year of frontline treatment initiation was 82.4 per 100 PYs for PI + IMiD regimens, 66.1 per 100 PYs for PI without IMiD regimens, and 57.7 per 100 PYs for non-PI regimens. For patients receiving second-line therapy, the incidence rate of SREs within 1 year of second-line treatment initiation was 133.5 per 100 PYs for PI + IMiD regimens, 73.2 per 100 PYs for PI without IMiD regimens, and 77.7 per 100 PYs for non-PI regimens. For patients receiving third-line therapy, the incidence rate of SREs within 1 year of third-line treatment initiation was 265.2 per 100 PYs for PI + IMiD regimens, 93.1 per 100 PYs for PI without IMiD regimens, and 81.6 per 100 PYs for non-PI regimens.
4. Discussion
In this study, we used a database of oncology EHRs linked with an administrative claims database to describe the incidence of SREs among patients with MM treated in a real-world setting based in the United States. We found that 34% of patients experienced an SRE after start of follow-up with a median time to first SRE of 167 days (among patients with a minimum of 1 year of follow-up). Pathologic fracture was the most common type of SRE in our study. Having a history of SRE was associated with an increased risk of SREs. Notably, for patients with relapsed disease, the higher rate of SREs was most pronounced at the beginning of second- or third-line therapy (time closest to relapse). The risk of SREs was present even when anti-MM regimens contained a PI. After adjustment for baseline covariates, the difference in SRE risk between patients who received continuous or non-continuous therapy was not substantial. These findings counter a common perception that once MM is treated and surveilled there are few additional SREs.
Other studies have assessed the real-world rate of SREs in patients with MM during the era of novel agents. A population-based study from the United Kingdom found a 32% rate of SREs among patients with MM who were newly diagnosed between 2004 and 2009 [30]. A 2016 chart audit of medical records of patients with newly diagnosed MM from France, Germany, Italy, Spain, and the United Kingdom found that 26% of patients had ≥1 new SRE between diagnosis and disease progression [31]. An analysis of the Truven Health MarketScan Commercial Claims and Medicare Supplemental databases found that 58% of United States patients with MM diagnosed between 2005 and 2011 experienced an SRE during the follow-up period [14]. The overall SRE rate in our study (34%) may have been underestimated due to selection bias or recording bias in the administrative claims database or shorter overall follow-up. Results from other studies, and our own clinical experience, suggest that bone events can be underreported in claims data. Some studies have found underreporting of fractures [32], [33], with one study noting that healthcare professionals cannot reliably code these events [33].
A notable finding from our study was that the risk of SREs continued to increase with each subsequent relapse, which could be a reflection of increasing bone destruction as the burden of disease grows in patients with relapsing MM. This finding highlights the need for continued surveillance of bone health beyond first-line therapy.
There is some evidence from laboratory and clinical studies that novel anti-MM agents such as PIs may reduce the risk of SREs in patients with MM. One study found that consolidation treatment with bortezomib, thalidomide, and dexamethasone (without administration of concomitant bisphosphonate therapy) improved levels of bone resorption biomarkers and was associated with a low rate of new SREs (n = 1) in patients with MM who had received autologous stem cell transplantation (n = 42) [26]. However, in our real-world study, the incidence of SREs was similar regardless of whether patients were treated with PIs or not.
Consistent with this, one retrospective, single-center study found that SREs remained a frequent complication with novel agents, with 22% of patients with MM presenting with fractures or requiring radiotherapy after frontline treatment with regimens based on bortezomib or IMiDs [27]. Another retrospective study reported 2-year SRE rates of 46–76% among patients who received first- and second-line treatment with novel agents, with or without zoledronic acid [34]. Firm conclusions regarding the effects of PIs and continuous therapy on the incidence of SREs cannot be made from our real-world study as it was limited by sample size and the influence of unmeasured confounding factors is a potential issue. As this study was descriptive in nature, direct comparisons cannot be made regarding differences in patients under one treatment regimen versus another. Further studies are warranted to evaluate the possible bone protective effects, if any, of novel agents used for the treatment of MM.
Our study has several limitations. First, follow-up was a minimum of 1 year and 58% of patients had 2 years of follow-up. Patients were required to have 1 year of follow-up because due to the nature of claims data, patients who die shortly after diagnosis are less likely to have their full medical claims billed and coded than patients who live longer, which results in an underreporting of SREs for patients with rapid mortality. However, this requirement may introduce a time bias whereby patients included in the analysis may have been healthier than those who died soon after diagnosis and were excluded. Our sensitivity analyses showed that patients who died soon after diagnosis had less recorded SREs (20.5% [n = 18] of patients who died within 1 year, n = 88) than those with at least 12 months of follow-up. The 42% of patients with less than 2 years of follow-up may be a contributor to why fewer patients had SRE in this study compared with other studies. Clinical guidelines from the National Comprehensive Care Network recommend that bisphosphonate therapy continue through 2 years past diagnosis [17], in recognition that the highest risk of SRE occurs during this time period. Second, the presence of bone lesions was unknown at baseline. This limited the ability to risk stratify patients at baseline. However, overall population level results should be representative of the treated MM population at large. Third, the true rate of SREs may be underestimated in our study, as administrative claims may not capture all SREs if they were not billed in insurance claims. Fourth, as noted previously, the population was selected with patients required to have matching records in the OSCER and MarketScan databases. This may have led to an overrepresentation of the working commercially insured population and an underrepresentation of the elderly retired Medicare population, leading to an overall healthier population as fewer elderly patients are represented. Fifth, there was some missing data: Eastern Cooperative Oncology Group performance status and International Staging System stage were unknown in 62% of patients. Finally, the effect of the use of bone-targeting agents on SRE risk was not evaluated in this study because a comparison would likely be too confounded by substantial differences in the population that received bone-targeting agents versus those who did not and further complicated by evaluating the effect of anti-myeloma therapy on SRE risk. The effect of bone-targeting agents on SRE risk merits additional study using more complex statistical methods which will be the subject of future on-going research.
5. Conclusions
In conclusion, this study demonstrates that most patients with MM experience their first SRE soon after diagnosis or at the beginning of each relapse. SREs became more common as patients progress through multiple lines of therapy and the incidence was similar regardless of whether patients were treated with a PI or not. Among patients experiencing an SRE, multiple SREs can occur, highlighting the importance of continued surveillance and proper management of MM-associated bone disease.
Acknowledgments
Acknowledgments
Medical writing assistance was provided by BlueMomentum, an Ashfield Company, part of UDG Healthcare plc, and supported by Amgen Inc.
Funding
This work was funded by Amgen Inc.
Author contribution
CK, SB, and RKH participated in the conception and design of the study and in the analysis and interpretation of data; LC participated in patient data collection/data acquisition; and RF participated in the analysis and interpretation of data. CK participated in the development of the first draft of the manuscript. All authors critically reviewed and revised the manuscript and approved of the final submitted version.
Conflict of interest
This study was supported by Amgen Inc. Amgen participated in the design of the study and the collection and analysis of the data and reviewed the final version of the manuscript before submission. CK, SB, and RKH report employment and stock ownership from Amgen Inc. LC reports employment by DOCS Global. RF reports consultancy with Amgen, BMS, Celgene, Takeda, Bayer, Jansen, Pharmacyclics, Merck, Sanofi, Kite and Juno; advisory board participation with Adaptive Biotechnologies; and a patent holding with Mayo Clinic for prognosticating myeloma using FISH.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jbo.2018.100215.
Appendix. Supplementary materials
References
- 1.Willan J., Eyre T.A., Sharpley F. Multiple myeloma in the very elderly patient: challenges and solutions. Clin. Interv. Aging. 2016;11:423–435. doi: 10.2147/CIA.S89465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.SEER Cancer Stat Facts: Myeloma. National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/statfacts/html/mulmy.html.
- 3.Costa L.J., Brill I.K., Omel J. Recent trends in multiple myeloma incidence and survival by age, race, and ethnicity in the United States. Blood. Adv. 2017;1:282–287. doi: 10.1182/bloodadvances.2016002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajkumar S.V., Dimopoulos M.A., Palumbo A. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet. Oncol. 2014;15:e538–e548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 5.Roodman G.D. Mechanisms of bone metastasis. N. Engl. J. Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 6.Silbermann R., Roodman G.D. Myeloma bone disease: pathophysiology and management. J. Bone. Oncol. 2013;2:59–69. doi: 10.1016/j.jbo.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Donnell E.K., Raje N.S. Myeloma bone disease: pathogenesis and treatment. Clin. Adv. Hematol. Oncol. 2017;15:285–295. [PubMed] [Google Scholar]
- 8.Terpos E., Szydlo R., Apperley J.F. Soluble receptor activator of nuclear factor kappaB ligand-osteoprotegerin ratio predicts survival in multiple myeloma: proposal for a novel prognostic index. Blood. 2003;102:1064–1069. doi: 10.1182/blood-2003-02-0380. [DOI] [PubMed] [Google Scholar]
- 9.Coleman R.E. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer. Treat. Rev. 2001;27:165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 10.Terpos E., Kanellias N., Moulopoulos L.A. Skeletal-related events in patients with multiple myeloma in the era of novel agents: low incidence of pathological fractures after treatment. Blood. 2013;122:3090. [Google Scholar]
- 11.Terpos E., Morgan G., Dimopoulos M.A. International Myeloma Working Group recommendations for the treatment of multiple myeloma-related bone disease. J. Clin. Oncol. 2013;31:2347–2357. doi: 10.1200/JCO.2012.47.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonmez M., Akagun T., Topbas M. Effect of pathologic fractures on survival in multiple myeloma patients: a case control study. J. Exp. Clin. Cancer. Res. 2008;27:11. doi: 10.1186/1756-9966-27-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jordan K., Proskorovsky I., Lewis P. Effect of general symptom level, specific adverse events, treatment patterns, and patient characteristics on health-related quality of life in patients with multiple myeloma: results of a European, multicenter cohort study. Support. Care. Cancer. 2014;22:417–426. doi: 10.1007/s00520-013-1991-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nash Smyth E., Conti I., Wooldridge J.E. Frequency of skeletal-related events and associated healthcare resource use and costs in US patients with multiple myeloma. J. Med. Econ. 2016;19:477–486. doi: 10.3111/13696998.2015.1132225. [DOI] [PubMed] [Google Scholar]
- 15.Mhaskar R., Kumar A., Miladinovic B., Djulbegovic B. Bisphosphonates in multiple myeloma: an updated network meta-analysis. Cochrane. Database. Syst. Rev. 2017;12 doi: 10.1002/14651858.CD003188.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson K., Ismaila N., Flynn P.J. Role of bone-modifying agents in multiple myeloma: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2018;36:812–818. doi: 10.1200/JCO.2017.76.6402. [DOI] [PubMed] [Google Scholar]
- 17.National Comprehensive Care Network. NCCN Clinical Practice Guidelines in Oncology – Multiple Myeloma Version 4.2018. 2018.
- 18.Moreau P., San Miguel J., Sonneveld P. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017;28:iv52–iv61. doi: 10.1093/annonc/mdx096. [DOI] [PubMed] [Google Scholar]
- 19.Berenson J.R., Lichtenstein A., Porter L. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N. Engl. J. Med. 1996;334:488–493. doi: 10.1056/NEJM199602223340802. [DOI] [PubMed] [Google Scholar]
- 20.Morgan G.J., Davies F.E., Gregory W.M. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet. 2010;376:1989–1999. doi: 10.1016/S0140-6736(10)62051-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.XGEVA . Amgen Inc.; Thousand Oaks, CA: 2018. (Denosumab) [package insert] [Google Scholar]
- 22.Raje N., Terpos E., Willenbacher W. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet. Oncol. 2018;19:370–381. doi: 10.1016/S1470-2045(18)30072-X. [DOI] [PubMed] [Google Scholar]
- 23.Kumar S.K., Dispenzieri A., Lacy M.Q. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28:1122–1128. doi: 10.1038/leu.2013.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Accardi F., Toscani D., Costa F. The proteasome and myeloma-associated bone disease. Calcif. Tissue. Int. 2018;102:210–226. doi: 10.1007/s00223-017-0349-1. [DOI] [PubMed] [Google Scholar]
- 25.Delforge M., Terpos E., Richardson P.G. Fewer bone disease events, improvement in bone remodeling, and evidence of bone healing with bortezomib plus melphalan-prednisone vs. melphalan-prednisone in the phase III VISTA trial in multiple myeloma. Eur. J. Haematol. 2011;86:372–384. doi: 10.1111/j.1600-0609.2011.01599.x. [DOI] [PubMed] [Google Scholar]
- 26.Terpos E., Christoulas D., Kastritis E. VTD consolidation, without bisphosphonates, reduces bone resorption and is associated with a very low incidence of skeletal-related events in myeloma patients post ASCT. Leukemia. 2014;28:928–934. doi: 10.1038/leu.2013.267. [DOI] [PubMed] [Google Scholar]
- 27.Terpos E., Kanellias N., Moulopoulos L.A. Low incidence of skeletal-related events at the time of first relapse in patients with multiple myeloma who received bortezomib-based regimens as first line treatment. Haematologica. 2016;101:87. [Google Scholar]
- 28.Aly A., Onukwugha E., Woods C. Measurement of skeletal related events in SEER-Medicare: a comparison of claims-based methods. BMC. Med. Res. Methodol. 2015;15:65. doi: 10.1186/s12874-015-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim C, Hernandez RK, Cyprien L, Liede A, Cheng PC. Patterns of bisphosphonate treatment among patients with multiple myeloma treated at oncology clinics across the USA: observations from real-world data. Support Care Cancer. 2018;26:2833–2841. doi: 10.1007/s00520-018-4133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashcroft J., Timothy B., Smith A. Skeletal-related events in myeloma: a population-based study. Blood. 2013;122:3158. [Google Scholar]
- 31.Mateos M., Cavo M., Fink L. Patient characteristics, skeletal related events (SRE) and renal impairment (RI) in patients with multiple myeloma (MM): a patient chart audit in EU5. Value. Health. 2017;20:A413. [Google Scholar]
- 32.Curtis J.R., Mudano A.S., Solomon D.H. Identification and validation of vertebral compression fractures using administrative claims data. Med. Care. 2009;47:69–72. doi: 10.1097/MLR.0b013e3181808c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lix L.M., Azimaee M., Osman B.A. Osteoporosis-related fracture case definitions for population-based administrative data. BMC. Public. Health. 2012;12:301. doi: 10.1186/1471-2458-12-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ria R., Reale A., Moschetta M. A retrospective study of skeletal and disease-free survival benefits of zoledronic acid therapy in patients with multiple myeloma treated with novel agents. Int. J. Clin. Exp. Med. 2013;6:30–38. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.