Abstract
Object: Many patients with Ewing sarcoma (ES) of the mobile spine present with neurologic symptoms leading to emergency decompressive surgery. Only rarely is optimal treatment involving neo-adjuvant chemotherapy followed by en bloc excision possible. The purpose of this study was to study treatment, neurologic and oncologic outcome in patients with ES of the mobile spine.
Methods: Twenty-four patients diagnosed between 1986 and 2012 were identified through the Scandinavian Sarcoma Group registry. Charts were reviewed in order to assess details in patient characteristics, neurologic status, treatment and outcome. Prognostic factors were analyzed with respect to local control, disease-free survival and overall survival.
Results: Neurologic symptoms were frequently observed at presentation, being present in 19/23 patients with documented neurologic status. Most (13/19) patients had a complete neurologic recovery regardless of whether or not emergency decompressive surgery was performed. The majority (18/24) of patients were treated with definitive radiotherapy. However, only 9/17 received the recommended dose of ≥ 50.4 Gy. The disease-free and overall survival rates at 10 years were 48% and 57%, respectively. The local recurrence rates were 19% and 27% at 5 and 10 years, respectively. Only year of diagnosis, categorized into periods with significant changes in chemotherapy protocols, was a significant factor for local recurrence, but there was a trend (p = 0.06) for an increased risk of a local recurrence if emergency decompressive surgery was performed.
Conclusion: Patients with ES of the mobile have a relatively favorable prognosis. Nonetheless, local recurrence rate is high for this group of patients for which local treatment mainly relies on definitive radiotherapy.
Emergency decompressive surgery may increase the risk for local recurrence.
Keywords: Ewing sarcoma, Ewing´s sarcoma, Spine, Oncology, Surgery, Radiation treatment
1. Introduction
Survival in Ewing sarcoma (ES) has substantially improved since the 1970s largely due to the introduction and development of aggressive multi-agent chemotherapeutic regimens [1, 2]. Advancements in radiotherapy (RT) planning and aggressive surgery have further improved local control which again contributes to improved overall survival [3]. For patients with localized disease the overall survival has now reached 65–75% [2]. However, for patients with axial or pelvic locations the outcome is not as favorable [1, [4], [5], [6], [7], [8]. Whether this is due to difficulties in achieving local control, or the fact that centrally located tumors tend to be larger, remains unclear [1]. It has also been suggested that axial ES has a more aggressive phenotype by virtue of the microenvironmental milieu of the axial skeleton [6, 8].
Depending on the anatomical location, the term “fixed spine” or “mobile spine” may be used when describing vertebral ES. In a previous study on pelvic ES, we published data on ES of the fixed spine (sacral vertebrae); therefore only ES of the mobile spine is presented in the current study [8].
Patients with ES of the mobile spine represent major local treatment challenges due to the close proximity to neurologic and vascular structures. En bloc surgery is often not possible without significant concurring morbidity. Furthermore, patients with ES of the mobile spine often present with neurologic symptoms, resulting in emergent decompressive surgery prior to standard complete diagnostic work-up and neo-adjuvant chemotherapy [9], [10], [11], [12], [13], [14]. How this affects the oncologic outcome is not well known as publications on local treatment and outcome for spinal ES are scarce and the cohorts studied are small [4, [14], [15], [16], [17].
The primary aim of this study was to study survival and local control in patients with ES of the mobile spine. The secondary aim of this study was to investigate the scale of neurologic symptoms at presentation as well as neurologic recovery with regards to surgical and non-surgical treatments. Whether emergency decompressive surgery had an effect on local recurrence was also investigated.
2. Materials and methods
The Scandinavian Sarcoma Group (SSG) Registry was established in 1986 and contains prospectively recorded data of patients from Sweden, Norway and Finland. The registry, which is population-based for Norway and for most of Sweden, is considered to be representative of sarcomas in Scandinavia [18].
In this study, patients with a histological diagnosis of ES of the mobile spine diagnosed between April 1986 and May 2012 were identified through the Scandinavian Sarcoma Group Registry and retrospectively reviewed by use of medical charts at each Institution. The study was approved by the Regional Ethical Review Board in Stockholm (Registration no. 2013/933-31/14).
As the vast majority of the patients were included in international trials, the histology was already peer-reviewed by a pathology committee within the SSG. The fusion gene analysis for ES has been in routine use since 1999. A total of 25 ES cases of the mobile spine with a minimum follow-up of 2 years were identified. One patient had metastasis to the spine and was wrongly classified and therefore excluded from the study. There were 24 patients in the final analysis. One patient was excluded from the analysis of neurologic function due to missing information on neurologic status both at presentation and at follow-up. Clinically relevant data for each case are shown in Table 1.
Table 1.
Baseline Characteristics, treatment and outcome of 24 Ewing sarcomas of the mobile spine.
| Patient | Age | Site/sizea | Frankelb | Metsc | Systemic treatment | Local treatment/microscopic resection margin | Total irradiation dose | Frankeld | LR | Metse | Statusf | Treatment complications |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 17 | L5/8 cm | D | No | SSG IX | Laminectomy + Radiotherapy/R2 | 44.8 Gy | E | Yes | No | Dead | None |
| 2 | 10 | Th4-5/8 cm | E | No | ISG/SSG III | Radiotherapy | 42 Gy | E | No | No | Dead | None |
| 3 | 15 | L4/5 cm | D | No | ISG/SSG III | Radiotherapy | 54 Gy | E | No | No | Dead | None |
| 4 | 22 | Th12-L1 | D | Yes | SSG IV | Laminectomy + Radiotherapy/R2 | Unknown | D | No | Yes | Dead | Cardiomyopathy |
| 5 | 16 | L5-S2 | Unknown | No | Yes | Radiotherapy | Unknown | Unknown | Yes (after 10 years) | Yes | Dead | None |
| 6 | 8 | C6 | D | No | SSG IX | Laminectomy + Radiotherapy/R2 | 42 Gy | E | No | No | NED | Secondary posterior spinal stabilization due to gibbus deformity. |
| Severe oesophageal stricture, Percutaneus Endoscopic Gastrostomy, hypothyreoidism, | ||||||||||||
| cardiomyopathy | ||||||||||||
| 7 | 18 | L4/6 cm | D | Yes | Euro Ewing 99 | Radiotherapy | 51 Gy | E | No | No | NED | None |
| 8 | 21 | C6/4 cm | E | No | SSG IX | Radiotherapy | 54 Gy | E | No | No | NED | Hypothyreoidism, hearing loss |
| 9 | 14 | L3 | Unknown | Yes | SSG IV | Laminectomy + Radiotherapy/R2 | 40 Gy | E | No | Yes | Dead | None |
| 10 | 20 | L5 | D | Yes | SSG IX | Radiotherapy only | 60 Gy | D improved | No | Yes | NED | None |
| 11 | 20 | C5-C7/9 cm | E (radiculo-pathy) | No | SSG IV | Laminectomy + Radiotherapy/R2 | 42 Gy | E (still radiculo-pathy, but improved) | Yes | No | Dead | None |
| 12 | 12 | L5 | E | No | SSG IX | Spondylectomy/R0 | No | E | No | Yes | NED | None |
| 13 | 43 | Th7 | Paresis, unknown grade | Yes | SSG IX | Laminectomy/posterior fixation + Radiotherapy/R2 | 52 Gy | Improved | No | Yes | Dead | None |
| 14 | 20 | L4/18 cm | B/C | No | ISG/SSG III | Spondylectomy + Radiotherapy/R1 | 42 Gy | E | No | No | NED | Revision spinal surgery due to pseudarthrosis and mechanical failure |
| 15 | 14 | Th3/7 cm | B | No | ISG/SSG III | Spondylectomy + Radiotherapy/R1 | 42 Gy | E | No | No | NED | Hypothyreoidism. Renal failure. Revision due to cerebrospinal fluid leakage |
| 16 | 4 | Th1 | D | No | ISG/SSG III | Laminectomy + Tumor Resection/R1 | No | E | No | No | NED | Revision surgery due to kyphosis |
| 17 | 52 | L1/13 cm | C | No | ISG/SSG III | Radiotherapy | 42 Gy | D | No | No | NED | Colostomy/ileostomy due to serious intestinal chemotherapy related toxicity |
| 18 | 14 | L3 | E | Yes | SSG IX | Spondylectomy/R0 | 42 Gy | E | No | Yes | NED | None |
| 19 | 20 | Th8-11/5 cm | C | No | SSG IV | Laminectomy + Radiotherapy/R2 | 48 Gy | D | Yes | No | Dead | None |
| 20 | 20 | L2/12 cm | C | No | SSG IX | Laminectomy + Radiotherapy/R2 | 60 Gy | E | Yes | Yes | Dead | None |
| 21 | 14 | C4/8 cm | E (radiculo-pathy) | No | SSG IX | Radiotherapy | 54 Gy | E | No | Yes | Dead | None |
| 22 | 6 | L1/8 cm | D | No | ISG/SSG III | Laminectomy + Radiotherapy/R2 | 54 Gy | E | No | No | NED | Multiple spinal revisions due to kyphosis |
| 23 | 26 | L4/4 cm | D | No | ISG/SSG III | Radiotherapy | 54 Gy | E | No | No | NED | None |
| 24 | 14 | Th4/7 cm | A | No | ISG/SSG III | Tumor Resection + Radiotherapy/R0 | 45 Gy | E | No | No | NED | Thoracic kyphosis treated non-operatively |
LR = Local recurrence.
NED = No evidence of disease.
ISG/SSG III = Italian Sarcoma Group/Scandinavian Sarcoma Group protocol III; SSG IV = Scandinavian Sarcoma Group protocol IV.
SSG IX = Scandinavian Sarcoma Group protocol IX; Euro Ewing 99 = Euro Ewing 99 trial.
R0 = Microscopic resection margin free of tumor cells.
R1 = Tumor cells microscopically present at the resection margin.
R2 = Tumor tissue grossly present (by the naked eye) at the resection margin.
Data not available for all patients.
Frankel = Neurologic status at presentation.
Mets = Metastasis at presentation.
Frankel = Neurologic status at last fo-up.
Mets = Metastasis at fo-up.
Status = Status at last fo-up.
The median follow-up time among ES survivors in this cohort was 11 years (mean 12 years, range 4–22 years). Excisional surgery versus definitive RT was assessed with local recurrence, disease-free survival and overall survival as end points.
Parameters included in the analyses were the presence of metastasis at time of diagnosis, tumor size, local treatment, treatment period and microscopic surgical margin. Tumor size was categorized into small (<= 8 cm) or large (>8 cm). Microscopic resection margin was defined as clear (R0) if the margin was reported as being wide or marginal, and as positive (R1 or R2) if the margin was assessed as intralesional. Most patients received chemotherapy according to Scandinavian protocols; SSG IV (1984–1990) [19] and SSG IX (1990–1999) [20], or more recently according to protocols based on collaboration with the Italian Sarcoma Group: ISG/SSG III and IV (1999-to the present) [21, 22] (Table 2). Year of diagnosis was categorized into the treatment periods concurring with changes in chemotherapy protocols. Follow-up was routinely done every 3–4 months the first 3 years after diagnosis, thereafter twice a year for another 2 years, and then annually for up to 10 years. This routine has been essentially unchanged since the start of the study period. The time to first recurrence, either local or distant, was calculated from the date of diagnosis.
Table 2.
Summary of patient demographics, tumor characteristics and local treatment for 24 patients with Ewing sarcoma of the mobile spine.
| Age at time of diagnosis (a) | 17(4–52) |
| Gender | |
| Maleb | 15(63) |
| Metastasis at time of diagnosisb | 5(21) |
| Treatment periodb | |
| 1984–1989 | 3(12) |
| 1990–1998 | 10(42) |
| 1999-present | 11(46) |
| Follow up time (years) (d) | 11(4–22) |
| Tumor size; mean ± SD (cm) (c) | 8 ± 4 |
| Local treatmentb | |
| Definitive radiotherapy | 18(75) |
| Excisional surgery | 2(8) |
| Excisional surgery + radiotherapy | 4(17) |
The values are given as the median, with the range in parenthesis.
The values are given as the number of patients, with the percentage in parenthesis.
The values are given as the mean and standard deviation.
The values are given as the median, with the range in parenthesis. Includes only ES survivors.
2.1. Patient demographics and local treatment
Patient demographics, tumor characteristics and local treatment are summarized in Table 2. Definitive RT of the primary tumor was the preferred local treatment for 18 patients, and excisional surgery for 6 patients. Four of the 6 surgically treated patients also received post-operative RT. Three patients underwent surgery of residual disease after neo-adjuvant chemotherapy. One patient with a predominant soft tissue mass extending into the spinal canal was treated with laminectomy and excision of all macroscopic tumor. This patient was regarded as having been treated with excisional surgery, albeit intralesional. Another 2 patients had intralesional surgical margins while 3 patients had their tumors resected with clear surgical margins (R0), of which 2 were evaluated as wide, and 1 as marginal. Hence, in total only 3 patients in this series were treated as Enneking appropriate, meaning that they were treated with en bloc excision with microscopic tumor-free margins.
Thirteen (54%) patients underwent spinal decompressive surgery due to spinal cord compression. For 9 of these patients, surgery was confined to emergency laminectomy only. The tumor border was violated for 8 of these cases, and margins are unknown for 1 case. The histologic diagnosis was not known prior to emergency laminectomy. Two patients were treated with emergency laminectomy and simultaneous tumor excision, 1 grossly intralesional and 1 with wide surgical margins. The remaining 2 patients who presented with significant neurologic symptoms received pre-operative chemotherapy prior to laminectomy and spondylectomy. The surgical margins were intralesional for the latter 2 patients.
Seventeen patients treated with definitive radiation treatment received a median radiation dose of 51.5 Gy, which is less than the recommended dose of 54 Gy in the ISG/SSG protocols, although the 45 Gy dose restraint to the spinal cord would entail compromised dose in the surrounding bone. In most recommendations, definitive radiation dose to ES of the spine is an exception of the rule since restricted to maximum 50.4 Gy [2, 23]. Nine of seventeen patients who received radiation treatment as definitive local treatment received the recommended dose of ≥ 50.4 Gy. One patient only received palliative radiation therapy because of disseminated disease and dismal prognosis. For one patient the radiation dose was unknown. For the majority of the patients (n = 17), RT was administered in a hyperfractionated regimen with 1.5 Gy given twice daily. Photon or electron radiation treatment was used for all patients.
2.2. Statistics
Statistics were calculated using IBM SPSS Statistics 23, Illinois. The rate of recurrence or death was estimated using the method of Kaplan and Meier, and the effect of each risk factor on the outcome was examined using the log-rank test.
The prognostic value of each covariate was analyzed using the Cox proportional hazards model for each end-point. Only clinical relevant and statistically significant variables were included in the multivariate analysis. None of the covariates used in the model were assumed to be time-dependent and therefore the fixed covariate Cox regression analysis was used.
3. Results
3.1. Local treatment and local control
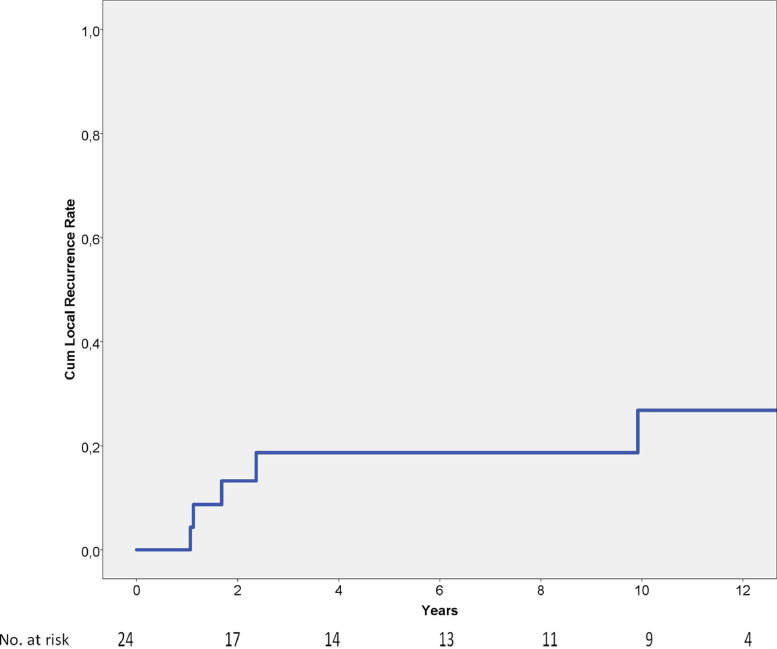
The cumulative local recurrence rate is depicted in Fig. 1, being 19% and 27%, at 5 respective 10 years. The median time to local recurrence was 2 years (range 1–10 years).
Fig. 1.
Local recurrence rate of 24 spinal Ewing sarcomas showing cumulative a 5-year local recurrence rate of 19%.
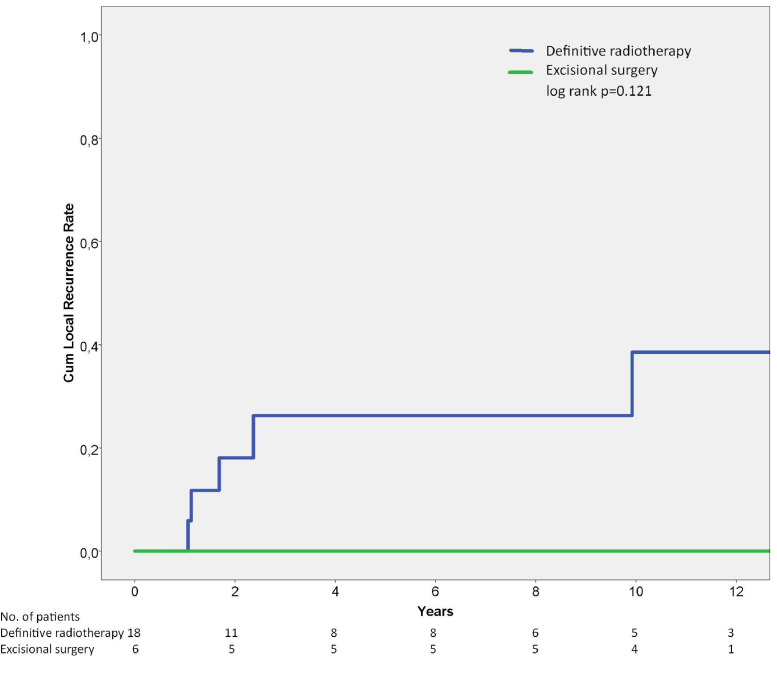
The cumulative local recurrence rate with regards to local treatment is presented in Fig. 2. There was no statistical difference between excisional surgery and definitive RT with regards to local recurrence rate (p = 0.121; Fig. 2), yet none of the 6 patients treated with tumor excision +/− adjuvant radiotherapy suffered from a local recurrence. One patient experienced a local recurrence 10 years after definitive radiation treatment. Univariate analysis of prognostic factors for local recurrence and overall survival of mobile spine tumors is depicted in Table 3. Treatment period was the only significant prognostic factor for local recurrence. However, there was a tendency for inferior local control among patients treated with emergency decompressive surgery without simultaneous tumor excision (p = 0.061). Radiation dose was not prognostic for local control.
Fig. 2.
Local recurrence rate of 24 patients with Ewing sarcoma of the mobile spine showing a 5-year local recurrence rate of 26% for patients treated with definitive radiation treatment and no local recurrences for patients treated with excisional surgery. Log rank p = 0.121.
Table 3.
Univariate analysis of prognostic factors for local recurrence and overall survival for ES of the mobile spine.
| Variable | No. of patients | No. of local recurrences | 10 year LR rate (%) | p-valuee | No. of deaths | 10 year OS rate (%) | p-valuee |
|---|---|---|---|---|---|---|---|
| Gender | 0.469 | 0.864 | |||||
| Male | 15 | 4 | 35 | 7 | 58 | ||
| Female | 9 | 1 | 14 | 4 | 56 | ||
| Age (years) | 0.180 | 0.213 | |||||
| <14 | 5 | 0 | 0 | 1 | 80 | ||
| >=14 | 19 | 5 | 36 | 10 | 51 | ||
| Metastasis at presentation | 0.312 | 0.962 | |||||
| No | 19 | 5 | 31 | 9 | 57 | ||
| Yes | 5 | 0 | 0 | 2 | 50 | ||
| Tumor sized | 0.267 | 0. 791 | |||||
| <=8 cm | 11 | 2 | 19 | 5 | 51 | ||
| >8 | 4 | 2 | 50 | 2 | 50 | ||
| Year of diagnosis | 0.030 | 0.098 | |||||
| 1986–1989 | 3 | 2 | – | 3 | 33 | ||
| 1990–1998 | 10 | 3 | 33 | 5 | 50 | ||
| 1999-to the present | 11 | 0 | 0 | 3 | 63 | ||
| Local treatment | 0.300 | 0.079 | |||||
| Excisional surgery | 2 | 0 | 0 | 0 | 100 | ||
| Surgery + Radiotherapy | 4 | 0 | 0 | 0 | 100 | ||
| Definitive radiotherapy | 18 | 5 | 39 | 11 | 43 | ||
| Excisional surgery (a) | 0.121 | 0.024 | |||||
| Yes | 6 | 0 | 0 | 0 | 100 | ||
| No | 18 | 5 | 39 | 11 | 43 | ||
| Margin (b) | N.A. | N.A. | |||||
| R1 | 3 | 0 | 0 | 0 | 100 | ||
| R0 | 3 | 0 | 0 | 0 | 100 | ||
| Radiation dose | 0.757 | 0.382 | |||||
| <45 Gy | 8 | 2 | 29 | 3 | 63 | ||
| >=45 Gy | 13 | 2 | 17 | 7 | 43 | ||
| Local recurrence | N.A. | 0.007 | |||||
| Yes | 5 | 5 | 20 | ||||
| No | 19 | 6 | 33 | ||||
| Decompressive surgery (c) | 0.061 | 0.105 | |||||
| Yes | 9 | 4 | 42 | 7 | 22 | ||
| No | 15 | 1 | 75 | 4 | 62 |
LR = Local recurrence.
OS = Overall survival.
N.A . = Not Applicable.
Excisional surgery +/− radiotherapy.
Includes only patients treated with excisional intent.
Does not include patients treated with simultaneous excisional surgery.
Data not available for all patients.
Log-rank test.
3.2. Disease-free and overall survival
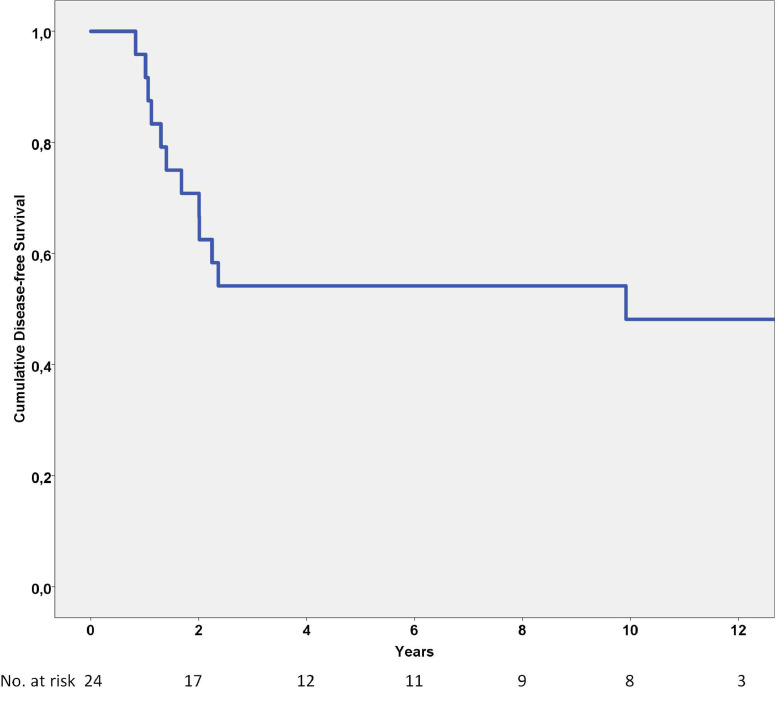
Disease-free survival was 54% and 48% at 5 and 10 years, respectively (Fig. 3). Treatment period and emergency decompressive surgery without tumor excision were significant prognostic factors in the univariate analysis (Table 4), but not in the multivariate analysis (Table 5).
Fig. 3.
Disease-free survival analysis of 24 spinal Ewing sarcomas showing a cumulative 5-year disease-free survival rate of 54%.
Table 4.
Univariate analysis of prognostic factors for disease-free survival of 24 ES of the mobile spine.
| Variable | No. of patients | No. of events | 10 year DFS rate (%) | p-valuee |
|---|---|---|---|---|
| Gender | 0.979 | |||
| Male | 15 | 8 | 43 | |
| Female | 9 | 4 | 56 | |
| Age (years) | 0.559 | |||
| <14 | 5 | 2 | 60 | |
| >=14 | 19 | 10 | 44 | |
| Metastasis at presentation | 0.586 | |||
| No | 19 | 9 | 51 | |
| Yes | 5 | 3 | 40 | |
| Tumor sized | 0.662 | |||
| <=8 cm | 11 | 4 | 64 | |
| >8 | 4 | 2 | 50 | |
| Year of diagnosis | 0.016 | |||
| 1986–1989 | 3 | 3 | 0 | |
| 1990–1998 | 10 | 7 | 30 | |
| 1999-to the present | 11 | 2 | 82 | |
| Local treatment | 0.520 | |||
| Excisional surgery | 2 | 1 | 50 | |
| Surgery + Radiotherapy | 4 | 1 | 75 | |
| Definitive radiotherapy | 18 | 10 | 42 | |
| Excisional surgery (a) | 0.287 | |||
| Yes | 6 | 2 | 67 | |
| No | 18 | 10 | 42 | |
| Margin (b) | 0.745 | |||
| R1 | 3 | 7 | 39 | |
| R0 | 3 | 2 | – | |
| Radiation dose | 0.803 | |||
| <45 Gy | 8 | 4 | 50 | |
| >=45 Gy | 13 | 6 | 54 | |
| Local recurrence | N.A. | |||
| Yes | 5 | |||
| No | 19 | |||
| Decompressive surgery (c) | 0.048 | |||
| Yes | 9 | 7 | 22 | |
| No | 9 | 3 | 58 |
DFS = Disease-free survival.
N.A . = Not Applicable.
Excisional Surgery +/− Radiotherapy.
Includes only patients treated with excisional intent.
Not included patients treated with excisional surgery.
Data not available for all patients.
Log-rank test.
Table 5.
Multivariate disease-free survival analysis for 24 Ewing sarcomas of the mobile spine (a).
| Variable | HR | 95% CI | p-value |
|---|---|---|---|
| Decompressive surgery (b) | 4.3 | 0.94–19.25 | 0.060 |
| Year diagnosed | |||
| 1986–1989 | 0.139 | ||
| 1990–1998 | 0.24 | 0.05–1.24 | 0.089 |
| 1999-present | 0.18 | 0.03–1.19 | 0.076 |
HR = hazard ratio, CI = Confidence Interval.
Without tumor excision.
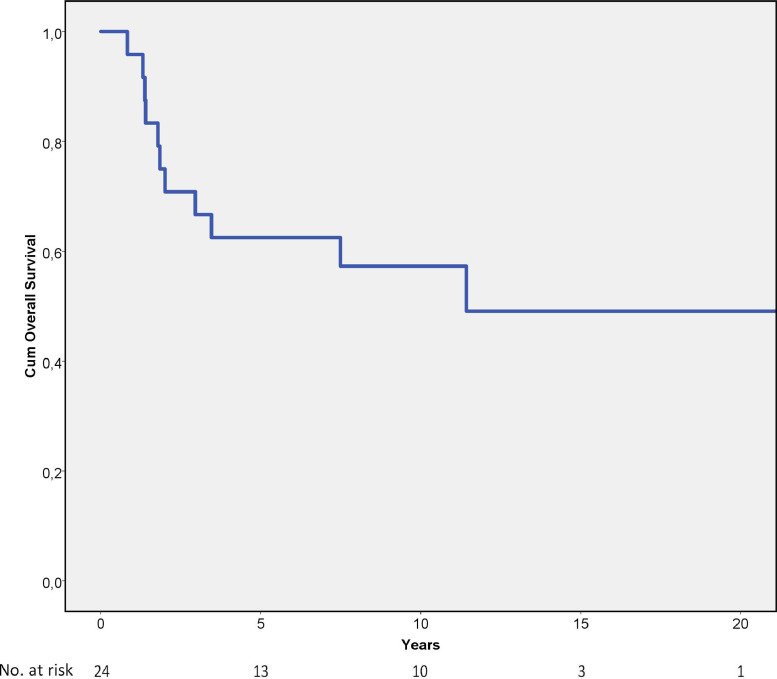
The 5-year and 10-year overall survival rates were 63% and 57%, respectively (Fig. 4). Excisional surgery and local recurrence were statistically significant prognostic factors for overall survival (Table 3), but none of these factors were significant in the multivariate analysis (Table 6).
Fig. 4.
Overall survival analysis of 24 spinal Ewing sarcomas showing cumulative 5-year overall survival rate of 63%.
Table 6.
Multivariate overall survival analysis for 24 Ewing sarcomas of the mobile spine (a).
| Variable | HR | 95% CI | p-value |
|---|---|---|---|
| Excisional surgery | 0.000 | 0.00–9.43E + 250 | 0.966 |
| Local recurrence | 2.82 | 0.85 to 9.33 | 0.089 |
HR = Hazard Ratio, CI = Confidence Interval.
3.3. Neurologic function
Nineteen out of 23 patients in our study presented with neurologic deficits. However, after local and systemic treatment only 6/23 patients had persistent neurologic deficits. One patient with unknown neurologic status at presentation underwent immediate decompressive surgery and had a nearly normal neurologic status at last follow-up. Six out of 19 patients with neurologic deficits were treated locally with radiation only, of whom 4 had a complete neurologic recovery. The remaining 2 had Frankel D grade deficits, one of whom also had radiculopathy. Thirteen out of 19 patients were treated with decompressive surgery, of whom 9 had a complete neurologic recovery. The remaining 4 patients improved after decompressive surgery, but still had minor neurologic sequelae. None had a worse neurologic grade than Frankel D. Altogether in the whole cohort, the Frankel grade mode at diagnosis was Frankel D, and at time of latest follow-up the mode was Frankel E.
3.4. Complications
There were 8 serious long-term treatment complications among the patients in this study. Five out of thirteen survivors suffered from late surgically-associated spinal complications requiring revision surgery. Four patients developed kyphotic deformities, 3 of whom required surgery at a later stage. They were all originally treated with laminectomy or tumor excision without internal fixation. One patient with a primary tumor of the C7 vertebrae treated with decompressive surgery and definitive RT is alive with a severe esophageal stricture requiring nutrition through a percutaneous endoscopic gastrostomy, and currently undergoing esophageal dilatations under general anesthesia every fourth week. Another patient with an L1 tumor suffered severe colitis and ileitis leading to a colostomy/ileostomy.
4. Discussion
ES are well known to be radiosensitive tumors [2]. Nevertheless, surgery has become the cornerstone of local treatment since it has proven to yield better local control than radiation treatment alone [2, [23], [24], [25], [26], [27], [28], [29]. Yet, no studies to our knowledge have been able to prove a benefit of one local treatment over another regarding local control of spinal ES [4, 14, 16, 17]. Surgical advancements in the field of spinal surgery have made it possible to resect tumors that were previously deemed to be non-resectable. Interestingly, Indelicatio et al. reported a 100% local control rate for six patients treated with excisional surgery with adjuvant RT [16]. Schuck et al. also documented a 100% local control rate for six patients treated with wide resection and RT, but only 50% local control rate in four patients treated with surgery exclusively [17]. In a cohort of 58 surgically treated spinal ES patients, Charest-Morin et al. found better overall survival, but not better local control for patients treated with en bloc excision and clear surgical margins (Enneking appropriate) if patients were not subject to prior spine surgery [15]. The inability in these studies, and in other studies, to prove better local control by surgical treatment of the primary tumor may be a matter of insufficient power. Conversely, the field of radiation therapy has also evolved, perhaps improving local control. Proton- or carbon ion radiation treatment was not used for any of the patients in this study; however its role in treatment of ES of the spine is likely to increase in the future as access to heavy ion treatment improves.
4.1. Local treatment and local control
The local recurrence rate in our study is comparable to what has been found in some other studies [4, 10, 13–15, 17]. However, the rate is higher than in the Children's Oncology Group study by Ahmed et al. which reported a 3.6% 5 year local recurrence rate, all local recurrences occurring in the group treated by definitive RT [30]. The excellent local control observed among patients treated with excisional surgery in our study may be attributed to improvements in chemotherapy rather than to the surgical treatment of the primary tumor. Our observation that treatment period was the only significant factor with regards to local control supports this explanation. Notably, improved local control attributed to systemic treatment was also evident in the Children´s Cancer Group-Pediatric Oncology Group cooperative study (INT-0091, 1988–1992). In their study, improved local control was the main reason for improved overall survival [31]. Furthermore, this study shows that urgent decompressive surgery in order to improve neurologic function has a cost as the procedure is likely to result in a higher local recurrence rate. A reasonable explanation could be that decompressive surgery most often results in tumor violation and contaminated surgical field. Hence, RT has to compensate for suboptimal surgery, in addition to a delay in chemotherapy due to the post-operative recovery period.
Local control is exceedingly important for overall survival. Only 22–24% of patients will survive 5 years after local relapse of ES [32]. The value of achieving local control is confirmed in the current study, as all of the five patients suffering from a local recurrence succumbed to the disease. The role of local treatment on local control and overall survival is a difficult issue because selection bias is inevitable. Our finding that excisional surgery of the primary tumor was a significant positive prognostic factor for overall survival may well be a result of selection bias.
4.2. Disease-free and overall survival
Axial and pelvic sites are associated with inferior overall survival and disease-free survival in several studies [1, 5–7, 33]. One problem reviewing the literature is the disparity in different studies in defining the axial skeleton [5, 6, 34, 35]. Although part of the axial skeleton, the overall survival- and disease-free survival rates in the present study, which are comparable to the results reported by other authors, are not inferior to extremity ES [5, 7, 13, 36, 37]. Indeed, ES of the mobile spine tend to be smaller in size than ES of other sites [13]. Due to the anatomic location, patients are likely to experience symptoms when the tumor is relatively small in size and thus seek medical consultation at an earlier stage. This may explain the relatively good prognosis in spite of the difficulties associated with local treatment. Nonetheless, the effect of tumor size on prognosis is debated [25, 38], and other explanations must be sought. There may be biological factors attributed to primary tumor site that affect the biological behavior of the tumor and its response to chemotherapy.
Compared with our previously published results of sacral ES, mobile spine ES seem to have a similar disease-free survival rate (66% and 54% respectively; p = 0.26) [8]. The largest comparative study between sacral and nonsacral vertebral was performed by the Mayo Clinic. They reported no statistical difference in disease-free survival between the two groups in a cohort of 51 patients. The 5-year disease-free survival rate in their study was 60% and 45% for sacral and nonsacral tumors, respectively [9]. These results concord with two other studies that reported a similar outcome between patients with ES located in the mobile spine and sacrum [11, 13]. Other studies have found better outcome in patients with ES of the mobile spine compared with sacral ES. Bacci et al., in a cohort of 43 non-metastatic patients, documented a better 5-year event-free survival rate in ES of the mobile spine compared with sacral ES [4]. However, this study only included 13 sacral tumors. Pilepich et al. also found a poorer disease-free survival among patients with sacral or coccygeal site compared to proximal spinal site in a study with 22 patients. Notably, the 7 sacral tumors were all very large, which may explain the poor prognosis [4, 12].
4.3. Neurologic function
As observed in this study and in other studies, neurologic deficits are frequently observed at time of diagnosis for patients with ES of the mobile spine [10, 13, 15, 16]. Patients treated with emergency decompressive surgery did have more severe neurologic deficits than patients receiving only RT as local treatment. However, as most ES respond well to chemotherapy with volume reduction even after one cycle, this may explain the apparent neurologic improvement among the patients in our study regardless of local treatment. This should be kept in mind when a patient with a possible ES of the mobile spine presents with myelopathy or radiculopathy.
4.4. Complications
The severe complications recorded in our study have also been described in previous studies. Radiation-induced esophageal stricture was described [16]. The typhlitis observed in the patient with an L1 tumor was a result of chemotherapy rather than RT-related toxicity [39]. Post-laminectomy kyphotic deformity is a known avoidable complication, which has also been described previously [10]. Posterior stabilization should therefore be performed when decompressive surgery or resection of ES of the posterior vertebral elements is done because the posterior tension band of the spine is disrupted [40].
4.5. Limitations and strengths
This study has certain limitations. The main limitation is the low number of patients, making analyses underpowered. Given the uncommon nature of the disease (1–3/million/year), this problem is obviously difficult to overcome [36]. Another limitation is its retrospective nature. However, data in the SSG registry have been prospectively collected since its inception. The long duration of follow-up is one of the strengths of the current study. On the contrary, as the study duration spans over a 26-year time period, the local and systemic treatment strategies have been subject to changes. The favorable outcome evident in surgically treated patients may thus be attributed to the fact that they were treated later in the study period, when systemic treatment had improved [2, 20, 31, 41].
5. Conclusion
In spite of the difficult anatomic location, patients with ES of the mobile spine have a relatively favorable prognosis. Nonetheless, local recurrence rate is high for this group of patients for which the vast majority is treated with definitive radiotherapy.
Neurologic symptoms leading to laminectomy are common at presentation. However, urgent decompressive surgery prior to obtaining histological diagnosis may increases the risk for local recurrence without providing a clear advantage over non-surgical treatment in terms of neurologic recovery. Progress in systemic therapy is likely the main reason for the improved local control observed in recent treatment eras.
Acknowledgments
Conflict of interest
Asle Charles Hesla has not received any financial support that might pose a conflict of interest in connection with the submitted article.
Øyvind Sverre Bruland has not received any financial support that might pose a conflict of interest in connection with the submitted article.
Nina Jebsen has not received any financial support that might pose a conflict of interest in connection with the submitted article.
Emelie Styring has not received any financial support that might pose a conflict of interest in connection with the submitted article.
Sigvard Eriksson has not received any financial support that might pose a conflict of interest in connection with the submitted article.
Panagiotis Tsagozis has not received any financial support that might pose a conflict of interest in connection with the submitted article.
Acknowledgments
The authors would like to thank all colleagues at the Scandinavian sarcoma centers for collecting data to the SSG Central Register (Karolinska University Hospital, Skane University Hospital in Lund, University Hospital of Umeaa, Linköping University Hospital, Sahlgrenska University Hospital in Gothenburg, Uppsala University Hospital, Oslo University Hospital, Haukeland University Hospital in Bergen, St. Olav's University Hospital in Trondheim, Tampere University Hospital in Tampere, Helsinki University Central Hospital and University Hospital of North Norway in Tromsø). Special thanks to Elisabeth Johansson, Maria Rejmyr and Eva-Mari Olofsson from the Regional Cancer Centre syd/SSG secretariat for help with data management and secretarial assistance and to Swedish Cancer Society for economical support. Thanks to Dr. Aarne Kivioja at Helsinki University Central Hospital for reviewing charts. Also thanks to Professor Henrik Bauer and Dr. Otte Brosjö at the Karolinska University Hospital for their invaluable support. Last but not least, thanks to Professor Robert Harris at the Karolinska Institute for language support and good advice.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jbo.2018.100216.
Contributor Information
Asle Charles Hesla, Email: asle.hesla@sll.se.
Øyvind Sverre Bruland, Email: OSB@ous-hf.no.
Nina Jebsen, Email: nina.louise.jebsen@helse-bergen.no.
Emelie Styring, Email: emelie.styring@med.lu.se.
Sigvard Eriksson, Email: sigvard.eriksson@vgregion.se.
Panagiotis Tsagozis, Email: panagiotis.tsagkozis@sll.se.
Appendix. Supplementary materials
References
- 1.Rodriguez-Galindo C., Liu T., Krasin M.J. Analysis of prognostic factors in Ewing sarcoma family of tumors: review of St. Jude Children's Research Hospital studies. Cancer. 2007;110(2):375–384. doi: 10.1002/cncr.22821. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 2.Gaspar N., Hawkins D.S., Dirksen U. Ewing sarcoma: current management and future approaches through collaboration. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015;33(27):3036–3046. doi: 10.1200/JCO.2014.59.5256. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Galindo C., Spunt S.L., Pappo A.S. Treatment of Ewing sarcoma family of tumors: current status and outlook for the future. Med. Pediatr. Oncol. 2003;40(5):276–287. doi: 10.1002/mpo.10240. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 4.Bacci G., Boriani S., Balladelli A. Treatment of nonmetastatic Ewing's sarcoma family tumors of the spine and sacrum: the experience from a single institution. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2009;18(8):1091–1095. doi: 10.1007/s00586-009-0921-0. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cotterill S.J., Ahrens S., Paulussen M. Prognostic factors in Ewing's tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing's Sarcoma Study Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2000;18(17):3108–3114. doi: 10.1200/JCO.2000.18.17.3108. [DOI] [PubMed] [Google Scholar]
- 6.Weiss K.R., Biau D.J., Bhumbra R. Axial skeletal location predicts poor outcome in Ewing's sarcoma: a single institution experience. Sarcoma. 2011;2011 doi: 10.1155/2011/395180. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craft A., Cotterill S., Malcolm A. Ifosfamide-containing chemotherapy in Ewing's sarcoma: the Second United Kingdom Children's Cancer Study Group and the Medical Research Council Ewing's Tumor Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1998;16(11):3628–3633. doi: 10.1200/JCO.1998.16.11.3628. [DOI] [PubMed] [Google Scholar]
- 8.Hesla A.C., Tsagozis P., Jebsen N. Improved prognosis for patients with Ewing sarcoma in the sacrum compared with the innominate bones: the Scandinavian sarcoma group experience. J. Bone Joint Surg. 2016;98(3):199–210. doi: 10.2106/JBJS.O.00362. American Volume[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 9.Ilaslan H., Sundaram M., Unni K.K. Primary Ewing's sarcoma of the vertebral column. Skelet. Radiol. 2004;33(9):506–513. doi: 10.1007/s00256-004-0810-x. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 10.Marco R.A., Gentry J.B., Rhines L.D. Ewing's sarcoma of the mobile spine. Spine. 2005;30(7):769–773. doi: 10.1097/01.brs.0000157755.17502.d6. [DOI] [PubMed] [Google Scholar]
- 11.Grubb M.R., Currier B.L., Pritchard D.J. Primary Ewing's sarcoma of the spine. Spine. 1994;19(3):309–313. doi: 10.1097/00007632-199402000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Pilepich M.V., Vietti T.J., Nesbit M.E. Ewing's sarcoma of the vertebral column. Int. J. Radiat. Oncol. Biol. Phys. 1981;7(1):27–31. doi: 10.1016/0360-3016(81)90056-0. [DOI] [PubMed] [Google Scholar]
- 13.Venkateswaran L., Rodriguez-Galindo C., Merchant T.E. Primary Ewing tumor of the vertebrae: clinical characteristics, prognostic factors, and outcome. Med. Pediatr. Oncol. 2001;37(1):30–35. doi: 10.1002/mpo.1159. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 14.Vogin G., Helfre S., Glorion C. Local control and sequelae in localised Ewing tumours of the spine: a French retrospective study. Eur. J. Cancer. 2013;49(6):1314–1323. doi: 10.1016/j.ejca.2012.12.005. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 15.Charest-Morin R., Dirks M.S., Patel S. Ewing Sarcoma of the spine: prognostic variables for survival and local control in surgically treated patients. Spine. 2018;43(9):622–629. doi: 10.1097/BRS.0000000000002386. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 16.Indelicato D.J., Keole S.R., Shahlaee A.H. Spinal and paraspinal Ewing tumors. Int. J. Radiat. Oncol. Biol. Phys. 2010;76(5):1463–1471. doi: 10.1016/j.ijrobp.2009.03.042. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 17.Schuck A., Ahrens S., von Schorlemer I. Radiotherapy in Ewing tumors of the vertebrae: treatment results and local relapse analysis of the CESS 81/86 and EICESS 92 trials. Int. J. Radiat. Oncol. Biol. Phys. 2005;63(5):1562–1567. doi: 10.1016/j.ijrobp.2005.05.036. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 18.Alvegard T., Sundby Hall K., Bauer H. The Scandinavian Sarcoma Group: 30 years' experience. Acta Orthop. 2009;80(Suppl 334):1–104. doi: 10.1080/17453690610046602. [DOI] [PubMed] [Google Scholar]
- 19.Nilbert M., Saeter G., Elomaa I. Ewing's sarcoma treatment in Scandinavia 1984–1990–ten-year results of the Scandinavian Sarcoma Group Protocol SSGIV. Acta Oncol. 1998;37(4):375–378. doi: 10.1080/028418698430601. [DOI] [PubMed] [Google Scholar]
- 20.Elomaa I., Blomqvist C., Saeter G. Chemotherapy in Ewing's sarcoma. The Scandinavian Sarcoma Group experience. Acta Orthop. Scand. 1999;285:69–73. Supplementum. [PubMed] [Google Scholar]
- 21.Ferrari S., Sundby Hall K., Luksch R. Nonmetastatic Ewing family tumors: high-dose chemotherapy with stem cell rescue in poor responder patients. Results of the Italian Sarcoma Group/Scandinavian Sarcoma Group III protocol. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol./ESMO. 2011;22(5):1221–1227. doi: 10.1093/annonc/mdq573. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 22.Luksch R., Tienghi A., Hall K.S. Primary metastatic Ewing's family tumors: results of the Italian Sarcoma Group and Scandinavian Sarcoma Group ISG/SSG IV Study including myeloablative chemotherapy and total-lung irradiation. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol./ESMO. 2012;23(11):2970–2976. doi: 10.1093/annonc/mds117. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 23.Donaldson S.S., Torrey M., Link M.P. A multidisciplinary study investigating radiotherapy in Ewing's sarcoma: end results of POG #8346. Pediatric Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 1998;42(1):125–135. doi: 10.1016/s0360-3016(98)00191-6. [DOI] [PubMed] [Google Scholar]
- 24.Granowetter L., Womer R., Devidas M. Dose-intensified compared with standard chemotherapy for nonmetastatic Ewing sarcoma family of tumors: a Children's Oncology Group Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009;27(15):2536–2541. doi: 10.1200/JCO.2008.19.1478. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krasin M.J., Rodriguez-Galindo C., Davidoff A.M. Efficacy of combined surgery and irradiation for localized Ewings sarcoma family of tumors. Pediatr. Blood Cancer. 2004;43(3):229–236. doi: 10.1002/pbc.20095. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 26.Craft A.W., Cotterill S.J., Bullimore J.A. Long-term results from the first UKCCSG Ewing's Tumour Study (ET-1). United Kingdom Children's Cancer Study Group (UKCCSG) and the Medical Research Council Bone Sarcoma Working Party. Eur. J. Cancer. 1997;33(7):1061–1069. doi: 10.1016/s0959-8049(97)00043-9. [DOI] [PubMed] [Google Scholar]
- 27.DuBois S.G., Krailo M.D., Gebhardt M.C. Comparative evaluation of local control strategies in localized Ewing sarcoma of bone: a report from the Children's Oncology Group. Cancer. 2015;121(3):467–475. doi: 10.1002/cncr.29065. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuck A., Ahrens S., Paulussen M. Local therapy in localized Ewing tumors: results of 1058 patients treated in the CESS 81, CESS 86, and EICESS 92 trials. Int. J. Radiat. Oncol. Biol. Phys. 2003;55(1):168–177. doi: 10.1016/s0360-3016(02)03797-5. [DOI] [PubMed] [Google Scholar]
- 29.Bacci G., Forni C., Longhi A. Long-term outcome for patients with non-metastatic Ewing's sarcoma treated with adjuvant and neoadjuvant chemotherapies. 402 patients treated at Rizzoli between 1972 and 1992. Eur. J. Cancer. 2004;40(1):73–83. doi: 10.1016/j.ejca.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed S.K., Randall R.L., DuBois S.G. Identification of patients with localized Ewing Sarcoma at higher risk for local failure: A report from the Children's Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 2017;99(5):1286–1294. doi: 10.1016/j.ijrobp.2017.08.020. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grier H.E., Krailo M.D., Tarbell N.J. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N. Engl. J. Med. 2003;348(8):694–701. doi: 10.1056/NEJMoa020890. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez-Galindo C., Billups C.A., Kun L.E. Survival after recurrence of Ewing tumors: the St Jude Children's Research Hospital experience, 1979–1999. Cancer. 2002;94(2):561. doi: 10.1002/cncr.10192. -9[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 33.Paulussen M., Ahrens S., Dunst J. Localized Ewing tumor of bone: final results of the cooperative Ewing's Sarcoma Study CESS 86. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2001;19(6):1818–1829. doi: 10.1200/JCO.2001.19.6.1818. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 34.Argon A., Basaran M., Yaman F. Ewing's sarcoma of the axial system in patients older than 15 years: dismal prognosis despite intensive multiagent chemotherapy and aggressive local treatment. Jpn. J. Clin. Oncol. 2004;34(11):667–672. doi: 10.1093/jjco/hyh122. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 35.Gupta A.A., Pappo A., Saunders N. Clinical outcome of children and adults with localized Ewing sarcoma: impact of chemotherapy dose and timing of local therapy. Cancer. 2010;116(13):3189–3194. doi: 10.1002/cncr.25144. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 36.Esiashvili N., Goodman M., Marcus R.B., Jr. Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: Surveillance Epidemiology and End Results data. J. Pediatr. Hematol./Oncol. 2008;30(6):425–430. doi: 10.1097/MPH.0b013e31816e22f3. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 37.Nesbit M.E., Jr., Gehan E.A., Burgert E.O., Jr. Multimodal therapy for the management of primary, nonmetastatic Ewing's sarcoma of bone: a long-term follow-up of the First Intergroup study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1990;8(10):1664–1674. doi: 10.1200/JCO.1990.8.10.1664. [DOI] [PubMed] [Google Scholar]
- 38.Oberlin O., Deley M.C., Bui B.N. Prognostic factors in localized Ewing's tumours and peripheral neuroectodermal tumours: the third study of the French Society of Paediatric Oncology (EW88 study) Br. J. Cancer. 2001;85(11):1646–1654. doi: 10.1054/bjoc.2001.2150. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nesher L., Rolston K.V. Neutropenic enterocolitis, a growing concern in the era of widespread use of aggressive chemotherapy. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2013;56(5):711–717. doi: 10.1093/cid/cis998. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 40.Tomita K., Kawahara N., Baba H. Total en bloc spondylectomy. A new surgical technique for primary malignant vertebral tumors. Spine. 1997;22(3):324–333. doi: 10.1097/00007632-199702010-00018. [DOI] [PubMed] [Google Scholar]
- 41.Balamuth N.J., Womer R.B. Ewing's sarcoma. Lancet Oncol. 2010;11(2):184–192. doi: 10.1016/S1470-2045(09)70286-4. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.