Abstract
Carnivorans are a highly diverse and successful group of mammals, found on the top of the food chain. They originated in the Palaeocene (ca. 60 Ma) and have developed numerous lifestyles, locomotion modes and hunting strategies during their evolutionary history. Mechanosensory organs, such as the inner ear (which houses senses of equilibrium and hearing), represent informative anatomical systems to obtain insights into function, ecology and phylogeny of extant and extinct vertebrates. Using µCT scans, we examined bony labyrinths of a broad sample of various carnivoran species, to obtain new information about hunting behaviours of ancient carnivorans. Bony labyrinths were digitally reconstructed and measurements were taken directly from these 3D models. Principal component analyses generally separated various hunting strategies (pursuit, pounce, ambush and occasional), but also support their phylogenetic relationships (Canoidea vs. Feloidea). The height, width and length of all three semicircular canals show functional morphological adaptations, whereas the diameter of the canals, the height of the cochlea and particularly the angle between the lateral semicircular canal and the cochlea indicate a phylogenetic signal. The results demonstrate that the bony labyrinth provides a powerful ecological proxy reflecting both predatory habits as well as phylogenetic relationships in extinct and extant carnivorans.
Introduction
Carnivorans are a highly diverse and well-documented group of mammalian top predators. They comprise two monophyletic clades, the Canoidea (dog–like carnivorans) and Feloidea (cat–like carnivorans)1 that can be distinguished based on morphological (e.g.2), and molecular data (e.g.3). However, the timing of the split between the two major clades has not been fully resolved4,5. These animals originated in the Palaeocene and distinctly diversified in the last 60 Million years1,6,7. During their evolutionary history, they have evolved to become morphologically disparate and have established an array of hunting strategies, behaviours and lifestyles, as well as a tremendous spatial range of habitats on almost all continents8. Comparatively large hunters pursue their prey over wide distances, whereas others are perfectly adapted to ambush or stalk their prey8. Pouncing on prey is more common in smaller carnivorans while specialised feeders on either fruits or insects rarely hunt at all8. However, determining if extinct carnivorans possessed similar variation in their hunting behaviours remains a challenge.
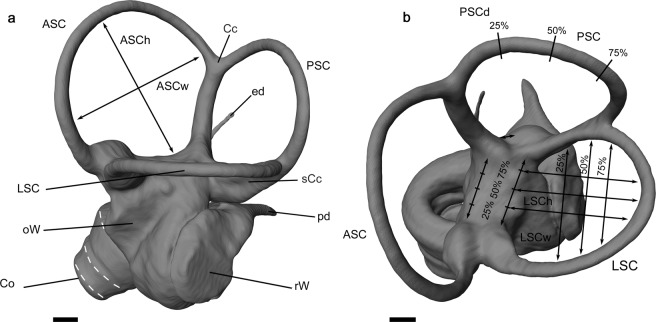
Previous studies attempted to infer the predatory niches of extinct carnivorans using dental (e.g.9–13), and limb morphologies (e.g.14–16). More recently however, there has been an increase in studies using the inner ear, the sensory organ responsible for equilibrium and hearing as a proxy for inferring ecological niche (Fig. 1). The bony labyrinth encloses the membranous system (together know as inner ear), including three semicircular ducts (SCCs; ASC – anterior semicircular canal; PSC – posterior semicircular canal, LSC – lateral semicircular canal) for angular acceleration, and the utricle and saccule within the vestibule for linear acceleration17. Located in the petrosal bone it comprises one of the best-preserved anatomical systems in the fossil record. The organ of equilibrium (vestibular system) reflects orientation in three dimensional space and hence mirrors functional morphological aspects and enables insights into ecological habits (e.g.18–30). It has already been proven that more agile animals, performing faster head movements, developed larger semicircular canals to improve their body balance while moving in complex 3D environments (e.g.23,30), than those animals having a slower mode of locomotion (e.g.18,20–28,31–33). Therefore bony labyrinth morphometry represents a powerful methodology to correlate cranial sensory changes with functional patterns and hence represents a proxy for ecological adaptations. The cochlea (Co), however, is responsible for sound detection and shows major morphological differences, which might represent phylogenetic correlations within vertebrates (e.g.33–36).
Figure 1.
Left bony labyrinth of Canis lupus (Grey wolf). (a) lateral view, (b) dorsal view. ASC, anterior semicircular canal; ASCh, height of the anterior semicircular canal; ASCw, width of the anterior semicircular canal; Cc, crus commune; Co, cochlea; ed, endolymphatic duct; LSC, lateral semicircular canal; LSCh, height of the lateral semicircular canal; LSCw, width of the lateral semicircular canal; oW, oval window; pd, perilymphatic duct; PSC, posterior semicircular canal; PSCd; diameter of the posterior semicircular canal; rW, round window; sCc, secondary crus commune. Scale 1 mm.
Here, we use the most diversified sample of carnivoran bony labyrinths yet assembled to predict hunting behaviours in extinct taxa, providing novel insights into the evolution, lifestyle and especially hunting strategies of carnivoran mammals.
Results
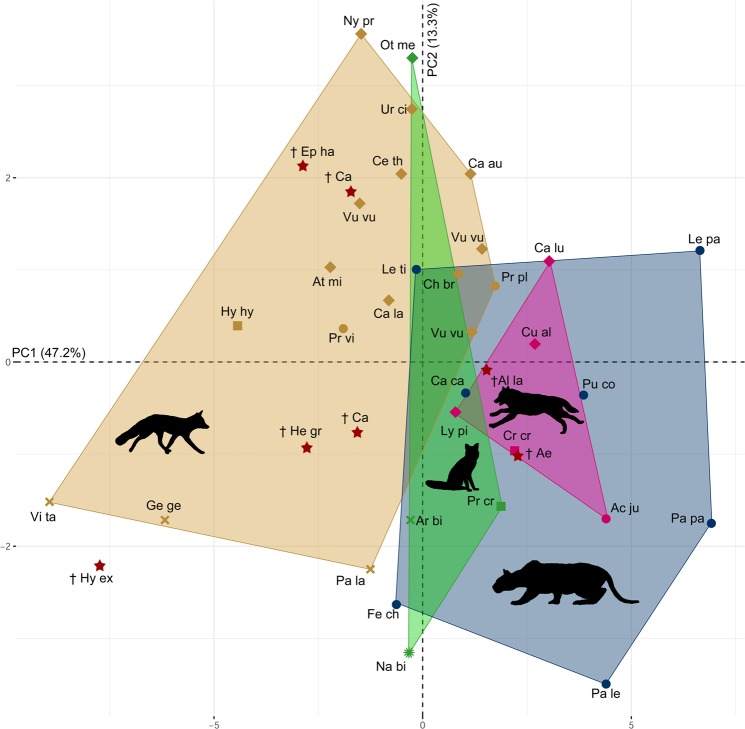
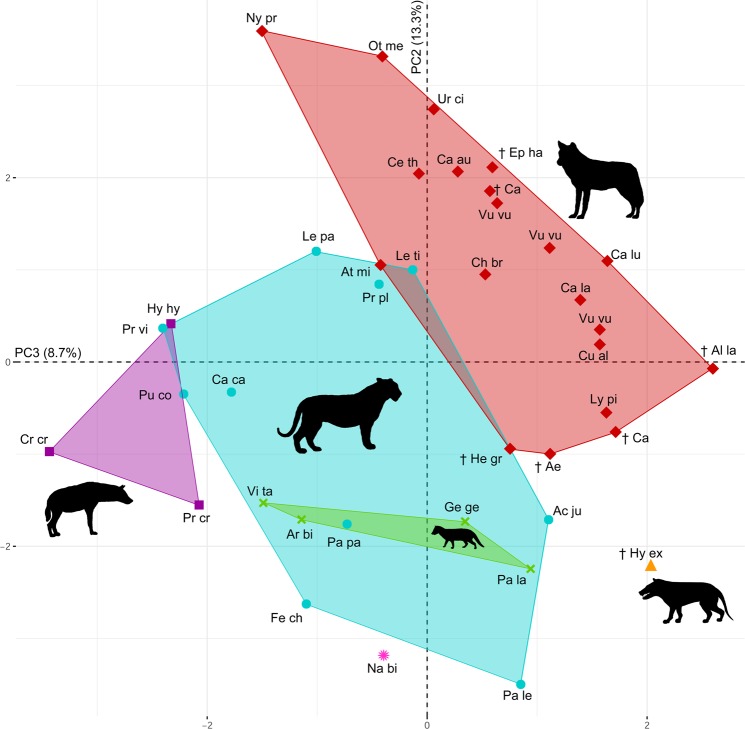
The functional morphological and phylogenetic signal was statistically investigated using standardised bony labyrinth measurements (Supplementary Dataset 1) and additionally a principal component analysis (PCA; Figs 2 and 3). The first axes of the PCA explains 47.2%, the second axis 13.3% and the third PC axis 8.7% of the shape variation.
Figure 2.
Principal component analysis (PCA) according to hunting strategies in carnivorans. Shape differences in the bony labyrinth. PC1 explains 47.2% of the variance; PC2 explains 13.3% of the variance. Coloured morphospace are defined as follows: brown: pounce; blue: ambush; pink: pursuit; darkgreen: occasional. Circles: Feloidea (Ac ju, Acinonyx jubatus; Ca ca, Caracal caracal; Fe ch, Felis chaus; Le pa, Leopardus pardalis; Le ti, Leopardus tigrinus; Pa le, Panthera leo; Pa pa, Panthera pardus; Pr pl, Prionailurus planiceps; Pr vi, Prionailurus viverrinus; Pu co, Puma concolor); squares: Hyaenidae (Cr cr, Crocuta crocuta; Hy hy, Hyaena hyaena; Pr cr, Proteles cristatus); cross: Viverridae (Ar bi, Arctictis binturong; Ge ge, Genetta genetta; Pa la, Paguma larvata; Vi ta, Viverra tangalunga); sun: Nandiniidae (Na bi, Nandinia binotata); diamonds: Canidae (At mi, Atelocynus microtis; Ca au, Canis aureus; Ca la, Canis latrans; Ca lu, Canis lupus; Ce th, Cerdocyon thous; Ch br, Chrysocyon brachyurus; Cu al, Cuon alpinus; Ly pi, Lycaon pictus; Ny pr, Nyctereutes procyonoides; Ot me, Otocyon megalotis; Ur ci, Urocyon cinereoargenteus; Vu vu, Vulpes vulpes); red/stars: extinct specimens (Ae, †Aelurodon sp.; Al la, †Alopex lagopus; Ca, †Canis sp.; Ep ha, †Epicyon haydeni; He gr, †Hesperocyon gregarius; Hy ex, †Hyaenodon exiguus).
Figure 3.
Principal component analysis (PCA) according to the family level of carnivorans. Shape differences in the bony labyrinth. PC2 explains 13.3% of the variance; PC3 explains 8.7% of the variance. Coloured morphospace are defined as follows: red/diamond: Canidae; cyan/circles: Felidae; purple/square: Hyaenidae; lightgreen/cross: Viverridae; pink/sun: Nandiniidae; orange/triangle: Hyaenodontidae. For data labels see Fig. 2.
The first principal component (PC1) correlates positively with the height, width, length and radius of all three SCCs; the length of the crus commune (Cc); the length, width and height of the Co and the ASC/LSC, LSC/PSC and LSC/Co angles, but correlates negatively with the ASC/PSC angles. The second principal component (PC2) correlates positively with the width and diameter of the ASC; the length and diameter of the PSC and LSC; the length of the Cc; the height, width and length of the Co and the angles LSC/PSC and LSC/Co, but correlates negatively with the height, length and radius of the ASC; the height, width and radius of the PSC and LSC and the angles ASC/PSC and ASC/LSC. The third principal component (PC3) correlates positively with the width and length of the ASC; the height and length of the PSC; the width, radius and length of the LSC; the length of the Cc; the height of the Co and all measured angles (ASC/PSC, ASC/LSC, LSC/PSC, LSC/Co), but correlates negatively with the height, diameter and radius of the ASC; the height, diameter and radius of the PSC; the height and diameter of the LSC and the width and length of the Co.A detailed list of the PCA scores is found in the electronic supplementary material (Supplementary Dataset 1).
The results of our phylogenetic test reveal that the size of all three semicircular canals and additionally PC1 (linked with ecology) are not influenced by phylogenetic information (detailed values in Supplementary Dataset 1 and Data 3). The cochlea however, is influenced by phylogenetic traits in carnivorans. In the CVA the different hunting strategies observed in the investigated carnivorans separate well, as also seen in the PCA. To test the significance of the morphological differences we used a MANOVA. This indicates an overall classification accuracy of 82.05% for the hunting strategies and 91.89% based on the family level of the carnivorans.
Discussion
Carnivorans have been a major research topic for decades (e.g.2,3,37,38). However, studies on their vestibular system have only recently come into focus, but are still restricted to specific groups (e.g. Feloidea28,30; Musteloidea27; Canis39). Nevertheless, it has been demonstrated that the vestibular system, representing the organ of equilibrium, provides a powerful proxy for reconstructing ecological preferences in vertebrates (e.g.18–20,22–24,26–28,30–33,40–45). Differences in ecology are related to morphological changes of the inner ear, particularly of the three semicircular canals, as they provide information for angular acceleration necessary to balance the body in complex 3D environments. During locomotion and predation, it is of major importance to stabilise the head and gaze (vestibulo-ocular and vestibulo-collic reflexes) especially for fast moving species46. In cetaceans, however, the cochlea can be correlated with habitat preferences, due to association with different echolocation abilities in differing habitats and additionally size is correlated with environment36.
Here, we investigated the bony labyrinth morphometry as a proxy for hunting behaviours and predatory niche adaptations in extinct carnivorans and additionally gain a better understanding of their evolutionary history. Correlations between size of the semicircular canals and hunting behaviour and speed are identified and visually shown in the PCA (Fig. 2). Previous studies demonstrate that high-speed hunting in cheetahs can be correlated with an enlargement of the vestibular system and elongation of the ASC and PSC30 and similarly, feloideans exhibit ecological bony labyrinth adaptations in the size of their semicircular canals28. We recognised distinct morphospaces, based on PC1, showing the highest loadings on the relative height, width and length of all three semicircular canals (Fig. 2). Four different hunting behaviours are present in the examined taxa (pounce, pursuit, ambush, occasional; defined after47, Table 1) showing an overall classification accuracy of 82% (Supplementary Data 2). Hence, carnivoran hunting behaviour is reflected in the size of the semicircular canals. A clear distinction is present between the hunting styles pounce and pursuit, and pounce and ambush, respectively. The range of the pounce and ambush morphospaces is much larger than of the pursuit and the occasional ones. Those species exhibiting an occasional diet and hence rarely hunt at all, are placed in between, overlapping all of the other hunting styles. Additionally, major distinctions are present in the overall hunting behaviour of feloids and canids. Generally, feloids are solitary, ambush hunters, able to retract their claws and developed more flexible forelimbs during their evolution to grapple with and hold their prey8,48,49. Canids, conversely, lack these features. Large canids, as the African hunting dog (Lycaon pictus) or the grey wolf (Canis lupus), need to organise in groups when hunting on large prey8. This is reflected in the morphospace reconstructions where they are assigned to the pursuit, comparative hunter niche48. However, it is assumed that an ambush hunting style is observed in large Borophaginae (an extinct canid subfamily), such as Epicyon haydeni14. Based on their strong teeth and robust skull50, most large extinct canids might have had a scavenging lifestyle and are known as ‘hyaenoid dogs’13. However, recent studies suppose a combined and unique predation strategy for fossil canids15. Viverridae and Nandiniidae however, are not that active and fast hunters as canids and feloids are, they generally have a solidary and omnivorous lifestyle8. Hyaenidae show the most variable hunting behaviour within carnivorans, the striped hyena (Hyaena hyaena) is a scavenger, the spotted hyena (Crocuta crocuta) is generally an active pack hunter and the Aardwolf (Proteles cristatus) is insectivore and specialised on termites8. This coincide with the PCA, as all three species plot in the respective morphospace. Here, ambush and pursuit predation as faster hunting strategies are reflected in larger semicircular canals represented in the relative height, width and length. This coincides with other carnivoran studies30,44, but contrasts with the functional morphological signal found in the diameter of the canals in the squirrel-related clade and marsupials26,28. However, based on carnivoran bony labyrinth morphometry our results clearly demonstrate that the extinct Hesperocyon gregarius, Epicyon haydeni, Canis sp., Alopex lagopus and Hyaenodon exiguus exhibited a pounce predation, whereas Aelurodon sp. developed a pursuit hunting strategy.
Table 1.
Extant specimens. Including five different carnivoran families: Canidae, Viverridae, Hyaenidae, Nandiniidae, Felidae.
| Family | Species | Common name | Hunting style |
|---|---|---|---|
| Canidae | Canis lupus | Grey wolf | pursuit |
| Canidae | Atelocynus microtis | Short eared dog | pounce |
| Canidae | Cerdocyon thous | Crab eating fox | pounce |
| Canidae | Chrysocyon brachyurus | Maned wolf | pounce |
| Canidae | Cuon alpinus | Dhole | pursuit |
| Canidae | Lycaon pictus | African wild dog | pursuit |
| Canidae | Nyctereutes procyonoides | Racoon dog | pounce |
| Canidae | Otocyon megalotis | Bat eared fox | occasional |
| Canidae | Urocyon cinereoargenteus | Grey fox | pounce |
| Canidae | Vulpes vulpes | Red fox | pounce |
| Canidae | Canis latrans | Coyote | pounce |
| Canidae | Canis aureus | Golden jackal | pounce |
| Felidae | Felis chaus | Jungle cat | ambush |
| Felidae | Panthera leo | Lion | ambush |
| Felidae | Acinonyx jubatus | Cheetah | pursuit |
| Felidae | Panthera pardus | Leopard | ambush |
| Felidae | Leopardus pardalis | Ocelot | ambush |
| Felidae | Leopardus tigrinus | Oncilla | pounce |
| Felidae | Caracal caracal | Caracal | ambush |
| Felidae | Puma concolor | Puma | ambush |
| Felidae | Prionailurus planiceps | Flat-headed cat | pounce |
| Felidae | Prionailurus viverrinus | Fishing cat | pounce |
| Hyaenidae | Hyaena hyaena | Striped hyena | pounce |
| Hyaenidae | Proteles cristatus | Aardwolf | occasional |
| Hyaenidae | Crocuta crocuta | Spotted hyena | pursuit |
| Nandiniidae | Nandinia binotata | African palm civet | occasional |
| Viverridae | Arctictis binturong | Binturong | occasional |
| Viverridae | Viverra tangalunga | Malay civet | pounce |
| Viverridae | Genetta genetta | Common genet | pounce |
| Viverridae | Paguma larvata | Masked palm civet | pounce |
The vestibular system, however, not only reflects ecological adaptations but also reveals information about phylogenetic relationships between the Canoidea and Feloidea (Fig. 4). Four carnivoran families (Hyaenidae, Viverridae, Nandiniidae and Canidae) can be clearly distinguished from each other (Fig. 3), and only the diverse Felidae show minor overlapping regions with other taxa. The extinct Hyaenodon exiguus fits in none of the defined phylogenetic morphospaces and has an intermediate position, which supports its position outside of the carnivorans51. Our results demonstrate that the most important trait bearing a phylogenetic signal in carnivorans is the bias angle26 between the LSC and the cochlea, and additionally the diameter of all three semicircular canals and the height of the cochlea, as it already was postulated for several feloidean families44. Canidae unambiguously developed a larger angle between LSC/Coc and additionally show a larger cochlea height compared to feloideans. However, the presence of a phylogenetic signal contrasts with investigations of both the squirrel-related clade (fossorial vs. arboreal26), as well as marsupials (saltorial vs. arboreal28), which show a functional morphological rather than a phylogenetic adaptation in the diameter of the SCCs. Additionally, the entire bony labyrinth shape infers phylogenetic relationships in various other mammalian families, such as Musteloidea, ruminants or cetaceans27,33,36,41.
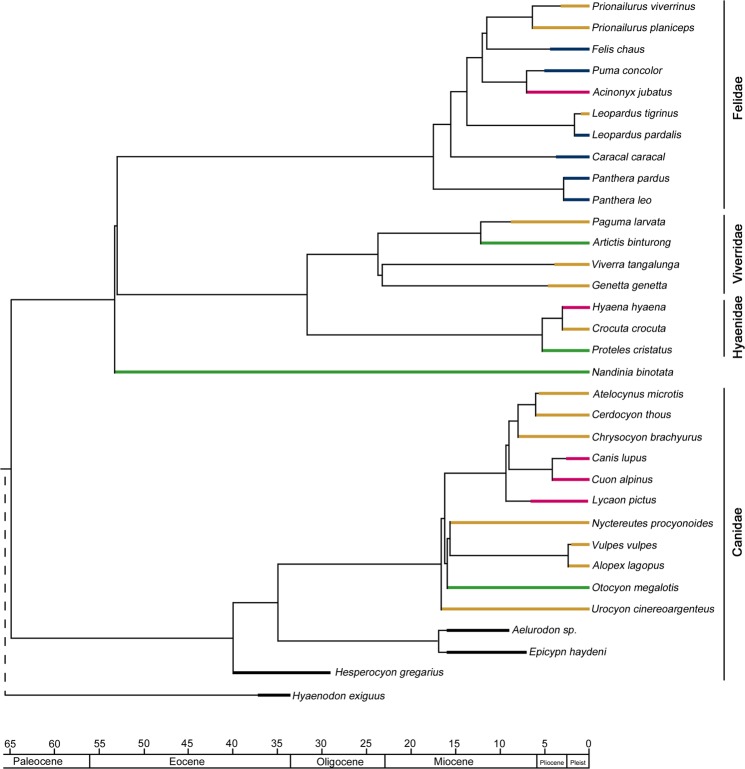
Figure 4.
Phylogeny of the examined specimens3. Colours represent the respective hunting behaviour: brown: pounce; blue: ambush; pink: pursuit; darkgreen: occasional.
In conclusion, the bony labyrinth morphometry clearly provides both a strong functional morphological signal as correlation with varying predation strategies, as well as a phylogenetic signal in carnivorans. Overall, the size of all three bony labyrinth semicircular canals have altered during the evolutionary history of carnivorans to adapt to different hunting strategies, with fast predators showing larger SCCs. Furthermore, the angle between the LSC and the cochlea, the diameter and the height of the cochlea contain phylogenetic information. Thus, the bony labyrinth morphology is a useful proxy for estimating predatory adaptations within a phylogenetic context in extinct carnivoran mammals.
Methods
Bony labyrinths of 36 specimens of five different carnivoran families and Hyaenodon exiguus (Tables 1 and 2) were used to estimate changes in hunting style during carnivoran evolutionary history, and additionally, to test for a phylogenetic signal. New data of canid specimens were combined with published measurements of feloideans44. Most specimens are housed in the collection of the Natural History Museum in Vienna (NHMW) and the collection of the Department of Palaeontology at the University of Vienna (IPUW), additional extinct canids are housed at the Field Museum of Natural History in Chicago (FMNH; Aelurodon sp., Hesperocyon gregarius), the American Museum of Natural History in New York (AMNH; Epicyon haydeni).
Table 2.
Extinct specimens. Including Canidae with three different subfamilies and Hyaenodontidae.
| Family | Subfamily | Species | Timeperiod |
|---|---|---|---|
| Canidae | Hesperocyoninae | † Hesperocyon gregarius | 40–29 Ma |
| Canidae | Borophaginae | † Epicyon haydeni | 16–7 Ma |
| Canidae | Borophaginae | †Aelurodon sp. | 16–9 Ma |
| Canidae | Caninae | †Canis sp. | 6 Ma - recent |
| Canidae | Caninae | † Alopex lagopus | 2.5 Ma - recent |
| Hyaenodontidae | Hyaenodontidae | † Hyaenodon exiguus | 37.2–33.8 Ma |
The skulls of the specimens were scanned non-invasively using µCT devices. The majority of the specimens were scanned at the Department of Palaeontology of the University of Vienna (SkyScan/Bruker 1173), the skulls of Canis lupus and Epicyon haydeni were scanned at the Department of Anthropology at the University of Vienna (Viscom X8060) and two fossil specimens (Hesperocyon gregarius and Aelurodon sp.) were scanned at the Department of Organismal Biology and Anatomy at the PaleoCT Luo Lab (GE v|tome|x scanner) at the University of Chicago. A detailed list of scanning settings is found in the electronic supplementary material (Supplementary Dataset 1).
Scanning images were visualised, bony labyrinths were segmented manually and virtually reconstructed three dimensionally using the software Amira 5.4.5 (Visualization Sciences Group). For comparison of morphological traits, only left labyrinths were reconstructed. Measurements were taken directly on the 3D labyrinths following the protocol of previous studies (Fig. 1)19,26,28,44,52. All measurements were scaled in millimetre related to the voxel (three-dimensional pixel) size. A detailed list of the 3D measurements is found in the electronic supplementary material (Supplementary Dataset 1).
Statistical analyses of the anatomy of the bony labyrinth were performed, using the software R version 1.0.13653. First, the mean of the measurements of the length, width and diameter of each canal was calculated. Additionally, linear regression and residuals of the measurements and the condylobasal length (CBL) were calculated for standardisation and to create size independent values26,28,44. A Principal Component Analysis (PCA; Figs 2 and 3) was performed and delimitable morphospaces were defined using the R packages FactoMineR54 and factoextra55, to correlate bony labyrinth morphology and hunting strategies as well as phylogenetic signals. A Canonical Variate Analysis (CVA; Supplementary Data 1) was calculated for the values of the PC axis and furthermore the Multivariate Analysis of Variance (MANOVA; Supplementary Data 2) using Morpho 2.656. A phylogenetic tree has been superimposed on the PCA using the R package phytools57 (Supplementary Data 5). Additionally, the phylogenetic influences in the vestibular system was calculated using the phylogenetic independent contrast (PIC), Blomberg’s K value and the Pagel’s lambda58–60 using Mesquite version 3.2, R 1.0.13653 and the R – packages ape 4.161, phylobase 0.8.262 and phylotools 0.6-0057. Phylogenetic analyses are based on the carnivoran supertree3. A detailed list of the calculated phylogenetic values is found in the electronic supplementary material (Supplementary Dataset 1).
The anatomical variance of the semicircular canals of the bony labyrinth was calculated for all specimens of the species Vulpes vulpes and additionally for all specimens of the genus Canis using the ‘coefficient of variability’63 (Supplementary Data 4).
Supplementary information
Acknowledgements
We are grateful to the following people for providing access to their collection and technical equipment: Alexander Bibl, Ursula B. Göhlich, Frank E. Zachos (Natural History Museum, Vienna), Karl Rauscher (Department of Palaeontology, University of Vienna), Martin Dockner (Department of Anthropology, University of Vienna), Carl M. Mehling (American Museum of Natural History, New York), William F. Simpson, Adrienne Stroup (Field Museum of Natural History, Chicago), Zhe-Xi Luo, April I. Neander (University of Chicago). We finally thank the editorial board member and two anonymous reviewers for their constructive comments, which greatly improved the manuscript. Open access funding is provided by the University of Vienna.
Author Contributions
J.A.S. participated in the design of the study, constructed the three-dimensional models, carried out the statistical analyses, prepared the figures and drafted the manuscript; J.K. participated in the design of the study, interpretation of the data and reviewed drafts of the paper; G.W. made the µCT scans and reviewed drafts of the paper; C.P. designed the study, made the µCT scans, interpreted the data and reviewed drafts of the paper. All authors gave final approval for publication.
Data Availability Statement
All data generated and analysed during this study are given in Supplementary data.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-37106-4.
References
- 1.Flynn, J. F. Early Cenozoic Carnivora (“Miacoidea”). In: Evolution of Tertiary Mammals of North America volume 1. terrestrial carnivores, ungulates, and ungulate like mammals (eds Janis, C. M., Scott, K. M. & Jacobs, L. L.) 110–123 (Cambridge University Press, 1998).
- 2.Flower WH. On the Value of the Characters of the Base of the Cranium in the Classification of the Order Carnivora, and on the Systematic Position of Bassaris and other disputed Forms. J. Zool. 1869;37:4–37. [Google Scholar]
- 3.Nyakatura K, Bininda-Emonds ORP. Updating the evolutionary history of Carnivora (Mammalia): a new species-level spertree complete with divergence time estimates. BMC Biol. 2013;10:1–31. doi: 10.1186/1741-7007-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wesley-Hunt GD, Flynn JJ. Phylogeny of the Carnivora: Basal relationships among the Carnivoramorphans, and assessment of the position of ‘Miacoidea’ relative to Carnivora. J. Syst. Palaeontol. 2005;3:1–28. doi: 10.1017/S1477201904001518. [DOI] [Google Scholar]
- 5.Tomiya S. A New Basal Caniform (Mammalia: Carnivora) from the Middle Eocene of North America and Remarks on the Phylogeny of Early Carnivorans. PLoS One. 2011;6:e2414. doi: 10.1371/journal.pone.0024146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunnell, G. F. Creodonta. In: Evolution of Tertiary Mammals of North America volume 1. terrestrial carnivores, ungulates, and ungulate like mammals (eds Janis, C. M., Scott, K. M. & Jacobs, L. L.) 91–109 (Cambridge University Press, 1998).
- 7.Janis, C. M. et al. Carnivorous mammals. In: Evolution of Tertiary Mammals of North America volume 1. terrestrial carnivores, ungulates, and ungulate like mammals (eds Janis, C. M., Scott, K. M. & Jacobs, L. L.) 73–90 (Cambridge University Press, 1998).
- 8.Wilson, D. E. & Mittermeier, R. A. Walker’s mammals of the world, 1st edn (John Hopkins University Press, 2009).
- 9.Baskin JA. The Generic Status of Aelurodon and Epicyon (Carnivora, Canidae) J. Paleontol. 1980;54:1349–1351. [Google Scholar]
- 10.Van Valkenburgh B. Iterative evolution of hypercarnivory in canids (Mammalia: Carnivora): evolutionary interactions among sympatric predators. Paleobiology. 1991;17:340–362. doi: 10.1017/S0094837300010691. [DOI] [Google Scholar]
- 11.Wang X. Phylogenetic Systematics of the Hesperocyoninae (Carnivora: Canidae) Bull. Am. Mus. Nat. Hist. 1994;221:1–207. [Google Scholar]
- 12.Van Valkenburgh, B. & Hertel, F. The Decline of North American Predators during the Late Pleistocene. Illinois State Museum Scientific Papers17 (1998).
- 13.Wang X, Tedford RH, Taylor BE. Phylogenetic Systematics of the Borophaginae (Carnivora: Canidae) Bull. Am. Mus. Nat. Hist. 1999;243:1–391. [Google Scholar]
- 14.Figueirido B, Martín-Serra A, Teng ZJ, Janis CM. Habitat changes and changing predatory habits in North American fossil canids. Nat. Commun. 2015;6:7976. doi: 10.1038/ncomms8976. [DOI] [PubMed] [Google Scholar]
- 15.Martín-Serra A, Figueirido B, Palmqvist P. In the Pursuit of the Predatory Behaviour of Borophagines (Mammalia, Carnivora, Canidae): Inferences from Forelimb Morphology. J. Mammal Evol. 2016;23:237–249. doi: 10.1007/s10914-016-9321-5. [DOI] [Google Scholar]
- 16.Panciroli E, Janis C, Stockdale M, Martín-Serra A. Correlates between calcaneal morphology and locomotion in extant and extinct carnivorous mammals. J. Morphol. 2017;278:1333–1353. doi: 10.1002/jmor.20716. [DOI] [PubMed] [Google Scholar]
- 17.de Burlet, H. M. Vergleichende Anatomie des stato-akustischen Organs. Handb. Vergl. Anat. Wirb. 2 (1934).
- 18.Spoor F, Wood B, Zonneveld F. Implications of early hominid labyrinthine morphology for evolution of human bipedal locomotion. Nature. 1994;369:645–648. doi: 10.1038/369645a0. [DOI] [PubMed] [Google Scholar]
- 19.Spoor F, Zonneveld F. Comparative review of the human bony labyrinth. Yearb. Phys. Anthropol. 1998;4:211–251. doi: 10.1002/(SICI)1096-8644(1998)107:27+<211::AID-AJPA8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 20.Spoor F, Bajpai S, Hussain ST, Kumar K, Thewissen JGM. Vestibular evidence for the evolution of aquatic behaviour in early cetaceans. Nature. 2002;417:163–166. doi: 10.1038/417163a. [DOI] [PubMed] [Google Scholar]
- 21.Spoor F, Hublin JJ, Braun M, Zonneveld F. The bony labyrinth of Neanderthals. J. Hum. Evol. 2003;44:14–165. doi: 10.1016/S0047-2484(02)00166-5. [DOI] [PubMed] [Google Scholar]
- 22.Hullar TE. Semicircular Canal Geometry, Afferent Sensitivity, and Animal Behaviour. Anat. Rec. 2006;288A:466–472. doi: 10.1002/ar.a.20304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spoor F, et al. The primate semicircular canal system and locomotion. Proc. Natl. Acad. Sci. USA. 2007;104:10 808–10 812. doi: 10.1073/pnas.0704250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malinzak MD, Kay RF, Hullar TE. Locomotor head movements and semicircular canal morphology in primates. Proc. Natl. Acad. Sci. USA. 2012;109:17914–17919. doi: 10.1073/pnas.1206139109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Georgi JA, Sipla JS, Forster CA. Turning Semicircular Canal Function on Its Head: Dinosaurs and a Novel Vestibular Analysis. PLoS ONE. 2013;8:e58517. doi: 10.1371/journal.pone.0058517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfaff C, Martin T, Ruf I. Bony labyrinth morphometry indicates locomotor adaptations in the squirrel-related clade (Rodentia, Mammalia) Proc. R. Soc. 2015;B282:20150744. doi: 10.1098/rspb.2015.0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grohé C, Tseng ZJ, Lebrun R, Boistel R, Flynn JJ. Bony labyrinth shape variation in extant Carnivora: a case study of Musteloidea. J. Anat. 2016;228:366–383. doi: 10.1111/joa.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfaff C, Czerny S, Nagel D, Kriwet J. Functional morphological adaptations of the bony labyrinth in marsupials (Mammalia, Theria) J. Morphol. 2017;278:742–749. doi: 10.1002/jmor.20669. [DOI] [PubMed] [Google Scholar]
- 29.Neenan JM, et al. Evolution of the Sauropterygian Labyrinth with Increasing Pelagic Lifestyle. Curr. Biol. 2017;27:3852–3858. doi: 10.1016/j.cub.2017.10.069. [DOI] [PubMed] [Google Scholar]
- 30.Grohé C, Lee B, Flynn JJ. Recent inner ear specialisation for high-speed hunting in cheetahs. Sci. Rep. 2018;8:2301. doi: 10.1038/s41598-018-20198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Billet G, et al. High morphological variation of vestibular system accompanies slow and infrequent locomotion in three-toed sloths. Proc. R. Soc. B. 2012;279:3932–3939. doi: 10.1098/rspb.2012.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Billet G, Germain D, Ruf I, de Muizon C, Hautier L. The inner ear of Megatherium and the evolution of the vestibular system in sloths. J. Anat. 2013;223:557–567. doi: 10.1111/joa.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mennecart B, et al. Bony labyrinth morphology clarifies the origin and evolution of deer. Sci. Rep. 2017;7:13176. doi: 10.1038/s41598-017-12848-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gray, A. A. The labyrinth of animals: including mammals, birds, reptiles and amphibians 1 (Churchill, J.A. 1907).
- 35.Ekdale EG. Comparative Anatomy of the Bony Labyrinth (Inner Ear) of Placental Mammals. PLoS ONE. 2013;8:e66624. doi: 10.1371/journal.pone.0066624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costeur L, et al. The bony labyrinth of toothed whales reflects both phylogeny and habitat preferences. Sci. Rep. 2018;8:7841. doi: 10.1038/s41598-018-26094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, X., Tedford, R. H., Van Valkenburgh, B. & Wayne, R. Ancestry: Evolutionary history, molecular systematics, and evolutionary ecology of Canidae. In: Biology and Conservation of Wild Canids (eds MacDonald, D. M. & Sillero-Zubiri, C.) 39–54 (Oxford University Press, Oxford, 2004).
- 38.Slater GJ, Dumont ER, Van Valkenburgh B. Implications of predatory specialization for cranial form and function in canids. J. Zool. 2009;278:181–188. doi: 10.1111/j.1469-7998.2009.00567.x. [DOI] [Google Scholar]
- 39.Schweizer AV, et al. Size Variation under Domestication: Conservatism in the inner ear shape of wolves, dogs and dingoes. Sci. Rep. 2017;7:13330. doi: 10.1038/s41598-017-13523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmelzle T, Sánchez-Villagra MR, Maier W. Vestibular labyrinth diversity in diprotodontian marsupial mammals. Mammal Study. 2007;32:83–97. doi: 10.3106/1348-6160(2007)32[83:VLDIDM]2.0.CO;2. [DOI] [Google Scholar]
- 41.Mennecart B, Costeur L. Shape variation and ontogeny of the ruminant bony labyrinth, an example in Tragulidae. J. Anat. 2016;229:422–35. doi: 10.1111/joa.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gunz P, Ramsier M, Kuhrig M, Hublin J, Spoor F. The mammalian bony labyrinth reconsidered, introducing a comprehensive geometric morphometric approach. J. Anat. 2012;220:529–543. doi: 10.1111/j.1469-7580.2012.01493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.David R, Stoessel A, Berthoz A, Spoor F, Bennequin D. Assessing morphology and function of the semicircular duct system: introducing new in-situ visualization and software toolbox. Sci. Rep. 2016;6:32772. doi: 10.1038/srep32772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfaff C, et al. Palaeobiology of Hyaenodon exiguus (Hyaenodonta, Mammalia) based on morphometric analysis of the bony labyrinth. J. Anat. 2016;230:282–289. doi: 10.1111/joa.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rae TC, Johnson PM, Yano B, Hirasaki E. Semicircular Canal Size and Locomotion in Colobine Monkeys: A Cautionary Tale. Folia Primatol. 2012;87:213–223. doi: 10.1159/000449286. [DOI] [PubMed] [Google Scholar]
- 46.Cox PG, Jeffery N. Morphology of the mammalian vestibulo-ocular reflex: the spatial arrangement of the human fetal semicircular canals and extraocular muscles. J. Morphol. 2007;268:878–890. doi: 10.1002/jmor.10559. [DOI] [PubMed] [Google Scholar]
- 47.Van Valkenburgh B. Locomotor Diversity within Past and Present Guilds of Large Predatory Mammals. Paleobiology. 1985;11:406–428. doi: 10.1017/S0094837300011702. [DOI] [Google Scholar]
- 48.Van Valkenburgh B, Koepfli KP. Cranial and dental adaptations to predation in canids. Symp. Zool. Soc. Lond. 1993;65:15–37. [Google Scholar]
- 49.Figueirido B, et al. Constraint and adaptation in the evolution of carnivoran skull shape. Paleobiology. 2011;37:490–518. doi: 10.1666/09062.1. [DOI] [Google Scholar]
- 50.Munthe K. The Skeleton of the Borophaginae (Carnivora, Canidae): Morphology and Function. University of California Publications in Geological Sciences. 1989;133:1–115. [Google Scholar]
- 51.Solé F. New proviverrine genus from the Early Eocene of Europe and the first phylogeny of Late Paleocene-Middle Eocene hyaenodontidans (Mammalia) J. Syst. Palaeontol. 2013;11:375–398. doi: 10.1080/14772019.2012.686927. [DOI] [Google Scholar]
- 52.Ekdale EG. Ontogenetic variation in the bony labyrinth of Monodelphis domestica (Mammalia: Marsupialia) following ossification of the inner ear cavities. Anat. Rec. 2010;293:1896–1912. doi: 10.1002/ar.21234. [DOI] [PubMed] [Google Scholar]
- 53.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing (2017).
- 54.Le S, Josse J, Husson F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Soft. 2008;25:1–18. doi: 10.18637/jss.v025.i01. [DOI] [Google Scholar]
- 55.Kassambara, A. & Mundt, F. factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R package version 1.0.4, https://CRAN.R-project.org/package=factoextra (2017).
- 56.Schlager, S. Morpho and Rvcg - Shape Analysis in R. In: Statistical Shape and Deformation Analysis (eds Zheng, G., Li, S. & Szekely, G.) 217–256 (Academic Press, 2017).
- 57.Revell L. J. phytools: An R package for phylogenetic comparative biology (and other things) Methods Ecol. Evol. 2012;3:217–223. doi: 10.1111/j.2041-210X.2011.00169.x. [DOI] [Google Scholar]
- 58.Felsenstein J. Phylogenies and the comparative method. Am. Nat. 1985;125:1–15. doi: 10.1086/284325. [DOI] [Google Scholar]
- 59.Pagel M. Detecting correlated evolutions on phylogenies: a general method for the comparative analysis of discrete characters. Proc. R. Soc. Lond. B. 1994;255:37–45. doi: 10.1098/rspb.1994.0006. [DOI] [Google Scholar]
- 60.Blomberg SP, Garland T, Ives AR. Testing for phylogenetic signal in comparative data: behavioural traits are more labile. Evolution. 2003;57:717–745. doi: 10.1111/j.0014-3820.2003.tb00285.x. [DOI] [PubMed] [Google Scholar]
- 61.Paradis E, Claude J, Strimmer K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- 62.Hackathon, R. et al. phylobase: Base Package for Phylogenetic Structures and Comparative Data. See, http://Phylobase.r-forge.r-project.org/ (2016).
- 63.Ekdale EG, Rowe T. Morphology and variation within the bony labyrinth in zhelestids (Mammalia, Eutheria) and other therian mammals. J. Vertebr. Paleontol. 2011;31:658–675. doi: 10.1080/02724634.2011.557284. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and analysed during this study are given in Supplementary data.