Abstract
Although naloxone has been documented to exert neuroprotection in animal model of cerebral ischemia, the mechanism is not well understood. In this present study we investigated whether naloxone affected the mitochondrial apoptotic pathway in ischemic brain injury of rats. SD rats were subjected to a permanent middle cerebral artery occlusion surgery, and received naloxone (0.5, 1, 2 mg/kg, i.v.) immediately after ischemia. Neurological deficits were evaluated 24 h after ischemia using the McGraw Stroke Index, and then the rats were killed, and the brains were collected for further analyses. We show that naloxone treatment dose-dependently decreased the infarction volume and morphological injury, improved motor behavioral function, and markedly curtailed brain edema. Furthermore, naloxone administration significantly inhibited the nuclear translocation of NF-κB p65 and decreased the levels of nuclear NF-κB p65 in the ischemic penumbra. Naloxone administration also dose-dependently increased the NF-κB inhibitory protein (IκBα) levels and attenuated phosphorylated NIK and IKKα levels in the ischemic penumbra. In addition, naloxone administration dose-dependently increased Bcl-2 levels, decreased Bax levels, stabilized the mitochondrial transmembrane potential, and inhibited cytochrome c release and caspase 3 and caspase 9 activation. These results indicate that the neuroprotective effects of naloxone against ischemic brain injury involve the inhibition of NF-κB activation via the suppression of the NIK/IKKα/IκBα pathway and the obstruction of the mitochondrial apoptotic pathway in neurons.
Keywords: cerebral ischemia, naloxone, NF-κB, mitochondrial apoptotic pathway, neuroprotection
Introduction
Ischemic stroke is one of the most common causes of mortality and morbidity worldwide [1]. It has been proposed that brain cells experience a dramatic reduction in blood supply during an ischemic stroke; this reduction diminishes their access to oxygen and glucose and induces the pathophysiological features of damaged cells, thereby promoting necrotic cell death [2–4]. The neuronal apoptosis effects of cerebral ischemia are now widely recognized and better understood [3, 5]. The ischemic penumbra, located on the periphery of the necrotic core, is rendered functionally silent by reduced blood flow but remains metabolically active [3, 6]. Neural cells in the ischemic penumbra will not undergo apoptosis until several hours or days later, creating a window of opportunity for recovery via post-stroke therapy [3]. We and other laboratories have found that inhibiting neuronal apoptosis may provide effective preventive or therapeutic interventions to mitigate ischemic brain injuries [5, 7].
Naloxone is an opioid receptor antagonist found to confer neuroprotective effects in animal models of focal brain ischemia; however, its mechanisms of action are obscure [4, 8, 9]. The administration of naloxone minimizes ischemic brain injuries by improving cerebral blood flow, reducing seizure activity, and enhancing survival rates [10]. Naloxone significantly increased microtubule-associated protein 2 (MAP-2) accumulation and reduced post-ischemic neuronal loss, astrogliosis, inflammatory cell infiltration, and cytokine/chemokine production [4]. A better understanding of the mechanisms of these effects may eventually lead to the discovery of a new pharmacological action of naloxone that will allow viable clinical translation. Using a rat model of permanent middle cerebral artery occlusion (pMCAO)-induced focal cerebral ischemia, we explored this possibility by investigating nuclear factor (NF)-κB signaling in neural cells.
A wide range of stimuli can activate NF-κB, and more than 100 genes are induced upon its activation [11]. Two distinct and evolutionarily conserved NF-κB signaling pathways have been described [12]. The canonical NF-κB pathway is normally triggered by numerous stimuli, including microbial stressors, viral infections, and cytokines. NF-κB activation occurs primarily through the IκB kinase (IKK) complex, which is composed of catalytic (IKKα and IKKβ) and regulatory (IKKγ) subunits; this complex phosphorylates the NF-κB inhibitory protein (IκBα), thereby triggering its ubiquitination and proteasomal degradation. NF-κB dimers, predominantly p50/p65 and p50/c-Rel, are thus liberated and translocated to the nucleus where they modulate the transcriptional activation of several hundred target genes [13]. Activation of the noncanonical NF-κB pathway involves different signaling molecules and leads to the selective activation of IKKα homodimers by the upstream NF-κB-inducing kinase (NIK), followed by the phosphorylation of the p100/NF-κB2 protein associated with RelB, which then becomes p52/NF-κB2 through proteolytic processing and forms a complex in conjunction with RelB. These p52/RelB dimers enter the nucleus and prompt the transcription of distinct sets of target genes to mediate different biological functions [14].
The involvement of NF-κB pathway activation in cerebral ischemia and neuronal cell death is well established [13]. In mice and human brain samples after ischemic stroke, the nuclear translocation of RelA can also be observed in the areas surrounding the necrotic infarct core [13, 15]. Activated NF-κB was detected in neurons after cerebral ischemia. However, interception using NF-κB inhibitors resulted in reduced infarct sizes and apoptotic cell counts [5, 15]. The ischemic effect is related to the known ability of NF-κB to activate genes with products that mediate these detrimental outcomes, implying an instrumental role of NF-κB in ischemic damage; [13] these findings support NF-κB inhibition as a useful strategy for ischemic stroke therapy [13, 15]. Here, we modified an experimental rat ischemic stroke model and demonstrated that the naloxone-mediated neuroprotective effects against cerebral ischemic brain injury are associated with the inhibition of NF-κB and apoptosis in neuronal cells.
Materials and methods
Photothrombosis model and treatments
Sprague Dawley (SD) male rats weighing 280–300 g were purchased from the Center for Experimental Animals, Tongji University. All animal experiments were carried out according to protocols approved by the Tongji University Ethics Committee in accordance with the NIH (National Institutes of Health) Guidelines for the Care and Use of Laboratory Animals. pMCAO was induced in the rats as previously described [16]. Briefly, the rats were anesthetized with 4% chloral hydrate by intraperitoneal injection. The rectal temperature was maintained at 37 ± 0.5 °C via a temperature-regulated heating pad. A 4-0 nylon monofilament with a heat-blunted end was used to permanently occlude the right MCA. Rats without neurological deficits (exhibiting flexion of the contralateral torso and forelimbs upon suspension of the whole body by the tail) following ischemia were excluded from further study.
Naloxone, purchased from Beijing Kawin Technology Co., Ltd, was injected intravenously (i.v.) at a dose of 0.5–2 mg/kg immediately after pMCAO. Nimodipine, which was kindly provided by ChiaTai Qingchunbao Pharmaceutical Co., Ltd, was dissolved in alcohol at 1 mg/kg for intravenous injection immediately after ischemia. Sham-operated animals received heparinized normal saline.
Assessment of the neurological deficit score
Neurological deficits were evaluated using the McGraw Stroke Index [17]. The neurological behavior of the rats was assessed 24 h after ischemia induction by an observer blind to the animal groups. This method included the following tests: spontaneous motor activity (normal, increased, decreased, absent); gait impairment (stiffness, disequilibrium, slowness of movement, disorientation); tail-flick reflex of the front and hind legs; reaction to sound; tremors; convulsions; muscle tone in the trunk and extremities (normal, increased, absent); and symptoms of ptosis (absent, one-sided, two-sided). Each indicator was scored as follows: 0 point (normal); 1 point (moderately pronounced changes); and 2 points (severely pronounced changes).
Infarct volume evaluation
Infarct volumes were evaluated as previously described [18]. At 24 h after ischemia, the rats were re-anesthetized with 4% chloral hydrate by intraperitoneal injection. Samples from the cerebellum, the olfactory bulb, and the remainder of the lower brain stem were rapidly extracted and sliced into 2 mm-thick coronal sections with the aid of a brain matrix. The sections were stained with a standard 2% 2,3,5-triphenyltetrazolium chloride solution (Sigma-Aldrich, St. Louis, MO, USA) for 30 min at 37 °C, followed by overnight immersion in 4% paraformaldehyde; the sections were then photographed with a digital camera (Sony, Wuxi, Jiangsu, China). The infarct volumes were analyzed with Adobe Photoshop 8.0CS by an investigator blind to the experimental groups. Relative infarct volumes were obtained after correction for edema as follows: infarct volume = 100% × (contralateral hemisphere volume − non-infarct ipsilateral hemisphere volume)/contralateral hemisphere volume.
Evaluation of brain edema
Brain edema was measured by the wet and dry weight method as described previously [19]. Briefly, the rats were killed at 24 h after ischemia, and their brains were removed to obtain the rhombencephalon and bulbus olfactorius, the wet weights of which were measured using an electronic analytic balance. The tissues were then dried in an oven at 107 °C for 72 h and weighed again to obtain the dry weight. The water content of each brain was calculated using the following formula: water content = [(wet weight − dry weight)/wet weight] × 100%.
Histology and immunohistochemistry
Following the behavioral examination of each treatment group at 12 h after ischemia, the rats were decapitated to collect the brains. The brain tissues were sliced quickly into five coronary brain sections: the second section was fixed in 4% paraformaldehyde and then prepared via ethanol gradient dehydration for serial paraffin embedding before being sliced into 4 μm-thick coronal sections. After routine dewaxing, the sections were stained with hematoxylin (Boster Bioengineering Inc., Wuhan, China) for 3 min and counterstained with eosin for 3 min, followed by mounting for observation and image collection with an Olympus CX23 microscope (Olympus, Tokyo, Japan).
For immunohistochemistry analysis of the ischemic penumbra, primary antibodies (a mouse monoclonal IgG against NF-κB p65, 1:200, Santa Cruz Biotechnology) were applied to deparaffinized and rehydrated sections after antigen retrieval by microwaving for 40 min at 160 W in 0.01 M citrate buffer, pH 6.0. Primary antibody binding was detected using a DAB detection kit according to the manufacturer’s recommendations (Dako, Carpinteria, CA, USA). For the TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) assays, a Dead End Colorimetric TUNEL System (Promega, Madison, WI, USA) was used according to the manufacturer’s recommendations.
For the immunostaining quantification of NF-κB p65 and TUNEL-positive cells, 10 microscopic fields (magnification ×400) in each section across the ischemic penumbra in the ipsilateral hemisphere were analyzed. Three sections were utilized for each animal. The number of cells with nuclear NF-κB p65 immunoreactivity and TUNEL-positive staining in each field was counted by an examiner blinded to the experimental conditions [20].
Western blot analysis
At the indicated time after ischemia, the animals were anesthetized, and the ipsilateral ischemic penumbras were dissected. The samples were homogenized in ice-cold buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 0.5% Triton X-100, 1 mM edetic acid, 1 mM phenylmethylsulfonyl fluoride, and 5 mM aprotinin) and then centrifugated at 10,000 × g and 4 °C for 10 min. Total protein from the supernatant was collected and stored at −80 °C. To measure cytochrome c release, the mitochondrial fraction was isolated from the total cell lysate using a mitochondria isolation kit for tissue (Pierce, Rockford, IL, USA) and used to determine cytochrome c levels. Protein concentrations were measured with Bradford assays. Western blot analyses were performed as previously described [21]. Briefly, samples were boiled in sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer for 5 min. Next, 40 μg aliquots were loaded onto 10–15% gradient precast SDS-PAGE gels and separated based on their molecular size. Prestained molecular size markers were used as molecular mass standards. The gels were then electroblotted onto polyvinylidene difluoride (PVDF) membranes. Immunodetection of the protein of interest was performed by blocking the membrane in 5% nonfat dry milk in Tris-buffered saline with Tween-20 and then adding the primary antibody at the specified dilution in blocking buffer. The PVDF membranes were then probed with horseradish peroxidase (HRP)-conjugated secondary antibodies. Antibody–antigen complexes were detected with ECL reagents (Amersham Bioscience, Buckinghamshire, UK). The primary antibodies included NF-κB (1:1000), IκBα (1:1000), cytochrome c (1:1000), GAPDH (1:2000), H3 (1:1000), cleaved caspase 3 (1:500), cleaved caspase 9 (1:1000), XIAP (1:1000) (all eight from Cell Signaling Technology, Danvers, MA, USA); p-IKKα (1:500) (Abcam, Cambridge, UK); and p-NIK (1:200) and Tom 20 (1:200) (both from Santa Cruz Biotechnology). HRP-conjugated goat anti-rabbit (1:2000; Santa Cruz Biotechnology) and rabbit anti-mouse (1:1000; Dako, Glostrup, Denmark) antibodies were chosen for the secondary antibodies.
RNA isolation and real-time PCR analysis
Total RNA was isolated from the ischemic cortex using an RNeasy plus mini kit (Qiagen, Santa Clarita, CA, USA) according to the manufacturer’s recommendations. The real-time PCR reaction mixtures used have been previously described [16]. Briefly, complementary DNA (cDNA) was synthesized by reverse transcription using a first-strand cDNA synthesis kit (Invitrogen, Carlsbad, CA, USA). Real-time PCR was performed using QPCR SYBR Green Mix (Bio-Rad, Hercules, CA, USA) and an AB 7500 real-time PCR system (AB Applied Biosystems, Singapore). The following PCR primers were used: GAPDH, 5′-GTCGGTGTGAACGGATTTG-3′ and 5′-TCCCATTCTCAGCCTTGAC-3′; Bcl-2, 5′-GGGATGCCTTTGTGGAAC-3′ and 5′-GTCTGCTGACCTCACTTG-3′; and Bax, 5′-GGACGCATCCACCAAGAAG-3′ and 5′-CTGCCACACGGAAGAAGAC-3′. The specificity of real-time PCR was verified by ‘no reverse transcription’ controls and melting curve analyses. Quantitative PCR results were obtained using the DDCT (cycle threshold) method. The data are normalized to the β-actin levels in each sample.
Statistical analysis
The data are presented as the mean ± SEM. Statistical analyses were performed by one-way analysis of variance. A P < 0.05 was considered statistically significant.
Results
Naloxone treatment reduced ischemia-induced motor deficits and brain injury in pMCAO rat models
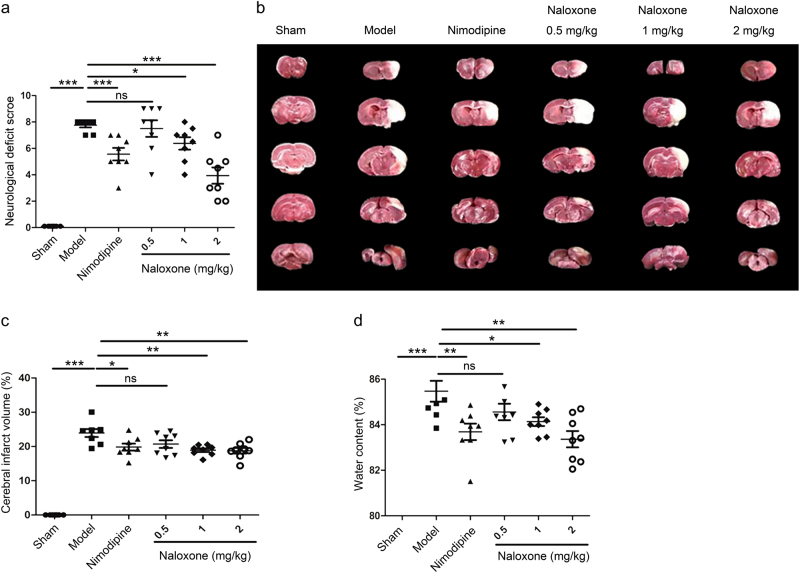
To assess the therapeutic potential of naloxone, the pMCAO model was utilized in SD rats. Naloxone was injected i.v. immediately after ischemic onset. Nimodipine was used as a positive control. Permanent MCAO in rats resulted in significant motor deficits, as measured by the neurological deficit score, 24 h after ischemic insult (Fig. 1a). Compared to that in the model group, motor recovery was significantly improved by naloxone treatment in a dose-dependent manner at 24 h following pMCAO (Fig. 1a). To correlate changes in motor deficits to brain injury, we next detected the infarct volume in the brain after focal cerebral ischemia. The infarct volume was significantly lower in the group treated with naloxone than in the model group (Fig. 1b, c), in addition to markedly decreased brain edema (Fig. 1d).
Fig. 1.
Effect of naloxone on ischemia-induced motor deficits and brain injury in rats. a Motor deficits measured 24 h after ischemic insult. Nimodipine was used as a positive control. b Representative photographs of coronal sections with 2% 2,3,5-triphenyltetrazolium chloride staining at 24 h after ischemia in the sham, model, nimodipine, and naloxone rat groups. The white regions are defined as infarct regions. c Quantitative analysis of the total lesion volume in the rat brains. d Water content measured 24 h after ischemic insult. The columns and bars represent the mean ± SEM; n = 8; *P < 0.05; **P < 0.01; ***P < 0.001. ns not significant
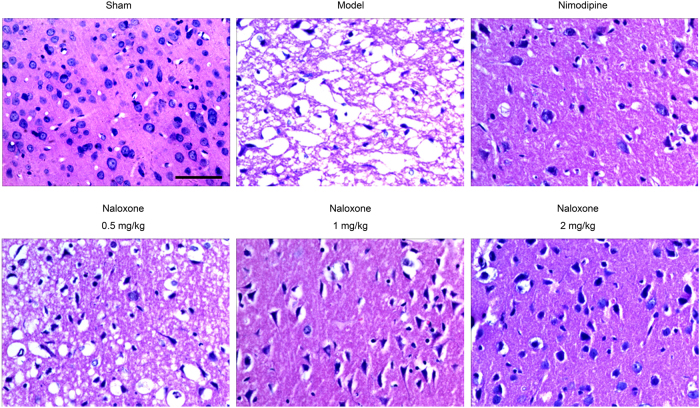
Further confirmation of the therapeutic potential of naloxone for treating cerebral ischemia was conducted, beginning again with the pMCAO model of SD rats injected i.v. with naloxone immediately after ischemic onset. Paraffin-embedded brain sections obtained thereafter underwent microscopic examinations to determine histopathological changes. In the sham group, the morphology of the neurons in the cortex showed intact features, including clearly dyed nuclei and cytoplasm, and the distribution of the neurons was normal (Fig. 2). In the pMCAO model of SD rats, however, obvious severe brain damage to the ipsilateral hemisphere of the penumbra cortex, including neuronal loss, karyopyknosis, and neuron vacuolization, was observed (Fig. 2). Upon naloxone administration, the pMCAO rats exhibited various degrees of recovery according to the penumbra cortex morphology; this recovery was evidenced by less neuronal loss compared to the model group, an increase in the number of normal neurons, and the diminution of vacuolization (Fig. 2).
Fig. 2.
Effect of naloxone on the histomorphology of brain tissue in pMCAO rats. Representative photomicrographs showing the histology in the ischemic penumbra in pMCAO rats. Scale bar = 50 μm
Naloxone inhibits NF-κB activation by blocking the nuclear translocation of NF-κB p65
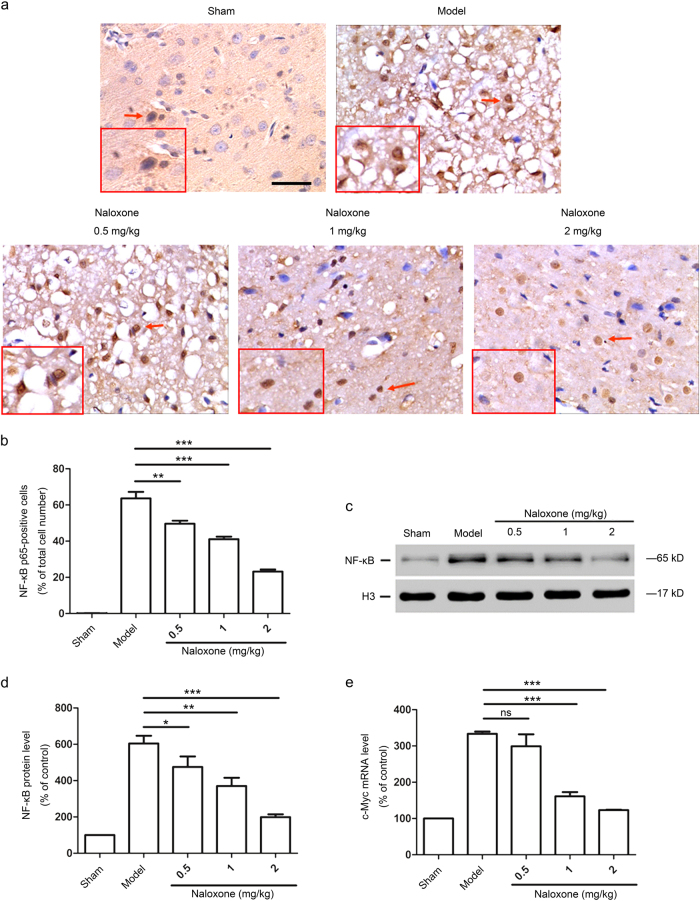
To elucidate the mechanisms of the neuroprotective effect of naloxone, we first compared the nuclear localization of the NF-κB subunit p65 in the dissected brains of rats treated with or without naloxone at 12 h after ischemia. Immunohistochemistry analyses showed that focal ischemia significantly induced the expression of the NF-κB subunit p65, which occurred predominantly in the nuclei of cortical cells (Fig. 3a, b). In contrast, the administration of naloxone to pMCAO rats resulted in a significant decrease in the proportion of cells positive for nuclear NF-κB p65 (Fig. 3a, b). This result was confirmed by immunoblot analyses for NF-κB p65 expression, the level of which was generally lower in naloxone-treated pMCAO rat cortical nuclear lysates (Fig. 3c, d). Additionally, cerebral ischemia led to increased expression levels of the NF-κB target gene c-Myc in isolated cortical cells, whereas a remarkable decrease in the expression levels was observed in the ischemic penumbra after naloxone treatment (Fig. 3e). Therefore, naloxone functions to block the nuclear translocation of NF-κB p65 in penumbra cortical cells, thus preventing the activation of NF-κB.
Fig. 3.
Naloxone blocked the nuclear translocation and expression of NF-κB p65 in the ischemic penumbra 12 h after ischemia. a Representative photomicrographs showing the immunohistochemistry of NF-κB p65 in the ischemic penumbra after pMCAO. Cells with positive NF-κB p65 immunoreactivity in the nuclei (arrowheads) were counted as nuclear NF-κB p65-positive cells. The insets show high-magnification views. Scale bar = 50 μm (b) Quantitative analysis of NF-κB p65-positive cells in the ischemic penumbra. The data are expressed as the percentage of the total number of cells. Results are mean ± SEM (n = 8 for each group); **P < 0.01; ***P < 0.001. c Western blot analysis of NF-κB p65 in the nuclear lysates from the ischemic penumbra after pMCAO. The expression of H3 was used as an internal protein loading control. d Quantitative analysis of the changes in NF-κB p65 protein levels in the nuclei from the ischemic penumbra. The data are normalized to the loading control H3 and are expressed as a percentage of the levels in sham-operated animals. The results are the mean ± SEM; n = 3; *P < 0.05; **P < 0.01; ***P < 0.001. e Real-time PCR analysis of c-Myc mRNA expression levels in the ischemic penumbra. The data are normalized to the loading control GAPDH and are expressed as a percentage of the levels in sham-operated animals (mean ± SEM, n = 3); ***P < 0.001. ns not significant
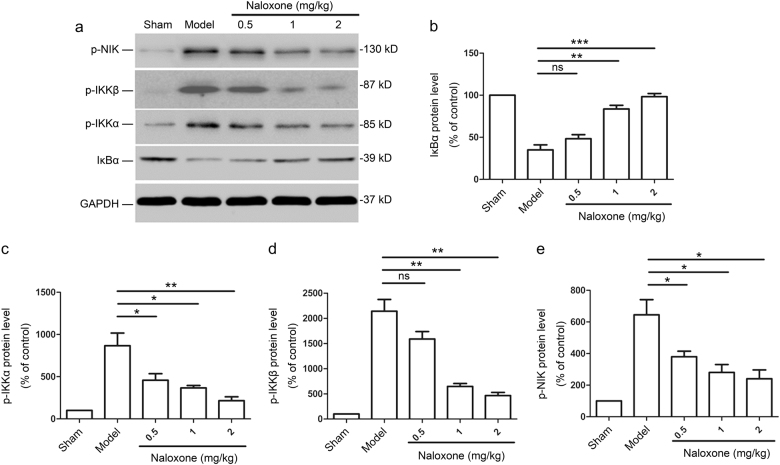
Next, we investigated whether the naloxone-induced inactivation of NF-κB in penumbra cortical cells was due to an upstream effect on the NF-κB signaling pathway. pMCAO rats were established as above, and the rats were killed 12 h after the administration of naloxone. The ischemic penumbra was lysed and analyzed by immunoblotting. The expression of IκBα was strongly downregulated in the penumbra 12 h after ischemia (Fig. 4a, b). Conversely, naloxone administration considerably increased IκBα levels in a dose-dependent manner (Fig. 4a, b). We found that the expression of phosphorylated IKKα (p-IKKα), an inducer of IκBα degradation, correlated with the NF-κB activation status of the cells, which was high in the ischemic penumbra but low in the penumbra from naloxone-treated rats (Fig. 4a, c). We also found that IKKβ, another enzymatic subunit of IKK, was activated by cerebral ischemia. With naloxone treatment, immunoblot analyses showed that IKKβ was efficiently reduced in the penumbra (Fig. 4a, d). We used Western blotting to determine the expression of phosphorylated NIK (p-NIK), an important upstream kinase involved in the phosphorylation/activation of IKK, in the ischemic penumbra of pMCAO rats that were treated with either naloxone or phosphate-buffered saline. We found that naloxone administration significantly reduced the protein levels of p-NIK in the ischemic penumbra in a dose-dependent manner (Fig. 4a, e). These results indicated that the NIK/IKKα/IκBα pathway is involved in the inhibition of NF-κB activation, subsequent to naloxone treatment.
Fig. 4.
The protective effect of naloxone is associated with the inhibition of NF-κB activation by suppressing the NIK/IKKα pathway. a Excised ischemic penumbras were analyzed for the expression of the indicated proteins by immunoblot 12 h after naloxone treatment. b–e Quantitative analysis of changes in the protein levels of IκBα, p-IKKα, p-IKKβ, and p-NIK. The data are normalized to the loading control GAPDH and are expressed as a percentage of that of the sham-operated animals (mean ± SEM, n = 3); *P < 0.05; **P < 0.01; ***P < 0.001. ns not significant
Naloxone inhibits ischemia-induced apoptosis in the penumbra through inactivating the mitochondrial pathway
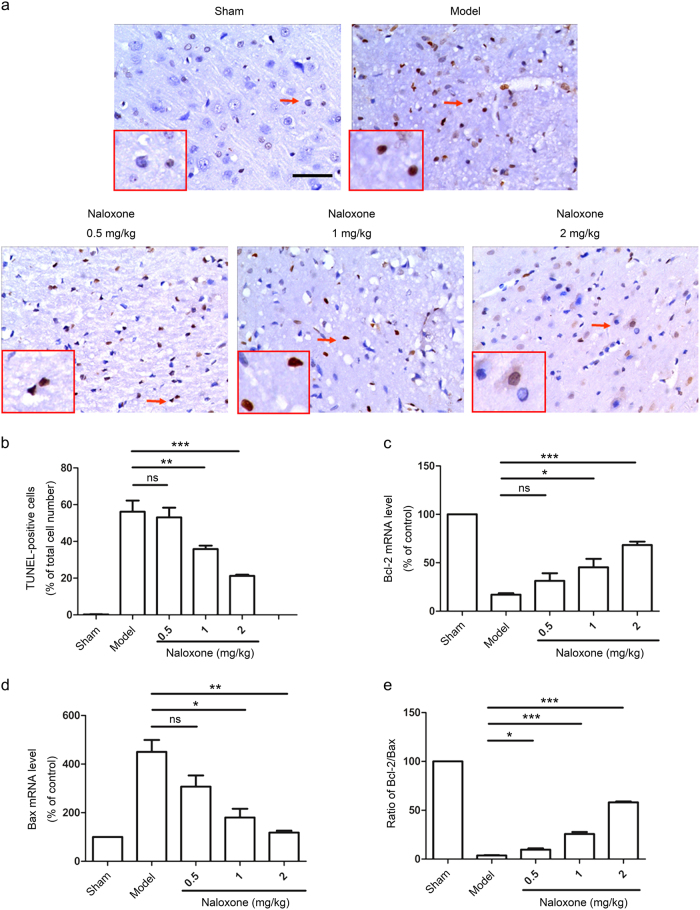
Cerebral ischemia is known to activate the apoptosis signaling pathway [2, 3]. Indeed, ischemia led to a marked induction of cellular apoptosis in the penumbra of pMCAO rats (Fig. 5a, b). We observed that naloxone significantly reduced the apoptotic response of the ischemic penumbra in a dose-dependent manner (Fig. 5a, b). It has been reported that the Bcl-2 family of proteins regulates apoptosis by controlling mitochondrial permeability [22]. We measured the messenger RNA (mRNA) expression levels of the antiapoptotic protein Bcl-2 and the proapoptotic protein Bax in the ischemic penumbra by real-time PCR, which revealed that ischemia downregulated Bcl-2 expression while upregulating Bax expression (Fig. 5c, d). pMCAO rats treated with naloxone exhibited an increase in Bcl-2 mRNA levels and a decrease in Bax mRNA levels, which resulted in a higher Bcl-2/Bax ratio (Fig. 5c–e).
Fig. 5.
Naloxone attenuated ischemia-induced cell apoptosis and increased the Bcl-2/Bax ratio in the ischemic penumbra. a Apoptotic cells (arrowheads) were identified by TUNEL staining 12 h after ischemia. The insets show high-magnification views. Scale bar = 50 μm. b Quantitative analysis of the number of TUNEL-positive cells. The data are expressed as a percentage of the total number of cells. c–e Quantitative analysis of changes in Bcl-2 and Bax mRNA levels. The data are normalized to the loading control GAPDH and are expressed as a percentage of the levels in sham-operated animals. Mean ± SEM; n = 5 for each group; *P < 0.05; **P < 0.01; ***P < 0.001. ns not significant
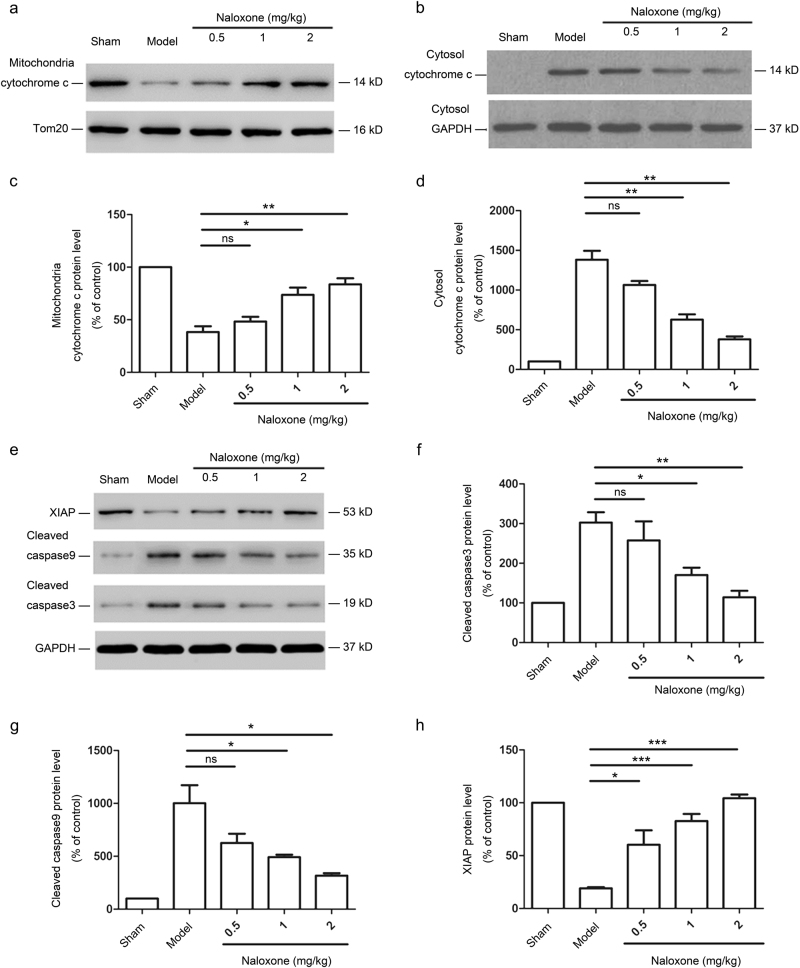
Mitochondrial apoptotic pathway initiation requires cytochrome c, a well-conserved electron transport protein normally present in the mitochondrial intermembrane space, to be released into the cytosol [23]. We observed increased cytochrome c release from the mitochondria to the cytosol in the penumbra after cerebral ischemia, whereas cytochrome c release in the mitochondria was significantly inhibited after naloxone treatment in a dose-dependent manner (Fig. 6a–d). We also noted the upregulation of cleaved caspases 3 and 9 in the penumbra of pMCAO rats; this upregulation is a key event downstream of the mitochondrial apoptotic pathway [24]. Accordingly, the administration of naloxone during cerebral ischemia attenuated cleaved caspase 3 and 9 levels in the penumbra (Fig. 6e–g). We also analyzed the protein expression of XIAP, the most potent natural cellular inhibitor of caspases; [25, 26] a large reduction in XIAP protein expression levels in the ischemic penumbra of pMCAO rats was observed, whereas the administration of naloxone caused a modest increase in XIAP protein levels (Fig. 6e, h). Thus, naloxone can influence the ultimate course of mitochondrial apoptosis if administered during the early stages of ischemic onset, thereby imparting neuroprotection.
Fig. 6.
Apoptosis protection by naloxone is mediated through the inactivation of the mitochondrial pathway. a, b Western blot analysis of cytochrome c release in the mitochondrial (a) and cytosolic (b) fractions. The expression of Tom 20 was used as a mitochondrial loading control. c, d Quantitative analysis of changes in the protein levels of cytochrome c in the mitochondrial (c) and cytosolic (d) fractions. e Excised ischemic penumbras were analyzed for the expression of the indicated proteins by immunoblot 12 h after naloxone treatment. f–h Quantitative analysis of changes in the protein levels of cleaved caspase 9, cleaved caspase 3, and XIAP. The data are normalized to the loading control GAPDH and expressed as a percentage of the levels in sham-operated animals; mean ± SEM; n = 3; *P < 0.05; **P < 0.01; ***P < 0.001. ns not significant
Discussion
In the present study, we used an experimental murine acute ischemic stroke model to investigate the mechanism of naloxone, an opioid receptor antagonist that can block apoptosis in neural cells, to yield a neuroprotective effect. It has been reported that post-conditioning with naloxone can reduce infarct sizes and protect against neuronal damage induced by focal cerebral ischemia in rats [9]. However, very little is known about the proximal signals that are responsible for the effects of naloxone in the context of cerebral ischemic insult. Our results indicate that the major signaling pathway responsible for the neuroprotective effect of naloxone is NF-κB. Although NF-κB has been reported to regulate the expression of multiple genes that play key roles in cell survival or apoptosis, NF-κB does not act as a cell survival mediator in this model of pMCAO; instead, it is responsible for increased cell death [13, 27]. Activated NF-κB was detected in neurons and glia, which may account for the ischemia-induced neuronal injury [16]. Interference with NF-κB activation in neurons has been reported to result in the significant abatement of cerebral ischemia-induced injury and infarct size [13, 28]. Here, we demonstrate that the neuroprotective activity of naloxone, exerted through the inhibition of NF-κB, inhibits cortical neuron injury in a rat model of pMCAO. We have also detected similar NF-κB inhibition in the ischemic cortex in pMCAO rats using a compound containing ginkgolides A and B that significantly reduces infarct sizes [5]. It has been proposed that naloxone attenuates cytokine/chemokine production during cerebral ischemia, an important property that is probably related to its NF-κB inhibition ability [4].
In their inactive form bound to their specific inhibitors, IκB proteins and NF-κB dimers are retained in the cytoplasm but translocate to the nucleus in response to IKK complex activation [29]. Our results suggest that IKKα is highly phosphorylated during cerebral ischemia-induced injury in pMCAO rats. IKK has been found to be activated in mouse/rat models of MCAO and myocardial infarction, although the associated mechanisms are obscure [1, 30]. IKK phosphorylates NF-κB-bound IκBs and targets them for ubiquitin-dependent degradation, allowing the consequently liberated NF-κB dimers to enter the nucleus where they induce the expression of genes important for proliferation (such as cyclin D and proliferating cell nuclear antigen) and apoptosis suppression (Bcl-xL, Bcl-2, and cIAP) [31, 32]. The deletion of IKK and the selective small-molecule inhibition of IKK reduce injury and have a neuroprotective effect in a mouse model of MCAO, suggesting a potential role for IKK inhibitors in stroke therapy [16]. In our case, the neuroprotective activity of naloxone is dependent on IKKα inhibition, which then results in the accumulation of IκBα and subsequent retention of NF-κB in the cytoplasm.
IKKα activation is driven by the upstream kinase NIK, an interaction representing a novel site for pharmacological intervention in a number of inflammatory conditions involving NF-κB [16, 33]. Our current results confirm our earlier observation that in cerebral ischemic insult, p-NIK is upregulated in the ischemic cortex, indicating that NIK is activated [16]. The NIK-induced IKKα/NF-κB2 pathway is a noncanonical NF-κB activation pathway that mediates the activation of the p52/RelB NF-κB complex, which regulates specific immunological processes [12, 14]. However, the role of the NIK/IKKα/NF-κB2 pathway in normal and ischemic brains has not been well defined. NIK may contribute to the activation of canonical NF-κB signaling [14]. By phosphorylating the IKKα subunit, NIK can activate the IKK complex, leading to IκBα degradation and NF-κB dimer (p50/p65) release [34]. The activation of the noncanonical pathway can also lead to the release of p50/p65 heterodimers for translocation into the nucleus [34]. Here, we demonstrated that naloxone treatment during cerebral ischemic insult interferes with NIK phosphorylation so that NIK activation is inhibited. Although the mechanism underlying the neuroprotective activity of naloxone could take several forms, our results strongly suggest that naloxone contributes to minimizing brain injury in cerebral ischemia by suppressing the NIK/IKKα/NF-κB pathway.
Apoptosis in neuronal degeneration after ischemic brain injury has been detected in animal models of stroke [2]. Increasing evidence demonstrates that the activation of the NF-κB signaling pathway contributes to neuronal apoptosis following cerebral ischemia [13]. NF-κB is thought to trigger the mitochondrial apoptotic pathway through regulating the Bcl-2/Bax ratio, which leads to cytochrome c release [35]. Mitochondria have been found to play a pivotal role in cerebral ischemic injury by releasing many apoptogenic mediators from the intermembrane space [24]. The Bcl-2 protein family is a central player in the regulation of apoptosis through the control of mitochondrial permeability. The Bcl-2 protein family comprises antiapoptotic proteins (Bcl-2, Bcl-xL, Mcl-1), proapoptotic proteins (Bax, Bak), and BH3-only proteins (Bim, Bad, Bid) [36]. The antiapoptotic protein Bcl-2 resides in the outer mitochondrial wall and lowers mitochondrial permeability by binding to membrane-inserted Bax monomers, thus preventing the functional oligomerization of Bax [22, 37]. In general, the relative ratio of Bcl-2 to Bax proteins signifies crucial cell apoptotic information [23]. It has previously been reported that naloxone exerts beneficial effects through preserving mitochondrial function, which manifests as a reduction in rat cerebral infarction [4]. We showed that naloxone increases the Bcl-2/Bax ratio, indicating an important antiapoptotic mechanism.
Cytochrome c is a well-conserved electron transport protein that is released from the mitochondrial intermembrane space through a channel formed by Bax in liposomes [3]. Cytochrome c binds to Apaf-1 and forms an activation complex with caspase 9 that triggers caspase 3 activation, eventually leading to apoptosis [38]. Our results demonstrate that naloxone reduced the cerebral ischemia-induced cytochrome c upregulation and caspase activation in the ischemic penumbra; these results are consistent with the Bcl-2/Bax ratio results. In addition to preventing the expression of apoptosis-related factors, naloxone also led to increased expression levels of XIAP, a member of the IAP protein family; these increased levels inhibit caspase 3 and caspase 9, thereby revealing the dual contribution of naloxone to inhibiting neuronal apoptosis [3].
Taken together, our results show that naloxone has an important function in delaying neuronal death induced by ischemia. Our research provides evidence that the inhibition of NF-κB activation caused by naloxone might protect injured brains during cerebral ischemia. Additionally, naloxone appears to attenuate neuronal apoptosis by inhibiting the mitochondrial apoptotic pathway. Further investigation regarding the pharmacological significance of naloxone would support its effective use in the treatment of cerebral ischemia.
Acknowledgements
This work was supported by the Research Program of the Committee of Science and Technology of Putuo District, Shanghai (PKW15102), National Natural Science Foundation of China Grants (81272603; 81472179), the Shanghai Shenkang Program (SHDC22014008) and the Three-year Planning for Strengthening the Construction of the Public Health System in Shanghai (2015-2017) (15GWZK0301).
Author contributions
DL and C-mJ designed the study and edited the manuscript; XW contributed to the analysis, interpretation, manuscript writing, and final approval of the manuscript; Z-jS, J-lW and W-qQ performed the research; W-dX and HC contributed new reagents, analytic tools, and manuscript writing; All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Cui-min Jiang, Email: 1098618044@qq.com.
Dong Li, Email: lidong@tongji.edu.cn.
References
- 1.Zhou N, Fu Y, Wang Y, Chen P, Meng H, Guo S, et al. p27 kip1 haplo-insufficiency improves cardiac function in early-stages of myocardial infarction by protecting myocardium and increasing angiogenesis by promoting IKK activation. Sci Rep. 2014;4:5978–88. doi: 10.1038/srep05978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattson MP, Culmsee C, Yu ZF. Apoptotic and antiapoptotic mechanisms in stroke. Cell Tissue Res. 2000;301:173–87. doi: 10.1007/s004419900154. [DOI] [PubMed] [Google Scholar]
- 3.Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:331–39. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- 4.Chen CJ, Liao SL, Chen WY, Hong JS, Kuo JS. Cerebral ischemia/reperfusion injury in rat brain: effects of naloxone. Neuroreport. 2001;12:1245–49. doi: 10.1097/00001756-200105080-00038. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Jiang CM, Wan HY, Wu JL, Quan WQ, Wu KY, et al. Neuroprotection against permanent focal cerebral ischemia by ginkgolides A and B is associated with obstruction of the mitochondrial apoptotic pathway via inhibition of c-Jun N-terminal kinase in rats. J Neurosci Res. 2014;92:232–42. doi: 10.1002/jnr.23306. [DOI] [PubMed] [Google Scholar]
- 6.Paciaroni M, Caso V, Agnelli G. The concept of ischemic penumbra in acute stroke and therapeutic opportunities. Eur Neurol. 2009;61:321–30. doi: 10.1159/000210544. [DOI] [PubMed] [Google Scholar]
- 7.Khoshnam SE, Winlow W, Farzaneh M, Farbood Y, Moghaddam HF. Pathogenic mechanisms following ischemic stroke. Neurol Sci. 2017;38:1167–86. doi: 10.1007/s10072-017-2938-1. [DOI] [PubMed] [Google Scholar]
- 8.Gooshe M, Abdolghaffari AH, Aleyasin AR, Chabouk L, Tofigh S, Hassanzadeh GR, et al. Hypoxia/ischemia a key player in early post stroke seizures: modulation by opioidergic and nitrergic systems. Eur J Pharmacol. 2015;746:6–13. doi: 10.1016/j.ejphar.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Liao SL, Chen WY, Raung SL, Chen CJ. Neuroprotection of naloxone against ischemic injury in rats: role of mu receptor antagonism. Neurosci Lett. 2003;345:169–72. doi: 10.1016/S0304-3940(03)00540-8. [DOI] [PubMed] [Google Scholar]
- 10.Chen CJ, Cheng FC, Liao SL, Chen WY, Lin NN, Kuo JS. Effects of naloxone on lactate, pyruvate metabolism and antioxidant enzyme activity in rat cerebral ischemia/reperfusion. Neurosci Lett. 2000;287:113–16. doi: 10.1016/S0304-3940(00)01151-4. [DOI] [PubMed] [Google Scholar]
- 11.D’Ignazio L, Bandarra D, Rocha S. NF-kappaB and HIF crosstalk in immune responses. FEBS J. 2016;283:413–24. doi: 10.1111/febs.13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cildir G, Low KC, Tergaonkar V. Noncanonical NF-kappaB signaling in health and disease. Trends Mol Med. 2016;22:414–29. doi: 10.1016/j.molmed.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Ridder DA, Schwaninger M. NF-kappaB signaling in cerebral ischemia. Neuroscience. 2009;158:995–1006. doi: 10.1016/j.neuroscience.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Sun SC. The noncanonical NF-kappaB pathway. Immunol Rev. 2012;246:125–40. doi: 10.1111/j.1600-065X.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang W, Potrovita I, Tarabin V, Herrmann O, Beer V, Weih F, et al. Neuronal activation of NF-kappaB contributes to cell death in cerebral ischemia. J Cereb Blood Flow Metab. 2005;25:30–40. doi: 10.1038/sj.jcbfm.9600004. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Qin ZH, Shi H, Savitz SI, Qin AP, Jiang Y, et al. Protective effect of Ginkgolids (A+B) is associated with inhibition of NIK/IKK/IkappaB/NF-kappaB signaling pathway in a rat model of permanent focal cerebral ischemia. Brain Res. 2008;1234:8–15. doi: 10.1016/j.brainres.2008.07.102. [DOI] [PubMed] [Google Scholar]
- 17.Mcgraw CP. Experimental cerebral infarctioneffects of pentobarbital in Mongolian gerbils. Arch Neurol. 1977;34:334–6. doi: 10.1001/archneur.1977.00500180028006. [DOI] [PubMed] [Google Scholar]
- 18.Hu B, Wang Q, Chen Y, Du J, Zhu X, Lu Y, et al. Neuroprotective effect of WIN 55,212-2 pretreatment against focal cerebral ischemia through activation of extracellular signal-regulated kinases in rats. Eur J Pharmacol. 2010;645:102–7. doi: 10.1016/j.ejphar.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Wang D, Wang H, Qu Y, Xiao X, Zhu Y. The protective effect of HET0016 on brain edema and blood-brain barrier dysfunction after cerebral ischemia/reperfusion. Brain Res. 2014;1544:45–53. doi: 10.1016/j.brainres.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 20.Scholzke MN, Potrovita I, Subramaniam S, Prinz S, Schwaninger M. Glutamate activates NF-kappaB through calpain in neurons. Eur J Neurosci. 2003;18:3305–10. doi: 10.1111/j.1460-9568.2003.03079.x. [DOI] [PubMed] [Google Scholar]
- 21.Xiao S, Li D, Zhu HQ, Song MG, Pan XR, Jia PM, et al. RIG-G as a key mediator of the antiproliferative activity of interferon-related pathways through enhancing p21 and p27 proteins. Proc Natl Acad Sci USA. 2006;103:16448–53. doi: 10.1073/pnas.0607830103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatok J, Racay P. Bcl-2 family proteins: master regulators of cell survival. Biomol Concepts. 2016;7:259–70. doi: 10.1515/bmc-2016-0015. [DOI] [PubMed] [Google Scholar]
- 23.Vaux DL. Apoptogenic factors released from mitochondria. Biochim Biophys Acta. 2011;1813:546–50. doi: 10.1016/j.bbamcr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Ola MS, Nawaz M, Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem. 2011;351:41–58. doi: 10.1007/s11010-010-0709-x. [DOI] [PubMed] [Google Scholar]
- 25.Kristiansen M, Ham J. Programmed cell death during neuronal development: the sympathetic neuron model. Cell Death Differ. 2014;21:1025–35. doi: 10.1038/cdd.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carter BZ, Mak DH, Schober WD, McQueen T, Harris D, Estrov Z, et al. Triptolide induces caspase-dependent cell death mediated via the mitochondrial pathway in leukemic cells. Blood. 2006;108:630–7. doi: 10.1182/blood-2005-09-3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harari OA, Liao JK. NF-kappaB and innate immunity in ischemic stroke. Ann N Y Acad Sci. 2017;1207:32–40. doi: 10.1111/j.1749-6632.2010.05735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guan T, Liu Q, Qian Y, Yang H, Kong J, Kou J, et al. Ruscogenin reduces cerebral ischemic injury via NF-kappaB-mediated inflammatory pathway in the mouse model of experimental stroke. Eur J Pharmacol. 2013;714:303–11. doi: 10.1016/j.ejphar.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Q, Lenardo MJ, Baltimore D. 30 years of NF-kappaB: a blossoming of relevance to human pathobiology. Cell. 2017;168:37–57. doi: 10.1016/j.cell.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malik S, Sharma AK, Bharti S, Nepal S, Bhatia J, Nag TC, et al. In vivo cardioprotection by pitavastatin from ischemic-reperfusion injury through suppression of IKK/NF-kappaB and upregulation of pAkt-e-NOS. J Cardiovasc Pharmacol. 2011;58:199–206. doi: 10.1097/FJC.0b013e31822002a6. [DOI] [PubMed] [Google Scholar]
- 31.Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF-kappaB in cancer cells converts inflammation- induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell. 2004;6:297–305. doi: 10.1016/j.ccr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Grivennikov SI, Karin M. Inflammation and oncogenesis: a vicious connection. Curr Opin Genet Dev. 2010;20:65–71. doi: 10.1016/j.gde.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–59. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 34.Razani B, Reichardt AD, Cheng G. Non-canonical NF-kappaB signaling activation and regulation: principles and perspectives. Immunol Rev. 2011;244:44–54. doi: 10.1111/j.1600-065X.2011.01059.x. [DOI] [PubMed] [Google Scholar]
- 35.Nijboer CH, Heijnen CJ, Groenendaal F, May MJ, van Bel F, Kavelaars A. Strong neuroprotection by inhibition of NF-kappaB after neonatal hypoxia- ischemia involves apoptotic mechanisms but is independent of cytokines. Stroke. 2008;39:2129–37. doi: 10.1161/STROKEAHA.107.504175. [DOI] [PubMed] [Google Scholar]
- 36.Birkinshaw RW, Czabotar PE. The BCL-2 family of proteins and mitochondrial outer membrane permeabilisation. Semin Cell Dev Biol. 2017;72:152–62. doi: 10.1016/j.semcdb.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Dlugosz PJ, Billen LP, Annis MG, Zhu W, Zhang Z, Lin J, et al. Bcl-2 changes conformation to inhibit Bax oligomerization. EMBO J. 2006;25:2287–96. doi: 10.1038/sj.emboj.7601126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shakeri R, Kheirollahi A, Davoodi J. Apaf-1: regulation and function in cell death. Biochimie. 2017;135:111–25. doi: 10.1016/j.biochi.2017.02.001. [DOI] [PubMed] [Google Scholar]