Abstract
Object recognition tasks detect cognitive deficits in transgenic Alzheimer’s disease (AD) mouse models. Object recognition, however, is not a unitary process, and there are many uncharacterized facets of object processing with relevance to AD. We therefore systematically evaluated object processing in 5xFAD and 3xTG AD mice to clarify the nature of object recognition-related deficits. Twelve-month-old male and female 5xFAD and 3xTG mice were assessed on tasks for object identity recognition, spatial recognition, and multisensory object perception. Memory and multisensory perceptual impairments were observed, with interesting dissociations between transgenic AD strains and sex that paralleled neuropathological changes. Overreliance on the widespread “object recognition” task threatens to slow discovery of potentially significant and clinically relevant behavioural effects related to this multifaceted cognitive function. The current results support the use of carefully designed object-based test batteries to clarify the relationship between “object recognition” impairments and specific aspects of AD pathology in rodent models.
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by cognitive dysfunction, as well as pathological accumulation of amyloid-β (Aβ) and hyperphosphorylated tau1. Transgenic mouse models of AD that express familial AD mutations recapitulate key features of Aβ and tau pathology and provide valuable insight into molecular and behavioural abnormalities in AD patients. 5xFAD transgenic mice express three mutations in the APP gene (K670N/M671L, I716V, and V717I) and two mutations in the PS1 gene (M146L and L286V), which induces aggressive amyloid pathology and neuronal loss2. 3xTG transgenic mice express APP (K670N/M671L) and PS1 (M146V) mutations associated with amyloid pathology in addition to a human tauopathy mutation (tauP301L)3. Because 5xFAD and 3xTG models have distinct neuropathological features and staging, comparing these complementary AD models could help clarify behavioural deficits associated with specific elements of AD pathology.
Object recognition (OR) is a rodent analogue of visual recognition memory tests in which human AD patients are impaired2,3. Rodent OR tasks have great translational potential because extensive training, reward and exposure to aversive stimuli are not required, and their one-trial nature is analogous to human episodic memory. Several studies have demonstrated OR memory deficits in 5xFAD4–9 and 3xTG mice10–24. However, the specific nature of OR deficits remains somewhat unclear, as some studies have failed to report OR impairments8,13,17,25–29. Differential performance on OR tasks is likely due to the lack of a standardized OR paradigm and systematic evaluation of sex, age and mnemonic delay. Furthermore, procedural differences can alter the conceptual nature of the task and thus tax distinct behavioural processes. Indeed, there are also many specific facets of object information processing that are affected by AD neuropathology in human patients. For example, AD patients have impaired spatial processing30, as well as multisensory integration and perception deficits31,32. While studies have described deficits in object spatial processing in transgenic AD mice33, multisensory integration has yet to be evaluated.
Here, we used a battery of specifically-designed cognitive tests to perform a systematic analysis of object processing in 12-month-old male and female 5xFAD and 3xTG mice to clarify the relationship between pathology and behavioural deficits across strains of AD mice. We report multifaceted impairment in object processing, with interesting strain and sex differences in object identity memory (OR), spatial memory (object location; OL), and multisensory perceptual integration (multisensory object oddity; MSO) that correspond with pathological changes in Aβ42, phosphorylated tau (P-tau) and other proteins associated with AD pathology.
Results
Object-based Memory Tasks
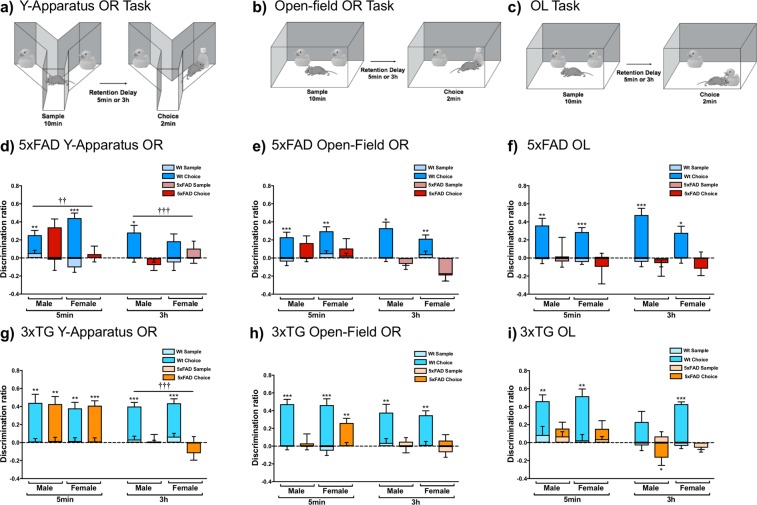
To specifically evaluate object identity processing, 5xFAD and 3xTG mice were tested using the Y-apparatus, which minimizes contextual cues. Y-apparatus OR was impaired in a delay-independent manner in 5xFAD mice (2 × 2 × 2 split-plot ANOVA: genotype (F1,36 = 19.198, p < 0.001), delay (F1,36 = 9.560, p = 0.004), and genotype x delay (F1,36 = 8.563; p = 0.006); Fig. 1d). Paired sample t-tests between sample and choice DR suggest intact memory in Wt males and Wt females at 5 min (Wt males, 5 min: t10 = −3.253, p = 0.009; Wt males, 3 h: t10 = −2.787, p = 0.019; Wt females, 5 min: t7 = −6.195, p < 0.001), but impaired memory in Wt females at 3 h (although approaching statistical significance; t7 = 2.071, p = 0.054), and 5xFAD mice in all conditions, as they failed to discriminate between the novel and sample objects (5xFAD males, 5 min: t9 = −2.062, p = 0.069 (although approaching significance); 5xFAD males, 3 h: t9 = 1.009, p = 0.339; 5xFAD females, 5 min: t10 = −0.407, p = 0.692; 5xFAD females, 3 h: t10 = 0.870, p = 0.405). 3xTG mice, however, were impaired in a delay-dependent manner (2 × 2 × 2 split-plot ANOVA: genotype (F1,36 = 26.260, p < 0.001), delay (F1,36 = 19.172, p < 0.001), and genotype x delay (F1,36 = 21.012, p < 0.001); Fig. 1g). Paired samples t-tests indicated intact memory in Wt mice (Wt males, 5 min: t10 = −4.453, p = 0.001; Wt males, 3 h: t10 = −6.851, p < 0.001; Wt females, 5 min: t11 = −4.013, p = 0.002; Wt females, 3 h: t11 = −6.851, p < 0.001), and 3xTG mice at 5 min (3xTG males: t6 = −4.321, p = 0.005; 3xTG females: t9 = −8.264, p < 0.001), but impaired memory in 3xTG mice at 3 h, as they failed to distinguish significantly between novel and sample objects (3xTG males: t6 = −0.135, p = 0.897; 3xTG females: t9 = 0.963, p = 0.361).
Figure 1.
5xFAD and 3xTG mice have impairments in object-based memory tasks. Schematic representations of the (a) Y-apparatus OR, (b) open-field OR, and (c) OL tasks. (d) 5xFAD mice were impaired on Y-apparatus OR (Wt male n = 11, Wt female n = 8, 5xFAD male n = 10, 5xFAD female n = 11; significant main effects of genotype (F1,36 = 19.198, p < 0.001) and delay (F1,36 = 9.560, p = 0.004), as well as a genotype x delay interaction (F1,36 = 8.563; p = 0.006) were observed), (e) open-field OR (Wt male n = 11, Wt female n = 8, 5xFAD male n = 10, 5xFAD female n = 11; significant main effects of genotype (F1,32 = 6.392, p = 0.017) and delay (F1,32 = 22.725, p < 0.001), as well as a genotype x delay interaction (F1,32 = 7.791, p = 0.009) were found) and (f) OL (Wt male n = 15, Wt female n = 12, 5xFAD male n = 10, 5xFAD male n = 10; a significant main effect of genotype was found (F1,43 = 20.451, p < 0.001) at both 5 min and 3 h retention delays. 3xTG mice had more selective memory impairments. (g) 3xTG mice were impaired on Y-apparatus OR at the 3 h, but not 5 min, delay (Wt male n = 11, Wt female n = 12, 3xTG male n = 7, 3xTG female n = 10; significant main effects of genotype (F1,36 = 26.260, p < 0.001) and delay (F1,36 = 19.172, p < 0.001), as well as a genotype x delay interaction (F1,36 = 21.012, p < 0.001) were observed). (h) 3xTG males were impaired on open-field OR at both the 5 min and 3 h delays, whereas females were only impaired at the 3 h delay (Wt male n = 11, Wt female n = 12, 3xTG male n = 7, 3xTG female n = 12; significant main effects of genotype (F1,36 = 26.260, p < 0.001) and delay (F1,36 = 19.172, p < 0.001), as well as a genotype x delay interaction (F1,36 = 21.012, p < 0.001) were demonstrated). (i) 3xTG mice were also impaired on OL at both delays (Wt male n = 12, Wt female n = 15, 3xTG male n = 14, 3xTG female n = 15; significant main effects of genotype (F1,51 = 43.417, p < 0.001) and delay (F1,51 = 16.387, p < 0.001) were found).Data are mean discrimination ratio ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 indicates significant differences between sample and choice DR, suggesting intact memory (paired-sample t-tests, two-tailed). ††p < 0.01, †††p < 0.001 indicates a significant overall difference between Wt and 5xFAD or 3xTG mice (independent samples t-test, two-tailed, Bonferroni correction).
We also evaluated OR in 5xFAD and 3xTG mice using a standard open-field, where spatial information is readily available. 5xFAD mice were also delay-independently impaired on open-field OR (2 × 2 × 2 split-plot ANOVA: genotype (F1,32 = 6.392, p = 0.017), delay (F1,32 = 22.725, p < 0.001), and genotype × delay (F1,32 = 7.791, p = 0.009); Fig. 1e). Paired samples t-tests between sample and choice DR indicated intact memory in Wt mice (Wt males, 5 min: t10 = −6.219, p < 0.001; Wt males, 3 h: t10 = −4.346, p = 0.002; Wt females, 5 min: t7 = −4.657, p = 0.002; Wt females, 3 h: t7 = −3.928, p = 0.006), but impaired memory in 5xFAD mice in all conditions, as they failed to distinguish significantly between novel and sample objects (5xFAD males, 5 min: t9 = −2.070, p = 0.068 (although approaching statistical significance); 5xFAD males, 3 h: t10 = t9 = −0.415, p = 0.689; 5xFAD females, 5 min: t10 = −0.668, p = 0.519; 5xFAD females, 3 h: t10 = 0.335, p = 0.746). Open-field OR was also impaired in 3xTG mice, as females and males were delay-dependently and delay-independently impaired, respectively (2 × 2 × 2 split-plot ANOVA: genotype (F1,36 = 26.260, p < 0.001), delay (F1,36 = 19.172, p < 0.001), and genotype × delay (F1,36 = 21.012, p < 0.001); Fig. 1h). Paired samples t-tests indicated intact memory in Wt mice (Wt males, 5 min: t10 = 3.918, p = 0.001; Wt males, 3 h: t10 = 3.000, p = 0.008; Wt females, 5 min: t11 = −7.094, p < 0.001; Wt females, 3 h: t11 = 4.835, p = 0.001) and 3xTG females at 5 min (3xTG females: t9 = −4.096, p = 0.003), but impaired memory in 3xTG males at both delays (5 min: t6 = −0.413, p = 0.694; 3 h: t6 = 0.708, p = 0.505), and 3xTG females at 3 h (t9 = −1.230, p = 0.250).
To evaluate spatial object processing directly, 5xFAD and 3xTG mice were tested on the OL task. 5xFAD mice had impaired spatial memory at both 5 min and 3 h delays (2 × 2 × 2 split-plot ANOVA: genotype (F1,43 = 20.451, p < 0.001); Fig. 1f). Paired samples t-tests between sample and choice DR indicated intact memory in Wt mice (Wt males, 5 min: t14 = −4.115, p = 0.001; Wt males, 3 h: t14 = −6.771, p < 0.0001; Wt females, 5 min: t11 = −4.013, p = 0.002; Wt females, 3 h: t11 = −2.663, p = 0.026), but impaired memory in 5xFAD mice in all conditions (5xFAD males, 5 min: t9 = −0.228, p = 0.825; 5xFAD males, 3 h: t9 = 0.200, p = 0.846; 5xFAD females, 5 min: t9 = 0.522, p = 0.613; 5xFAD females, 3 h: t9 = 0.200, p = 0.846). Similarly, OL was delay-independently impaired in 3xTG mice (2 × 2 × 2 split-plot ANOVA: genotype (F1,51 = 43.417, p < 0.001), and delay (F1,51 = 16.387, p < 0.001); Fig. 1i). Paired samples t-tests suggest intact memory in Wt females and Wt males at 5 min (Wt males, 5 min: t11 = −3.850, p = 0.002; Wt females, 5 min: t14 = −5.264, p < 0.001; Wt females, 3 h; t14 = 10.856, p < 0.001), but impaired memory in Wt males at 3 h and 3xTG mice in all conditions (Wt males, 3 h: t11 = 1.978, p = 0.076 (although approaching significance); 3xTG males, 5 min: t13 = −1.251, p = 0.233; 3xTG females, 5 min: t14 = −1.149, p = 0.271; 3xTG females, 3 h: t14 = −0.576, p = 0.575). 3xTG males displayed a familiarity preference at 3 h (t13 = 2.518, p = 0.027), instead of the novelty preference from which rodent object memory is typically inferred. A familiarity preference may imply intact memory, and could reflect abnormities in habituation, dishabituation or novelty preference. However, given the impairment observed at the 5-min delay, in this case it is possible that the familiarity preference is the result of a sample DR greater than zero. Indeed, a single-sample t-test comparing the 3xTG male choice DR at 3 h to 0 was not significant (t13 = −2.103, p = 0.06).
Perceptual Object Oddity Tasks
Finally, object oddity tasks were used to evaluate multisensory integration, as well as unimodal tactile and visual object perception. Wt males and 5xFAD mice had irregular performance on object oddity (2 × 2 × 3 split-plot ANOVA: task (F2,78 = 3.740, p = 0.035) and task × sex (F2,78 = 4.302, p = 0.022); Fig. 2b). One-sample t-tests suggested intact perception in Wt females and Wt males (Wt male, visual: t9 = 2.749, p = 0.023; Wt female, MSO: t9 = 6.018, p < 0.001; Wt female, visual: t9 = 4.4092, p = 0.003; Wt female, tactile: t9 = 3.062, p = 0.014), with the exception of Wt males on the MSO and tactile tasks (Wt male, MSO: t9 = −0.409, p = 0.692; Wt male, tactile: t9 = 2.123, p = 0.066 (although approaching statistical significance)). 5xFAD female mice had intact unimodal perception (visual: t10 = 3.366, p = 0.007; tactile: t10 = 4.502, p = 0.001), but not MSO (t10 = −0.058, p = 0.955). 5xFAD males had impaired perception on all tasks (MSO: t11 = −1.978, p = 0.073 (although approaching significance); visual: t11 = 1.619, p = 0.134; tactile: t11 = 1.127, p = 0.286). 3xTG mice demonstrated a selective impairment in tactile-visual multisensory perception, while unimodal tasks were generally unimpaired (2 × 2 × 3 split-plot ANOVA: task (F2,104 = 4.795, p = 0.010) and task x genotype (F1,104 = 6.967, p = 0.001); Fig. 2c). One-sample t-tests demonstrated perception above chance in Wt mice (males, MSO: t12 = 4.572, p = 0.001; males, tactile; t12 = 4.535, p = 0.001; females, MSO: t14 = 5.333, p < 0.001; females, visual: t14 = 5.627, p < 0.001; females, tactile: t14 = 6.460, p < 0.001), with the exception of visual perception in Wt males (visual: t12 = 1.274, p = 0.220). 3xTG mice had intact perception (3xTG males, visual: t13 = 3.609, p = 0.003; 3xTG males, tactile: t13 = 5.065, p < 0.001; 3xTG females, visual: t13 = 4.027, p = 0.001; 3xTG females, tactile t13 = 3.788, p = 0.002), except on the MSO task (males: t13 = 0.401, p = 0.695; females: t13 = 2.156, p = 0.050 (although very close to significance)).
Figure 2.
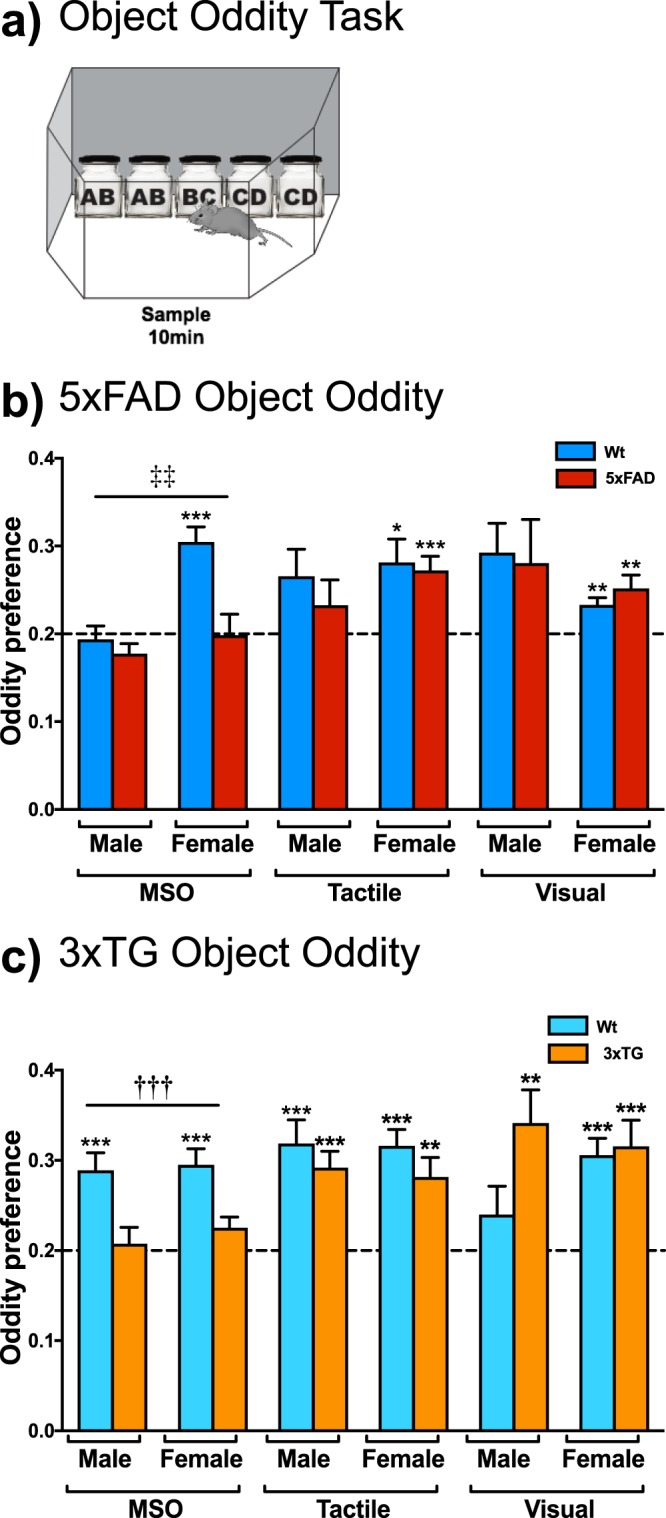
5xFAD and 3xTG mice have impairments in multisensory object oddity. (a) Schematic of multisensory and unimodal object oddity tasks. Wt and 5xFAD male mice had irregular performance on multisensory and unimodal tactile and visual object oddity. (b) 5xFAD female mice had a deficit in multisensory but not unimodal tactile or visual oddity (Wt male n = 10, Wt female n = 10, 5xFAD male n = 12, 5xFAD female n = 11; a significant main effect of task (F2,78 = 3.740, p = 0.035) and a task x sex interaction (F2,78 = 4.302, p = 0.022) were observed). (c) 3xTG mice had deficits in multisensory but not unimodal tactile or visual object oddity (Wt male n = 13, Wt female n = 15, 3xTG male n = 14, 3xTG female n = 14; a significant main effect of task (F2,104 = 4.795, p = 0.010) and task x genotype interaction (F1,104 = 6.967, p = 0.001) were found). Data are mean oddity preference ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 indicates significant one-sample t-tests, suggesting intact perception (two-tailed). †††p < 0.001 Indicates a significant difference between Wt and 3xTG mice (independent samples t-test, two-tailed, Bonferroni correction). ‡‡p < 0.01 Indicates a significant difference between males and females (independent samples t-test, two-tailed, Bonferroni correction).
ELISA
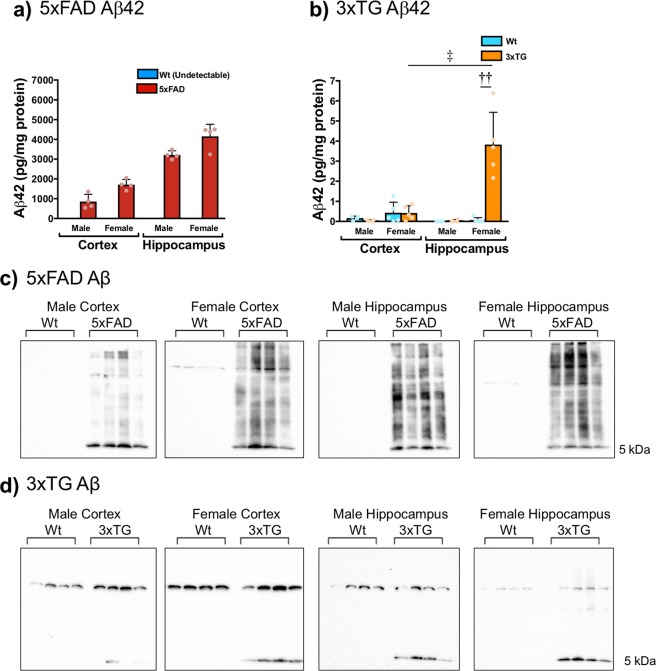
5xFAD mice have extensive Aβ42 deposition in the cortex and HPC (2 × 2 × 2 ANOVA: genotype (F1,24 = 632.278, p < 0.0001), sex (F1,24 = 20.770, p < 0.0001), brain region (F1,24 = 147.648, p < 0.0001), genotype x brain region (F1,24 = 147.648, p < 0.0001), and genotype × sex (F1,24 = 20.770, p < 0.0001); Fig. 3a). Note: Aβ42 was undetectable in all Wt samples (<10 pg/mL); values of 0 were assigned to undetectable samples for analyses. Only 3xTG female mice demonstrate sizeable Aβ42 deposition in the HPC (2 × 2 × 2 ANOVA: genotype (F1,28 = 17.329, p < 0.0001), sex (F1,28 = 25.974, p < 0.0001), brain region (F1,28 = 10.650, p = 0.003), genotype × sex (F1,28 = 20.093, p < 0.0001), genotype x brain region (F1,28 = 20.093, p < 0.0001), sex × brain region (F1,28 = 20.093, p < 0.001), and genotype x sex x brain region (F1,28 = 16.816, p < 0.0001); Fig. 3b). Independent samples t-tests revealed significantly higher Aβ42 levels in the 3xTG female HPC than the Wt HPC (t8 = −5.222, p < 0.0001) and 3xTG female cortex (t8 = 4.624, p = 0.001). Note: Aβ42 was undetectable (<1 pg/mL) in 4 Wt male HPC samples, 4 Wt female HPC samples, 2 3xTG male cortical samples, 1 3xTG female cortical sample, and two 3xTG male HPC samples. Although significant Aβ42 was undetectable by ELISA in 3xTG males, total Aβ (37,38,39,40,42) could be detected in both 3xTG females and males, above Wt levels, using western blotting (Fig. 3d). Densitometry was not performed because Aβ was undetectable in Wt samples.
Figure 3.
Aβ42 deposition in 13 month old 5xFAD and 3xTG mice. (a) There is extensive Aβ42 accumulation in the cortex and HPC of 5xFAD mice (n = 4 per group; main effects of genotype (F1,24 = 632.278, p < 0.0001), sex (F1,24 = 20.770, p < 0.0001), brain region (F1,24 = 147.648, p < 0.0001), as well as genotype x brain region (F1,24 = 147.648, p < 0.0001), and genotype × sex (F1,24 = 20.770, p < 0.0001) interactions were observed). (b) Only 3xTG female mice demonstrate sizeable Aβ42 deposition in the HPC (male n = 4 per group, female n = 5 per group; significant main effects of genotype (F1,28 = 17.329, p < 0.0001), sex (F1,28 = 25.974, p < 0.0001), brain region (F1,28 = 10.650, p = 0.003), as well as genotype x sex (F1,28 = 20.093, p < 0.0001), genotype x brain region (F1,28 = 20.093, p < 0.0001), sex x brain region (F1,28 = 20.093, p < 0.001), and genotype x sex x brain region (F1,28 = 16.816, p < 0.0001) interactions were observed). Total Aβ (37, 38, 39, 40, 42), however, could be detected in both (c) 5xFAD and (d) 3xTG mice, above Wt levels, using western blotting. ELISA data are mean Aβ42 pg/ug protein ± SEM. †††p < 0.001 Indicates a significant difference between Wt and 5xFAD or 3xTG mice (independent samples t-test, two-tailed, Bonferroni correction).
Western Blots
Aged 5xFAD and 3xTG mice have several significant changes to a number of proteins associated with AD pathology.
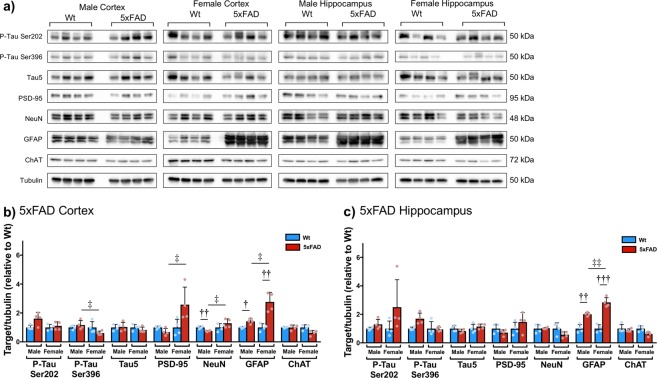
5xFAD mice have abnormal expression of P-tau Ser202, P-tau Ser396, PSD-95, NeuN, GFAP, and ChAT, relative to Wt, in the cortex (2 × 2 ANOVA: P-tau Ser202 genotype (F1,16 = 5.633, p = 0.035); P-tau Ser396 sex (F1,16 = 5.635, p = 0.035), genotype x sex (F1,16 = 4.770, p = 0.050); PSD-95 sex (F1,16 = 9.742, p = 0.009); NeuN sex (F1,16 = 6.702, p = 0.024), genotype x sex (F1,16 = 7.344, p = 0.019); GFAP genotype (F1,16 = 48.595, p < 0.0001), sex (F1,16 = 13.261, p = 0.003), genotype x sex (F1,16 = 13.995, p = 0.003); ChAT genotype (F1,16 = 6.432, p = 0.026), sex (F1,16 = 5.954, p = 0.031), genotype x sex (F1,16 = 5.612, p = 0.035) Fig. 4b) and/or HPC (2 × 2 ANOVA: P-tau Ser202 genotype F1,16 = 143.914, p < 0.0001; GFAP genotype (F1,16 = 100.387, p < 0.0001), sex (F1,16 = 5.095, p = 0.043), genotype x sex (F1,16 = 5.090, p = 0.044); ChAT genotype (F1,16 = 7.109, p = 0.021) Fig. 4c)). Independent samples t-tests revealed a significant difference between cortical levels of P-tau Ser396 (t6 = 3.685, p = 0.010), PSD-95 (t6 = −4.206, p = 0.006), NeuN (t6 = −3.810, p = 0.009), and GFAP (t6 = −4.460, p = 0.004). There was also a significant difference between hippocampal levels of P-tau Ser396 in 5xFAD males and females (t6 = −5.117, p = 0.002). Independent samples t-tests revealed a significant difference between cortical levels of NeuN (t6 = 5.278, p = 0.002) in Wt and 5xFAD males. There was also a significant difference between Wt and 5xFAD levels of GFAP in the cortex (males: t6 = −4.930, p = 0.003; females: t6 = −5.669, p = 0.001) and HPC (males: t6 = −5.855, p = 0.001; females: t6 = −8.198, p < 0.0001).
Figure 4.
13 month old 5xFAD mice have abnormal expression of phospho-tau (Ser202, Ser396), PSD-95, NeuN, GFAP, and ChAT in the cortex and/or HPC. (a) Representative western blots (n = 4 per group). The approximate molecular weights for each identified protein, in kilodaltons (kDa) are shown to the right of the bands. Corresponding densitometric measurement in the (b) cortex (P-tau Ser202 genotype (F1,16 = 5.633, p = 0.035); P-tau Ser396 sex (F1,16 = 5.635, p = 0.035), genotype × sex (F1,16 = 4.770, p = 0.050); PSD-95 sex (F1,16 = 9.742, p = 0.009); NeuN sex (F1,16 = 6.702, p = 0.024), genotype x sex (F1,16 = 7.344, p = 0.019); GFAP genotype (F1,16 = 48.595, p < 0.0001), sex (F1,16 = 13.261, p = 0.003), genotype x sex (F1,16 = 13.995, p = 0.003); ChAT genotype (F1,16 = 6.432, p = 0.026), sex (F1,16 = 5.954, p = 0.031), genotype × sex (F1,16 = 5.612, p = 0.035) and (c) HPC (P-tau Ser202 genotype F1,16 = 143.914, p < 0.0001; GFAP genotype (F1,16 = 100.387, p < 0.0001), sex (F1,16 = 5.095, p = 0.043), genotype x sex (F1,16 = 5.090, p = 0.044); ChAT genotype (F1,16 = 7.109, p = 0.021). All values for 5xFAD mice are presented as a fold change in band intensity relative to Wt mice on the same blot; therefore, all Wt values are presented with a mean of 1. Data are mean expression ± SEM. ††p < 0.01 Indicates a significant difference between Wt and 5xFAD (independent samples t-test, two-tailed, Bonferroni correction). ‡p < 0.05 Indicates a significant difference between males and females (independent samples t-test, two-tailed, Bonferroni correction). Blots have been cropped for conciseness; full length blots are presented in Supplementary Figures 1–4.
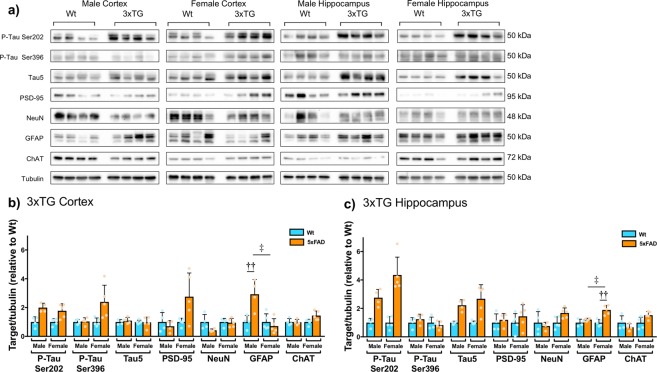
3xTG mice have abnormal protein expression of phospho-tau (Ser202, Ser396 and total Tau5), PSD-95, NeuN, GFAP and ChAT in the cortex (2 × 2 ANOVA: P-tau Ser202 genotype (F1,18 = 25.208, p < 0.0001); P-tau Ser396 genotype (F1,18 = 6.794, p = 0.021), sex (F1,18 = 5.855, p = 0.030), genotype x sex (F1,18 = 6.103, p = 0.027); PSD-95 sex (F1,18 = 5.876, p = 0.029), genotype x sex (F1,18 = 4.894, p = 0.044); NeuN genotype (F1,18 = 5.179, p = 0.039); GFAP sex (F1,18 = 10.885, p = 0.005), genotype x sex (F1,18 = 10.434, p = 0.006); Fig. 5b) and/or HPC (2 × 2 ANOVA: P-tau Ser202 genotype (F1,18 = 77.628, p < 0.0001); tau5 genotype (F1,18 = 35.523, p < 0.0001); GFAP genotype (F1,18 = 23.200, p < 0.0001), sex (F1,18 = 7.261, p = 0.017), genotype x sex (F1,18 = 7.210, p = 0.018); ChAT sex (F1,18 = 6.468, p = 0.023), genotype x sex (F1,18 = 6.142, p = 0.027); Fig. 5c). Independent samples t-tests revealed significant differences between cortical GFAP levels in Wt and 3xTG males (t6 = −3.561, p = 0.012), as well as male and female 3xTG mice (t6 = 4.394, p = 0.003). There was also a significant difference between hippocampal levels of GFAP in Wt and 3xTG females (t6 = −5.502, p = 0.001), as well as male and female 3xTG mice (t6 = −4.409, p = 0.003).
Figure 5.
13 month old 3xTG mice have abnormal protein expression of phospho-tau (Ser202, Ser396 and total Tau5), PSD-95, NeuN, GFAP and ChAT in the cortex and/or HPC. (a) Representative western blots (male n = 4 per group, female n = 5 per group). Corresponding densitometric measurement in the (b) cortex (P-tau Ser202 genotype (F1,18 = 25.208, p < 0.0001); P-tau Ser396 genotype (F1,18 = 6.794, p = 0.021), sex (F1,18 = 5.855, p = 0.030), genotype x sex (F1,18 = 6.103, p = 0.027); PSD-95 sex (F1,18 = 5.876, p = 0.029), genotype x sex (F1,18 = 4.894, p = 0.044); NeuN genotype (F1,18 = 5.179, p = 0.039); GFAP sex (F1,18 = 10.885, p = 0.005), genotype x sex (F1,18 = 10.434, p = 0.006) and (c) HPC (P-tau Ser202 genotype (F1,18 = 77.628, p < 0.0001); tau5 genotype (F1,18 = 35.523, p < 0.0001); GFAP genotype (F1,18 = 23.200, p < 0.0001), sex (F1,18 = 7.261, p = 0.017), genotype × sex (F1,18 = 7.210, p = 0.018); ChAT sex (F1,18 = 6.468, p = 0.023), genotype × sex (F1,18 = 6.142, p = 0.027). All values for 3xTG mice are presented as a fold change in band intensity relative to Wt mice on the same blot; therefore, all Wt values are presented with a mean of 1. Data are mean expression ± SEM. ††p < 0.01 Indicates a significant difference between Wt and 3xTG (independent samples t-test, two-tailed, Bonferroni correction). ‡p < 0.05 Indicates a significant difference between males and females (independent samples t-test, two-tailed, Bonferroni correction). Blots have been cropped for conciseness; full length blots are presented in Supplementary Figures 5–8.
Discussion
This study demonstrates impairments in object memory and perception in male and female 5xFAD and 3xTG mice, and these deficits parallel AD-like pathological changes. Specifically, tasks that directly probe different aspects of object processing (object identity memory, spatial location memory, and perceptual discrimination) reveal distinctive patterns of impairment in different strains and sexes. The pattern of impairments we observed in 5xFAD and 3xTG mice are consistent with both the large literature demonstrating impairments in AD mice4–24, as well as the lack of impairments8,13,17,25–29 seen in some studies which have used short retention delays or younger animals.
Object recognition impairments in 5xFAD and 3xTG mice were observed in both the Y-apparatus and open-field tasks. 5xFAD mice had OR impairments at the 5-min and 3-h retention delays, although a few 5xFAD groups appeared to be trending towards statistical limits that would demonstrate intact memory. The widespread impairments observed in 5xFAD mice are consistent with their severe pathology and possibly indicate the presence of attentional deficits in addition to memory impairment. 3xTG mice had selective impairments in Y-apparatus OR at 3 h, suggestive of a more selective long-term object memory deficit. However, when OR was evaluated in the open-field, 3xTG females were also selectively impaired at 3 h but 3xTG males were impaired at both 5 min and 3 h. This suggests that OR impairments can become more severe in some strains when the spatial nature of the task is increased. The Y-apparatus could increase the focus on objects and provides fewer potentially interfering contextual stimuli than the open-field. Perhaps AD mice have insufficient mnemonic resources for both object and environmental information present in the open-field OR task. While the HPC seems to be necessary for basic OR in mice34,35, the open-field OR task likely places even greater demand on the HPC36,37, which has substantial amyloid accumulation.
In OR, we also observed a subtle sex difference between 3xTG males and females, where 3xTG females had a less severe OR deficit than males. Specifically, short-term OR memory was intact in 3xTG females in open-field and Y-apparatus OR, whereas 3xTG males had short-term open-field OR deficits that were not observed at short retention delays in the Y-apparatus. This pattern is consistent with sexual dimorphism in working memory, such that 3xTG males have a more severe behavioural deficit beginning at two-months-of-age38. Although our data, and others39–41, demonstrate a more aggressive amyloid pathology in 3xTG females, activational estrogens are believed to be neuroprotective against Alzheimer’s pathology. Ovariectomy-induced depletion of sex hormones in 3xTG females increases Aβ accumulation and impairs HPC-dependent memory, an effect that can be attenuated by estrogen treatment42–45. It is also possible that differences in male and female longevity contributed to sex differences in 3xTG mice, since 3xTG females have a longer lifespan than males46. This sex difference was not observed in 5xFAD mice at this age, where a more aggressive amyloid pathology may block the beneficial effects of estrogens in easier testing conditions.
Object location memory was impaired across sex and AD strain, suggesting that spatial processing of objects may be more difficult for AD mice than the processing of object identity. Impaired OL memory is consistent with previous work17,33,47–49, and as OL is a HPC-dependent task this finding provides additional support for susceptibility of HPC-dependent processes to AD pathology. Since Wt males for the 3xTG strain had difficulty performing the OL task, it is possible that some OL deficits in males may be related to general aging rather than AD. Indeed, OL is often impaired in aged mice50,51.
Multisensory integration (MSI) is a relatively neglected facet of cognition in AD, despite findings from patients demonstrating abnormal MSI31,32. Multisensory integration involves the binding of information across sensory modalities, which can require connectivity of modality-specific sensory processing areas52,53. Given the disruption of cortical connectivity in human patients54,55 and APP/PS1 mice56, impaired connectivity between cortical regions may impair binding of visual and tactile object information. The current results represent the first demonstration of impaired MSI in rodent models of AD. While 5xFAD female and 3xTG mice show a selective deficit on tactile-visual MSO, 5xFAD and Wt male mice from the same background also had impairments in unimodal tactile and visual object oddity tasks. Unimodal tactile and visual object oddity performance also informs the interpretation of results from object memory tasks, in which mice must also encode object features perceptually. 5xFAD mice display delay-independent impairments on all mnemonic object tasks and 5xFAD males have impairments on tactile and visual oddity; therefore, impaired OR and OL in 5xFAD males could be most parsimoniously attributed to deficits in object perception rather than memory. Again, the impairment in 5xFAD Wt males may be related to accelerated aging or other abnormalities in the 5xFAD background strain. Conversely, 5xFAD females and 3xTG mice had selective multisensory oddity impairments, with spared unimodal perception; thus, it is unlikely that OR and OL impairments can be explained by perceptual deficits. In this case, the object test battery approach provides a great advantage over standard testing methods in this field by strengthening the conclusions that can be drawn about specific cognitive impairments.
Atypical motor and anxiety-like behaviours have been reported in both 5xFAD and 3xTG strains. Aged 3xTG mice have increased anxiety-like behaviours including increased restlessness, startle responses, and freezing23,57–59, whereas motor behaviour is more complex with accounts of hypoactivity59 or normal locomotion in the open-field23, as well as enhanced performance on other motor tasks16,24. Conversely, 5xFAD mice have reduced anxiety-like behaviours and motor deficits60,61. However, there were no consistent differences between genotypes or sex in terms of general object exploratory behaviour in any of the tests used; thus, it is unlikely that gross changes to motor or anxiety-related behaviors significantly impacted the current findings.
Abnormalities in several proteins associated with AD were seen in both 5xFAD and 3xTG mice. A pattern of amyloid deposition that is more severe in the HPC than cortex was observed in both mouse strains. 5xFAD mice have much more Aβ42 deposition than 3xTG mice, consistent with multiple APP and PS1 mutations acting in an additive fashion to accelerate amyloid deposition and cognitive impairment in the former62. While Aβ42 likely contributes to the observed cognitive impairments, especially in HPC-dependent object tasks, Aβ42 deposition is minimal in 3xTG mice at this advanced age. Indeed, Aβ42 was not elevated in the cortex or HPC of 3xTG males. However, we did detect increased levels of total Aβ, suggesting that 3xTG males may have different proportions of Aβ 37, 38, 39, 40, and 42 at 12-months-of-age. Despite the lack of robust changes in Aβ42, we have demonstrated significant behavioural impairments in male 3xTG mice, highlighting the importance of other pathological changes on behavioural phenotypes. Both strains had abnormalities in tau phosphorylation and astrogliosis, other pathological hallmarks of AD associated with cognitive impairment63,64. Consistent with previous observations we report elevations in tau phosphorylation in both 5xFAD65–67 and 3xTG42,68–71 mice, with novel strain, sex, and regional differences on phosphorylation at specific tau residues. Extensive gliosis (GFAP) was also observed in both 5xFAD62,72 and 3xTG68–70,73 mice, also with interesting differences between strain, sex and brain region. Paradoxically, PSD-95 was elevated in 5xFAD and 3xTG females which may result from the loss of PSD-95 at apical dendrites but accumulation in cell bodies in advanced AD74. Contrary to the existing literature75, ChAT was also elevated in 3xTG females and may reflect a compensatory response as female sex hormones can modulate AD pathology and cholinergic neurotransmission42,76. The opposite pattern was observed for ChAT in 5xFAD mice77.
The current study illustrates the subtle but potentially clinically significant differences in behavioural characterization possible when using a testing battery to probe different aspects of object information processing, not just “object recognition” as defined by the most commonly chosen task. We recommend such a systematic approach moving forward to better evaluate the cognitive phenotypes of transgenic models, as well as potential therapeutic interventions, in this context.
Methods
Animals
5xFAD wild-type (B6SJLF1/J; male n = 20, female n = 33) and transgenic (B6SJLTg(APPSwF1Lon,PSEN1*M146L*L286V)6799Vas/Mmjax; male n = 23, female n = 33) mice and 3xTG wild-type (B6129SF2/J; male n = 24, female n = 35) and transgenic (B6;129Psen1tm1Mpm Tg(APPSwe, tauP301L)1Lfa/Mmjax; male n = 21, female n = 35) mice were obtained from Jackson Laboratory (Bar Harbor, USA). Because we were primarily comparing WT and TG mice, there was no random assignment to groups. A minimum of 7 mice per group were used for behavioural experiments; our previous work indicates power of 0.80 can be achieved with a sample size of 6 mice (α = 0.05). Differences in sample sizes between sexes was a result of attrition46. Mice were housed in polyethylene cages (16 × 12 × 26 cm) with corncob bedding, crink-l’Nest and cotton nest squares and food (Tekland Global 16% Protein Rodent Maintenance Diet, Harlan Tekland, USA) and water available ad libitum. Mice were tested during the light phase of a 12 h light/dark cycle (0800 lights on; 2000h lights off). All procedures followed the guidelines of the Canadian Council on Animal Care and were approved by the Animal Care Committee at the University of Guelph and the University of Western Ontario (2006-103 and 2006-104). Object recognition testing started when mice were 12 months of age after they were tested in different touchscreen tasks.
Object-based Memory Tasks
Object recognition was run in a modified Y-apparatus with walls 30.5 cm high, and arms 15 cm long and 7 cm wide constructed from white Plexiglas (Fig. 1a). The start arm of the Y-apparatus has a guillotine door 11 cm from the back of the arm that was closed at the beginning of each trial. When OR is conducted in the Y-apparatus, spatial information is minimized, allowing for direct evaluation of object identity processing37. Objects were visually and tactually distinct, approximately 5–15 cm in height and 3–6 cm wide, made of glass, metal or plastic and were previously found to produce no obvious preference biases in mice. In the sample phase, mice were presented two identical objects to explore for 10 min. Following the sample phase, there was a 5-min or 3-h retention delay, to assess short- and long-term memory, respectively. At the end of the retention delay, mice underwent a 2-min choice phase, in which mice were presented with one object from the sample phase and a novel object. Object recognition was also evaluated with the same parameters in a square open-field arena (45 × 45 × 30 cm) made of white corrugated plastic (Fig. 1b). When OR is evaluated in the open-field, spatial and contextual cues in the testing room are readily available; this task might therefore be more hippocampus-dependent than Y-apparatus OR37. In both OR tasks, the order of object pairs, the designated sample and choice novel object within each pair, and the side of the apparatus (left or right) where the novel object was placed during the choice phase were counterbalanced. The object location task was used to evaluate spatial object memory in the open-field using the same testing parameters as OR, except that one object from the sample phase was moved to an adjacent corner of the arena in the choice phase (Fig. 1c); in this case, the location of one object, rather than its identity, is novel. In OL, the order of object pairs and the side of the apparatus (left or right) where the novel object was placed during the choice phase were counterbalanced. For all object memory tasks, the novelty preference was quantified by calculating a discrimination ratio (DR = (novel object exploration – familiar object exploration)/(total object exploration)). Repeated measures ANOVAs were used to analyze DRs with retention delay as a within-subjects factor and sex and genotype as between-subjects factors. Where appropriate, post-hoc t-tests were used to analyze group differences. In the sample phase, objects should be equally novel and a DR of approximately zero is expected. In the choice phase, a DR significantly greater than zero indicates novelty preference, from which we infer intact memory. Paired samples t-tests were used to compare sample and choice DRs, as a significant increase in the DR from sample to choice is indicative of intact memory. Outliers (>2 SD ± mean; 33 data points total) and mice that spent less than 3% of the choice phase exploring78 (19 data points) were excluded. General exploratory measures are reported in Supplementary Tables 3 and 4.
Perceptual Object Oddity Tasks
Oddity tasks were run in a modified trapezoid-like open-arena (front wall 39 cm, side walls 14 cm, angled side walls 10 cm, back wall 28 cm; Fig. 2a). In MSO, mice explored two pairs of objects that shared combinations of tactile and visual features (e.g., AB/AB and CD/CD), as well as a dissimilar object that comprised a unique (i.e. ‘odd’) combination of those features (e.g., BC) for 10 min. Tactile object features were manipulated using varieties of sandpaper with different grades, while visual object features were manipulated using 2-D stickers with distinctive visual markings. Unimodal control trials were also run according to the same format, except the objects were distinguished by configurations of features from within the same sensory modality (i.e., visual or tactile)52. Placement of the ‘odd’ object was counterbalanced across trials and experiments.
An oddity preference for the ‘odd’ object was calculated (OP = (exploration of the odd object)/(total object exploration)). Repeated measures ANOVAs were used to analyze OPs with task as a within-subjects factor and genotype as a between-subjects factor. Where appropriate, post-hoc t-tests were used to analyze between group differences. With five objects, an OP significantly greater than 0.2 (‘chance’) indicates an oddity preference, from which we infer intact perceptual discrimination; this analysis was performed using one sample t-tests. Outliers (>2 SD ± mean) and mice that spent less than 3% of the sample phase exploring were excluded.
General Behavioural Procedure
All behavioural testing was conducted under white fluorescent light in a 190 cm by 145 cm room with white walls and an orange door. A television, computer modem, and computer monitor sat atop metal shelving. A few additional visual cues were fixed to the walls. The apparatuses were placed on the floor away from the walls.
Prior to behavioural testing all mice were extensively handled and habituated to an empty testing apparatus for 10 min on two consecutive days. Behavioural testing began at least 24 h after the second habituation day. Immediately prior to testing, mice were brought into the testing room in their home cage. Mice were placed in either the start arm of the Y-apparatus or in a start box placed in the center of the open field. The trial began when the start arm/box was opened or removed, respectively, and the mouse began to explore. During testing, an experimenter blind to the experimental condition, viewed the mouse on a television screen and pressed a key corresponding to a given object at the onset and end of an exploratory bout (sniffing within 1 cm of the object and/or touching the object with the nose). Between trials, objects were wiped with 50% ethanol (to eliminate olfactory cues), the testing apparatus was wiped with dry paper towel and the mouse was returned to its home cage.
Western Blots and ELISA
Immediately after behavioural testing (~13 months of age), cortices and hippocampi were homogenized in Triton X-100 lysis buffer containing 1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride, 10 μM leupeptin, 25 μM aprotinin, 10 μM pepstatin A, and 700 units DNase. Lysate protein concentrations were determined using a Bradford assay.
Total Aβ (37, 38, 39, 40, 42), phospho-tau, total tau, NeuN, postsynaptic density (PSD)-95, glial fibrillary acidic protein (GFAP), and choline acetyltransferase (ChAT) levels were evaluated using Western blot analysis. For western blots protein samples (25 μg/well) were run on 10% SDS-PAGE using a Mini-PROTEAN Tetra cell system. Samples were transferred to nitrocellulose membranes, blocked in 3% BSA (Fisher Scientific; with or without milk) in tris-buffered saline 0.1% Tween20 (TBS-T) for 1.5 h, and incubated in primary antibodies overnight (see Supplementary Table 1). Blots were subsequently rinsed with TBS-T, incubated in secondary antibody in BSA, rinsed with TBS-T, and visualized using Luminata Forte chemi solution and the ChemiDoc MP imaging system. Densitometry was performed using Image Labv4.1 software comparing levels of each target to α-tubulin. To account for variability between blots the densitometric value for each individual protein band was expressed as a fraction of the total amount of that protein on the same blot. Each protein of interest was normalized to α-tubulin values from the same sample and the normalized protein levels in the transgenic mice were presented as the fold change relative to values from the Wt mice from the same blot. Two-way ANOVAs were used to analyze genotype and sex effects on each target protein. Log (x + 1) transformations were performed to address heterogeneity of variance.
ELISA measurements of Aβ42 were conducted using the standard or ultrasensitive amyloid beta 42 human ELISA kits from ThermoFisher Scientific for 5xFAD and 3xTG lysates, respectively. Three-way ANOVAs were used to analyze strain, genotype and sex effect on Aβ42.
Statistical Analysis
All statistical analyses were performed with α = 0.05 using SPSS. Where appropriate, the Bonferroni correction was applied. All data met assumptions of normality and homogeneity.
Supplementary information
Acknowledgements
Funding was provided by a Transformational Program Grant in Neuroscience from the Weston Brain Institute to V.F.P., M.A.M.P., and B.D.W., as well as NSERC to B.E.K. and NSERC to N.J.M.
Author Contributions
S.D.C. and B.D.W. conceived and directed behavioural experiments; S.D.C. and D.P. performed behavioural experiments; S.D.C., A.L.M., B.E.K., N.J.M. V.F.P., M.A.M.P. and B.D.W. conceived and directed molecular experiments; S.D.C., A.L.M. and B.E.K. performed western blots and ELISA; S.D.C. and B.D.W. wrote the manuscript; all authors revised the manuscript.
Data Availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-37312-0.
References
- 1.Alzheimer A. Uber eigenartige Krankheitsfalle des spateren Alters. Z Ges Neurol Psychiatr. 1911;4:356–385. doi: 10.1007/BF02866241. [DOI] [Google Scholar]
- 2.Didic M, et al. Impaired visual recognition memory predicts Alzheimer’s disease in amnestic mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 2013;35:291–299. doi: 10.1159/000347203. [DOI] [PubMed] [Google Scholar]
- 3.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-X. [DOI] [PubMed] [Google Scholar]
- 4.Giannoni P, et al. Early administration of RS 67333, a specific 5-HT4 receptor agonist, prevents amyloidogenesis and behavioral deficits in the 5XFAD mouse model of Alzheimer’s disease. Front. Aging Neurosci. 2013;5:96. doi: 10.3389/fnagi.2013.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tohda C, Nakada R, Urano T, Okonogi A, Kuboyama T. Kamikihi-to (KKT) rescues axonal and synaptic degeneration associated with memory impairment in a mouse model of Alzheimer’s disease, 5XFAD. Int. J. Neurosci. 2011;121:641–8. doi: 10.3109/00207454.2011.602809. [DOI] [PubMed] [Google Scholar]
- 6.Tohda C, Urano T, Umezaki M, Nemere I, Kuboyama T. Diosgenin is an exogenous activator of 1,25D3-MARRS/Pdia3/ERp57 and improves Alzheimer’s disease pathologies in 5XFAD mice. Sci. Rep. 2012;2:535. doi: 10.1038/srep00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joyashiki E, Matsuya Y, Tohda C. Sominone improves memory impairments and increases axonal density in Alzheimer’s disease model mice, 5XFAD. Int. J. Neurosci. 2011;121:181–90. doi: 10.3109/00207454.2010.541571. [DOI] [PubMed] [Google Scholar]
- 8.Fragkouli A, Tsilibary EC, Tzinia AK. Neuroprotective role of MMP-9 overexpression in the brain of Alzheimer’s 5xFAD mice. Neurobiol. Dis. 2014;70:179–89. doi: 10.1016/j.nbd.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 9.Frydman-Marom A, et al. Orally administrated cinnamon extract reduces B-amyloid oligomerization and corrects cognitive impairment in Alzheimer’s disease animal models. PLoS One. 2011;6:1–11. doi: 10.1371/journal.pone.0016564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arsenault D, Julien C, Tremblay C, Calon F. DHA improves cognition and prevents dysfunction of entorhinal cortex neurons in 3xTg-AD mice. PLoS One. 2011;6:e17397. doi: 10.1371/journal.pone.0017397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanchard J, et al. Pharmacologic reversal of neurogenic and neuroplastic abnormalities and cognitive impairments without affecting Aβ and tau pathologies in 3xTg-AD mice. Acta Neuropathol. 2010;120:605–21. doi: 10.1007/s00401-010-0734-6. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, et al. intracerebroventricular streptozotocin exacerbates Alzheimer-like changes of 3xTg-AD mice. Mol. Neurobiol. 2014;49:547–62. doi: 10.1007/s12035-013-8539-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis KE, Easton A, Eacott MJ, Gigg J. Episodic-like memory for what-where-which occasion is selectively impaired in the 3xTgAD mouse model of Alzheimer’s disease. J. Alzheimers. Dis. 2013;33:681–98. doi: 10.3233/JAD-2012-121543. [DOI] [PubMed] [Google Scholar]
- 14.Davis K, Eacott M, Easton A, Gigg J. Episodic-like memory is sensitive to both Alzheimer’s-like pathological accumulation and normal ageing processes in mice. Behav. Brain Res. 2013;254:73–82. doi: 10.1016/j.bbr.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Feld M, et al. Decrease of ERK/MAPK overactivation in prefrontal cortex reverses early memory deficit in a mouse model of Alzheimer’s disease. J. Alzheimers. Dis. 2014;40:69–82. doi: 10.3233/JAD-131076. [DOI] [PubMed] [Google Scholar]
- 16.Filali M, et al. Cognitive and non-cognitive behaviors in the triple transgenic mouse model of Alzheimer’s disease expressing mutated APP, PS1, and Mapt (3xTg-AD) Behav. Brain Res. 2012;234:334–42. doi: 10.1016/j.bbr.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Gulinello M, et al. Validation of a 2-day water maze protocol in mice. Behav. Brain Res. 2009;196:220–7. doi: 10.1016/j.bbr.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guzmán-Ramos K, et al. Restoration of dopamine release deficits during object recognition memory acquisition attenuates cognitive impairment in a triple transgenic mice model of Alzheimer’s disease. Learn. Mem. 2012;19:453–60. doi: 10.1101/lm.026070.112. [DOI] [PubMed] [Google Scholar]
- 19.Kazim SF, et al. Disease modifying effect of chronic oral treatment with a neurotrophic peptidergic compound in a triple transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis. 2014;71:110–30. doi: 10.1016/j.nbd.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Masciopinto F, et al. Effects of long-term treatment with pioglitazone on cognition and glucose metabolism of PS1-KI, 3xTg-AD, and wild-type mice. Cell Death Dis. 2012;3:e448. doi: 10.1038/cddis.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onishi T, et al. A novel glycogen synthase kinase-3 inhibitor 2-methyl-5-(3-{4-[(S)-methylsulfinyl]phenyl}-1-benzofuran-5-yl)-1,3,4-oxadiazole decreases tau phosphorylation and ameliorates cognitive deficits in a transgenic model of Alzheimer’s disease. J. Neurochem. 2011;119:1330–40. doi: 10.1111/j.1471-4159.2011.07532.x. [DOI] [PubMed] [Google Scholar]
- 22.Parachikova A, Vasilevko V, Cribbs DH, LaFerla FM, Green KN. Reductions in amyloid-beta-derived neuroinflammation, with minocycline, restore cognition but do not significantly affect tau hyperphosphorylation. J. Alzheimers. Dis. 2010;21:527–42. doi: 10.3233/JAD-2010-100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.St-Amour I, et al. IVIg protects the 3xTg-AD mouse model of Alzheimer’s disease from memory deficit and Aβpathology. J. Neuroinflammation. 2014;11:54. doi: 10.1186/1742-2094-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stover KR, Campbell Ma, Van Winssen CM, Brown RE. Early detection of cognitive deficits in the 3xTg-AD mouse model of Alzheimer’s disease. Behav. Brain Res. 2015;289:29–38. doi: 10.1016/j.bbr.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 25.Cheng D, Low JK, Logge W, Garner B, Karl T. Chronic cannabidiol treatment improves social and object recognition in double transgenic APPswe/PS1∆E9 mice. Psychopharmacology (Berl). 2014;231:3009–17. doi: 10.1007/s00213-014-3478-5. [DOI] [PubMed] [Google Scholar]
- 26.Good MA, Hale G, Staal V. Impaired ‘episodic-like’ object memory in adult APPswe transgenic mice. Behav. Neurosci. 2007;121:443–8. doi: 10.1037/0735-7044.121.2.443. [DOI] [PubMed] [Google Scholar]
- 27.Hale G, Good M. Impaired Visuospatial Recognition Memory but Normal Object Novelty Detection and Relative Familiarity Judgments in Adult Mice Expressing the APPswe Alzheimer’ s Disease Mutation. Behav. Neurosci. 2005;119:884–891. doi: 10.1037/0735-7044.119.4.884. [DOI] [PubMed] [Google Scholar]
- 28.Karl T, Bhatia S, Cheng D, Kim WS, Garner B. Cognitive phenotyping of amyloid precursor protein transgenic J20 mice. Behav. Brain Res. 2012;228:392–7. doi: 10.1016/j.bbr.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 29.Yassine N, et al. Detecting spatial memory deficits beyond blindness in tg2576 Alzheimer mice. Neurobiol. Aging. 2013;34:716–30. doi: 10.1016/j.neurobiolaging.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 30.Caterini F, et al. Object recognition and object orientation in Alzheimer’s disease. Neuropsychology. 2002;16:146–155. doi: 10.1037/0894-4105.16.2.146. [DOI] [PubMed] [Google Scholar]
- 31.Wu J, et al. Delayed audiovisual integration of patients with mild cognitive impairment and Alzheimer’s disease compared with normal aged controls. J. Alzheimer’s Dis. 2012;32:317–328. doi: 10.3233/JAD-2012-111070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delbeuck X, Collette F, Van der Linden M. Is Alzheimer’s disease a disconnection syndrome?. Evidence from a crossmodal audio-visual illusory experiment. Neuropsychologia. 2007;45:3315–3323. doi: 10.1016/j.neuropsychologia.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Dodel R, et al. Naturally occurring autoantibodies against beta-amyloid: investigating their role in transgenic animal and in vitro models of Alzheimer’s disease. J. Neurosci. 2011;31:5847–54. doi: 10.1523/JNEUROSCI.4401-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammond RS, Tull LE, Stackman RW. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol. Learn. Mem. 2004;82:26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Cohen S, et al. The rodent hippocampus is essential for non-spatial object memory. Curr Biol. 2013;23:1685–1690. doi: 10.1016/j.cub.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winters B, Saksida L, Bussey T. Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci. Biobehav. Rev. 2008;32:1055–70. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Winters B, Forwood S, Cowell R, Saksida L, Bussey T. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J. Neurosci. 2004;24:5901–8. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevens LM, Brown RE. Reference and working memory deficits in the 3xTg-AD mouse between 2 and 15-months of age: A cross-sectional study. Behav. Brain Res. 2015;278:496–505. doi: 10.1016/j.bbr.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 39.Carroll JC, et al. Sex differences in B-amyloid accumulation in 3xTg-AD mice: Role of neonatal sex steroid hormone exposure. Brain Res. 2010;1366:233–245. doi: 10.1016/j.brainres.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clinton LK, et al. Age-dependent sexual dimorphism in cognition and stress response in the 3xTg-AD mice. Neurobiol. Dis. 2007;28:76–82. doi: 10.1016/j.nbd.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirata-Fukae C, et al. Females exhibit more extensive amyloid, but not tau, pathology in an Alzheimer transgenic model. Brain Res. 2008;1216:92–103. doi: 10.1016/j.brainres.2008.03.079. [DOI] [PubMed] [Google Scholar]
- 42.Carroll JC, et al. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J. Neurosci. 2007;27:13357–13365. doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carroll JC, Pike CJ. Selective estrogen receptor modulators differentially regulate Alzheimer-like changes in female 3xTg-AD mice. Endocrinology. 2008;149:2607–2611. doi: 10.1210/en.2007-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng H, et al. Modulation of A(beta) peptides by estrogen in mouse models. J. Neurochem. 2002;80:191–6. doi: 10.1046/j.0022-3042.2001.00690.x. [DOI] [PubMed] [Google Scholar]
- 45.Levin-allerhand JA, Lominska CE, Wang J, Smith JD. 17α-estradiol and 17β-estradiol treatments are effective in lowering cerebral amyloid-β levels in AβPPSWE transgenic mice. J. Alzheimer’s Dis. 2002;4:449–457. doi: 10.3233/JAD-2002-4601. [DOI] [PubMed] [Google Scholar]
- 46.Rae EA, Brown RE. The problem of genotype and sex differences in life expectancy in transgenic AD mice. Neurosci. Biobehav. Rev. 2015;57:238–251. doi: 10.1016/j.neubiorev.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Frye C, Walf A. Effects of progesterone administration and APPswe + PSEN1Deltae9 mutation for cognitive performance of mid-aged mice. Neurobiol. Learn. Mem. 2008;89:17–26. doi: 10.1016/j.nlm.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 48.Bollen E, et al. 7,8-Dihydroxyflavone improves memory consolidation processes in rats and mice. Behav. Brain Res. 2013;257:8–12. doi: 10.1016/j.bbr.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 49.Bergin DH, Liu P. Agmatine protects against beta-amyloid25-35-induced memory impairments in the rat. Neuroscience. 2010;169:794–811. doi: 10.1016/j.neuroscience.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 50.Wang W, Li S, Dong HP, Lv S, Tang YY. Differential impairment of spatial and nonspatial cognition in a mouse model of brain aging. Life Sci. 2009;85:127–135. doi: 10.1016/j.lfs.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 51.Wimmer ME, Hernandez PJ, Blackwell J, Abel T. Aging impairs hippocampus-dependent long-term memory for object location in mice. Neurobiol. Aging. 2012;33:2220–2224. doi: 10.1016/j.neurobiolaging.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cloke JM, et al. A Novel Multisensory Integration Task Reveals Robust Deficits in Rodent Models of Schizophrenia: Converging Evidence for Remediation via Nicotinic Receptor Stimulation of Inhibitory Transmission in the Prefrontal Cortex. J. Neurosci. 2016;36:12570–12585. doi: 10.1523/JNEUROSCI.1628-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winters BD, Reid JM. A Distributed Cortical Representation Underlies Crossmodal Object Recognition in Rats. J. Neurosci. 2010;30:6253–6261. doi: 10.1523/JNEUROSCI.6073-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de LaCoste M-C, White Iii CL. The role of cortical connectivity in Alzheimer’s disease pathogenesis: A review and model system. Neurobiol. Aging. 1993;14:1–16. doi: 10.1016/0197-4580(93)90015-4. [DOI] [PubMed] [Google Scholar]
- 55.Delbeuck X, Van der Linden M, Collette F. Alzheimer’s disease as a disconnection syndrome. Neuropsychol. Rev. 2010;20:191–208. doi: 10.1007/s11065-010-9128-8. [DOI] [PubMed] [Google Scholar]
- 56.Delatour B, Blanchard V, Pradier L, Duyckaerts C. Alzheimer pathology disorganizes cortico-cortical circuitry: Direct evidence from a transgenic animal model. Neurobiol. Dis. 2004;16:41–47. doi: 10.1016/j.nbd.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 57.Sterniczuk R, Antle MC, Laferla FM, Dyck RH. Characterization of the 3xTg-AD mouse model of Alzheimer’s disease: Part 2. Behavioral and cognitive changes. Brain Res. 2010;1348:149–155. doi: 10.1016/j.brainres.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 58.Espana J, et al. Intraneuronal B-Amyloid Accumulation in the Amygdala Enhances Fear and Anxiety in Alzheimer’s Disease Transgenic Mice. Biol. Psychiatry. 2010;67:513–521. doi: 10.1016/j.biopsych.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 59.García-Mesa Y, et al. Melatonin plus physical exercise are highly neuroprotective in the 3xTg-AD mouse. Neurobiol. Aging. 2012;33:1124.e13–1124.e29. doi: 10.1016/j.neurobiolaging.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 60.Jawhar S, Trawicka A, Jenneckens C, Bayer Ta, Wirths O. Motor deficits, neuron loss, and reduced anxiety coinciding with axonal degeneration and intraneuronal A?? aggregation in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol. Aging. 2012;33:196.e29–196.e40. doi: 10.1016/j.neurobiolaging.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 61.Schneider F, Baldauf K, Wetzel W, Reymann KG. Behavioral and EEG changes in male 5xFAD mice. Physiol. Behav. 2014;135:25–33. doi: 10.1016/j.physbeh.2014.05.041. [DOI] [PubMed] [Google Scholar]
- 62.Oakley H, et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J. Neurosci. 2006;26:10129–40. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roberson E, et al. Reducing Endrogenous Tau Ameliorates Amyloid B-Induced Deficits in an Alzheimer’s Disease Mouse Model. Science (80−.). 2007;316:750–755. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 64.Girard D, et al. Onset of Hippocampus-Dependent Memory Impairments in 5XFAD Transgenic Mouse Model of Alzheimer’ s Disease St. Hippocampus. 2014;24:762–772. doi: 10.1002/hipo.22267. [DOI] [PubMed] [Google Scholar]
- 65.Kanno T, Tsuchiya A, Nishizaki T. Hyperphosphorylation of Tau at Ser396 occurs in the much earlier stage than appearance of learning and memory disorders in 5XFAD mice. Behav. Brain Res. 2014;274C:302–306. doi: 10.1016/j.bbr.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 66.Shukla V, et al. A truncated peptide fromp35, a Cdk5 activator, prevents Alzheimer’s disease phenotypes in model mice. FASEB J. 2013;27:174–186. doi: 10.1096/fj.12-217497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Griñán-Ferré C, et al. Epigenetic mechanisms underlying cognitive impairment and Alzheimer disease hallmarks in 5XFAD mice. Aging (Albany NY) 2016;8:664–684. doi: 10.18632/aging.100906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oddo S, et al. Triple-Transgenic Model of Alzheimer’s Disease with Plaques and Tangles: Intracellular AB and Synaptic Dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/S0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 69.Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol. Aging. 2003;24:1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 70.Mastrangelo Ma, Bowers WJ. Detailed immunohistochemical characterization of temporal and spatial progression of Alzheimer’s disease-related pathologies in male triple-transgenic mice. BMC Neurosci. 2008;9:81. doi: 10.1186/1471-2202-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosario ER, Carroll J, Pike CJ. Testosterone regulation of Alzheimer-like neuropathology in male 3xTg-AD mice involves both estrogen and androgen pathways. Brain Res. 2010;1359:281–290. doi: 10.1016/j.brainres.2010.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ohno M, et al. BACE1 gene deletion prevents neuron loss and memory deficits in 5XFAD APP/PS1 transgenic mice. Neurobiol. Dis. 2007;26:134–45. doi: 10.1016/j.nbd.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Caruso D, et al. Age-related changes in neuroactive steroid levels in 3xTg-AD mice. Neurobiol. Aging. 2013;34:1080–1089. doi: 10.1016/j.neurobiolaging.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shao CY, et al. Postsynaptic degeneration as revealed by PSD-95 reduction occurs after advanced AB and tau pathology in transgenic mouse models of Alzheimer’s disease. Acta Neuropathol. 2011;122:285–292. doi: 10.1007/s00401-011-0843-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perez SE, et al. Cholinotrophic basal forebrain system alterations in 3xTg-AD transgenic mice. Neurobiol. Dis. 2011;41:338–352. doi: 10.1016/j.nbd.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simpkins JW, et al. Role of Estrogen Replacement Therapy in Memory Enhancement and the Prevention of Neuronal Loss Associated With Alzheimer’ s Disease. Am. J. Med. 1997;103:19–25. doi: 10.1016/S0002-9343(97)00260-X. [DOI] [PubMed] [Google Scholar]
- 77.Devi, L. & Ohno, M. Phospho-eIF2α level is important for determining abilities of BACE1 reduction to rescue cholinergic neurodegeneration and memory defects in 5XFAD mice. PLoS One5 (2010). [DOI] [PMC free article] [PubMed]
- 78.Phan A, Lancaster KE, Armstrong JN, MacLusky NJ, Choleris E. Rapid effects of estrogen receptor a and b selective agonists on learning and dendritic spines in female mice. Endocrinology. 2011;152:1492–1502. doi: 10.1210/en.2010-1273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.