Abstract
Due to the surge of natural gas production, feedstocks for chemicals shift towards lighter hydrocarbons, particularly methane. The success of a Gas-to-Chemicals process via synthesis gas (CO and H2) depends on the ability of catalysts to suppress methane and carbon dioxide formation. We designed a Co/Mn/Na/S catalyst, which gives rise to negligible Water-Gas-Shift activity and a hydrocarbon product spectrum deviating from the Anderson–Schulz–Flory distribution. At 240 °C and 1 bar, it shows a C2-C4 olefins selectivity of 54%. At 10 bar, it displays 30% and 59% selectivities towards lower olefins and fuels, respectively. The spent catalyst consists of 10 nm Co nanoparticles with hcp Co metal phase. We propose a synergistic effect of Na plus S, which act as electronic promoters on the Co surface, thus improving selectivities towards lower olefins and fuels while largely reducing methane and carbon dioxide formation.
The conversion of natural gas to chemicals and fuels via the Fischer-Tropsch synthesis process, depends on the ability of catalyst materials to suppress the formation of methane and carbon dioxide. Here, the authors develop a Co1Mn3-Na2S catalyst, which has a hydrocarbon product spectrum deviating from the Anderson–Schulz–Flory distribution.
Introduction
The abundant availability of methane feedstock due to the shale gas revolution decreases the dependence on crude oil, however new technologies have to be developed to utilize its potential1,2. Methane may be converted to synthesis gas (syngas, a mixture of H2 and CO), which can then be used to produce chemicals and fuels via the Fischer-Tropsch synthesis (FTS) process3. FTS is a surface polymerization reaction so the product selectivity is governed by the Anderson–Schulz–Flory (ASF) distribution4. Deviation of the ASF distribution to suppress methane formation is critical to attain high fractions of lower olefins (ethylene, propylene, and butylenes), and this is possible with promoted Fe-carbide-based5–7 and promoted Co-carbide-based catalysts8,9. However, most carbide-based catalysts are also active for the water-gas-shift (WGS) reaction10, thereby producing CO2 and rendering them inefficient for methane-derived H2-rich syngas. Similarly, the bifunctional oxide-zeolite catalysts, which convert syngas directly to lower olefins, showed high activity for WGS and are thus only suitable for CO-rich syngas11,12. The importance of decreasing CO2 production during the FT step was recently highlighted by Wang et al. in their development of phase pure, stable and low-CO2 selective ε-iron carbide FT catalysts for the coal-to-liquids process13.
To be active for FTS but not for WGS, Co has to be in the metallic state during catalysis. Metallic Co-based catalysts are used commercially for the gas-to-liquids process in which long-chain saturated hydrocarbon products are produced that are subsequently cracked to valuable transportation fuels in particular kerosene and diesel14–17. The direct production of lower olefins from H2-rich syngas is advocated, but this poses two challenges, specifically the suppression of methane and of CO2 formation during FTS.
Till now, Co-based catalysts for the direct conversion of syngas to lower olefins focused on MnO as promoter, but the product spectrum was still dictated by the ASF distribution18–22. Adding alkali promoters to Co/MnO catalysts stimulates formation of Co-carbide, which inhibits methane, but promotes CO2 production23. Besides acting as structural promoters, alkali metals were established to decrease activity for metallic Co-based catalysts and it was proposed to be correlated to the element electronegativity24,25. These alkali metals including Na or K, exist as oxides Na2O or K2O during catalysis, yet the oxygen counter-ion was often overlooked. The importance of counter-ions to alkali metal promoters was demonstrated previously for Fe-based catalysts26, particularly the combination of Na and S was found to give a synergistic effect27–29. S is generally perceived to be a poison for Co-based catalysts in terms of activity and selectivity towards long-chain hydrocarbons (C5+)30, however it was also shown to decrease chain growth probability and improve olefins selectivity depending on its concentration31–33. Nonetheless, the influence of alkali metal and its counter-ion has not been considered for Co-based catalysts.
In this work, we demonstrate that the presence of Na plus S inhibits WGS and suppresses methane formation for metallic Co-based catalysts. We present an efficient metallic Co-based catalyst consisting of Co/Mn/Na/S, which has a product spectrum deviating from ASF distribution yet is inactive for WGS. The catalytic performance of this catalyst is evaluated over a range of reaction temperatures, 240–280 °C, and reaction pressures, 1–10 bar. H2/CO feed ratio is kept constant at 2, a stoichiometric ratio relevant for methane feedstock. At industrially relevant conditions of 240 °C and 10 bar, Co1Mn3–Na2S shows superior product selectivities towards lower olefins and fuels in comparison to other Co-based catalysts. Detailed characterization of the spent catalysts using X-ray diffraction (XRD) and transmission electron microscopy (TEM) reveal 10 nm Co nanoparticles with hcp Co metal phase. Preliminary DFT calculations indicate the importance of the counter-ion for sodium and the consequences to catalysis. The approach of dispersing metallic Co nanoparticles on the MnO support, and utilizing alkali metal Na and its counter-ion S as electronic promoters is effective in reducing CO2 and methane formation, hence creating new opportunities in gas-to-chemicals processes.
Results
Catalysts
Co1Mn3 catalysts with an atomic ratio Co/Mn = 1/3 were synthesized via co-precipitation, and the calcined catalysts were impregnated with Na2CO3, (NH4)2SO4, Na2S2O3 or Na2S precursors followed by another calcination step. These catalysts were named Co1Mn3–Na2O, Co1Mn3,−SO42−, Co1Mn3–Na2S2O3 and Co1Mn3–Na2S, respectively. As a comparison, Co3Mn1 catalysts were also synthesized and named in a similar fashion. An overview of calcined catalysts and their elemental loadings of Mn, Co, Na and S are included in Supplementary Table 1. The XRD pattern of calcined Co1Mn3–Na2S (Supplementary Figure 1) consisted of Mn2O3, MnO2 and CoMnO3 phases, and the addition of promoters did not result in change of crystalline phases. An SEM image (Supplementary Figure 2) of calcined Co1Mn3–Na2S, showed its morphology and the homogeneity of Co and Mn elemental loadings was confirmed by scanning electron microscopy-energy-dispersive X-ray spectroscopy (SEM-EDX, Supplementary Table 2). Scanning transmission electron microscopy-energy-dispersive X-ray spectroscopy (STEM-EDX) mapping (Supplementary Figure 3) also showed mixing of Co and Mn, and no isolated Co nanoparticles were observed.
Catalytic performance
Catalytic performance was evaluated at a range of reaction conditions (240–280 °C, 1–10 bar, H2/CO = 2). At mild conditions of 240 °C, 1 bar, H2/CO = 2, 1% CO conversion, Co1Mn3–Na2S displayed a high C2–C4 olefins selectivity of 54% with a C2–C4 olefin/paraffin ratio of 17. Moreover, methane selectivity at 17% was lower than what was predicted by the ASF distribution (Supplementary Figure 4 and Supplementary Table 3). While the addition of Na2S improved selectivity, it also decreased activity which is in agreement with literature that S is detrimental to activity of metallic Co catalysts30,32. In a control experiment, addition of sulfur only (without Na) was shown to decrease activity, while increasing methane selectivity (Supplementary Figure 4 and Supplementary Table 3).
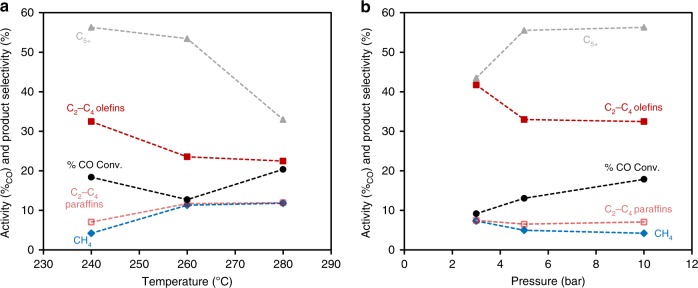
The effects of reaction pressures and temperatures on the catalytic performance of Co1Mn3–Na2S are shown in Fig. 1 and detailed information can be found in Supplementary Tables 4–7. From Fig. 1a, at 10 bar, H2/CO = 2, 13–30% CO conversion, an increase in temperature from 240 to 280 °C corresponded to decrease in C2–C4 olefins and C5+ selectivities, but increase in methane and C2–C4 paraffins selectivities. For Co1Mn3–Na2S 21% CO2 selectivity was attained at 280 °C and 10 bar. From Fig. 1b, at 240 °C, H2/CO = 2, 10–18% CO conversion, an increase in pressure from 3 to 10 bar corresponded to an increase in C5+ selectivity together with a decrease of selectivity towards C1–C4 hydrocarbon products. For Co1Mn3–Na2S, no CO2 production was detected at 3–10 bar, 240 °C, H2/CO = 2. The increase in chain growth probability, α, due to increase in pressure was confirmed by the ASF distribution plot in Supplementary Figure 5. Notably, the methane fraction was always lower than expected from the ASF distribution for Co1Mn3–Na2S. Since a high olefin/paraffin ratio was attained and no C1 olefin exists, a lowered C1 fraction is to be expected. Nonetheless, only with Na2S promotion this is actually achieved, while in literature catalysts always produce more methane than expected.
Fig. 1.
Catalytic performance of Co1Mn3–Na2S at different reaction temperatures or pressures. a Activity and selectivity at 240–280 °C, 10 bar, and H2/CO = 2, and b activity and selectivity at 240 °C, 3–10 bar, and H2/CO = 2. Activity is shown here as % CO conversion and product selectivity is shown in terms of methane, CH4 (blue solid diamonds), C2–C4 olefins (red solid squares), C2–C4 paraffins (light red open squares), and C5+ (grey solid triangles) which include all other products except CO2 and C1–C4 hydrocarbons
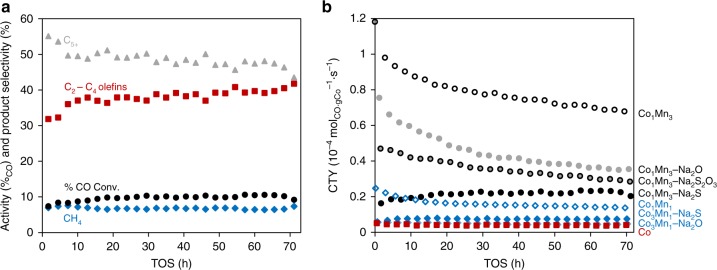
Catalytic stability is an important consideration hence the catalytic performance of Co1Mn3–Na2S over 70 h is shown in Fig. 2a. The activity of Co1Mn3–Na2S showed an initial increase and remained then constant over 70 h. Methane selectivity remained stable over time, while C5+ (all products except CO2 and C1–C4 hydrocarbons) and C2–C4 olefin selectivities also stabilized after 10 h. The activity and stability of Co1Mn3–Na2S were also compared with other Co-based catalysts in Fig. 2b. As shown in Fig. 2b, the addition of Mn increased activity for Co-based catalysts, which is in agreement with literature34,35. Catalysts with Co/Mn ≈0.3 showed highest activity per gram Co (cobalt-time-yield, CTY), and the addition of Na2O, Na2S2O3 or Na2S decreased activity. Nonetheless, the activity of Co1Mn3–Na2S was still higher than the remaining Co-based catalysts. In terms of stability, Co1Mn3, Co1Mn3–Na2O, Co1Mn3–Na2S2O3 and Co3Mn1 showed deactivation but all other catalysts remained stable over 70 h.
Fig. 2.
Catalytic performance over 70 h time-on-stream. Reaction conditions: 240 °C, 3 bar, and H2/CO = 2. a Activity in terms of %CO conversion (black solid circles) and selectivity towards methane, CH4 (blue solids diamonds), C2–C4 olefins (red solid squares), and C5+ (grey solid triangles) of Co1Mn3–Na2S over time, and b activity in terms of cobalt-time-yield, CTY, of various Co-based catalysts, namely Co1Mn3 (black open circles), Co1Mn3–Na2O (grey solid circles), Co1Mn3–Na2S2O3 (grey solid with black outline circles), Co1Mn3–Na2S (black solids circles), Co3Mn1 (blue open diamonds), Co3Mn1–Na2S (blue solid diamonds), Co3Mn1–Na2O (light blue solid diamonds), and bulk Co (red squares) over time
At more industrially relevant conditions of 240 °C, 10 bar, H2/CO = 2, 18–30% CO conversion, the catalytic performance of Co1Mn3–Na2S was compared to other Co-based catalysts (Table 1). Co1Mn3–Na2S displayed the highest selectivity towards lower olefins and lowest selectivities towards undesired methane and lower paraffins (C2–C4 olefin/paraffin ratio = 4.2). Remarkably, CO2 selectivity was below 3% (below detection limit, see Supplementary Figure 6 for chromatograms), suggesting the absence of WGS activity and making it an attractive catalyst for H2-rich syngas. CO2 selectivity was consistently below detection limit for all catalysts except where less Mn is present, i.e. Co3Mn1–Na2O and Co3Mn1–Na2S. Even so, the suppression of WGS activity by Na2S instead of Na2O addition was evident by the CO2 selectivity of Co3Mn1–Na2S compared to Co3Mn1–Na2O, i.e. 13 versus 28%, respectively. The precursor of Na/S and loading of Na were varied (Na2S and Na2S2O3) and the favourable effects on selectivity remain (Supplementary Table 1 and Table 1). Further optimization of precursor and loadings of the promoters is however outside the scope of this study.
Table 1.
Catalytic performance at 240 °C, 10 bar, H2/CO = 2, 18–30% CO conversion
| CO conv., X (%) | CTY (10−4 molCO. gCo−1 s−1) | C1, S (%) | C2–C4 olefins, S (%) | C2–C4 paraffins, S (%) | C5+, S (%) | CO2, S (%) | O/P C2–C4 | α | |
|---|---|---|---|---|---|---|---|---|---|
| Co | 32 | 0.13 | 12 | 6 | 7 | 75 | <2 | 0.8 | 0.69 |
| Co3Mn1 | 31 | 0.14 | 11 | 17 | 10 | 62 | <2 | 1.8 | 0.63 |
| Co3Mn1–Na2O | 20 | 0.09 | 9 | 14 | 7 | 42 | 28 | 1.9 | 0.53 |
| Co3Mn1–Na2S | 25 | 0.12 | 5 | 20 | 7 | 56 | 13 | 2.9 | 0.56 |
| Co1Mn3 | 31 | 0.56 | 15 | 12 | 13 | 61 | <2 | 0.9 | 0.67 |
| Co1Mn3–Na2O | 27 | 0.46 | 14 | 11 | 12 | 63 | <2 | 0.9 | 0.65 |
| Co1Mn3–Na2S | 18 | 0.40 | 4 | 30 | 7 | 59 | <3 | 4.2 | 0.53 |
| Co1Mn3−Na2S2O3 | 22 | 0.42 | 7 | 25 | 12 | 56 | <3 | 2.1 | 0.50 |
CO conversion (X, %), activity per gram of Co (CTY), product selectivity (S, %), The detection limit of CO2 selectivity is 0.5% yield, equivalent to 3% CO2 selectivity at 18% CO conversion.
Bulk Co catalyst had the highest α, therefore its main product was C5+ hydrocarbons. The addition of Mn–Co resulted in a lower α, and the addition of Na2O or Na2S further decreased α. Bulk Co catalyst showed typical ASF distribution deviation, whereby the C1 fraction is higher and the C2 fraction is lower than predicted. The addition of Na2O suppressed the C1 fraction, but this suppression was most prominent with the addition of Na2S (Supplementary Figure 5).
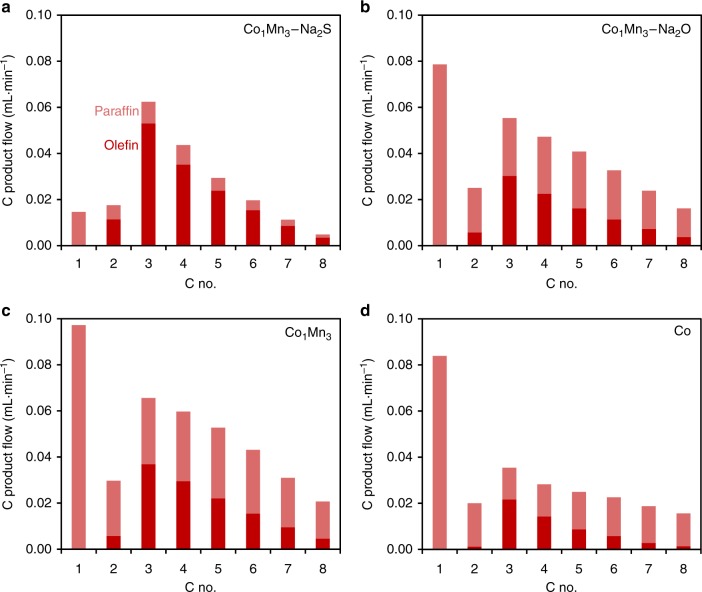
To obtain further mechanistic insights into the various catalytic systems, the detailed C product flow of 1-olefin and n-paraffin for each C number product is shown in Fig. 3. The mechanistic considerations of metallic Co FT catalysts include chain growth, chain branching, primary olefin/paraffin formation and olefin secondary reactions, such as secondary hydrogenation and isomerization36. From Fig. 3, Co1Mn3–Na2S produced significantly more primary olefins than linear paraffins for each C containing hydrocarbon product. This suggests that β-H elimination was the dominant termination pathway for Co1Mn3–Na2S and secondary hydrogenation of olefins was also suppressed. Besides, the lower fraction of 2-butene in the C4 hydrocarbon product spectrum of Co1Mn3–Na2S implied the suppression of secondary isomerization of olefins (Supplementary Table 9 and Supplementary Figure 7). This is in agreement with the presumption that secondary hydrogenation and isomerization of olefins take place at identical sites36. In addition, the low methane and C2 hydrocarbon products from Co1Mn3–Na2S point to the blocking of sites for surface methyl, methylene and H species37.
Fig. 3.
Distribution of 1-olefins and n-paraffins of C1–C8 hydrocarbon products. Reaction conditions: 240 °C, 10 bar, H2/CO = 2, 18–30% CO conversion. a Co1Mn3–Na2S, b Co1Mn3–Na2O, c Co1Mn3 and d Co. Red bar corresponds to olefin product flow and light red bar corresponds to paraffin product flow
Structure analysis of spent catalysts
In order to understand the catalytic performance, the spent catalysts after being exposed to industrially relevant FTS conditions were characterized. Fig. 4a compares the XRD patterns of spent Co1Mn3, Co1Mn3–Na2S and Co3Mn1–Na2O and their crystalline phase compositions are summarized in Fig. 4b. Additional Rietveld QPA results for the spent catalysts are given in Supplementary Table 10. The diffraction patterns of crystallized wax were observed in Fig. 4a, and the wax present on the spent catalysts served to prevent oxidation of the spent catalysts. Co1Mn3 and Co1Mn3–Na2S consisted predominantly of a Mn0.95O phase, and a mixed MnxCoyO4 phase was observed which both contributed most likely not to any form of FT activity. Crucially, the hexagonal (hcp) metallic Co phase was present in both spent Co1Mn3 and Co1Mn3–Na2S. The average crystallite size for the hcp Co phase was 9.2 nm with a standard deviation of 1.9 nm. Small contributions from a MnCO3 phase were also noted in both spent Co1Mn3 and Co3Mn1–Na2O. In addition to the Mn0.95O, MnxCoyO4, Co (hcp), MnCO3 phases, a Co2C phase was present in spent Co3Mn1–Na2O in line with the work of Sun et al8.
Fig. 4.
XRD analysis of spent Co1Mn3, Co1Mn3–Na2S, Co3Mn1–Na2O. Reaction conditions: 240–280 °C, 10 bar, and H2/CO = 2. a Background corrected XRD patterns and b rietveld QPA-based crystalline phase compositions, which shows the MnxCoyO4 phase (blue), Mn0.95O (cyan), MnCO3 (green), hcp Co (violet) and Co2C (brown)
Fig. 5 shows the electron microscopy images and particle size distribution of spent Co1Mn3–Na2S after industrially relevant conditions (240–280 °C, 10 bar, and H2/CO = 2), and STEM-EDX mappings were carried out to differentiate Co and Mn. From Fig. 5a, wax/amorphous carbon (indicated with arrows) was observed, which is in agreement with the XRD analysis in Fig. 4a. The Co particle size distribution from TEM revealed the average Co particle size to be 9.6 nm with a standard deviation of 4.4 nm, in agreement with the Co crystallite size of 9.2 nm with a standard deviation of 1.9 nm from XRD analysis. The elemental maps of Co and Mn in Fig. 5f confirmed that spent Co1Mn3–Na2S consisted of Co nanoparticles well dispersed on the MnO support.
Fig. 5.
Electron microscopy images of spent Co1Mn3–Na2S. Reaction conditions: 240–280 °C, 10 bar, and H2/CO = 2. a bright-field TEM image with a scale bar corresponding to 100 nm, and blue arrows to point out the presence of wax, b dark-field TEM images with a scale bar corresponding to 50 nm, c particle size distribution of Co nanoparticles supported on MnO, and d–f STEM-EDX maps of Co and Mn, and the scale bars correspond to 200 nm
Theoretical calculations on Na2S vs. Na2O
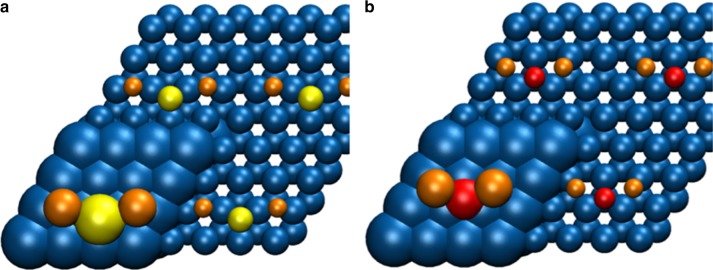
To gain further understanding of the difference in Na2S and Na2O, DFT calculations of both species on metallic Co (0001) surface were performed. Please note that these calculations are of a preliminary nature and further work is needed to arrive at full reaction pathway analysis which is outside the scope of this work. Pederson et al. recently performed DFT calculations on CoMnO systems for the production of light olefins and they found that selectivities can be attributed to an inhibited hydrogenation activity demonstrated by the increased barriers for CH3 and CH4 formation22. Strømsheim et al. recently showed that the restructuring of Co surface under CO exposure with K pre-adsorbed proceeded on the terraces rather than from the step edges38. Other notable theoretical studies on multiple elemental surfaces include ZnO/Cu, Co2C/Co, Cu/Co39–41. While these studies are highly relevant, they are insufficient to explain current findings. As it is shown that the combination of Na2S is critical for product selectivity, the theoretical calculations were focused on Na2S and Na2O promotion. The function of the sodium promoter, as any alkali metal promoter, is to donate charge to the cobalt metal. For the manganese-containing catalysts studied here, this turns out to increase olefin formation. However, for good effect another counter-ion is needed, i.e., sulfur. As shown by DFT calculations (Fig. 6), the function of the sulfur promoter is to increase the charge donation from the Na promoter ions to the cobalt surface. When no specific counter-ions are added, sodium binds in the form of Na2O and a considerable part of the sodium charge donation is taken up by the oxygen atom. With sulfur it is suggested to form Na2S instead, resulting in a higher charge donation to the cobalt surface. The DFT calculations show that every Na2O moiety donates a total charge of −0.51 to the cobalt surface (Na becomes +0.39 and O becomes −0.28), whereas Na2S donates a total charge of −0.62 (Na becomes +0.36 and S becomes −0.10). We tentatively interpret these results of higher charge donation to coincide with lower hydrogen coverages thus leading to lower methane selectivity in FTS similar to what we have reported for iron carbide29.
Fig. 6.
DFT-calculated binding geometries of Na2S and Na2O on the Co (0001) surface. a Na2O and b Na2S bind in a very similar fashion, although the O atom ends up above a subsurface cobalt atom and the S atom above an empty site. Atoms outside the calculation unit cell are depicted as smaller spheres; blue is Co, orange is Na, yellow is S and red is O
Structure-performance relations of metallic Co vs. Co2C
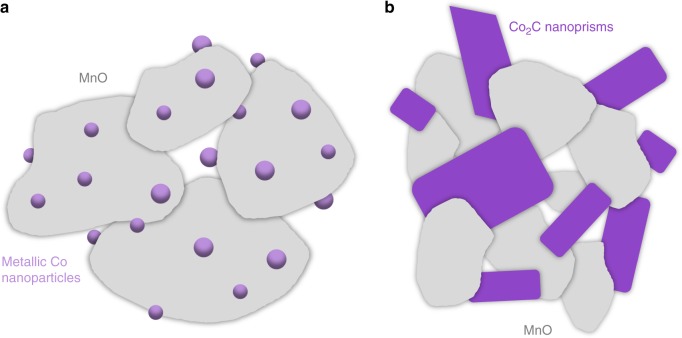
In Table 1, CO2 selectivity was negligible for most catalysts except Co3Mn1–Na2O and Co3Mn1–Na2S. From detailed XRD structural analysis of the spent catalysts, it was revealed that the active Co phase in Co1Mn3 and Co1Mn3–Na2S was metallic Co, but Co2C was present as an active phase in Co3Mn1–Na2O. For classic Co-based FT catalysts (i.e. bulk Co and CoMn) with appropriate reduction/ activation procedure and reaction conditions, metallic Co is widely accepted to be the active phase16,42. As metallic Co catalysts are not active for WGS, it was expected that no CO2 selectivity was observed for these catalysts. Upon the addition of Na2O or Na2S, the ratio of Co/Mn apparently played a critical role in influencing the crystal structure of the Co phase during FT as CO2 selectivities were significantly higher for Co3Mn1 than Co1Mn3. Li et al. recently concluded that Mn has a controlling effect on Co2C morphology and the formation of Co2C nanoprisms or nanospheres was dependent on the synthesis method43. In this study, the results of Co3Mn1–Na2O and Co3Mn1–Na2S were in agreement with Li et al. as Co content was higher than Mn in both cases. However, for catalysts with more Mn than Co, Co2C was not formed and Co remained in metallic phase. It is believed that when Co/Mn ≈ 0.3, MnOx served as a support for the metallic Co nanoparticles thereby ensuring a good dispersion and stabilization of these nanoparticles (Fig. 5). MnO is also known to act as an electronic and structural promoter and the promoting effects of MnO are strongly dependent on its location and amount. For instance, Morales et al. showed that CO preferentially bonded linearly to surface metal sites when MnO loading was increased44. It is noted, however, that in mentioned literature, the MnOx loading was much lower than the Co loading and the promoting effects of using MnO as a support are not yet clear. These findings are illustrated in Fig. 7.
Fig. 7.
Schematic drawing of the structure of the active catalysts based on TEM and XRD analysis. a Co1Mn3–Na2S consists of metallic Co nanoparticles of ~10 nm dispersed on MnO support and b Co3Mn1–Na2O consists of Co2C nanoprisms of ~10–50 nm from Zhong et al8. Whereas the former catalyst restricts WGS and thus CO2 formation, the latter leads to large amounts of CO2
While similar hydrocarbon product selectivities were reported earlier by Sun et al. for Na-promoted Co2Mn1 catalytic systems8,23, it is noteworthy to point out that in their work CO2 selectivity was almost 50% of CO converted due to high WGS activity. The addition of Na (most likely in Na2O state) served as a structural promoter and appeared to facilitate the formation of Co2C nanoprisms, which displayed high C2–C4 olefins and CO2 selectivities. In our Co1Mn3–Na2S catalytic system, the active phase appeared to be metallic Co and Na2S seemed to be an electronic promoter for product selectivity. Sun et al. showed the effect of Na2O loading on Co2Mn1 catalytic systems, and here we presented the importance of the counter-ion for Na (Fig. 6) using theoretical DFT calculations.
Besides the loadings and counter-ions for Na, the activation procedure is an important parameter for catalytic performance and structure-performance relations. For instance, de Smit et al. demonstrated that different Fe-carbide phases may be synthesized during catalyst pretreatment by controlling carbon chemical potential45. Claeys et al. concluded that while cobalt carbide is relatively stable at typical reaction conditions, it would decompose rapidly into hcp Co with hydrogen at 150 °C46. Davis et al. also showed that reaction conditions played a significant role in formation of cobalt carbide or metallic cobalt47. To induce the formation of metallic Co, calcined catalysts in our study were reduced at 350 °C and 1 bar under diluted H2 flow for 8 h, followed by introduction of syngas at a temperature of 180 °C and a pressure of 10 bar. This activation procedure to form metallic Co is different from that of Sun et al. to form Co2C8. To show the effect of activation procedure on catalytic performance, the same catalysts were reduced at 300 °C and 1 bar under diluted H2 flow for 5 h, followed by introduction of syngas at 250 °C and 10 bar. With this activation procedure, CO2 selectivity increased to 6% (Supplementary Table 13) possibly related to cobalt carbide formation. Nonetheless, Na2S was still the most effective promoter (Supplementary Tables 11–13).
In summary, we have designed a catalytic system Co1Mn3–Na2S, which showed negligible WGS activity and suppression of methane formation in FTS. Structure analysis of the spent catalyst revealed 10 nm metallic Co nanoparticles as the active phase supported on MnO during reaction. Theoretical calculations revealed the importance of counter-ion S for Na, and Na2S was more efficient in tuning hydrocarbon product selectivity than Na2O. Tentatively the addition of Na2S to Co1Mn3 was proposed to deactivate sites for secondary olefin hydrogenation and isomerization and for methanation, whereas the lower degree of alkalization as compared with Na2O is insufficient to promote the WGS reaction. For this complex catalytic system, further studies on the effects of various elements on structure-performance relations and advanced characterization are advocated.
The state-of-the-art processes and catalysts for direct production of lower olefins from synthesis gas are compared in Supplementary Table 14. While all catalysts showed favourable selectivity towards lower olefins, Co1Mn3–Na2S is the only catalyst, which combined lower olefin selectivity with negligible CO2 production. This comparison suggests that Co1Mn3–Na2S is a promising catalyst which is capable of producing chemicals and fuels directly from H2-rich syngas derived from natural gas. This gas-to-chemicals process would greatly reduce CO2 emissions, thereby contributing prevention of climate change.
Methods
Synthesis of CoMn catalysts
Two grams of Co(NO3)2•6H2O (99 +%, Acros) and 5.7 g Mn(NO3)2•4H2O (97.5 +%, Acros), were dissolved in 40 mL deionized water at room temperature in a 100 mL round-bottom flask. After 1 h of stirring at room temperature, the round-bottom flask was heated to 60 °C in a water bath. Twenty microliters of 1.0 M aqueous (NH4)2CO3 (30 +% (NH3), Acros) was added dropwise to the mixed nitrate solution using a mechanical pump set at 1 mL/min and pH was kept at ~8. The resulting pink powder was aged for 30 min at room temperature, followed by decanting and washing with deionized water thrice. The precipitate was then dried at 120 °C under static air for 2 h with stirring every 0.5 h and calcined at 400 °C (2 °C/min) under air flow for 2 h. The synthesized Co1Mn3 was then impregnated with either Na2CO3 anhydrous (99.5%, Fisher Scientific), (NH4)2SO4 (≥99.0%, Sigma-Aldrich), Na2S2O3 anhydrous (≥98.0%, Sigma-Aldrich) or Na2S nonahydrate (≥98.0%, Sigma-Aldrich) precursor's dissolved in deionized water, followed by calcination at 400 °C (2 °C/min) under air flow for 2 h. The Co3Mn1 catalysts were synthesized with the identical procedure but different Co and Mn precursors mass loadings.
Catalyst characterization
Elemental loading of Co, Mn, Na and S were determined with a Thermo Jarrell Ash model ICAP 61E trace analyzer inductively coupled plasma-atomic emission spectrometer (ICP-AES). Scanning electron microscopy (SEM) images were taken using a FEI XL30 FEG SEM instrument in backscattering electron mode at an acceleration voltage of 15 kV. SEM samples were prepared on carbon grids followed by Pt-coating to improve electron conductivity. STEM-HAADF images and EDX analysis were obtained with an FEI Talos F200X transmission electron microscope, operated at 200 kV and equipped with a high-brightness field emission gun (X-FEG) and a Super-X G2 EDX detector. More than 150 particles were measured to obtain a particle size distribution. XRD patterns were measured with a Bruker D8 Discover instrument in Debye-Scherrer transmission (capillary) geometry with a Mo (Kα1 0.709 Å) source. A Göbel-mirror was used to focus a near-parallel X-ray beam on the 1000 µm (OD, wall thickness 10 µm) capillary. Energy dispersive LynxEye XE Position Sensitive Detector (PSD) was used, only accepting diffracted X-ray photons originating from Mo Kɑ emission lines. Details on the instrument can be found in a recent publication48. Measurement parameters used were 2θ 5–48° with step size of 0.032° and exposure time of 18 s per step, for each measurement. Rietveld Quantitative Phase Analysis (Rietveld QPA) was performed on the measured diffractograms using Bruker TOPAS (v5) software. Details and discussion on the Rietveld refinement procedure are given in Supplementary Methods. Phase identification from diffractograms was done using ICDD PDF-4+2016 database and structures used in the Rietveld QPA were obtained from the same database and are listed in Supplementary Table 10.
Catalytic tests at mild conditions
Low pressure tests were carried out at 240 °C, 1 bar, H2/CO = 2 v/v, <3% CO conversion. A fixed-bed reactor was loaded with 0.02 g (75–150 μm) catalyst and 0.20 g SiC (212–425 μm) for bed dilution. The catalysts were reduced prior to reaction at 350 °C (5 °C /min) under diluted H2 flow (33 vol.% H2, 67 vol.% He, 60 mL/min total flow) for 2 h. After reduction, temperature was decreased to 240 °C (2 °C /min) under 40 mL/min He flow. At 240 °C and 1 bar, the feed flow was switched to a mixture of H2 and CO (H2/CO = 2 v/v, 9 mL/min total flow). Hydrocarbons (C1–C16) from the product stream were analysed online with gas chromatography (Varian CP3800), and CO2 was not measured. The line from the reactor to GC was heated to at least 150 °C to prevent hydrocarbon condensation. Activities and product selectivities were calculated on a carbon atom basis. Activity is reported as moles of CO converted per gram Co per second, and moles of CO converted is based on moles of C in the hydrocarbon product stream. Product selectivity was calculated as equivalent of carbon atoms in a product with respect to the total carbon atoms present in the hydrocarbons produced (% C).
Catalytic tests at industrially relevant conditions
Medium pressure tests were performed using a high throughput 16 parallel fixed-bed reactors set-up (Flowrence, Avantium). Each reactor was loaded with 50 mg catalyst (75–150 μm) and 100 μL SiC (212–425 μm) as diluent. The catalysts were first dried at 100 °C (5 °C /min) under He flow for 2 h and subsequently reduced at 350 °C (1 °C/min) under dilute H2 flow (25 vol.% H2, 75 vol.% He) for 8 h. After reduction, temperature was decreased to 180 °C (1 °C /min) and pressure was increased to 10 bar under H2 flow. At 180 °C and 10 bar, the feed flow was switched to syngas mixture (H2/CO/He = 60/30/10, 6.6 mL/min total flow per reactor) and subsequently the temperature was raised to 240 °C (1 °C /min). The product stream was analysed using online gas chromatography (Agilent 7890A) with Ar as carrier gas. Hydrocarbons (C1–C9) were separated on an Agilent J&W PoraBOND Q column, detected using an FID detector and quantified against the TCD signal of the internal standard He. The permanent gases (CO, H2, He, CO2 and CH4) were separated on a ShinCarbon ST (#19043) column and quantified against He as an internal standard using a TCD detector. CO2 was also measured and the detection limit of CO2 was determined to 0.5% yield, which was 3% CO2 selectivity and 18% CO conversion (Supplementary Figure 5 and Supplementary Table 8). Catalytic activity and product selectivities were measured at 240–280 °C, 10 bar, H2/CO = 2, 10–70% CO conversion. To show the effect of activation procedure on catalytic performance, the same catalysts were reduced at 300 °C and 1 bar under diluted H2 flow for 5 h, followed by introduction of syngas at 250 °C and 10 bar. Definitions of the selectivity and activity, expressed as CO conversion and cobalt-time-yield (CTY) are included as Supplementary Methods.
DFT calculations
DFT modelling was performed with the ADF-BAND package (version 2016.102)49,50, using the rPBE functional51 and Grimme D3 corrections52. A TZP basis set with small frozen cores, a “good” k-space, and otherwise “normal” settings were used. For efficiency, the SCF was converged to only 5 × 10−4 Hartree. Gradients were converged to 0.001 Hartree/Å. The bulk cobalt unit cell vectors were reoptimized, giving a = 2.43 Å (experimental 2.51 Å) and c = 3.91 Å (experimental 4.07 Å). The (0001) surface was modelled with 6 atomic layers, giving a slab of 12 Å thick, of which the bottom two layers were frozen and calculated at minimal settings (SZ basis set with large frozen core, orbital confinement to 4 bohr, and “basic” settings for the Becke grid and zlm-fit parameters). The surface unit cell consisted of 4 × 4 atoms. Since ADF-BAND uses true 2D periodicity, no vacuum spacing nor dipole corrections were needed. Atomic charges were calculated with Hirshfeld’s method53.
Supplementary information
Acknowledgements
This research received funding from the Netherlands Organisation for Scientific Research (NWO) in the framework of the TASC Technology Area “Syngas, a Switch to Flexible New Feedstock for the Chemical Industry (TA-Syngas)”. Dow and Johnson Matthey (JM) are also acknowledged for the funding received. K.P.d.J. acknowledges the European Research Council, EU FP7 ERC Advanced Grant no. 338846. J.J. Mulder (ICP-AES), Y. Wei (N2-physisorption) M. Versluijs-Helder (SEM-EDX), and H. Meeldijk (TEM-EDX) are acknowledged for the respective measurements. Dr. M. Ruitenbeek (Dow), Dr. M. Watson (JM) and Dr. L. van der Water (JM) are thanked for fruitful discussions.
Author contributions
J.X. and K.P.d.J conceived, coordinated the research and designed the experiments. J.X. synthesized, characterized and tested catalysts. T.W.v.D contributed to the design of experiments and evaluation of catalyst performance. B.M.W. and P.P. conceptualized and performed XRD characterization, including Rietveld analysis. M.J.L. performed DFT calculations. All authors contributed to analysis and discussion on the data. The manuscript was primarily written by J.X. and K.P.d.J with input from all authors.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-08019-7.
References
- 1.Mitchell SF, Shantz DF. Future feedstocks for the chemical industry - where will the carbon come from? AIChE. 2015;61:2374–2384. doi: 10.1002/aic.14910. [DOI] [Google Scholar]
- 2.Dieterle M, Schwab E. Raw material change in the chemical industry. Top. Catal. 2016;59:817–822. doi: 10.1007/s11244-016-0554-6. [DOI] [Google Scholar]
- 3.Sousa-Aguiar EF, Noronha FB, Faro A., Jr. The main catalytic challenges in GTL (gas-to-liquids) processes. Catal. Sci. Technol. 2011;1:698. doi: 10.1039/c1cy00116g. [DOI] [Google Scholar]
- 4.Zhang Q, Kang J, Wang Y. Development of novel catalysts for Fischer-Tropsch synthesis: tuning the product selectivity. ChemCatChem. 2010;2:1030–1058. doi: 10.1002/cctc.201000071. [DOI] [Google Scholar]
- 5.Torres Galvis HM, et al. Supported iron nanoparticles as catalysts for sustainable production of lower olefins. Science. 2012;335:835–838. doi: 10.1126/science.1215614. [DOI] [PubMed] [Google Scholar]
- 6.Santos VP, et al. Metal organic framework-mediated synthesis of highly active and stable Fischer-Tropsch catalysts. Nat. Commun. 2015;6:6451. doi: 10.1038/ncomms7451. [DOI] [PubMed] [Google Scholar]
- 7.Zhai P, et al. Highly tunable selectivity for Syngas-derived alkenes over zinc and sodium-modulated Fe5C2 catalyst. Angew. Chem. Int. Ed. 2016;55:9902–9907. doi: 10.1002/anie.201603556. [DOI] [PubMed] [Google Scholar]
- 8.Zhong L, et al. Cobalt carbide nanoprisms for direct production of lower olefins from syngas. Nature. 2016;538:84–87. doi: 10.1038/nature19786. [DOI] [PubMed] [Google Scholar]
- 9.Xiang Y, Kruse N. Tuning the catalytic CO hydrogenation. Nat. Commun. 2016;7:1–6. doi: 10.1038/ncomms13058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumaran M, et al. Low temperature water – gas shift reaction over alkali metal promoted cobalt carbide catalysts. Top. Catal. 2014;57:612–618. doi: 10.1007/s11244-013-0219-7. [DOI] [Google Scholar]
- 11.Jiao F, et al. Selective conversion of syngas to light olefins. Science. 2016;351:1065–1068. doi: 10.1126/science.aaf1835. [DOI] [PubMed] [Google Scholar]
- 12.Cheng K, et al. Direct and highly selective conversion of synthesis gas into lower olefins: design of a bifunctional catalyst combining methanol synthesis and carbon – carbon coupling. Angew. Chemie Int. Ed. 2016;55:4725–4728. doi: 10.1002/anie.201601208. [DOI] [PubMed] [Google Scholar]
- 13.Wang P, et al. Synthesis of stable and low-CO2 selective ε-iron carbide Fischer-Tropsch catalysts. Sci. Adv. 2018;4:eaau2947. doi: 10.1126/sciadv.aau2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iglesia E. Design, synthesis, and use of cobalt-based Fischer-Tropsch synthesis catalysts. Appl. Catal. A. 1997;161:59–78. doi: 10.1016/S0926-860X(97)00186-5. [DOI] [Google Scholar]
- 15.Van Steen E, et al. Stability of nanocrystals: thermodynamic analysis of oxidation and re-reduction of cobalt in water/hydrogen mixtures. J. Phys. Chem. B. 2005;109:3575–3577. doi: 10.1021/jp045136o. [DOI] [PubMed] [Google Scholar]
- 16.Khodakov AY, Chu W, Fongarland P. Advances in the development of novel cobalt Fischer-Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels. Chem. Rev. 2007;107:1692–1744. doi: 10.1021/cr050972v. [DOI] [PubMed] [Google Scholar]
- 17.Navarro V, van Spronsen MA, Frenken JWM. In situ observation of self-assembled hydrocarbon Fischer–Tropsch products on a cobalt catalyst. Nat. Chem. 2016;8:929–934. doi: 10.1038/nchem.2613. [DOI] [PubMed] [Google Scholar]
- 18.Mirzaei Aa, Faizi M, Habibpour R. Effect of preparation conditions on the catalytic performance of cobalt manganese oxide catalysts for conversion of synthesis gas to light olefins. Appl. Catal. A Gen. 2006;306:98–107. doi: 10.1016/j.apcata.2006.03.036. [DOI] [Google Scholar]
- 19.Dinse A, Aigner M, Ulbrich M, Johnson GR, Bell AT. Effects of Mn promotion on the activity and selectivity of Co/SiO2 for Fischer–Tropsch Synthesis. J. Catal. 2012;288:104–114. doi: 10.1016/j.jcat.2012.01.008. [DOI] [Google Scholar]
- 20.Zhou W, Liu J, Wu X, Chen J, Zhang Y. An effective Co/MnOx catalyst for forming light olefins via Fischer – Tropsch synthesis. Catal. Commun. 2015;60:76–81. doi: 10.1016/j.catcom.2014.10.027. [DOI] [Google Scholar]
- 21.Morales, F. & Weckhuysen, B. M. Promotion Effects in Co-based Fischer – Tropsch Catalysis. Catalysis19, Spivey, J.J. (Ed.), Royal Society of Chemistry, Cambridge, p. 1–40 (RSC Publishing, 2006).
- 22.Pedersen EOslash, Svenum IH, Blekkan EA. Mn promoted Co catalysts for Fischer-Tropsch production of light olefins – an experimental and theoretical study. J. Catal. 2018;361:23–32. doi: 10.1016/j.jcat.2018.02.011. [DOI] [Google Scholar]
- 23.Li Z, et al. Effects of sodium on the catalytic performance of CoMn catalysts for Fischer–Tropsch to Olefin reactions. ACS Catal. 2017;7:3622–3631. doi: 10.1021/acscatal.6b03478. [DOI] [Google Scholar]
- 24.Borg Oslash, et al. Effect of biomass-derived synthesis gas impurity elements on cobalt Fischer–Tropsch catalyst performance including in situ sulphur and nitrogen addition. J. Catal. 2011;279:163–173. doi: 10.1016/j.jcat.2011.01.015. [DOI] [Google Scholar]
- 25.Lillebø AH, Patanou E, Yang J, Blekkan EA, Holmen A. The effect of alkali and alkaline earth elements on cobalt based Fischer–Tropsch catalysts. Catal. Today. 2013;215:60–66. doi: 10.1016/j.cattod.2013.03.030. [DOI] [Google Scholar]
- 26.Huo C, et al. The mechanism of potassium promoter: enhancing the stability of active surfaces. Angew. Chemie Int. Ed. 2011;50:7403–7406. doi: 10.1002/anie.201007484. [DOI] [PubMed] [Google Scholar]
- 27.Torres Galvis HM, et al. Effects of sodium and sulfur on catalytic performance of supported iron catalysts for the Fischer–Tropsch synthesis of lower olefins. J. Catal. 2013;303:22–30. doi: 10.1016/j.jcat.2013.03.010. [DOI] [Google Scholar]
- 28.Botes, G. F. et al. Development of a chemical selective iron Fischer Tropsch catalyst. Catal. Today (2016). 10.1016/j.cattod.2015.11.044
- 29.Xie J, et al. Size and promoter effects in upported Iron Fischer–Tropsch catalysts: insights from experiment and theory. ACS Catal. 2016;6:3147–3157. doi: 10.1021/acscatal.6b00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsakoumis NE, Rønning M, Borg Oslash, Rytter E, Holmen A. Deactivation of cobalt based Fischer–Tropsch catalysts: a review. Catal. Today. 2010;154:162–182. doi: 10.1016/j.cattod.2010.02.077. [DOI] [Google Scholar]
- 31.Curtis V, Nicolaides CP, Coville NJ, Hildebrandt D, Glasser D. The effect of sulfur on supported cobalt Fischer–Tropsch catalysts. Catal. Today. 1999;49:33–40. doi: 10.1016/S0920-5861(98)00405-2. [DOI] [Google Scholar]
- 32.Visconti CG, Lietti L, Forzatti P, Zennaro R. Fischer–Tropsch synthesis on sulphur poisoned Co/Al2O3 catalyst. Appl. Catal. A Gen. 2007;330:49–56. doi: 10.1016/j.apcata.2007.07.009. [DOI] [Google Scholar]
- 33.Barrientos J, Montes V, Boutonnet M, Järås S. Further insights into the effect of sulfur on the activity and selectivity of cobalt-based Fischer–Tropsch catalysts. Catal. Today. 2016;275:119–126. doi: 10.1016/j.cattod.2015.10.039. [DOI] [Google Scholar]
- 34.Bezemer GL, et al. Investigation of promoter effects of manganese oxide on carbon nanofiber-supported cobalt catalysts for Fischer–Tropsch synthesis. J. Catal. 2006;237:152–161. doi: 10.1016/j.jcat.2005.10.031. [DOI] [Google Scholar]
- 35.Johnson GR, Werner S, Bell AT. An investigation into the effects of Mn promotion on the activity and selectivity of Co/SiO2 for Fischer–Tropsch synthesis: evidence for enhanced CO adsorption and dissociation. ACS Catal. 2015;5:5888–5903. doi: 10.1021/acscatal.5b01578. [DOI] [Google Scholar]
- 36.Schulz H. Selforganization in Fischer–Tropsch synthesis with iron- and cobalt catalysts. Catal. Today. 2014;228:113–122. doi: 10.1016/j.cattod.2013.11.060. [DOI] [Google Scholar]
- 37.Fontenelle AB, Fernandes FAN. Comprehensive polymerization model for Fischer-Tropsch synthesis. Chem. Eng. Technol. 2011;34:963–971. doi: 10.1002/ceat.201000498. [DOI] [Google Scholar]
- 38.Strømsheim MD, et al. Effects of K adsorption on the CO-induced restructuring of Co(11-20) Catal. Today. 2018;299:37–46. doi: 10.1016/j.cattod.2017.05.086. [DOI] [Google Scholar]
- 39.Kattel S, Ramírez PJ, Chen JG, Rodriguez JA, Liu P. Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts. Science. 2017;355:1296–1299. doi: 10.1126/science.aal3573. [DOI] [PubMed] [Google Scholar]
- 40.Zhang R, et al. C2 oxygenate synthesis via Fischer-Tropsch synthesis on Co2C and Co/Co2C interface catalysts: how to control the catalyst crystal facet for optimal selectivity. ACS Catal. 2017;7:8285–8295. doi: 10.1021/acscatal.7b02800. [DOI] [Google Scholar]
- 41.Voss JM, et al. Characterization of CoCu- and CoMn-based catalysts for the Fischer–Tropsch reaction toward chain-lengthened oxygenates. Top. Catal. 2018;61:1016–1023. doi: 10.1007/s11244-018-0938-x. [DOI] [Google Scholar]
- 42.Price SWT, et al. Chemical imaging of Fischer-Tropsch catalysts under operating conditions. Sci. Adv. 2017;3:e1602838. doi: 10.1126/sciadv.1602838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Z, et al. Mechanism of the Mn Promoter via CoMn Spinel for Morphology Control: Formation of Co2C Nanoprisms for Fischer-Tropsch to Olefins Reaction. ACS Catal. 2017;7:8023–8032. doi: 10.1021/acscatal.7b02144. [DOI] [Google Scholar]
- 44.Morales F, de Smit E, de Groot FMF, Visser T, Weckhuysen BM. Effects of manganese oxide promoter on the CO and H2 adsorption properties of titania-supported cobalt Fischer-Tropsch catalysts. J. Catal. 2007;246:91–99. doi: 10.1016/j.jcat.2006.11.014. [DOI] [Google Scholar]
- 45.de Smit E, et al. Stability and reactivity of E- χ- θ iron carbide catalyst phases in Fischer–Tropsch synthesis: Controlling µ C. J. Am. Chem. Soc. 2010;132:14928–14941. doi: 10.1021/ja105853q. [DOI] [PubMed] [Google Scholar]
- 46.Claeys M, et al. In situ magnetometer study on the formation and stability of cobalt carbide in Fischer–Tropsch synthesis. J. Catal. 2014;318:193–202. doi: 10.1016/j.jcat.2014.08.002. [DOI] [Google Scholar]
- 47.Mohandas JC, et al. Fischer–tropsch synthesis: characterization and reaction testing of cobalt carbide. ACS Catal. 2011;1:1581–1588. doi: 10.1021/cs200236q. [DOI] [Google Scholar]
- 48.Cats KH, Weckhuysen BM. Combined operando X-ray diffraction/Raman Spectroscopy of catalytic solids in the laboratory: the Co/TiO2 Fischer–Tropsch synthesis catalyst showcase. ChemCatChem. 2016;8:1531–1542. doi: 10.1002/cctc.201600074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.te Velde G, et al. Chemistry with ADF. J. Comput. Chem. 2001;22:931–967. doi: 10.1002/jcc.1056. [DOI] [Google Scholar]
- 50.Fonseca Guerra, C., Visser, O., Snijders, J. G., te Velde, G. & Baerends, E. J. Methods and Techniques for Computational Chemistry. Methods and Techniques for Computational Chemistry (STEF, Cagliari, 1995). 303–395.
- 51.Hammer B, Hansen LB, Nørskov JK. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals. Phys. Rev. B. 1999;59:7413–7421. doi: 10.1103/PhysRevB.59.7413. [DOI] [Google Scholar]
- 52.Grimme S, Antony J, Ehrlich S, Krieg H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010;132:154104–154119. doi: 10.1063/1.3382344. [DOI] [PubMed] [Google Scholar]
- 53.Hirshfeld FL. Bonded-atom fragments for describing molecular charge densities. Theor. Chim. Acta. 1977;138:129–138. doi: 10.1007/BF00549096. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.