Abstract
The theory of syndemics has been used to explain elevated HIV risk facing men who have sex with men (MSM). However, few studies have employed suitable analytical methods to test this theory. Using data from a probability-based sample of MSM in India, we tested three proposed models linking the co-occurring epidemics of violence victimisation, drug use, and frequent alcohol use to HIV risk: 1) the syndemic model of synergistically interacting epidemics; 2) the “chains of risk” model; and 3) the model of mutually causal epidemics. The primary outcome was inconsistent condom use with male or hijra (transgender women) partners in the past month. For the syndemic model, we included product terms between the exposures and assessed for interaction on the additive (linear probability regression) and multiplicative (logistic regression) scales. Path analysis was used to test the models of serially causal epidemics and mutually causal epidemics. Among 22,297 HIV-negative MSM, violence victimisation (24.7%), frequent alcohol use (27.5%), and drug use (10.9%) frequently co-occurred. We found evidence for a three-way interaction between violence victimisation, drug use and frequent alcohol use on both the multiplicative (semi-elasticity = 0.28; 95% CI 0.10, 0.47) and additive (b = 0.14; 95% CI 0.01, .27) scales. We also estimated statistically significant two-way interactions between violence victimisation and frequent alcohol use on the multiplicative (semi-elasticity = .10; 95% CI 0.008, 0.20) and additive (b = 0.05, 95% CI 0.002, 0.107) scales, and between drug use and frequent alcohol use on the multiplicative (semi-elasticity = 0.13, 95% CI 0.02, 0.24) and additive (b = 0.06, 95% CI 0.007, 0.129) scales. Thus, we found strong evidence for the syndemic model. The models of serially causal and mutually causal epidemics were partially supported. These findings highlight the need to sharpen how syndemic models are specified so that their empirical predictions can be adequately tested and distinguished from other theories of disease distribution.
Keywords: Syndemic, Syndemics, Men who have sex with men, Interactions, HIV, India, Condom use, Violence victimisation, Drug use, Alcohol use
Highlights
-
•
We studied HIV transmission risk behavior among Indian men who have sex with men.
-
•
We tested three different models of co-occurring epidemics.
-
•
The findings were most consistent with a syndemic model of synergistic interaction.
-
•
Findings partly supported models of serially causal and mutually causal epidemics.
-
•
Observed synergies offer possibilities to effectively reduce HIV risk.
Introduction
Men who have sex with men (MSM) in India and worldwide face a disproportionate HIV burden. Two large-scale, multi-site surveys conducted in India have shown a national average HIV prevalence of 4–7% among MSM (Mehta et al., 2015, NACO, 2015), about 20 to 30 times higher than that of the general population (0.26%) (NACO, 2017). The annualized HIV incidence has been reported to be 0.87%, with some sites reporting incidence rates as high as 2.20% (Solomon et al. 2015). In recent years, there has been an increase in HIV prevalence among MSM in certain regions of the country (NACO, 2015). HIV prevention interventions supported by the National AIDS Control Organisation (NACO) have focused on HIV education, condom promotion, and free condom distribution. Recent studies have shown that knowledge about HIV and about the effectiveness of condoms in reducing HIV transmission risk are quite high in the general population and among marginalised groups, including MSM. For example, >90% of MSM were found to have accurate knowledge about three modes of HIV transmission and about the role of condoms in preventing HIV transmission (NACO, 2017). Despite this knowledge, condom use among MSM remains inconsistent (NACO, 2015).
The theory of syndemics has been used to explain the clustering and concentration of diseases in certain populations or settings due to harmful social conditions such that they mutually reinforce each other and synergistically amplify disease burden (Singer, 1996). Syndemics of non-communicable diseases in India are becoming increasingly important (Mendenhall et al., 2017, Mendenhall et al., 2012, Weaver and Mendenhall, 2014). The prevalence of psychosocial health conditions, which include non-communicable diseases, is relatively high among MSM in India: depression, 11–35% (Chakrapani et al., 2017, Safren et al., 2009); problematic alcohol use, 15–40% (Tomori et al., 2018, Yadav et al., 2014); and violence victimisation (experience of physical and sexual violence), 18–50% (Chakrapani et al., 2017, Shaw et al., 2012). The prevalence of injecting drug use (0.1% to 3.4%) has been reported to be relatively low (Tomori et al. 2016). These conditions have been associated with elevated HIV risk among MSM in India and globally. Moreover, HIV among MSM in India has become epidemic in the context of large-scale social forces and structural factors such as negative societal attitudes towards same-sex attracted people (Chakrapani, Newman, Shunmugam, McLuckie, & Melwin, 2007) that are also reflected in enacted policy such as criminalisation of adult consensual same-sex relations until recently (September 2018). However, limited evidence is available from studies of MSM in India on the extent to which these health risks co-occur (Chakrapani et al., 2017) and/or are synergistically reinforcing (Tomori et al., 2018). Understanding the presence and nature of synergy is of key theoretical and practical significance as such knowledge may provide clues for understanding the causal mechanisms and potential public health solutions to prevent or mitigate the effects of epidemic disease (Tsai & Burns, 2015).
In their systematic review, Tsai and Burns (2015) showed that the majority of studies claiming to have tested the theory of syndemics had not used appropriate analytical strategies. The literature showed little improvement two years later (Tsai, Mendenhall, Trostle, & Kawachi, 2017). Most of these analyses included the number of exposures as a cumulative count to predict HIV transmission risk, a specification that requires implausible assumptions for interpretation and which has little to do with the interaction concept embedded in syndemic theory (Tsai & Venkataramani, 2016). One recent study of MSM in India (Tomori et al., 2018) followed the preferred way of showing synergy, namely by estimating the relative excess risk due to interaction (RERI), which can be calculated from adjusted regression models. Out of the five exposures examined by Tomori et al. (2018), they reported a synergistic interaction on the additive scale between intimate partner violence and depression in predicting condomless anal intercourse, and between alcohol dependence and illicit drug use in predicting syphilis.
Several other theories of disease distribution have described how diseases ‘interact’. Drawing on these theories, Tsai (2018) provided a typology of three potential ways of conceptualising co-occurring epidemics: mutually causal and synergistically interacting epidemics, which are statistically distinct ways of understanding co-occurring epidemics (Rothman, Greenland, & Walker, 1980) but which are often used interchangeably in the literature on syndemics (Singer, 1996, Singer and Clair, 2003) and serially causal epidemics, which are also statistically distinct from the other two models (Kuh, Ben-Shlomo, Lynch, Hallqvist, & Power, 2003) but are nonetheless often described as being consistent with the original formulation of syndemic theory (Safren et al., 2010, Stall et al., 2008, Stall et al., 2003, Stall et al., 2001) (Table 1). This lack of conceptual clarity in the literature – what is meant by epidemic “interaction”? What is meant by “synergy”? – has contributed to tremendous inconsistency in the empirical literature on syndemics. Most studies of HIV-related syndemics, especially among MSM, have attempted – and failed – to test the hypothesis that co-occurring epidemics (i.e., of alcohol use, violence victimisation, etc.) synergistically interact (Guadamuz et al., 2014, Stall et al., 2003, Tomori et al., 2018). No studies have simultaneously tested multiple models of co-occurring epidemics.
Table 1.
Models of co-occurring epidemics.
| Model | Representative text | Candidate modelling strategy | Policy or programmatic implication |
|---|---|---|---|
| Mutually causal epidemics | “AIDS, drug use, and violence are conceived not as distinct ‘things in the world’ but as phenomena in tandem, the essence of each being significantly shaped by the presence, nature and influence of the others.” (Singer et al. 2006, p.50) | Path analysis | In a setting of 3 mutually causal epidemic exposures, a single component intervention designed to eliminate a single exposure will not reduce health risk because of the mutually reinforcing effects of other exposures |
| Synergistically interacting epidemics | “…the term syndemic refers to two or more epidemics (i.e., notable increases in the rate of specific diseases in a population), interacting synergistically and contributing, as a result of their interaction, to excess burden of disease in a population” (Singer & Clair, 2003, p. 425) | Product terms | A single component intervention designed to eliminate a single exposure will reduce health risk to a greater degree than would be expected if no interactions were present |
| Serially causal epidemics | “…accumulation of these stressors leads to development of psychosocial health problems which in turn snowball to increase the likelihood of HIV risk-taking behaviors, such as condomless anal sex” (Ferlatte, Hottes, Trussler, & Marchand, 2014, pp. 1257–1258) | Mediation analysis | Interventions targeting exposures earlier in the life course will reduce health risk by preventing subsequent cascades of psychosocial problems (See also Stall et al., 2008) |
To address these gaps in the literature, we analysed data from MSM in India to test the three models of co-occurring epidemics described by Tsai (2018) (Fig. 1): 1) the model of synergistically interacting epidemics, in which we evaluated the joint associations between violence victimisation, drug use, and frequent alcohol on HIV transmission risk behaviour and assessed for synergistic interaction on both the additive and multiplicative scales; 2) the “chains of risk” model (Coie et al. 1993), in which alcohol and drug use were conceptualised as potential mediators of the effect of violence victimisation on HIV transmission risk behaviour; and 3) the model of mutually causal epidemics (Singer, 1996), in which two of the three exposures that contribute to HIV transmission risk behaviours were conceptualised as mutually causal, with violence victimisation leading to drug use and drug use leading to violence victimisation.
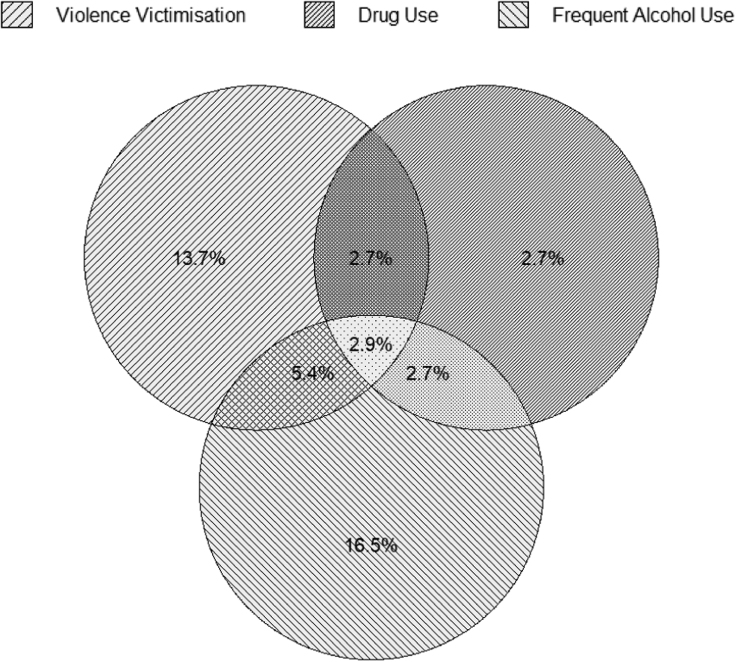
Fig. 1.
Co-occurrence of violence victimisation, drug use, and frequent alcohol use (N = 22,297 MSM).
Methods
Study design
The data for these analyses were drawn from a population-based, cross-sectional survey of MSM recruited in the Integrated Bio-Behavioural Surveillance (IBBS) study (NACO, 2015). The IBBS was conducted by India’s National AIDS Control Organisation in 2014/15 to generate evidence on HIV transmission risk behaviours and HIV prevalence among “key populations” (i.e., MSM, transgender people, and people who inject drugs) to plan and prioritise programme efforts at the district, state and national levels. Eligible participants were: men aged 15 years and above who reported anal or oral sex with a man or hijra (transgender women) partner in the past month. The unit of survey under IBBS was a ‘domain’ - a single district or group of socio-culturally similar districts. In each domain, first a list of hotspots or cruising sites (where MSM meet other potential male partners) was prepared, and the functional status of those sites was determined by field visits. New hotspots were also identified by searching the entire domain. The information collected from this assessment was then used to develop a sampling frame of primary sampling units, or clusters. For the time-location clusters, each hotspot was divided into 4 clusters: peak day-peak time, peak day-lean time, lean day-peak time and lean day-lean time. The final selection of clusters was random. All regression estimates make use of sample weights provided by NACO (2015) to account for the complex survey design (Solon, Haider, & Wooldridge, 2015). More details on the sampling strategy are available in the online report (NACO, 2015). A total of 23,081 MSM were interviewed across 61 domains in 24 States and Union Territories, with a response rate of 85%.
Measures
Primary exposures of interest
Any violence victimisation
Participants were asked two questions about physical and sexual violence: 1) how frequently they had experienced physical abuse in the last 12 months (hurt, hit, slapped, pushed, kicked, punched, choked or burned); and 2) any experience of forced sex in the last 12 months. Those who reported any experience of physical or sexual violence were defined as experienced ‘any violence victimisation’.
Frequent alcohol use
Participants were asked about the number of days they had consumed alcohol in the previous week. Responses were dichotomised at the upper quartile of consumption for ‘frequent alcohol use’ (>2 days/week vs. ≤ 2 days/week).
Any drug use
Two questions assessed whether the participants used non-injection (e.g., ganja, cocaine) or injection drugs (e.g., heroin) in the last 12 months. Consumption of any drug (injection or not) was incorporated into the dichotomous measure of ‘any drug use’.
Outcome measure - Inconsistent condom use
Inconsistent condom use during anal intercourse with 5 different types of partners (hijra/trans women regular partners, and male regular, casual, paying and paid partners) in the past month was assessed using this question: In the last one month, how often have you used condoms when you had anal sex with your [type of] male/hijra partner? The options included: every time, most of the time, sometimes, and never. Those who reported ‘every time’ were coded as consistently using condoms (0), while those who reported ‘most of the time’, ‘sometimes,’ or ‘never’ were coded as inconsistently using condoms (1). A single outcome variable ‘inconsistent condom use with any type of male/hijra partner’ was then created by combining the condom use responses for the different types of partners.
Other covariates
Sociodemographic characteristics
These included: age (in years), years of education, marital status (e.g., single, married, separated, divorced), and sexual role-based identities (kothi - feminine/receptive role; panthi - masculine/insertive role; double-decker - versatile role) (Chakrapani et al., 2007).
HIV risk knowledge
Study participants were asked about four behaviours associated with HIV transmission risk (condomless sex, sharing needles, blood transfusion, and mother-to-child transmission) and about one misconception related to HIV transmission risk (mosquito bites) (Chan & Tsai, 2018). For the analysis, each correct response was coded as 1, otherwise ‘zero’. The total score ranged from 0 to 5.
HIV programme exposure
Participants were asked to report their level of exposure in the past year to 8 HIV-related services provided through non-governmental organisations (e.g., information on HIV or sexually transmitted infections [STIs], condom distribution, and referrals to STI services and HIV testing). The total programme exposure score ranged from 0 to 8.
Social support score
This composite score was calculated from 3 items: whether participants received help and support when faced with physical or sexual violence (yes or no); and whether they were a member of a self-help group (yes or no) or MSM collective (yes or no). The score (range 0–3) was then used as a proxy for social support, because support from others has been shown to be a resilience resource for coping with psychosocial stressors (Woodward, Banks, Marks, & Pantalone, 2017).
Forced sex experience during adolescence
While some studies in the literature on syndemics have conceptualised childhood sexual abuse as a primary exposure of interest (Biello et al., 2014, Mimiaga et al., 2015), we specified this variable as an early life adversity/adverse childhood experience, in line with the position taken in some other studies (Felitti et al., 1998, Tulloch et al., 2015). The variable ‘forced sex experience during adolescence’ was derived from two questions: 1) ‘How old were you when you had your first sex with a male/hijra?’; and 2) ‘Were you forced to have sex during the first sexual encounter with a male/hijra?’ Participants who reported first sex with a male/hijra when they were less than 18 years (age of consent for heterosexual intercourse in India is 18 years, but adult same-sex relations were criminalised at the time of survey) and who reported forced sex during the first sexual encounter with a male/hijra were categorised as having experienced forced sex during adolescence. In addition, participants who were 15–18 years old at the time of the survey and who had reported forced sex in the previous year were also categorised as having experienced forced sex during adolescence.
Analyses
The present analysis was restricted to HIV-negative MSM (n = 22,297). The other 784 (3.4%) HIV-positive MSM who participated in the IBBS were excluded from this analysis. To take into account both the design effect (as the primary sampling units were time-location and conventional clusters) and sample weights, the svyset command of Stata was used before fitting regression models and path models (Heeringa, Berglung, & West, 2010). To test the model of synergistically interacting epidemics, we assessed for interactions on both the additive and multiplicative scales (Rothman, 1974).
Interactions on the additive scale were assessed using multivariable linear probability regression models, with standard errors corrected for heteroskedasticity. The dichotomous outcome measure was inconsistent condom use with any type (regular, casual, paying and paid) of male/hijra partner. Regression models were adjusted for the following variables: age, education, marital status, sexual identity, forced sex experience during adolescence, HIV risk perception, HIV knowledge, social support and HIV programme exposure. We included product terms representing two-way and three-way interactions between violence victimisation, drug use, and frequent alcohol use. The estimated regression coefficients on the product terms were interpreted directly as measures of interaction on the additive scale. All comparisons of excess risk were in relation to those who reported neither frequent alcohol use nor any drug use, and who reported no experience of violence victimisation.
Interactions on the multiplicative scale were assessed using multivariable logistic regression. Similar to the linear probability models, we included product terms representing two-way and three-way interactions between violence victimisation, drug use, and frequent alcohol use and estimated the multiplicative interaction parameters (Knol and VanderWeele, 2012, VanderWeele, 2015). After fitting the logistic regression models, which estimate multiplicative interaction on the odds scale, we used the Stata margins command to estimate multiplicative interaction on the probability scale. If the estimated semi-elasticities are non-zero and statistically significant, this implies interaction is present on the multiplicative scale. The estimated semi-elasticities can also be interpreted as the proportional change in the expected value of the outcome that is associated with a one unit change in the covariate (e.g. age expressed in years). For example, a semi-elasticity of.10 is interpreted as a 10 percent relative change in the expected outcome associated with a one unit change in the covariate. The semi-elasticity of a product term of binary exposures (“interaction term”) needs to be interpreted as the percent relative change in the expected value of the outcome (inconsistent condom use) that is associated with the interaction - i.e., the percent relative change in the outcome that can be attributed to the joint effect of two (or more) exposures, above and beyond their independent associations with the outcome. The logistic regression models were adjusted for the same covariates as in the linear probability regression models described above.
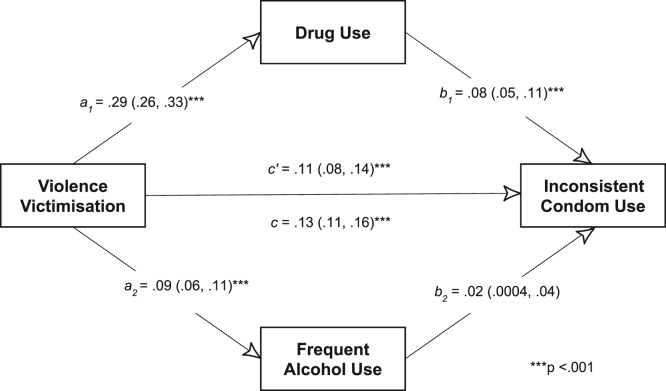
To test the model of serially causal epidemics (“chains of risk”, described by Coie et al. (1993)), we conducted a mediation analysis of a path model (Fig. 2) using the sem command in Stata (version 14; College Station, Texas, USA). For path analyses, we specified continuous variables for the psychosocial exposures and outcome. The alcohol use score ranged between 0 and 7 (the number of days of alcohol use in the past week). The drug use score ranged from 0–3 (where 0 = no drug use; 1 = non-injection drug use alone; 2 = injection drug use alone; 3 = both non-injection and injection drug use). The violence victimisation score ranged from 0–3 (where 0 = no experience of violence; 1 = experience of physical violence alone; 2 = experience of sexual violence alone; 3 = experience of physical and sexual violence). The inconsistent condom use score ranged from 0 to 5, with one point for inconsistent condom use with each type of partner. Given the evidence from qualitative studies that violence victimisation could lead to alcohol use and sexual risk (Chakrapani et al., 2018, Shaw et al., 2012), we tested the extent to which the association between violence victimisation and sexual risk was mediated through alcohol and drug use. The confidence intervals of the direct, indirect and total effects were estimated using the delta method.
Fig. 2.
Testing the model of serially causal epidemics using mediation analysis [Standardised estimates (95% CI)].
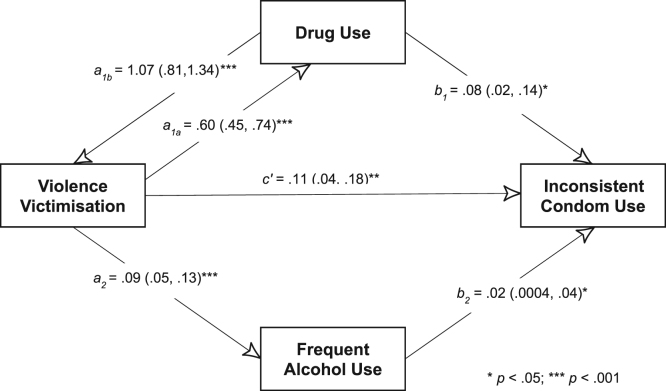
To test the model of mutually causal epidemics, we conducted path analyses using the sem command in Stata, and estimated direct, indirect and total effects. Similar to the path analyses for the serially causal models, we specified continuous variables for the psychosocial exposures and outcome. The cyclic or non-recursive model (with reciprocal arrows or feedback loops) tested is shown in Fig. 3. Ideally, to test the model of mutually causal epidemics, all three exposures should be considered as causative of each another. However, given that the exposures were elicited with different reference periods, bidirectional arrows were specified only between violence victimisation (past year) and drug use (past year). It was assumed that cause is prior to effect, that both cause and effect took place within the reference window (past year), and that the values of the mutually ‘causal variables’ (here violence victimisation and drug use) are more stable than their effects. For meaningful substantive interpretation of parameters in a cyclic model, one key condition that must be met is that the sum of loops is defined (as the regression coefficients in a loop are interpreted as resulting from an infinite sum of loops) (Blunch, 2013). This condition can be tested by estimating a stability index for two-way causation. If the stability index is < 1, then the condition is considered to be satisfied (Blunch, 2013). The fit values of the path model were also estimated, with a standardized root mean squared residual (SRMR) of <0.08 (Hu & Bentler, 1999) and coefficient of determination close to 1 interpreted as indices of good fit. Given that domains were the clusters under which participants were sampled, robust standard errors were used. All analyses were conducted using Stata (version 14; College Station, Texas, USA).
Fig. 3.
Testing the model of mutually causal epidemics using path analysis [Estimates (95% CI)].
As noted previously, several studies have used the ‘sum score’ approach to test the theory of syndemics. The sum score is entered into a regression model either as the continuous sum of the number of exposures (0 to 3) or as a categorical variable (4 categories: 0, 1, 2 and 3 conditions). Although this approach is inappropriate for testing the theory of syndemics (Tsai & Venkataramani, 2016), we did the same for the sake of comparison with previous studies.
To confirm that our findings were not driven by arbitrary choices in categorisation thresholds, we conducted several different sensitivity analyses for the syndemic model. First, we dichotomised alcohol use as ‘any’ (1–7 days/week vs. none). Second, we specified the exposures as continuous variables rather than binary variables, i.e., number of days of alcohol use ranged from 0 to 7 days/week, the drug use score ranged from 0 to 3 (where 0 = no drug use; 1 = non-injection drug use alone; 2 = injection drug use alone; 3 = both non-injection and injection drug use), and the violence victimisation score ranged from 0 to 3 (where 0 = no experience of violence; 1 = experience of physical violence alone; 2 = experience of sexual violence alone; 3 = experience of physical and sexual violence). In the latter model, for the alcohol use variable, we additionally explored inclusion of a quadratic term (Osborne, 2014) to test the hypothesis that alcohol consumption may have a curvilinear effect on HIV transmission risk behaviour (Simons, Simons, Maisto, Hahn, & Walters, 2018).
Ethics
Written informed consent was obtained from all participants, who were compensated with INR 200 (approximately 3 USD at the time of the study) for their time and travel. The questionnaire was administered in 15 different regional languages (Assamese, Bengali, Gujarati, Hindi, Kannada, Khashi, Malayalam, Manipuri, Marathi, Mizo, Nagamese, Oriya, Punjabi, Tamil and Telugu). The primary study, which focused on estimating the prevalence of HIV and HIV transmission risk behaviours among key populations, was approved by the ethics committee constituted by the National AIDS Control Organisation. This manuscript describes the findings of a secondary analysis of the data from this study (Table 2).
Table 2.
Sociodemographic and other characteristics of the participants (N = 22,297).
| Characteristics | Median (Interquartile range) |
|---|---|
| Age (in years) | 26 (22–31) |
| Number of years of education | 10 (8–12) |
| HIV knowledge score | 5 (4–5) |
| HIV programme exposure score | 4 (2–7) |
| Resilience/agency score | 2 (0–3) |
| n (%) | |
| Marital status | |
| Currently single | 15,024 (67.4) |
| Currently married | 7103 (31.9) |
| Sexual role-based identities | |
| Kothi (feminine, receptive role) | 9962 (44.7) |
| Double-decker (versatile role) | 6886 (30.9) |
| Panthi (masculine, insertive role) | 5419 (24.3) |
| Engagement in sex work | |
| Yes | 8338 (37.4) |
| No | 13953 (62.6) |
| HIV risk perceptiona | |
| Low | 12582 (56.4) |
| High | 8760 (39.3) |
| Forced sex experience during adolescence | |
| Yes | 2563 (11.1) |
% may not add to 100 due to missing values.
Results
Sociodemographic and related characteristics
Participants had a median age of 26 years (interquartile range [IQR], 22–31). About three-fifths (63.3%) were currently single and about one-third (31.7%) were currently married. Most participants self-identified as kothi (44.7%), while 30.9% identified as double-deckers and 24.3% identified as panthi. While only 4.4% reported sex work as their main occupation, more than one-third (37.4%) of the participants reported having had sex in exchange for money in the past 12 months. A little more than one-tenth (11.1%) reported forced sex during adolescence. Inconsistent condom use with male partners was 54.9%, among those who reported anal sex in the past month.
Co-occurrence of adverse psychosocial exposures
Among the 22,297 HIV-negative MSM, frequent alcohol use (n = 6137; 27.5%), drug use (n = 2441; 11.0%), and violence victimisation (n = 5570; 24.7%) frequently co-occurred: 595 (2.6%) reported both alcohol and drug use, 1210 (5.4%) reported both alcohol use and violence victimisation, 604 (2.7%) reported both drug use and violence victimisation, and 644 (2.9%) reported all three exposures. More than one-half of the sample reported no adverse psychosocial exposures (11,901 [53.3%]) (See Table 3 and Fig. 1).
Table 3.
Adverse psychosocial exposures – frequent alcohol use, drug use and violence victimisation, and their combinations among HIV-negative MSM (N = 22,297).
| Adverse psychosocial exposures and their combinations | n (%)a |
|---|---|
| Violence victimisation (V)b | 5510 (24.7) |
| Drug use (D)b | 2441 (10.9) |
| Frequent alcohol use (A)b | 6137 (27.5) |
| No syndemic conditions | 11901 (53.3) |
| V alone | 3052 (13.7) |
| D alone | 598 (2.7) |
| A alone | 3687 (16.5) |
| V and D | 604 (2.7) |
| V and A | 1210 (5.4) |
| D and A | 595 (2.7) |
| V and D and A | 644 (2.9) |
% may not add to 100 due to missing values
Irrespective of the presence of other two psychosocial conditions
Model of synergistically interacting epidemics
In a multivariable logistic regression model without any product terms, violence victimisation (aOR = 1.51, 95% CI 1.34–1.72) and drug use (aOR = 1.52, 95% CI 1.34–1.72) were independently associated with inconsistent condom use, while frequent alcohol use was not (aOR = .93, 95% CI 0.83–1.03). We used multivariable logistic regression models with product terms to assess for departures from multiplicativity on the probability scale. In order to compare models with the same degrees of freedom, we first fitted three logistic regression models (Models 1, 2, and 3 in Table 4) with one product term at a time. In all three models, the main effects of violence victimisation and drug use had statistically significant associations with inconsistent condom use. In addition, we estimated a multiplicative interaction between violence victimisation and frequent alcohol use: the joint effect of violence victimisation and frequent alcohol use, above and beyond their individual associations with inconsistent condom use, was associated with a 10.7 percent relative increase in the outcome (95% CI, 8.9–20.5, p = .03). Similarly, we estimated a multiplicative interaction between drug use and frequent alcohol use: the joint effect of drug use and frequent alcohol use, above and beyond their individual associations with inconsistent condom use, was associated with a 13.5 percent relative increase in the outcome (95% CI, 2.2–24.8, p = .01). In a logistic regression model including the main effects and all two-way product terms (Model 4 in Table 4), we did not find evidence of multiplicative interaction; for example, the joint effect of violence victimisation and drug use, above and beyond their individual associations with inconsistent condom use (and the other main/joint effects), was associated with a 11.5 percent relative increase in the outcome, but this association was not statistically significant (95% CI, -0.4–23.3, p = .05). In a logistic regression model including the main effects, two-way product terms, and three-way product term (Model 5 in Table 4), we found evidence for a three-way interaction on the multiplicative scale between violence victimisation, drug use and frequent alcohol use: their joint effect, above and beyond their individual and two-way joint associations with inconsistent condom use, was associated with a 28.7 percent relative increase in the outcome (95% CI, 10.1–47.4, p = .003).
Table 4.
Effect of adverse psychosocial exposures on HIV transmission risk behaviour (inconsistent condom use with male and hijra partners): Multiplicative two-/three-way interactions between violence victimisation, drug use, and frequent alcohol use (N = 22,297).
| Adverse psychosocial exposures and product terms |
Model 1: Two-way product term (V × D) |
Model 2: Two-way product term (V × A) |
Model 3: Two-way product term (D × A) |
Model 4: All two-way product terms |
Model 5: All two- and three-way product terms |
|---|---|---|---|---|---|
| Estimated semi-elasticity (95% CI), p value | Estimated semi-elasticity (95% CI), p value | Estimated semi-elasticity (95% CI), p value | Estimated semi-elasticity (95% CI), p value | Estimated semi-elasticity (95% CI), p value | |
| V × D | 0.07 (−0.05, 0.20), p = .26 | 0.05 (−0.07, 0.18), p = .42 | −0.12 (−0.33, 0.09), p = 26 | ||
| V × A | 0.10 (0.008, 0.20), p = .03 | 0.08 (−0.01, 0.18), p = .10 | 0.03 (−0.08, 0.15), p = .61 | ||
| D × A | 0.13 (0.02, 0.24), p = .01 | 0.11 (−0.004, 0.23), p = .05 | −0.04 (−0.22, 0.14), p = .64 | ||
| V × D × A | 0.28 (0.10, 0.47), p = .003 |
Note. 1) The models were adjusted for covariates such as age, education, marital status, sexual identity, forced sex experience during adolescence, HIV risk perception, HIV knowledge, social support and HIV programme exposure. 2) The estimates of the main effects are not shown. The semi-elasticity here is to be interpreted as the percent relative change in the expected value of the outcome (inconsistent condom use) that is associated with the interaction - i.e., the percent relative change in the outcome that can be attributed to the joint effect of two or more exposures, above and beyond their independent associations with the outcome. For example, a semi-elasticity of .10 is interpreted as a 10 percent relative increase in the expected outcome that is associated with the interaction.
Among the other covariates included in the multivariable logistic regression model that included main effects and both two- and three-way product terms, higher odds of HIV transmission risk behaviour were estimated for participants who reported forced sex experience during adolescence (aOR = 1.25, 95% CI 1.06–1.47), who engaged in sex work (aOR = 1.56, 95% CI 1.40–1.74), and who identified as double-decker (aOR = 1.39, 95% CI 1.23–1.58) or panthi (aOR = 1.23, 95% CI 1.08–1.41). Lower odds of HIV transmission risk behaviour were associated with higher levels of education (aOR = .98, 95% CI 0.96–0.99) and higher scores in programme exposure (per point, from 0–8) (aOR = 0.88, 95% CI 0.86–0.90), social support (per point, from 0–3) (aOR = 0.95, 95% CI 0.91–0.99) and HIV transmission knowledge (per point, from 0–5) (aOR = .83, 95% CI 0.77–0.88).
We used linear probability regression models to estimate interactions on the additive scale. In linear probability regression models that included main effects and one two-way product term at a time, we found evidence for departures from additivity for the two-way product terms of violence victimisation and frequent alcohol use (b = .05, 95% CI 0.002–0.107), and drug use and frequent alcohol use (b = 0.06, 95% CI 0.007–0.129) (Table 5). As shown in VanderWeele (2015), these non-zero, positive, and statistically significant estimates imply relative excess risk due to interaction or RERI > 0. In a linear probability regression model that included the main effects and all possible two-way product terms only, we found no evidence of departures from additivity for violence victimisation and frequent alcohol use. In a linear probability regression model that included the main effects, all possible two-way product terms, and three-way product term, we found evidence of a three-way interaction on the additive scale (b = 0 .14, 95% CI 0.01–0.27) (Model 5 in Table 5).
Table 5.
Effect of adverse psychosocial exposures on HIV transmission risk behaviour (inconsistent condom use with male and hijra partners): Additive two-/three-way interactions between violence victimisation, drug use, and frequent alcohol use (N = 22,297).
| Adverse psychosocial exposures and product terms |
Model 1: Two-way product term (V × D) |
Model 2: Two-way product term (V × A) |
Model 3: Two-way product term (D × A) |
Model 4: Two-way product terms alone |
Model 5: All two- and three-way product terms |
|---|---|---|---|---|---|
| b (95% CI), p value | b (95% CI), p value | b (95% CI), p value | b (95% CI), p value | b (95% CI), p value | |
| Violence victimisation (V) x Drug use (D) | 0.02 (−0.03, 0.09), p = .42 | 0.01 (−0.05, 0.07), p = .67 | −0.05 (−0.14, 0.03), p = .21 | ||
| V × A | 0.05 (0.002, 0.107), p = .04 | 0.04 (−0.01, 0.09), p = .11 | 0.01 (−0.04, 0.08), p = .56 | ||
| D × A | 0.06 (0.007, 0.129), p = .02 | 0.05 (−.008, 0.11), p = .08 | −0.01 (−0.10, 0.07), p = .69 | ||
| V × D × A | 0.14 (0.01, 0.27), p = .03 |
Note. 1) The models were adjusted for covariates such as age, education, marital status, sexual identity, forced sex experience during adolescence, HIV risk perception, HIV knowledge, social support and HIV programme exposure. 2) The estimates of the main effects are not shown, and ‘b’ represents the estimated regression coefficient on the product term (use to assess additive interaction).
In sensitivity analyses where the exposures were specified in different ways, we obtained qualitatively similar results. Dichotomising alcohol use as “any” vs none yielded a similar pattern of two- and three-way interactions, as did specifying the exposures as continuous variables (Table 6). Finally, in the multivariable logistic regression model specifying the exposures as continuous variables, we added a quadratic term for the alcohol use variable (model not shown). In this regression model, the main effect for alcohol use was statistically significant (aOR = 1.10, 95% CI 1.02–1.18) as well as its quadratic term (aOR = 0 .97, 95% CI 0.96–0.99).
Table 6.
Sensitivity analyses of the syndemic model: Multiplicative interactions between violence victimisation, drug use, and frequent alcohol use in predicting inconsistent condom use with male and hijra partners (N = 22,297), based on alternative categorisation of alcohol use or with exposures specified as continuous variables (multivariable logistic regression).
|
With alcohol use categorised as any vs none |
All exposures specified as continuous variables (scores) |
|||
|---|---|---|---|---|
| Adverse psychosocial exposures and product terms | Adjusted ORa(95% CI), p value | Adverse psychosocial exposures and product terms | Mean (SD) | Adjusted ORa(95% CI), p value |
| Violence victimisation (V) | 1.46 (1.20, 1.78), p <.001 | Violence victimisation score (0–3)a | 0.43 (.84) | 1.21 (1.11, 1.33), p <0.001 |
| Drug use (D) | 1.74 (1.22, 2.48), p = .002 | Drug use score | 0.16 (.55) | 1.13 (0.93, 1.36), p = .20 |
| (0–3)b | ||||
| Frequent alcohol use (A) | 1.07 (.94, 1.22), p = .28 | Alcohol use score | 1.56 (2.02) | .98 (0.95, 1.01), p = .27 |
| (0– 7)c,d | ||||
| V × D | .63 (.35, 1.13), p = .12 | V × D | .94 (0.83, 1.05), p = .30 | |
| V × A | 1.00 (.77, 1.30), p = .96 | V × A | 1.00 (0.52, .98), p = .52 | |
| D × A | .71 (.47, 1.09), p = .12 | D × A | 1.00 (0.81, .95), p = .24 | |
| V × D × A | 2.23 (1.13, 4.40), p = .02 | V × D × A | 1.03 (1.00, 1.07), p = .03 | |
Based on the scoring system: 0 = No experience of violence; 1 = experience of physical violence alone; 2 = experience of sexual violence alone; 3 = experience of physical and sexual violence
Based on the scoring system: 0 = No drug use; 1 = Non-injection drug use alone; 2 = Injection drug use alone; 3 = Both non-injection and injection drug use
Alcohol consumption in number of days/week
When a quadratic term for alcohol use was added to this model, the main effect of alcohol became statistically significant (aOR = 1.10; 95% CI, 1.02–1.18) in addition to its significant quadratic term (aOR = .97; 95% CI, .96 to .99).
Model of serially causal epidemics (“chains of risk”)
We estimated statistically significant direct (c′ = 0.11, 95% CI 0.08–0.14) and total (c = 0.13, 95% CI 0.11–0.51) effects of violence victimisation on inconsistent condom use (Fig. 2). Similarly, the direct effects of violence victimisation on drug use (a1 = 0.29; 95% CI 0.26–0.33) and frequent alcohol use (a2 = 0.09, 95% CI 0.06–0.11) were also statistically significant. The direct effect of drug use (b1 = 0.08, 95% CI 0.05–0.11), but not the direct effect of frequent alcohol use (b2 = 0 .02, 95% CI 0.0004–0.04), on inconsistent condom use was statistically significant. Using bootstrapping, we found that the combined mediated effect (0.02, 95% CI 0.01–0.03) of drug use and frequent alcohol use was 15.3% of the total effect of violence victimisation on inconsistent condom use.
Model of mutually causal epidemics
In testing the model of mutually causal epidemics, violence victimisation had a statistically significant association with drug use (a1a = 0.60, 95% CI 0.45–0.74) and drug use had a statistically significant association with violence victimisation (a1b = 1.07, 95% CI 0.81–1.34) (Fig. 3). Violence victimisation also had a statistically significant association with frequent alcohol use (a2 = 0 .09, 95% CI 0.05–0.13). The estimated direct effects of violence victimisation, drug use and frequent alcohol use on HIV transmission risk behaviour were statistically significant. The stability index was 0.80. Model fit was adequate, with SRMR = 0.04, and CD = 0.88.
Sum score approach
When the count of exposures was included in a multivariable logistic regression model as a continuous variable, it had a statistically significant association with HIV transmission risk behaviour (aOR = 1.26, 95% CI 1.18–1.34). The categorical specification obtained similar results: when compared to no exposures, there were elevated odds of HIV transmission risk behaviour for one exposure (aOR = 1.14, 95% CI 1.01–1.27), two exposures (aOR = 1.41, 95% CI 1.20–1.67), and three exposures (aOR = 3.20, 95% CI 2.41–4.26). Of note, the sum score approach – either the continuous or categorical specification – assumes that the associations between the exposures and outcome are equivalent to each other. In this instance, such a specification would be problematic because: a) theoretically, the sum score approach bears no relation to the theory of syndemics; and b) the estimates displayed in Model 1 of Table 4a show clearly that the estimated associations between the exposures and outcome are not equivalent to each other. (As a contrasting example, Anda et al. (1999) showed that different adverse childhood experiences had approximately equivalent associations with smoking behaviour and used this empirical approach to justify their sum score analysis.).
Discussion
In this cross-sectional, probability-based sample of 22,297 MSM in India, we found that violence victimisation, drug use and alcohol use frequently co-occurred, and that violence victimisation and drug use had robust, statistically significant associations with inconsistent condom use. We tested three models of co-occurring epidemics (Tsai, 2018): a model of synergistically interacting epidemics, in which the joint effects of violence victimisation, drug use and frequent alcohol use on inconsistent condom use were evaluated on both the additive and multiplicative scales; a model of serially causal epidemics, in which drug use and frequent alcohol use were tested as potential mediators of the effect of violence victimisation on inconsistent condom use; and a model of mutually enhancing epidemics, in which violence victimisation and drug use were conceptualised as mutually enhancing each other, and independently and jointly with frequent alcohol use contributing to inconsistent condom use. On both the multiplicative and additive scales, we found evidence of a three-way synergistic interaction between violence victimisation, drug use and frequent alcohol use; and we found evidence of two-way synergistic interactions between violence victimisation and frequent alcohol use, and between drug use and frequent alcohol use. Thus, the evidence was strong for the model of synergistically interacting epidemics. The evidence was less strong for the model of serially causal epidemics; there was some evidence that the effect of violence victimisation on inconsistent condom use was mediated through alcohol and drug use, but the proportion of the mediated effect was relatively low. Finally, using path analyses we also found some evidence that the exposures were mutually enhancing. Taken together, our findings are most strongly consistent with the model of synergistically interacting epidemics.
The study most relevant to ours is the recent analysis by Tomori et al. (2018), who examined the joint effects of alcohol dependence, illicit drug use, depression, intimate partner violence and childhood sexual abuse in a sample of MSM in India recruited using a respondent-driven sampling design. They reported, out of 26 different interaction terms tested, only two statistically significant two-way additive interactions: between intimate partner violence and depression on condomless anal sex, and between alcohol dependence and illicit drug use on syphilis. In contrast, we analysed the joint effects of violence victimisation, drug use and frequent alcohol use on HIV transmission risk behaviour and found evidence of interaction on the multiplicative and additive scales. Our findings are not directly comparable to theirs given the differences in the nature and number of exposures examined, and the differences in how the exposures were categorised. However, the empirical approach is similar given that both of our analyses serve as a more appropriate test of syndemic theory than has been adopted in the literature to date (Tsai & Burns, 2015; Tsai et al. Lancet 2017).
One point of similarity between the present study and Tomori et al. (2018) is that we also adopted the sum score approach solely for the sake of comparing our findings with previously published studies (Tsai & Venkataramani, 2016). We found that the sum score had statistically significant associations with HIV transmission risk behaviour whether the sum score was specified as a continuous or categorical variable. This finding is similar to what has been used in classic studies in the literature on syndemics (Mustanski et al., 2007, Stall et al., 2003). These studies, which were among the earliest of their kind in the HIV literature, demonstrated the importance of understanding how cumulative disadvantage/cumulative adversity influence HIV risk, highlighted the need to emergently address multiple co-occurring epidemics among MSM in the U.S., and paved the way for a large scientific literature to follow and describe a complex and changing epidemic in a vulnerable population (Stall et al., 2001). Had we limited our analysis to use of the sum score approach, our analysis would have been incomplete in describing the extent to which there is strong evidence of a syndemic in this population. There is little consensus about how syndemics should be operationalised and tested in epidemiological studies, with contradictory language used even by seminal contributors to the field (Singer, 1996, Singer and Clair, 2003). Our study demonstrates that the extent to which the evidence is consistent with a syndemic is contingent upon how a syndemic is conceptualised (Tsai, 2018). Had we limited our investigation to studying the model of serially causal epidemics, sometimes referred to as the “snowball” effect (Stall et al., 2008), we might have concluded that a syndemic was not present. Further conceptual research is necessary to distinguish between the theory of syndemics and other theories of disease distribution so that these theories can be empirically tested to form the basis for clinical, policy, or programmatic recommendations.
Interpretation of our findings is subject to several limitations. First, the cross-sectional nature of the data preclude causal inference. However, theoretical considerations and differing recall windows for the adverse psychosocial exposures of interest (violence victimisation – one year; drug use – one year, and alcohol use – one week), suggest that the three models we tested are plausible. Second, self-reported alcohol use and drug use may have underestimated actual consumption. Despite this possibility, the reported prevalence of (any) alcohol use in this study (48.6%) was higher than that reported in the general male population (29.2%) (MoHFW & IIPS, 2016), suggesting that under-reporting may have been less likely. Third, the survey questions on alcohol and drug use had different recall windows (one week for alcohol use and one year for drug use). Differing recall windows were used because a much larger proportion of participants were expected to report alcohol use compared with drug use (Sivasubramanian et al., 2011, Tomori et al., 2018). Given that the condom use questions preceded the alcohol and drug use questions in order in the administered survey, differential reporting of use of alcohol and illicit drugs based solely on prior responses to the condom use questions is unlikely. Fourth, the models for synergistically interacting epidemics, serially causal epidemics, and mutually causal epidemics were non-nested models. Support for the model of serially causal epidemics appeared to be less strong than the other two, based on the magnitudes of the estimated associations and mediated effects. However, we were unable to make statistical comparisons to adjudicate between the modelling approaches. Fifth, in the mutually causal model, we could not test for a mutually reinforcing effect of alcohol use (past week) on violence victimisation (past year) due to the difference in the timeframes in which the data on these two exposures were captured. Sixth, we emphasise that our analysis only examines co-occurrence and interactions between exposures at the individual level. A syndemic, like an epidemic, is a population-level phenomenon. An empirical finding, based on data from individuals, that alcohol & drug use and violence victimisation synergistically interact to amplify HIV risk at the individual level is not equivalent to a finding that the epidemics of alcohol and drug use, and violence victimisation synergistically interact to worsen the HIV epidemic at the population level. It would be most appropriate to analyse a syndemic using data that have to do with populations. Such data could be solely ecological or could be both ecological and individual (i.e., multilevel). Multilevel analyses could potentially be used, for example, to test the extent to which structural forces give rise to psychosocial problems that compound over the life course and exacerbate HIV risk. In the systematic review by Tsai and Burns (2015) subsequently updated by Tsai et al. (2017), none of the empirical studies in the syndemics literature were based on ecological or multilevel data. Future work should draw on these other study designs to better understand the effects of syndemics on population health.
The present study has certain strengths as well. Our study represents the largest probability-based study of syndemics among MSM (Tsai & Burns, 2015). The non-response rate was low (15%). In line with the usual practice, to compute the statistical indices for assessing additive and multiplicative interactions, we dichotomised the adverse psychosocial exposures (VanderWeele, 2015). However, sensitivity analyses for multiplicative interactions revealed that similar findings (including the statistically significant three-way interaction) were obtained if we used different ways of dichotomising alcohol use, or if we specified the exposures on the continuous scale.
Implications for practice, policy and theory
The study findings suggest that drug use and violence victimisation independently, and in concert with alcohol use synergistically, increase HIV transmission risk behaviour. Inconsistent condom use was observed even if either violence victimisation or drug use alone were present, or in a particular range of frequency of consumption of alcohol use; thus, addressing even one or two of these three exposures could potentially reduce HIV transmission risk behaviour. While integrated, multicomponent interventions could nonetheless be effective in reducing sexual risk, our findings suggest that in the setting of synergistic interactions, single-component interventions can still have an incremental impact on reducing HIV transmission risk behaviour. Although addressing all key exposures in such situations could more substantially reduce risk, single-component interventions may be of interest especially if there are budgetary constraints in resource-limited settings; formal cost-effectiveness analyses may guide such decisions. The partial support found for the model of serially causal epidemics points out the complexity of how exposures may be related to each other in the real world. For exposures that lead to other exposures (e.g., violence victimisation leading to alcohol and drug use), interventions at different levels might be beneficial (e.g., stigma reduction and violence prevention efforts at the societal level, and screening and management of alcohol and drug use at the individual level). These programmatic recommendations, which are more nuanced, evidence-informed and pragmatic, stand in contrast to existing calls for integrated, multicomponent interventions, which predominate in the literature and which are based on findings from studies based on the sum score specification (Tsai & Burns, 2015).
It should be noted here that there are often situations of public health emergency, or perhaps even public health urgency, particularly in vulnerable populations, in which there is a need for interventions to be developed, piloted, field tested and potentially even deployed without requiring definitive evidence validating the conceptual framework motivating the intervention. Here we are in agreement with the admonition provided by Stall, Coulter, Friedman, and Plankey (2015), who usefully caution: “It is untenable to halt the development of interventions that rely on syndemics theory until the question of synergy is settled” (p.130). We add here that even if it turns out that the clustering of psychosocial problems within vulnerable populations does not (empirically) line up with how syndemics have formally been defined in theory, such a scenario would not obviate the need to address social determinants in vulnerable populations, nor would such a scenario obviate the need to address the multiple health risks in such populations. Neither of these conceptual features are unique to the theory of syndemics. Certainly interventions targeting health risks that are clustered in the context of harmful social conditions can be motivated by other theories of disease causation and disease distribution (e.g., structural violence (Farmer, 1996)), fundamental cause theory (Link & Phelan, 1995), ecosocial theory (Krieger, 1994), and/or multimorbidity (Melis et al., 2017, van den Akker et al., 1996), either alone or in combination, that also provide useful conceptual alternatives to the theory of syndemics for policy and programmatic interventions.
Although alcohol use was not found to be a statistically significant predictor of HIV transmission risk behaviour, we found that, in the presence of violence victimisation, or in the presence of both drug use and violence victimisation, alcohol use substantially increased HIV transmission risk behaviour. The non-governmental agencies that implement HIV prevention interventions in India should establish efficient referral systems within public health settings that offer treatment for alcohol and drug dependence, or post-violence support services. In addition, prevention efforts at the individual level in terms of education or counselling about the ill effects of alcohol and drug use (particularly their use prior to engaging in sexual intercourse), and stigma reduction campaigns to reduce the stigma and violence faced by MSM, are needed. Given that larger social forces like negative attitudes toward same-sex attracted persons and criminalisation of consensual adult same-sex intercourse contribute to violence victimisation and indirectly lead to alcohol and drug use through internalised homonegativity and depression (Chakrapani et al., 2018, Meyer, 2003, Stall et al., 2008, Starks et al., 2013), addressing these structural/social forces are also important.
The presence of co-occurring adverse psychosocial exposures (with prevalence rates ranging from 10% to 25%) and both additive and multiplicative synergistic interactions in the data, in the setting of harmful social conditions in India that give rise to this concentration of disease, supports the syndemic model of synergistically interacting epidemics (Singer & Clair, 2003). The data also support the possibility that the relations between these exposures may be serially causal, but the mediated effect was relatively small in magnitude. A few studies have tested whether the effect of violence victimisation on sexual risk is mediated by the number of adverse psychosocial exposures (Herrick et al., 2014, Tulloch et al., 2015), but not with the individual exposures as mediators. A longitudinal study, especially one that uses both quantitative and qualitative components, would help in deciphering the causal mechanisms as well as provide further evidence for the presence of synergy among these and other identified psychosocial exposures (e.g., internalised homonegativity, sexual compulsivity) that were not measured in this study. Long-term studies, with data on exposures at different life stages, could provide further support for the “snowball” hypothesis of cascading health risks that has been elaborated in several influential treatises on the subject (Dyer et al., 2012, Egan et al., 2011, Herrick et al., 2013, Mayer et al., 2012, Stall et al., 2008).
Conclusion
This study offers empirical support to three models of co-occurring epidemics: synergistically interacting epidemics, serially causal epidemics, and mutually causal epidemics. Independent exposures to violence victimisation (which often results from structural discrimination) and drug use increase HIV-related sexual risk. We found evidence for synergistic (additive and multiplicative) interactions between violence victimisation, drug use, and frequent alcohol use on HIV transmission risk behaviour. Evidence for the model of serially causal epidemics suggests that prevention of violence victimisation could potentially prevent some of the alcohol and drug use burden, thereby decreasing sexual risk, but this evidence was less robust. Similarly, the evidence for the model of mutually causal epidemics suggests that simultaneously preventing and addressing both violence victimisation and drug use through integrated interventions may be needed to effectively decrease HIV transmission risk behaviour. Future work should more thoroughly elaborate the harmful social forces that contribute to syndemics, and employ longitudinal mixed methods and conduct cross-level (multi-level) analyses to further characterize the nature of interactions between adverse psychosocial exposures, contributing to both theory and practice.
Acknowledgements
The Integrated Behavioural and Biological Assessment (IBBS) was primarily funded by the Government of India, Ministry of Health and Family Welfare, with complementary funding from CDC-DGHA (CDC’s Division of Global HIV/AIDS) India through FHI 360, Public Health Foundation of India and WHO India. Dr. Venkatesan Chakrapani was in part supported by the Wellcome Trust/DBT India Alliance Senior Fellowship (IA/CPHS/16/1/502667).
Acknowledgments
Data statement
Researchers who are interested in examining the data need to write to the National AIDS Control Organisation (NACO), India. The fifth author can be contacted in relation to accessing datasets. Access will be granted to de-identified datasets on a case-by-case basis.
Ethics statement
The primary study, called ‘Integrated Behavioural and Biological Assessment [IBBS]’, was approved by the ethics committee constituted by the National AIDS Control Organisation, India. This manuscript is based on the secondary analyses of the data collected from this study.
Declaration of interest
None.
References
- Anda R.F., Croft J.B., Felitti V.J., Nordenberg D., Giles W.H., Williamson D.F. Adverse childhood experiences and smoking during adolescence and adulthood. JAMA. 1999;282:1652–1658. doi: 10.1001/jama.282.17.1652. [DOI] [PubMed] [Google Scholar]
- Biello K.B., Colby D., Closson E., Mimiaga M.J. The syndemic condition of psychosocial problems and HIV risk among male sex workers in Ho Chi Minh City, Vietnam. AIDS and Behavior. 2014;18:1264–1271. doi: 10.1007/s10461-013-0632-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blunch N.J. SAGE; Los Angeles: 2013. Introduction to structural equation modeling using IBM SPSS statistics and AMOS. [Google Scholar]
- Chakrapani V., Kaur M., Newman P.A., Mittal S., Kumar R. Syndemics and HIV-related sexual risk among men who have sex with men in India: Influences of stigma and resilience. Culture, Health & Sexuality. 2018:1–16. doi: 10.1080/13691058.2018.1486458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrapani V., Newman P.A., Shunmugam M., Logie C.H., Samuel M. Syndemics of depression, alcohol use, and victimisation, and their association with HIV-related sexual risk among men who have sex with men and transgender women in India. Global Public Health. 2017;12:250–265. doi: 10.1080/17441692.2015.1091024. [DOI] [PubMed] [Google Scholar]
- Chakrapani V., Newman P.A., Shunmugam M., McLuckie A., Melwin F. Structural violence against Kothi-identified men who have sex with men in Chennai, India: A qualitative investigation. AIDS Education and Prevention. 2007;19:346–364. doi: 10.1521/aeap.2007.19.4.346. [DOI] [PubMed] [Google Scholar]
- Chan B.T., Tsai A.C. HIV knowledge trends during an era of rapid antiretroviral therapy scale-up: An analysis of 33 sub-Saharan African countries. Journal of the International AIDS Society. 2018;21:e25169. doi: 10.1002/jia2.25169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coie J.D., Watt N.F., West S.G., Hawkins J.D., Asarnow J.R., Markman H.J. The science of prevention. A conceptual framework and some directions for a national research program. American Psychologist. 1993;48:1013–1022. doi: 10.1037//0003-066x.48.10.1013. [DOI] [PubMed] [Google Scholar]
- Dyer T.P., Shoptaw S., Guadamuz T.E., Plankey M., Kao U., Ostrow D. Application of syndemic theory to black men who have sex with men in the Multicenter AIDS Cohort Study. Journal of Urban Health. 2012;89:697–708. doi: 10.1007/s11524-012-9674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan J.E., Frye V., Kurtz S.P., Latkin C., Chen M., Tobin K. Migration, neighborhoods, and networks: Approaches to understanding how urban environmental conditions affect syndemic adverse health outcomes among gay, bisexual and other men who have sex with men. Aids and Behavior. 2011;15(Suppl 1):S35–S50. doi: 10.1007/s10461-011-9902-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer P. Women, poverty, and AIDS. In: Farmer P., Connors M., Simmons J., editors. Women, poverty and AIDS: Sex, drugs, and structural violence. Common Courage Press; Monroe: 1996. pp. 3–38. [Google Scholar]
- Felitti V.J., Anda R.F., Nordenberg D., Williamson D.F., Spitz A.M., Edwards V. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Ferlatte O., Hottes T.S., Trussler T., Marchand R. Evidence of a syndemic among young Canadian gay and bisexual men: Uncovering the associations between anti-gay experiences, psychosocial issues, and HIV risk. Aids and Behavior. 2014;18:1256–1263. doi: 10.1007/s10461-013-0639-1. [DOI] [PubMed] [Google Scholar]
- Guadamuz T.E., McCarthy K., Wimonsate W., Thienkrua W., Varangrat A., Chaikummao S. Psychosocial health conditions and HIV prevalence and incidence in a cohort of men who have sex with men in Bangkok, Thailand: Evidence of a syndemic effect. Aids and Behavior. 2014;18:2089–2096. doi: 10.1007/s10461-014-0826-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeringa S.G., Berglung, West . CRC Press; Boca Raton: 2010. Applied survey data analysis. [Google Scholar]
- Herrick A., Lim S.H., Plankey M.W., Chmiel J.S., Guadamuz T.E., Kao U. Adversity and syndemic production among men participating in the multicenter AIDS cohort study: A life-course approach. American Journal of Public Health. 2013;103:79–85. doi: 10.2105/AJPH.2012.300810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick A., Stall R., Egan J., Schrager S., Kipke M. Pathways towards risk: Syndemic conditions mediate the effect of adversity on HIV risk behaviors among young men who have sex with men (YMSM) Journal of Urban Health. 2014;91:969–982. doi: 10.1007/s11524-014-9896-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Lt, Bentler P.M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6:1–55. [Google Scholar]
- Knol M.J., VanderWeele T.J. Recommendations for presenting analyses of effect modification and interaction. International Journal of Epidemiology. 2012;41:514–520. doi: 10.1093/ije/dyr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N. Epidemiology and the web of causation: Has anyone seen the spider? Social Science & Medicine. 1994;39:887–903. doi: 10.1016/0277-9536(94)90202-x. [DOI] [PubMed] [Google Scholar]
- Kuh D., Ben-Shlomo Y., Lynch J., Hallqvist J., Power C. Life course epidemiology. Journal of Epidemiology and Community Health. 2003;57:778–783. doi: 10.1136/jech.57.10.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link B.G., Phelan J. Social conditions as fundamental causes of disease. Journal of Health and Social Behavior. 1995 (Spec No, 80-94) [PubMed] [Google Scholar]
- Mayer K.H., Bekker L.-G., Stall R., Grulich A.E., Colfax G., Lama J.R. Comprehensive clinical care for men who have sex with men: An integrated approach. Lancet. 2012;380:378–387. doi: 10.1016/S0140-6736(12)60835-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S.H., Lucas G.M., Solomon S., Srikrishnan A.K., McFall A.M., Dhingra N. HIV care continuum among men who have sex with men and persons who inject drugs in India: Barriers to successful engagement. Clinical Infectious Diseases. 2015;61:1732–1741. doi: 10.1093/cid/civ669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis R.J., Gijzel S.M., Olde Rikkert M.G. Moving beyond multimorbidity as a simple count of diseases. Journal of Evaluation in Clinical Practice. 2017;23:216–218. doi: 10.1111/jep.12693. [DOI] [PubMed] [Google Scholar]
- Mendenhall E., Kohrt B.A., Norris S.A., Ndetei D., Prabhakaran D. Non-communicable disease syndemics: Poverty, depression, and diabetes among low-income populations. Lancet. 2017;389:951–963. doi: 10.1016/S0140-6736(17)30402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendenhall E., Narayanan G., Prabhakaran D. Depression and diabetes in India: Perspectives and recommendations. Diabetic Medicine. 2012;29:e308–e311. doi: 10.1111/j.1464-5491.2012.03708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer I.H. Prejudice, social stress, and mental health in lesbian, gay, and bisexual populations: Conceptual issues and research evidence. Psychology Bulletin. 2003;129:674–697. doi: 10.1037/0033-2909.129.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimiaga M.J., O’Cleirigh C., Biello K.B., Robertson A.M., Safren S.A., Coates T.J. The effect of psychosocial syndemic production on 4-year HIV incidence and risk behavior in a large cohort of sexually active men who have sex with men. Journal of Acquired Immune Deficiency Syndromes. 2015;68:329–336. doi: 10.1097/QAI.0000000000000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MoHFW, IIPS . Ministry of Health and Family Welfare, International Institute for Population Sciences; New Delhi: 2016. National family health survey - 4, 2015-16: India fact sheet. [Google Scholar]
- Mustanski B., Garofalo R., Herrick A., Donenberg G. Psychosocial health problems increase risk for HIV among urban young men who have sex with men: Preliminary evidence of a syndemic in need of attention. Annals of Behavioral Medicine. 2007;34:37–45. doi: 10.1080/08836610701495268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NACO . NACO; New Delhi: 2015. National Integrated Biological and Behavioural Surveillance (IBBS), India 2014–2015. [Google Scholar]
- NACO (2017). Annual Report - 2016-17. National AIDS Control Organization (NACO). New Delhi.
- Osborne J.W. SAGE; Los Angeles: 2014. Best practices in logistic regression. [Google Scholar]
- Rothman K.J. Synergy and antagonism in cause-effect relationships. American Journal of Epidemiology. 1974;99:385–388. doi: 10.1093/oxfordjournals.aje.a121626. [DOI] [PubMed] [Google Scholar]
- Rothman K.J., Greenland S., Walker A.M. Concepts of interaction. American Journal of Epidemiology. 1980;112:467–470. doi: 10.1093/oxfordjournals.aje.a113015. [DOI] [PubMed] [Google Scholar]
- Safren S., Reisner S.L., Herrick A., Mimiaga M.J., Stall R.D. Mental health and HIV risk in men who have sex with men. Journal of Acquired Immune Deficiency Syndromes. 2010;55(Suppl 2):S74–S77. doi: 10.1097/QAI.0b013e3181fbc939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safren S., Thomas B.E., Mimiaga M.J., Chandrasekaran V., Menon S., Swaminathan S. Depressive symptoms and human immunodeficiency virus risk behavior among men who have sex with men in Chennai, India. Psychology, Health & Medicine. 2009;14:705–715. doi: 10.1080/13548500903334754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S.Y., Lorway R.R., Deering K.N., Avery L., Mohan H.L., Bhattacharjee P. Factors associated with sexual violence against men who have sex with men and transgendered individuals in Karnataka, India. PLoS One. 2012;7:e31705. doi: 10.1371/journal.pone.0031705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons J.S., Simons R.M., Maisto S.A., Hahn A.M., Walters K.J. Daily associations between alcohol and sexual behavior in young adults. Experimental and Clinical Psychopharmacology. 2018;26:36–48. doi: 10.1037/pha0000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M. A dose of drugs, a touch of violence, a case of AIDS: Conceptualizing the SAVA syndemic. Free Inquiry in Creative Sociology. 1996;24:99–110. [Google Scholar]
- Singer M., Clair S. Syndemics and public health: Reconceptualizing disease in bio-social context. Medical Anthropology Quarterly. 2003;17:423–441. doi: 10.1525/maq.2003.17.4.423. [DOI] [PubMed] [Google Scholar]
- Singer M., Erickson P.I., Badiane L., Diaz R., Ortiz D., Abraham T. Syndemics, sex and the city: Understanding sexually transmitted diseases in social and cultural context. Social Science & Medicine. 2006;63:2010–2021. doi: 10.1016/j.socscimed.2006.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasubramanian M., Mimiaga M.J., Mayer K.H., Anand V.R., Johnson C.V., Prabhugate P. Suicidality, clinical depression, and anxiety disorders are highly prevalent in men who have sex with men in Mumbai, India: Findings from a community-recruited sample. Psychology, Health & Medicine. 2011;16:450–462. doi: 10.1080/13548506.2011.554645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon S.S., Mehta S.H., Srikrishnan A.K., Vasudevan C.K., McFall A.M., Balakrishnan P. High HIV prevalence and incidence among MSM across 12 cities in India. AIDS. 2015;29:723–731. doi: 10.1097/QAD.0000000000000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon G., Haider S.J., Wooldridge J.M. What are we weighting for? Journal of Human Resources. 2015;50:301–316. [Google Scholar]
- Stall R., Coulter R.W., Friedman M.R., Plankey M.W. Commentary on “Syndemics of psychosocial problems and HIV risk: A systematic review of empirical tests of the disease interaction concept” by A. Tsai and B. Burns. Social Science & Medicine. 2015;145:129–131. doi: 10.1016/j.socscimed.2015.07.016. [DOI] [PubMed] [Google Scholar]
- Stall R., Friedman M., Catania J.A. Interacting epidemics and gay men’s health: A theory of syndemic production among urban gay men. In: Wolitski R.J., Stall R., Valdiserri R.O., editors. Unequal opportunity: health disparities affecting gay and bisexual men in the United States. Oxford University Press; New York: 2008. [Google Scholar]
- Stall R., Mills T.C., Williamson J., Hart T., Greenwood G., Paul J. Association of co-occurring psychosocial health problems and increased vulnerability to HIV/AIDS among urban men who have sex with men. American Journal of Public Health. 2003;93:939–942. doi: 10.2105/ajph.93.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stall R., Paul J.P., Greenwood G., Pollack L.M., Bein E., Crosby G.M. Alcohol use, drug use and alcohol-related problems among men who have sex with men: The Urban Men’s Health Study. Addiction. 2001;96:1589–1601. doi: 10.1046/j.1360-0443.2001.961115896.x. [DOI] [PubMed] [Google Scholar]
- Starks T.J., Rendina H.J., Breslow A.S., Parsons J.T., Golub S.A. The psychological cost of anticipating HIV stigma for HIV-negative gay and bisexual men. Aids and Behavior. 2013;17:2732–2741. doi: 10.1007/s10461-013-0425-0. [DOI] [PubMed] [Google Scholar]
- Tomori C., McFall A.M., Solomon S.S., Srikrishnan A.K., Anand S., Balakrishnan P. Is there synergy in syndemics? Psychosocial conditions and sexual risk among men who have sex with men in India. Soc Sci Med. 2018;206:110–116. doi: 10.1016/j.socscimed.2018.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomori C., McFall A.M., Srikrishnan A.K., Mehta S.H., Nimmagadda N., Anand S. The prevalence and impact of childhood sexual abuse on HIV-risk behaviors among men who have sex with men (MSM) in India. Bmc Public Health. 2016;16:784. doi: 10.1186/s12889-016-3446-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai A.C. Syndemics: A theory in search of data or data in search of a theory? Social Science & Medicine. 2018 doi: 10.1016/j.socscimed.2018.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai A.C., Burns B.F. Syndemics of psychosocial problems and HIV risk: A systematic review of empirical tests of the disease interaction concept. Social Science & Medicine. 2015;139:26–35. doi: 10.1016/j.socscimed.2015.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai A.C., Mendenhall E., Trostle J.A., Kawachi I. Co-occurring epidemics, syndemics, and population health. Lancet. 2017;389:978–982. doi: 10.1016/S0140-6736(17)30403-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai A.C., Venkataramani A.S. Syndemics and health disparities: A methodological note. Aids and Behavior. 2016;20:423–430. doi: 10.1007/s10461-015-1260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulloch T.G., Rotondi N.K., Ing S., Myers T., Calzavara L.M., Loutfy M.R. Retrospective reports of developmental stressors, syndemics, and their association with sexual risk outcomes among gay men. Archives of Sexual Behavior. 2015;44:1879–1889. doi: 10.1007/s10508-015-0479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Akker M., Buntinx F., Knottnerus J.A. Comorbidity or multimorbidity. European Journal of General Practice. 1996;2:65–70. [Google Scholar]
- VanderWeele T.J. Oxford University Press; New York: 2015. Explanation in causal inference: Methods for mediation and interaction. [Google Scholar]
- Weaver L.J., Mendenhall E. Applying syndemics and chronicity: Interpretations from studies of poverty, depression, and diabetes. Medical Anthropology. 2014;33:92–108. doi: 10.1080/01459740.2013.808637. [DOI] [PubMed] [Google Scholar]
- Woodward E.N., Banks R.J., Marks A.K., Pantalone D.W. Identifying resilience resources for HIV prevention among sexual minority men: a systematic review. AIDS and Behavior. 2017;21:2860–2873. doi: 10.1007/s10461-016-1608-2. [DOI] [PubMed] [Google Scholar]
- Yadav D., Chakrapani V., Goswami P., Ramanathan S., Ramakrishnan L., George B. Association between alcohol use and HIV-related sexual risk behaviors among men who have sex with men (MSM): Findings from a multi-site bio-behavioral survey in India. Aids and Behavior. 2014;18:1330–1338. doi: 10.1007/s10461-014-0699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]