Abstract
Germline stem cells (GSCs) self-renew and differentiate to sustain a continuous production of gametes. In the female Drosophila germ line, two differentiation factors, bag of marbles (bam) and benign gonial cell neoplasm (bgcn), work in concert in the stem cell daughter to promote the generation of eggs. In GSCs, bam transcription is repressed by signaling from the niche and is activated in stem cell daughters. In contrast, bgcn is transcribed in both the GSCs and stem cell daughters, but little is known about how bgcn is transcriptionally modulated. Here we find that the conserved protein Nipped-A acts through the Tat interactive protein 60-kDa (Tip60) histone acetyl transferase complex in the germ line to promote GSC daughter differentiation. We find that Nipped-A is required for efficient exit from the gap phase 2 (G2) of cell cycle of the GSC daughter and for expression of a differentiation factor, bgcn. Loss of Nipped-A results in accumulation of GSC daughters. Forced expression of bgcn in Nipped-A germline-depleted ovaries rescues this differentiation defect. Together, our results indicate that Tip60 complex coordinates cell cycle progression and expression of bgcn to help drive GSC daughters toward a differentiation program.
INTRODUCTION
Germ line gives rise to sperm and eggs that function as links between generations by passing information from parent to offspring. In the adult gonads, germ cells can acquire germline stem cell (GSC) fate that allows them to both self-renew and differentiate to ensure a steady supply of gametes. Failure to regulate GSC self-renewal and differentiation programs leads to infertility. Thus, understanding how GSCs divide and differentiate is critical to understanding the biological basis of reproductive success (Spradling et al., 1997, 2008, 2011; Boyle et al., 2007; Morrison and Spradling, 2008; Lehmann, 2012).
Drosophila female GSCs are an excellent model system to study stem cell dynamics due to precise characterization of early events in GSC differentiation and availability of mutants and markers (Dansereau and Lasko, 2008; Spradling et al., 2011; Flora et al., 2017). Drosophila female GSCs are housed in a structure called the germarium (Figure 1A). The germarium consists of both germ line and somatic cells. The somatic cells constitute the niche for the GSCs that divide asymmetrically, giving rise to self-renewed GSCs and daughters called precystoblasts (pre-CBs) (Chen and McKearin, 2003a). Both GSCs and their daughters are marked by endoplasmic reticulum (ER)-rich structures called spectrosomes (de Cuevas et al., 1996). The pre-CB will then differentiate to become a CB by expressing the differentiation factor Bag of marbles (Bam) that acts in concert with Benign gonial cell neoplasm (Bgcn) (McKearin and Spradling, 1990; Ohlstein and McKearin, 1997). The CB then undergoes four incomplete divisions and gives rise to cysts, marked by branched structures called fusomes (de Cuevas and Spradling, 1998). Following the fourth division, a 16-cell cyst is made, where one cell will develop into the oocyte and the other 15 will become support cells called nurse cells. The 16-cell cyst migrates, buds off from the germarium to form egg chambers, and eventually generates mature eggs (Huynh and St Johnston, 2000; Narbonne-Reveau et al., 2006).
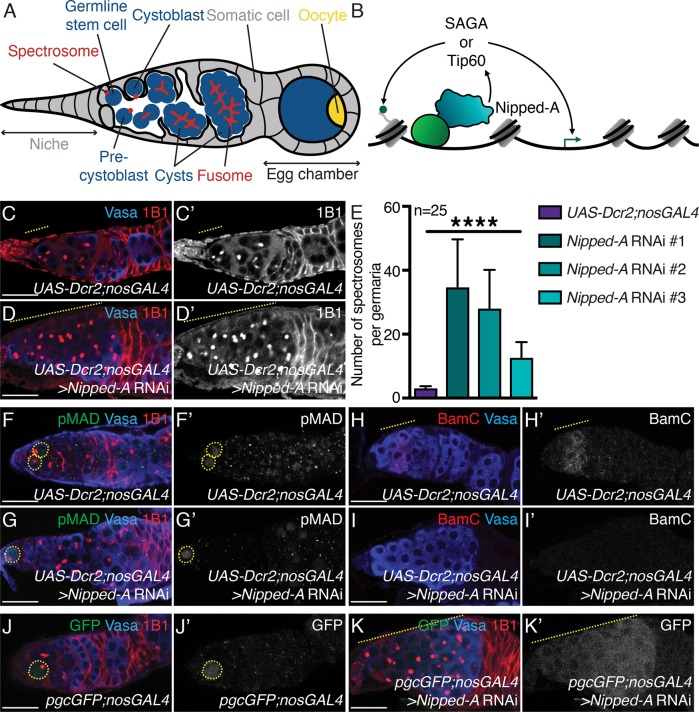
FIGURE 1:
Nipped-A is required in the germ line for differentiation of the germline stem cell daughter. (A) Schematic representation of a Drosophila germarium where germ cells (blue) are surrounded by somatic cells (gray). The germline stem cells (GSCs) reside in the germarium and are maintained by a somatic niche. The GSC divides to give rise to a daughter, called a precystoblast (pre-CB). The pre-CB turns on differentiation factors and is then called the cystoblast (CB). The CB undergoes incomplete mitotic divisions to give rise to 2-, 4-, 8-, and 16-cell cysts. Single cells are marked by punctate structures called spectrosomes (red), and cysts are marked by the elongated, branched spectrosomes called fusomes (red). The 16-cell cyst migrates, buds off from the germarium, and is encapsulated by the soma (gray) to generate egg chambers. Developing egg chambers will have one germ cell that becomes the oocyte (yellow), and the other germ cells will be support cells (blue). (B) Schematic of Nipped-A function. Nipped-A (teal) can associate with transcriptional activators (light green) to recruit SAGA and Tip60 complexes. These complexes can acetylate lysines on histones (dark green circle) to regulate transcription (dark green arrow). Nipped-A cartoon is based on the Cryo-EM structure of Tra1 in SAGA complex in Pichia pastoris yeast (Sharov et al., 2017). (C, C′) Control and (D, D′) germline-depleted Nipped-A (RNAi line #1) germaria stained with Vasa (blue) and 1B1 (red). Germaria depleted of Nipped-A show accumulation of single cells (yellow dashed line). 1B1 channel is shown in C′ and D′. (E) Quantitation of the number of single cells in germaria of control and germline-depleted Nipped-A using three RNAi lines (34.64 ± 15.04 in Nipped-A RNAi #1, 27.96 ± 12.17 in Nipped-A RNAi #2, and 12.56 ± 4.94 in Nipped-A RNAi #3 compared with 3.04 ± 0.68 in UAS-Dcr2;nosGAL4 control; n = 25 for all, ****P < 0.0001). (F, F′) Control and (G, G′) germline-depleted Nipped-A germaria stained with pMAD (green), Vasa (blue), and 1B1 (red). Germaria depleted of Nipped-A do not accumulate pMAD-positive germ cells (yellow dashed circle) (n = 20 for both, P < 0.0001). pMAD channel is shown in F′ and G′. (H, H′) Control and (I, I′) germline-depleted Nipped-A germaria stained with BamC (red) and Vasa (blue). Germaria depleted of Nipped-A do not accumulate BamC-positive germ cells (yellow dashed line in control) (n = 25 for both). BamC channel is shown in H′ and I′. (J, J′) Control and (K, K′) pgcGFP with Nipped-A germline-depletion germaria stained with GFP (green), Vasa (blue), and 1B1 (red). Germaria depleted of Nipped-A accumulate a higher number of Pgc-positive germ cells (n = 25 for both, P < 0.0001). Pgc expression is marked by GFP (yellow dashed circle/line in control and knockdown, respectively). GFP channel is shown in J′ and K′. Statistical analysis performed with Student’s t test for all except for Chi-square for H–I′. Scale bar for all images is 20 μm.
GSC self-renewal and differentiation is exquisitely balanced by both extrinsic and intrinsic factors (Xie and Spradling, 2000; Pan et al., 2007; Slaidina and Lehmann, 2014; Upadhyay et al., 2016; Flora et al., 2017). One such extrinsic factor is the signaling from the somatic niche, which promotes GSC self-renewal. GSCs remain in contact with the somatic niche and receive Decapentaplegic (Dpp) signaling, while the pre-CB will be displaced from the niche after asymmetric division (Xie and Spradling, 1998, 2000; Kai and Spradling, 2003; Casanueva, 2004). The Dpp ligand binds to Thick veins (TKV) and Saxophone (Sax) receptors on GSCs, leading to the phosphorylation of Mothers-against-Dpp (pMAD) (Brummel et al., 1994; Penton et al., 1994; Xie et al., 1994; Letsou et al., 1995; Sekelsky et al., 1995). pMAD translocates to the nucleus to directly suppress the expression of the differentiation factor, bam, to promote GSC maintenance (Kai and Spradling, 2003; Chen and McKearin, 2003a,b). Once the pre-CB is displaced from the niche it is removed away from the source of Dpp signaling and is now able to express bam (Chen and McKearin, 2003b; Xia et al., 2010). Additionally, transient transcriptional silencing in the pre-CB helps reprogram the cell cycle of the pre-CB to promote the expression of Bam (Flora et al., 2018). TKV receptors are turned over, and Bam complexes with Bgcn, which is expressed from GSC stage onwards, to down-regulate GSC maintenance factors (Li et al., 2009; Kim et al., 2010a). Like Bgcn in Drosophila, its homologue in mice, YTH Domain Containing 2 (YTHDC2) is required for a meiotic cell fate and gamete production (Bailey et al., 2017; Soh et al., 2017; Jain et al., 2018). However, it is not yet known what regulates bgcn expression in the GSCs and pre-CBs.
Extensive remodeling of the GSC epigenome occurs during differentiation (Chen et al., 2008; Rangan et al., 2011; Flora et al., 2017). While several repressive mechanisms have been identified in GSCs, that down-regulate the differentiation program, little is known about how differentiation-promoting genes are positively regulated. One class of enzymes that can activate transcription is histone acetyltransferases (HATs). There are five classes of HATs, two of which are general control of nuclear-5–(Gcn5)-related N-acetyltransferases (GNATs) and “MOZ, Ybf2/Sas3, Sas2, and Tip60” (MYST)-related HATs (Roth et al., 2001; Carrozza et al., 2003; Lee and Workman, 2007). GNATs and MYST HATs are members of Spt-Ada-Gcn5-acetyltransferase (SAGA) and Tat interactive protein 60-kDa (Tip60) complexes, respectively (Grant et al., 1998; Saleh et al., 1998; Allard et al., 1999). Nipped-A is a conserved member of both Tip60 and SAGA complexes (Grant et al., 1998; Allard et al., 1999; Ikura et al., 2000; Kusch et al., 2004). In yeast and humans, Nipped-A interacts with transcriptional activators to recruit Tip60 and SAGA complexes (Grant et al., 1998; Brown et al., 2001; Carrozza et al., 2003) (Figure 1B). Thus, Nipped-A plays an important role in targeting those HAT-containing complexes to promoters (Vassilev et al., 1998; Allard et al., 1999). SAGA complex’s HAT module includes Gcn5 that in Drosophila is required for oogenesis but does not seem to affect the earliest stages of GSC daughter differentiation (Li et al., 2017). And while Tip60 complex HAT activity has been shown to regulate GSC maintenance and differentiation in Drosophila testis (Feng et al., 2017, 2018), its role in promoting female germline differentiation both intrinsically and extrinsically is not known.
Tip60 complex acetylates (ac) lysines (K) 5, 8, 12, and 16 on histone H4, among other targets, to maintain cellular homeostasis (Steunou et al., 2013; Jacquet et al., 2016). By acetylating histones and transcription factors, Tip60 complex regulates expression of proto-oncogenes, cell cycle regulators, and stress responses (Patel et al., 2004; Avvakumov and Côté, 2007a,b; Ikeda et al., 2017) (Figure 1B). Furthermore, human Tip60 complex has been implicated in several diseases, most notably cancer (Avvakumov and Côté, 2007b; Voss and Thomas, 2009). But how Tip60 complex regulates normal development of stem cells and how this can become misregulated to lead to cancer has not been well characterized.
Here we have shown that Nipped-A is a crucial regulator of germline differentiation in female Drosophila. We find that Nipped-A, in conjunction with Tip60 complex members, is required for oogenesis. We report that Tip60 HAT activity is required for proper differentiation and loss of Tip60 complex members or its HAT activity leads to pre-CB accumulation and failure to generate mature eggs. We find Nipped-A to be required for expression of bgcn, a crucial component for differentiation. We propose that Tip60 complex coordinates cell cycle progression and expression of differentiation factors to drive germ cells into a differentiated cell fate.
RESULTS
Nipped-A is required in the germ line for proper germline stem cell daughter differentiation
To identify critical regulators of germline differentiation, we depleted epigenetic modifiers in the germ line utilizing a germline driver, nanosGAL4 (nosGAL4) and RNA interference (RNAi), then assayed for defects by staining for Vasa and 1B1 (Navarro-Costa, McCarthy, et al., 2016). Vasa marks the germ line and 1B1 marks specrosomes, fusomes, and somatic cell membranes (Figure 1A). We found depletion of Nipped-A in the germ line, leads to an accumulation of single germ cells with spectrosomes compared with control (Figure 1, C–E). Furthermore, we used two other RNAi lines targeting Nipped-A, and similarly observed accumulation of single cells on depletion (Figure 1E; Supplemental Figure 1, A–C′). To validate the RNAi line, we made use of a GFP-trap inserted into the Nipped-A locus (Nipped-AMI10513) and observed GFP intensity in control and Nipped-A–depleted germaria. We found Nipped-A–depleted germ cells had diminished levels of GFP intensity compared with control germaria (Supplemental Figure 1, D–E′). To determine whether Nipped-A has a role in somatic cells, we drove Nipped-A RNAi in the soma by using a traffic jamGAL4 (tjGAL4) driver and then stained for Vasa and 1B1. On depletion of somatic Nipped-A, we found that ovaries failed to form properly (100% in Nipped-A RNAi compared with 0% in tjGAL4 control; n = 10 ovary pairs for both, P < 0.0001) (Supplemental Figure 1, F and G). Thus, Nipped-A is required both in the germ line and the surrounding soma for germline development. Here, we focus on the germline role of Nipped-A that promotes GSC daughter differentiation.
We next asked at which step during the transition from GSCs to cysts does Nipped-A promote in the germ line. Tumors of single germ cells with spectrosomes can be either GSCs or GSC daughters. To test whether these accumulating germ cells are GSCs, we stained for the GSC marker, pMAD, and found no significant difference in the number of pMAD-positive germ cells in Nipped-A–depleted germaria compared with control (1.90 ± 0.85 in Nipped-A RNAi compared with 2.25 ± 0.44 in UAS-Dcr2;nosGAL4 control; n = 20 for both, P = 0.1117) (Figure 1, F–G′). We then asked whether these accumulating germ cells are GSC daughters. Immediate GSC daughters that have yet to express the differentiation factor Bam are pre-CBs. On Bam expression, the GSC daughter is then referred to as a CB. To identify whether these accumulating cells are either pre-CBs or CBs, we stained for BamC and found that only 4% of Nipped-A–depleted germaria were BamC-positive, while 100% of control germaria stained positive for BamC (n = 25 for both, P < 0.0001) (Figure 1, H–I′). These accumulating germ cells were neither pMAD nor BamC positive, suggesting that these cells are pre-CBs. Polar granule component (Pgc) is a transcriptional silencer expressed in gap phase 2 (G2) of the pre-CB cell cycle (Flora et al., 2018). To test whether the accumulating GSC daughters express Pgc, we utilized a fly line that expresses a pgcGFP reporter and nosGAL4 element (Flora et al., 2018). We found an average of 28.4 ± 7.11 germ cells of Nipped-A–depleted germaria were GFP positive compared with only an average of 0.05 ± 0.22 GFP-positive germ cells in control germaria (n = 20 for both, P < 0.0001) (Figure 1, J–K′). Thus, we conclude that Nipped-A in the germ line promotes differentiation of Pgc-positive pre-CBs.
Loss of Nipped-A in the germ line leads to accumulation of germ cells expressing Pgc that fail to differentiate. Pgc is required to promote expression of Cyclin B (CycB), which then promotes expression of Bam (Flora et al., 2018). We asked whether Nipped-A is required for up-regulation of CycB post-Pgc expression by staining for both Cyclin A (CycA) and CycB, markers of early and late G2, respectively (Jia et al., 2015). If Nipped-A–depleted germ cells fail to properly express CycB, then as a consequence they would not differentiate in a timely manner. To study the transient developmental pre-CB stage, we and others used genetic tools to enrich for pre-CBs by arresting development prior to differentiation (Kai and Spradling, 2003; Tastan et al., 2010; Flora et al., 2018). When we compared Nipped-A RNAi to bam RNAi germaria, we found that like bam-depleted germaria, Nipped-A–depleted germaria also accumulated cells in G2 phase of the cell cycle. However, Nipped-A–depleted germaria had a significantly higher number of CycB-positive cells compared bam-depleted germaria (Supplemental Figure 2, A–E). These cells are not arrested, as they eventually enter the cell cycle and divide (2.36 ± 3.34% phosphohistone– 3 (pH3) positive germ cells germ cells in Nipped-A RNAi compared with 3.92 ± 4.77% pH 3–positive germ cells in bam RNAi control; n = 25 for both, P = 0.2366). Taken together, we conclude that Nipped-A is required in the germ line after both Pgc and CycB expression to drive undifferentiated pre-CBs into a CB differentiation program.
Nipped-A acts as part of the Tip60 complex to promote differentiation
How does Nipped-A promote the transition from pre-CB to Bam-expressing CB state? Nipped-A is a member of both Tip60 and SAGA complexes that can activate transcription (Grant et al., 1998; Allard et al., 1999; Ikura et al., 2000; Kusch et al., 2004). To determine through which complex Nipped-A regulates early germline development, we systematically depleted Tip60 and SAGA complex members in the germ line. After depletion of 13 of the 16 Tip60 complex members in the germ line, we found loss of 11 of the members leads to an accumulation of germ cells with spectrosomes, depletion of reptin (rept) leads to complete loss of germ line, and depletion of Actin 87E (Act87E) failed to give any phenotype (Figure 2, A–G; Supplemental Figure 3, A–H′). When we depleted six of the 21 SAGA complex members in the germ line, including the HAT Gcn5 and SAGA-specific Ada2b, we found no significant difference in the number of spectrosome-containing cells compared with the control; consistent with what has been previously reported (Supplemental Figure 3I) (Li et al., 2017). Altogether, these data suggest that Nipped-A and members of the Tip60 complex in the germ line promote differentiation. Depletion-of-Tip60-complex members phenocopy loss of Nipped-A in the germ line. But accumulation of spectrosome-containing germ cells could also arise if Nipped-A and Tip60 complex work in parallel pathways. To test whether Nipped-A works in the same pathway as Tip60 complex, we genetically reduced one copy of Nipped-A and Tip60 in the same fly and stained for Vasa and 1B1. Tip60E0239;Nipped-ANC116 trans-heterozygote germaria accumulated more spectrosome-containing germ cells as compared with Nipped-ANC116heterozygotes and Tip60E0239heterozygotes alone (Figure 2, H–J). These results suggest that Nipped-A works as a part of the Tip60 complex to promote proper differentiation.
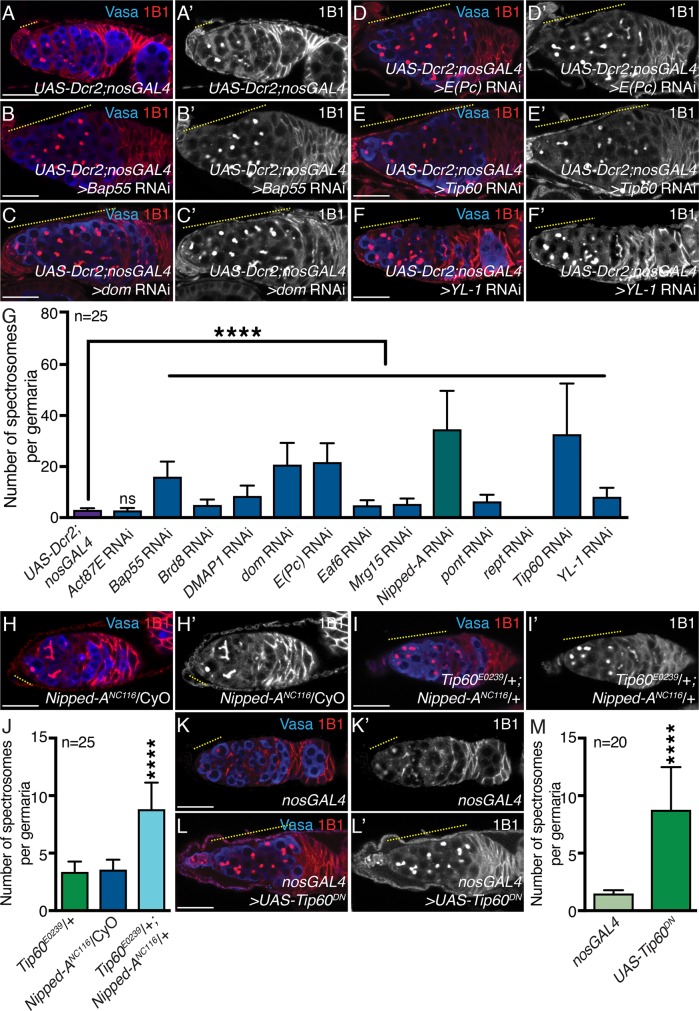
FIGURE 2:
Tip60 complex members and HAT activity are required for timely differentiation. (A, A′) Control and germline-depleted Tip60 complex members (B,B′) Bap55, (C, C′) dom, (D, D′) E(Pc), (E, E′) Tip60, and (F, F′) YL-1 germaria stained with Vasa (blue) and 1B1 (red). Tip60 complex member–depleted germaria show accumulation of single cells (yellow dashed line). 1B1 channel is shown in A′, B′, C′, D′, E′, and F′. (G) Quantitation of the number of single cells in control and germline-depleted Tip60 complex members Act87E, Bap55, Brd8, DMAP1, dom, E(Pc), Eaf6, Mrg15, Nipped-A, pont, rept, Tip60, and YL-1 germaria (3.20 ± 1.50 in Act87E, 16.04 ± 5.93 in Bap55 RNAi, 4.96 ± 2.15 in Brd8 RNAi, 8.52 ± 4.02 in DMAP1 RNAi, 20.76 ± 8.54 in dom RNAi, 21.76 ± 7.42 in E(Pc) RNAi, 4.80 ± 2 in Eaf6 RNAi, 5.36 ± 2.16 in Mrg15 RNAi, 34.64 ± 15.04 in Nipped-A RNAi, 6.36 ± 2.58 in pont RNAi, 0 ± 0 in rept RNAi, 32.72 ± 19.86 in Tip60 RNAi, and 8.16 ± 3.57 in YL-1 RNAi compared with 3.04 ± 0.68 in UAS-Dcr2;nosGAL4 control; n = 25 for all, P = 0.3110 for Act87E; ****P < 0.0001). (H, H′) Nipped-A heterozygote and (I, I′) Tip60;Nipped-A trans-heterozygote germaria stained with Vasa (blue) and 1B1 (red). Trans-heterozygote germaria show accumulation of single cells in (yellow dashed line). 1B1 channel is shown in H′ and I′. (J) Quantitation of the number of single cells in germaria of Tip60 heterozygote, Nipped-A heterozygote, and Tip60;Nipped-A trans-heterozygote (8.80 ± 2.33 in Tip60;Nipped-A trans-heterozygote compared with 3.36 ± 0.91 in Tip60 heterozygote control and 3.56±0.87 in Nipped-A heterozygote control; n = 25 for both; ****P < 0.0001). (K, K′) Control and (L, L′) nosGAL4-driven UAS-Tip60DN germaria stained with Vasa (blue) and 1B1 (red). Germaria with overexpression of Tip60DN show accumulation of single cells (yellow dashed line). 1B1 channel is shown in K′ and L′. (M) Quantitation of the number of single cells in control and nosGAL4-driven UAS-Tip60DNgermaria (17.5 ± 7.47 in UAS-Tip60DN compared with 2.95 ± 0.60 in nosGAL4 control; n = 20 for both; ****P = 0.0009). Statistical analysis performed with Student’s t test. Scale bar for all images is 20 μm.
One of Tip60 complex’s major functions is its HAT activity, so we next asked whether HAT activity is required for timely differentiation. To test this, we expressed a dominant negative HAT-defective Tip60 mutant (UAS-Tip60DN; Lorbeck et al., 2011) in the germ line and found a significantly higher number of spectrosome-containing germ cells compared with the control (Figure 2, K–M). To determine whether the accumulated cells are also pre-CBs, we used the pgcGFP;nosGAL4 reporter fly in conjunction with the Tip60 dominant negative conditional mutant. Upon expression of Tip60DN in the germ line, in conjunction with the pgcGFP reporter, we found an average of 0.97 ± 1.40 GFP-positive germ cells per germaria compared with 0.07 ± 0.25 GFP-germ cells in control germaria (n = 30 for both, P = 0.001) (Supplemental Figure 3, J–K′). Thus, we conclude that the HAT activity of the Tip60 complex is required for GSC daughter differentiation.
Tip60 complex localizes to promoters of highly transcribed genes and acetylates histone H4, including lysine 16 (H4K16ac), to promote decompaction (Sánchez-Molina et al., 2014; Lau et al., 2016). We find that loss of Tip60 complex HAT activity alone is sufficient to cause a differentiation defect, so we next asked whether global levels of H4K16ac decrease on depletion of Nipped-A or expression of Tip60DN in the germ line. To test for this, we stained for H4K16ac in UAS-Dcr2;nosGAL4, bam RNAi, Nipped-A RNAi, and UAS-Tip60DN germaria. We quantified the intensity of fluorescence per nuclei of single germ cells one cell diameter away from the niche and normalized this to somatic levels. We found that, while H4K16ac somatic levels were not significantly different between conditions (Figure 3E), Nipped-A RNAi and single cells of UAS-Tip60DNgermaria have significantly lower intensity of normalized H4K16ac levels compared with bam RNAi and single cells in UAS-Dcr2;nosGAL4 control (Figure 3, A–D′ and E′). Thus, we conclude that Nipped-A works in the same pathway as the Tip60 complex and that Tip60 complex’s HAT activity is required to modulate H4K16ac to support normal germline differentiation.
FIGURE 3:
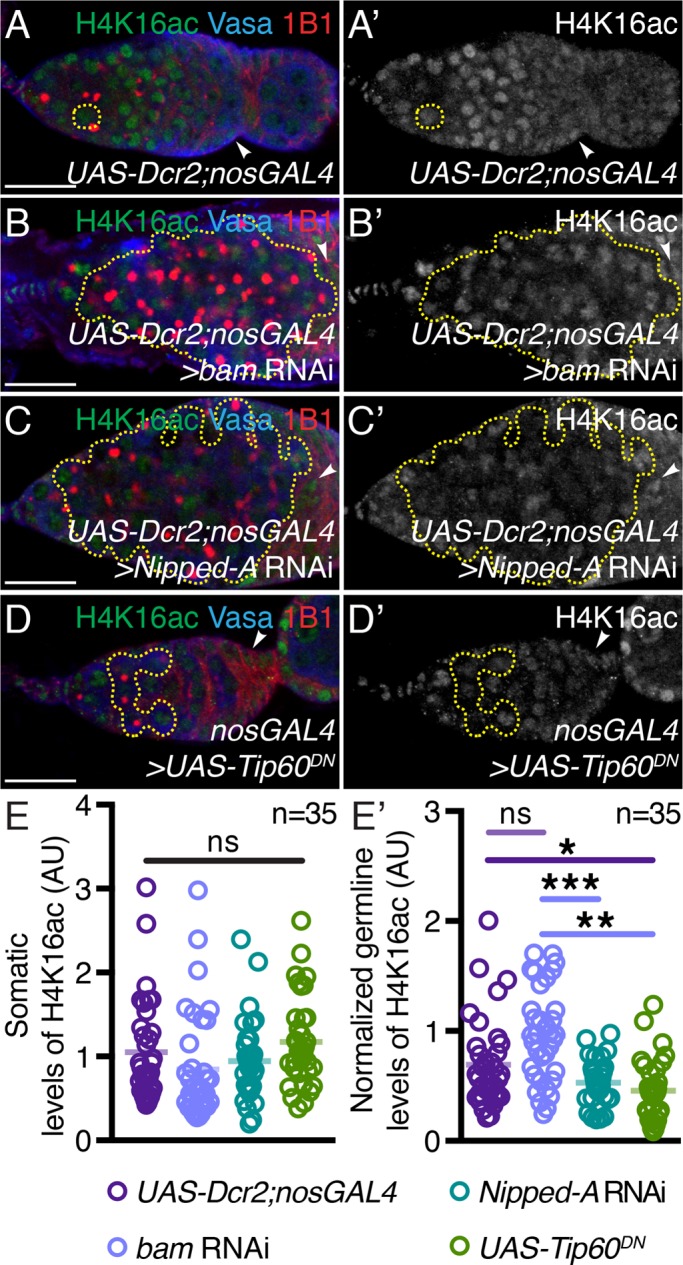
Tip60 complex members are required for acetylation of H4K16. (A, A′) UAS-Dcr2;nosGAL4, (B, B′) germline-depleted bam, (C, C′) germline-depleted Nipped-A, and (D, D′) nosGAL4-driven UAS-Tip60DN germaria stained with H4K16ac (green), Vasa (blue), and 1B1 (red). Germaria with depletion of Nipped-A or Tip60 HAT activity show lower levels of H4K16ac levels in the germ line. Stem cell daughters are outlined with yellow dashed lines in all images. H4K16ac channel is shown in A′, B′, C′, and D′. (E) Quantitation of somatic H4K16ac intensity in UAS-Dcr2;nosGAL4, germline-depleted bam and Nipped-A, and nosGAL4-driven UAS-Tip60DN(1.05 ± 0.60 in UAS-Dcr2;nosGAL4, 0.85 ± 0.65 in bam RNAi, 0.94 ± 0.47 in Nipped-A RNAi, and 1.18 ± 0.09 in UAS-Tip60DN; n = 35 for all; P = 0.0924). (E′) Quantitation of normalized germline H4K16ac intensity in UAS-Dcr2;nosGAL4, germline-depleted bam and Nipped-A, and nosGAL4-driven UAS-Tip60DNgerm cells (0.52 ± 0.20 in Nipped-A RNAi and 0.45 ± 0.27 in UAS-Tip60DNcompared with 0.69 ± 0.41 in UAS-Dcr2;nosGAL4 and 0.96 ± 0.43 in bam RNAi controls; n = 35 for all; *P < 0.03, **P = 0.0093, and ***P = 0.004). Statistical analysis performed with Student’s t test for all except for one-way analysis of ANOVA for E. Scale bar for all images is 20 μm.
Tip60 complex–mediated differentiation is independent of DNA damage and nucleolar stress
Tip60 complex is known to regulate tumor suppressors, such as p53, and DNA damage response (DDR) proteins to promote DNA double strand break (DSB) repair (Avvakumov and Côté, 2007a; Rossetto et al., 2010; Kim et al., 2010b; Jeong et al., 2011; Ravens et al., 2015). Upon DSB formation in Drosophila, the histone variant, H2Av, is phosphorylated (pH2Av) and initiates a signaling cascade to recruit DDR proteins (Sibon et al., 1999; Laurençon et al., 2003; Jaklevic and Su, 2004; Shiloh, 2006; Joyce and McKim, 2009; Joyce et al., 2011). Tip60 complex remodels chromatin by acetylating near the lesion and exchanging pH2Av with unphosphorylated H2Av, thus resolving DDR (Kusch et al., 2004; Joyce et al., 2011). To ensure that damaged DNA is repaired, DDR is activated and p53 orchestrates quality control mechanisms, such as cell cycle arrest or apoptosis (Levine, 1997; Vousden and Lu, 2002). Cell cycle progression is also influenced by levels of ribosomal proteins, as ribosome biogenesis defects can lead to cell cycle arrest or apoptosis (Pestov et al., 2001; Deisenroth and Zhang, 2010; James et al., 2014; Russo and Russo, 2017). In human cell lines TRRAP (Nipped-A) can modulate ribosomal transcription, implicating Nipped-A in regulating nucleolar size and stress response (Arabi et al., 2005; Sanchez et al., 2016). Altogether, this suggests that HAT complexes play a role in sensing and regulating ribosomal biogenesis.
We find that loss of Tip60 complex leads to accumulation of cells in late G2 phase. During cell cycle transitions, cells with DNA damage fail to proceed through cell cycle in a timely manner as a DDR checkpoint is activated (Tang et al., 2006; Sun et al., 2010; Kaidi and Jackson, 2013). We asked whether Tip60 complex–depleted germ cells accumulate pre-CBs that cannot differentiate because of cell cycle checkpoint activation. We hypothesize that if loss of Tip60 complex leads to accumulation of DSBs in early oogenesis, then there would be a higher number of pH2Av-positive germ cells in Tip60 complex member–depleted germaria as compared with control. To determine the frequency of pre-CBs that are pH2Av-positive, we stained for pH2Av in UAS-Dcr2;nosGAL4 ovaries. Only 8% of observed single cells one cell diameter removed from the niche are positive for pH2Av, suggesting that DDR in these cells is a rare event (n = 25) (Supplemental Figure 4, A and A′). We then stained for pH2Av in Nipped-A–depleted germaria and quantitated the percentage of germ cells that are pH2Av-positive compared with single germ cells in bam-depleted germaria (15.90 ± 8.76% germ cells per germaria in Nipped-A RNAi compared with 14.20 ± 6.27% germ cells per germaria in bam RNAi control; n = 20 for both, P = 0.4380). We found no significant difference between the percentage of H2Av-positive germ cells in Nipped-A–depleted germaria compared with bam-depleted germaria (Supplemental Figure 4, B–C′). Thus, perturbed differentiation is not due to the persistence of DDR pathway signaling.
To test whether a p53-dependent DNA damage checkpoint is activated in Tip60 complex–depleted germ cells, we utilized a p53 bio-sensor fly expressing nosGAL4 (Wylie et al., 2014). Expectedly, the bio-sensor was active in 16-cell cyst stages of the positive control, during meiotic recombination that induces DSBs (Brodsky et al., 2000; Lu et al., 2010; Wylie et al., 2014) (Supplemental Figure 4, D and D′). As Nipped-A–depleted germ cells fail to differentiate and thus enter meiosis, any GFP expression will be a consequence of recombination-independent p53 activation. To test this, we stained for GFP, Vasa, and 1B1 in Nipped-A- and bam-depleted germaria that also carry the p53 bio-sensor. We found no significant difference in the percentage of germ cells with active p53 in Nipped-A–depleted germaria compared with bam-depleted germaria (3.47 ± 6.73% germ cells in Nipped-A RNAi compared with 2.73 ± 4.28% germ cells in bam RNAi control; n = 20 for both, P = 0.6464) (Supplemental Figure 4, E–F′). Altogether, this suggests that the accumulation of undifferentiated cells in Nipped-A–depleted germaria is independent of DDR.
Differentiation can also be impeded by nucleolar stress. Nipped-A has been implicated in regulating nucleolar size, stress response, and cell cycle progression (Arabi et al., 2005; Sanchez et al., 2016; Russo and Russo, 2017). We hypothesized that if loss of Nipped-A leads to nucleolar stress response, inhibiting differentiation, then Nipped-A–depleted germ cells would have altered nucleolar volume (James et al., 2013; Falahati et al., 2016). To test for this, we utilized a nucleolar marker, Fibrillarin (Supplemental Figure 4, G and G′). When we compared Nipped-A- and bam-depleted germ cells’ nucleolar-to-nuclear area ratios, we found no significant difference (0.19 ± 0.82 in Nipped-A RNAi compared with 0.22 ± 0.79 in bam RNAi control; n = 20 for both, P = 0.2383) (Supplemental Figure 4, H–I′). Altogether, these data suggest that loss of Nipped-A does not lead to nucleolar stress, suggesting that Tip60 complex regulates differentiation independent of DDR and nucleolar stress.
Tip60 complex regulates expression of the differentiation factor, bgcn
To identify the regulators of Tip60 complex-mediated differentiation in an unbiased manner, we utilized high-throughput RNA sequencing (RNAseq). Comparing transcriptomes of Nipped-A–depleted ovaries to bam-depleted ovaries, both of which accumulate pre-CBs, we identified 750 differentially expressed genes (Figure 4A). Of those genes, 470 genes were up-regulated and 280 were down-regulated in Nipped-A RNAi compared with bam RNAi. Among those significantly up-regulated were genes related to cell cycle regulation, such as string, twine, CycA, and CycB (false discovery rates [FDRs] = 1.38 × 10–2, 1.51 × 10–2, 1.63 × 10–2, and 1.87 × 10–2, respectively). Furthermore, there was an enrichment for genes involved in chitin-based cuticle development among the down-regulated data set (Gene Ontology [GO]: 0040003) and endopeptidase activity among the up-regulated data set (GO: 0004175), suggestive of stunted development and protein turnover, respectively (Figure 4B). Interestingly, we found that bgcn was among the significantly down-regulated genes (sixfold change, FDR = 7.65 × 10–3) (Figure 4C). Collectively, our transcriptome analysis is consistent with our immunohistochemistry data, which showed loss of Tip60 complex leads to accumulation of G2 phase cells that fail to differentiate.
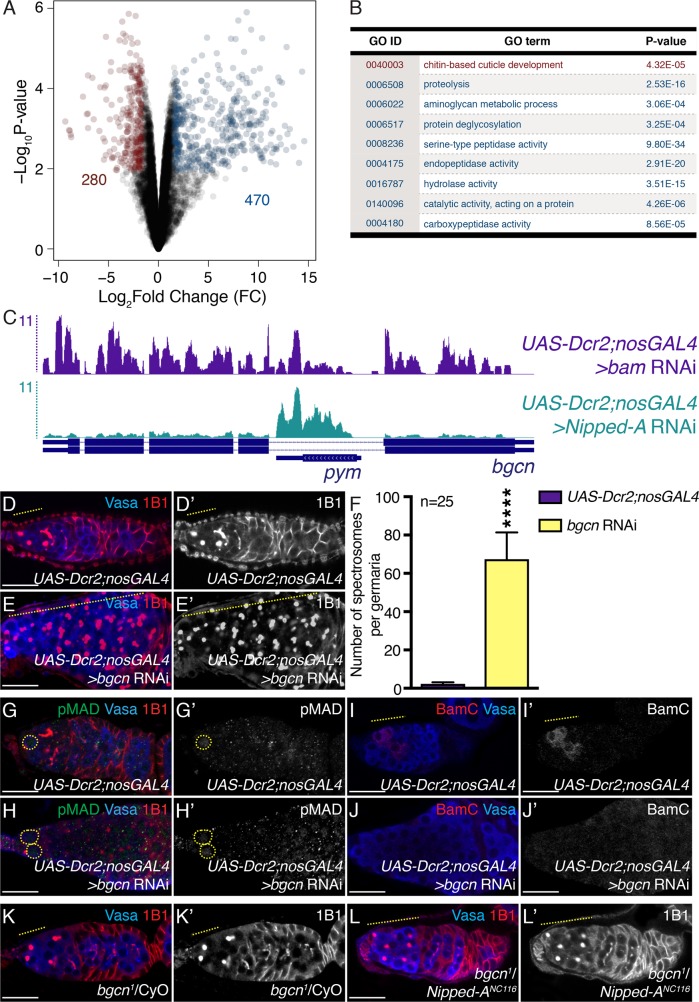
FIGURE 4:
Nipped-A regulates bgcn levels. (A) Volcano plot of log2 fold change (FC) vs. –log10 P value. Red dots represent significantly down-regulated transcripts, and blue dots represent significantly up-regulated transcripts in Nipped-A RNAi ovaries compared with bam RNAi ovaries (FDR = 0.05). Genes with threefold or higher change were considered significant. Two-hundred eighty genes were significantly down-regulated while 470 genes were significantly up-regulated. (B) Table representing the Gene Ontology analysis carried out on differentially expressed genes in Nipped-A–depleted ovaries compared with bam-depleted ovaries. GO: 0040003 was identified from significantly down-regulated genes (red), while GO: 0006508, GO: 0006022, GO: 0006517, GO: 0008236, GO: 0004175, GO: 0016787, GO: 0140096, and GO: 0004180 were identified from significantly up-regulated genes (blue). (C) UCSC genome browser view of bgcn locus and pym intervening locus. Germline-depleted bam and Nipped-A reads shown on top (purple and teal, respectively). RefSeq annotations (bottom, blue) indicate that germline-depleted Nipped-A has reads mapping to Partner of Y14 and Mago (pym); pym reads do not change but reads mapping to bgcn are lower in germline-depleted Nipped-A. (D, D′) Control and (E, E′) germline-depleted bgcn germaria stained with Vasa (blue) and 1B1 (red). Germaria depleted of bgcn show accumulation of single cells (yellow dashed line). 1B1 channel is shown in D′ and E′. (F) Quantitation of the number of single cells in control and germline-depleted bgcn germaria (67.36 ± 13.99 in bgcn RNAi compared with 2.28 ± 0.84 in UAS-Dcr2;nosGAL4 control; n = 25 for both, ****P < 0.0001). (G, G′) Control and (H, H′) germline-depleted bgcn germaria stained with pMAD (green), Vasa (blue), and 1B1 (red). Germaria depleted of bgcn do not accumulate pMAD-positive germ cells (yellow dashed circle) (n = 25, P = 0.1189). pMAD channel is shown in G′ and H′. (I, I′) Control and (J, J′) germline-depleted bgcn germaria stained with BamC (red) and Vasa (blue). Germaria depleted of bgcn do not accumulate BamC-positive germ cells (yellow dashed line in control) (n = 25 for both). BamC channel is shown in I′ and J′. (K, K′) bgcn heterozygote and (L, L′) bgcn/Nipped-A trans-heterozygote germaria stained with Vasa (blue) and 1B1 (red). Trans-heterozygote shows accumulation of single cells (yellow dashed line) (n = 25). 1B1 channel is shown in K′ and L′. Statistical analysis performed with Student’s t test for all except for Chi-square for I–J′. Scale bar for all images is 20 μm.
Bam and its partner, Bgcn, play a critical role in germline differentiation as differentiation fails to occur if either is absent in GSC daughters (McKearin and Ohlstein, 1995; Ohlstein and McKearin, 1997; Ohlstein et al., 2000; Lavoie et al., 1999). We hypothesized that if significantly lower levels of bgcn in Nipped-A–depleted germaria are impeding differentiation, then ectopic expression of bam in Nipped-A–depleted germaria should not rescue the defect. To test whether bam is limiting, we induced bam expression under control of a heat shock (hs) promoter (Ohlstein and McKearin, 1997). Ectopic expression of bam in nosGAL4 or in bam RNAi background alone lead to a conversion to cysts post–heat shock (Supplemental Figure 5, A–D′ and G). However, this is not observed post–heat shock in Nipped-A–depleted germaria (Supplemental Figure 5, E–G). As ectopic expression of bam alone cannot rescue Nipped-A, we then asked whether bgcn levels limit pre-CB differentiation in Nipped-A RNAi. If this is the case, then loss of bgcn should phenocopy loss of Tip60 complex members in the germ line. As previously reported, we found bgcn-depleted germaria accumulated a significantly higher number of germ cells with spectrosomes compared with the control (Figure 4, D–F) (Gateff, 1982; Lavoie et al., 1999). These accumulated cells are also negative for pMAD (1.76 ± 0.44 in bgcn RNAi compared with 1.96 ± 0.45 in UAS-Dcr2;nosGAL4 control; n = 25 for both, P = 0.1189) (Figure 4, G–H′) and BamC (0% germaria BamC-positive in bgcn RNAi compared with 100% germaria BamC-positive in UAS-Dcr2;nosGAL4 control; n = 25 for both, P < 0.0001) (Figure 4, I–J′). Next, we asked whether Nipped-A works in the same pathway as Bgcn. To test for this, we genetically reduced one copy of Nipped-A and Bgcn in the same fly and stained for Vasa and 1B1. Trans-heterozygote germaria accumulated more spectrosome-containing germ cells compared with bgcn1 and Nipped-ANC116 heterozygotes alone (6.76 ± 1.45 in bgcn1;Nipped-ANC116 transheterozygotes compared with 3.92 ± 1.26 in bgcn1 heterozygotes and 3.44 ± 1.00 in Nipped-ANC116heterozygotes; n = 25 for all, P < 0.0001 for both) (Figure 4, K–L′). Taken together, these data demonstrate that Nipped-A regulates expression of bgcn.
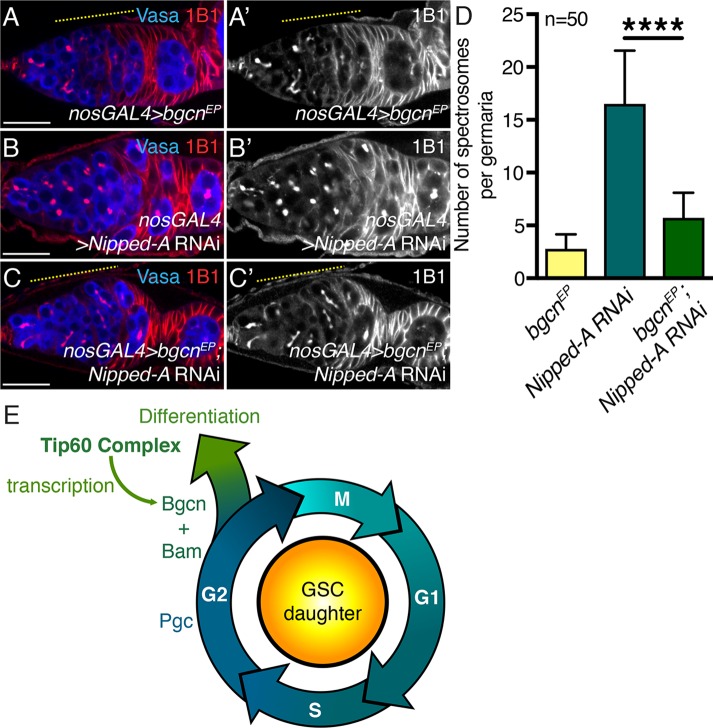
We hypothesized that if Nipped-A regulates bgcn expression, then overexpression of bgcn could rescue the differentiation defect in germline-depleted Nipped-A ovaries. To determine this, we made use of an EP line (bgcnEY00974), which carries a UAS element and basal core promoter upstream of the bgcn gene. We found that overexpression of bgcn in the germ line did not lead to an increase in the number of single cells per germaria or later-stage defects (Figure 5, A, A′, and D; Supplemental Figure 6, A and A′). Depletion of Nipped-A alone in the germ line leads to accumulation of single cells, whereas overexpression of bgcn in Nipped-A–depleted germaria resulted in a significant reduction in the number of single cells and the appearance of fusomelike structures (Figure 5, B–D). Furthermore, we found that 78% of rescue germaria had subsequent egg chambers compared with only 10% of Nipped-A–depleted germaria (n = 50 for both; P < 0.05) (Supplemental Figure 6, B–C′). However, rescue egg chambers died mid-oogenesis (Supplemental Figure 6, A, A′, C, and C′). Thus, we conclude that Tip60 complex controls GSC daughter differentiation via expression of the differentiation factor Bgcn (Figure 5E).
FIGURE 5:
Ectopic expression of bgcn rescues Nipped-A RNAi early defect. (A, A′) Germline-driven bgcnEP, (B, B′) depletion of Nipped-A, and (C, C′) rescue germaria stained with Vasa (blue) and 1B1 (red). Germline-driven bgcnEPand rescue germaria show fusome formation (yellow dashed line). Germaria depleted of Nipped-A accumulate only spectrosome-containing cells (white arrow). 1B1 channel is shown in A′, B′, and C′. (D) Quantitation of the number of single cells in germline-driven bgcnEP, depletion of Nipped-A, and rescue germaria (2.78 ± 1.38 in bgcnEP; 5.72 ± 2.37 in rescue compared with 16.52 ± 5.02 in Nipped-A RNAi; n = 50 for all ****P < 0.0001). (E) Schematic showing Tip60 complex regulates differentiation in the precystoblast (GSC daughter). In G2 phase, Pgc is expressed prior to differentiation, we postulate that Tip60 complex modulates bgcn mRNA levels to promote differentiation. Bgcn and Bam proteins then complex to stimulate differentiation in the GSC daughter. Statistical analysis performed with Student’s t test. Scale bar for all images is 20 μm.
DISCUSSION
Here we find that Nipped-A regulates female Drosophila germline differentiation through the Tip60 complex. We observe that Nipped-A plays a role in both the germ line and soma of the gonad to regulate oogenesis (Figure 1; Supplemental Figure 1, F and G). In the germ line, we show that Tip60 complex members and Tip60 HAT activity is required for timely differentiation and loss of either leads to accumulation of pre-CBs (Figure 2; Supplemental Figure 3, A–H′ and J–K′). Furthermore, we demonstrate that Tip60 complex regulates differentiation downstream of Pgc-mediated CycB accumulation (Figure 1, J–K′; Supplemental Figure 2, C–E; Supplemental Figure 3, J–K′) (Flora et al., 2018). We find that Nipped-A–depleted germ cells fail to differentiate independently of DDR (Supplemental Figure 4, A–F′) and is also separate from nucleolar stress (Supplemental Figure 4, H–I′) (Sanchez et al., 2016; Feng et al., 2018). Consistent with these findings, we saw no significant difference in mRNA levels of DDR genes p53, reaper, hid, grim, or sickle between Nipped-A- and bam-depleted germaria (Song et al., 2004b; Bi et al., 2005; Klattenhoff et al., 2007). Additionally, there was no significant difference in cell cycle arrest regulators grapes (chk1) and loki (chk2) (Fogarty et al., 1997; Abdu et al., 2002). Instead, we find that Nipped-A is required for expression of the differentiation factor bgcn (Figure 4, C and K–L′). Thus, Tip60 complex drives germ cells into a differentiation program by regulating bgcn, a critical component of differentiation.
While Bam and Bgcn are both required for differentiation, previous studies have primarily focused on elucidating the molecular mechanism of Bam and its role in germline maintenance and differentiation (Chen and McKearin, 2003a,b; Song et al., 2004a). Bgcn has been well characterized posttranslationally and predominantly in the context of protein–protein interactions, but its transcriptional modulators had yet to be identified (Ohlstein and McKearin, 1997; Ohlstein et al., 2000; Lavoie et al., 1999; Li et al., 2009; Kim et al., 2010a). We have identified Tip60 complex as a regulator of bgcn in the female germ line. We find that Nipped-A is required for expression of the differentiation factor bgcn, and overexpression of bgcn, but not bam, suppresses the early differentiation defect we observe in Nipped-A–depleted germaria (Figure 5, A–D; Supplemental Figures 5 and 6). While overexpression of bgcn resulted in egg chamber formation in Nipped-A RNAi, these egg chambers did not give rise to fertile eggs, suggesting that Nipped-A is also required at late stages of oogenesis to regulate yet-unknown targets (Supplemental Figure 6, C and C′). Intriguingly, bgcn mRNA is expressed only in the undifferentiated stages. While our data show that Nipped-A is required for activation of bgcn expression, it does not explain what restricts bgcn expression postdifferentiation. Feng et al. has described Tip60 complex’s role in Drosophila testis to promote differentiation via bam (Feng et al., 2018). They find that on depletion of Enhancer of Polycomb (E(Pc)), germ cells fail to properly differentiate and that while bam mRNA levels are unperturbed, they suggest E(Pc) is required for posttranscriptional regulation of this differentiation factor (Feng et al., 2018). Together with Feng et al.’s findings, we suggest that Tip60 complex may have developed different strategies to promote differentiation in the male and female germ line.
The Bgcn mammalian homologue YTHDC2 is required for meiotic progression in mice (Bailey et al., 2017; Soh et al., 2017; Jain et al., 2018). YTHDC2 partners with a protein similar to Bam, MEIOC, to regulate this developmental switch. Loss of either YTHDC2 or MEIOC lead to a failure to execute a more specialized program, strikingly reminiscent to what has been observed in Drosophila development. Prior work in other stem cell systems suggests that Tip60 complex regulation of differentiation factors at the transcriptional level may not be unique to just Drosophila GSCs (Fazzio et al., 2008a,b; Acharya et al., 2017). To investigate this, we made use of publically available Tip60 ChIPseq data (Ravens et al., 2015). When we interrogated whether Tip60 was found enriched at promoters of YTH family genes (YTHDC1, YTHDC2, YTHDF1, YTHDF2, and YTHDF3) in mouse embryonic stem cells, we found that indeed there was enrichment at their promoter regions (Fold changes = 17.6, 12.46, 19.33, 17.09, and 9.60, respectively; FDR = 0.05). However, we do not know whether Tip60 complex regulates YTHDC2 or bgcn directly in the mouse and Drosophila gonads, respectively.
We, and others, have identified Nipped-A as being a regulator of differentiation (Tapias et al., 2014; Flegel et al., 2016; Sanchez et al., 2016; Tauc et al., 2017). Flegel et al. show that loss of Tip60 complex does not affect terminal differentiation of Drosophila wing cells but rather the cells ectopically express cell cycle markers and lose cell identity (Flegel et al., 2016). Across many species, Tip60 complex regulates cell cycle genes, presumably directly by histone acetylation (Fazzio et al., 2008a,b; Tapias et al., 2014; Feng et al., 2018). We find that loss of Nipped-A leads to an up-regulation of G2 phase regulators (string, twine, CycA, and CycB) (Supplemental Figure 2). These results are consistent with Feng et al.’s data, where they similarly showed that germline Tip60 complex is required for proper levels of CycB (Feng et al., 2017, 2018). As we can rescue the differentiation defects by expressing bgcn, this suggests that the cell cycle defects we observe could be a consequence of lack of differentiation factor. Altogether, we show that Tip60 complex mediates a critical decision in germline differentiation, and we suggest that this is coordinated at the level of chromatin structure and transcriptional modulation of differentiation factors (Figure 5E).
MATERIALS AND METHODS
Fly lines
The following RNAi stocks were used in this study; if more than one line is listed, then only the first was used for quantitation and shown in figures, unless otherwise stated in the text: Act87E RNAi (Bloomington #42652), Bap55 RNAi (VDRC #v24704), Brd8 RNAi (Bloomington #42658 and VDRC #v104879), DMAP1 RNAi (Bloomington #63666), dom RNAi (Bloomington #31054 and #40914), Eaf6 RNAi (Bloomington #33905 and VDRC #101457), E(Pc) RNAi (Bloomington #28686 and VDRC #v35271), Mrg15 RNAi (Bloomington #35241), Nipped-A RNAi (Bloomington #34849 [line #1], VDRC #v52486 [line #2], and Bloomington #31255 [line #3]), pont RNAi (Bloomington #50972 and VDRC #v105408), rept RNAi (Bloomington #32415 and VDRC #v103483), Tip60 RNAi (Bloomington #35243), YL-1 RNAi (Bloomington #31938), Ada2b RNAi (Bloomington #31347 and #35334), Ada3 RNAi (Bloomington #28905 and #32451), Ataxin7 RNAi (VDRC #v102078), Gcn5 RNAi (Bloomington #33981 and #35601), Sgf29 RNAi (Bloomington #36636 and #39000), Spt3 RNAi (Bloomington #35148 and #57733), bam RNAi (Bloomington #58178), and bgcn RNAi (VDRC #v25591).
The following tissue-specific drivers were used in this study: UAS-Dcr2;nosGAL4 (Bloomington #25751), nosGAL4;MKRS/TM6 (Bloomington #4442), pgcGFP;nosGAL4 (Rangan Lab; Flora et al. [2018]), tjGAL4 (Lehmann Lab), and nosGAL4;p53R-GFP (Abrams Lab).
The following mutant and overexpression stocks were used in this study: Nipped-ANC116cn1bw1/CyO (Bloomington #7188), w1118,Tip60e02395 (Bloomington #18052), bgcn1/CyO (Bloomington #6054), bgcnEY00974 (Bloomington #20106), Nipped-AMI10513/SM6a (Bloomington #54566), hs-bam (Bloomington #24637), and UAS-Tip60DN (UAS-Tip60E431Q, Elefant Lab; Lorbeck et al. [2011]).
Dissection and immunostaining
Two- to 3-d-old ovaries were dissected in 1x phosphate-buffered saline (PBS), fixed for 15 min in PBS plus 5% methanol-free formaldehyde. The ovaries were washed in PBT (1x PBS, 0.5% Triton X-100, and 0.3% BSA) three times for 10 min each. Primary antibodies were added in PBT and incubated at 4°C overnight. The ovaries were washed three times for 10 min each in PBT and then washed once in PBT supplemented with 2% donkey serum (Sigma) for 10 min. Secondary antibodies were added in PBT supplemented with 4% donkey serum and incubated at room temperature for 4 h. Ovaries were washed four times for 10 min each in 1x PBST (1x PBS with 0.2% Tween 20) and mounted using Vectashield with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories). The following primary antibodies were used: mouse anti-1B1 (1:20, DSHB), Rabbit anti-Vasa (1:1000, Rangan Lab), Chicken anti-Vasa (1:1000, Rangan Lab (Upadhyay et al., 2016)), Rabbit anti-GFP (1:2000, abcam, ab6556), Rabbit anti-pMAD (1:200, abcam, ab52903), Mouse anti-BamC (1:200, DSHB, Supernatant), Mouse anti-CycA (1:20, DSHB), Rabbit anti-CycB (1:200, Santa Cruz Biotechnology, 25764), Rabbit anti-pH2Av (1:500, Rockland, 600-401-914), Rabbit anti-H4K16ac (1:500, Millipore, 07-329), and Mouse anti-Fibrillarin (1:50, Fuchs Lab). The following secondary antibodies were used: Alexa 488 (Molecular Probes), Cy3 and Cy5 (Jackson Labs) were used at a dilution of 1:500. All experiments were conducted at least three times for each experimental condition.
Fluorescence imaging
The tissues were visualized under 10× dry and 40× oil objective lenses, and images were acquired using a Zeiss LSM-710 confocal microscope under the 20×, 40×, and 63× oil objectives. Images of whole ovaries were visualized and acquired under 4× and 10× objectives using the EVOS FL Cell Imaging System.
RNA extraction and RNAseq library preparation
Ovaries from UAS-Dcr2;nosGAL4>bam RNAi and UAS-Dcr2;nosGAL4>Nipped-A RNAi flies were dissected in 1x PBS. RNA was isolated using TRIzol (Invitrogen, 15596026), treated with DNase (TURBO DNA-free Kit, Life Technologies, AM1907), and then run on a 1% agarose gel to check integrity of the RNA. To generate mRNA-enriched libraries, total RNA was treated with poly(A)tail selection beads and then following the manufacturer’s instructions of the NEXTflex Rapid Directional RNAseq Kit (Bioo Scientific Corp., NOVA-5138-08), except that RNA was fragmented for 13 min. Single-end mRNA sequencing (75 base pair) was performed on biological duplicates from each genotype on an Illumina NextSeq500 by the Center for Functional Genomics (CFG). After quality assessment, the sequenced reads were aligned to the Drosophila melanogaster genome (UCSCdm6) using HISAT2 (version 2.1.0) with the RefSeq-annotated transcripts as a guide (Kim et al., 2015). Raw counts were generated using featureCounts (version 1.6.0.4) (Liao et al., 2014). Differential gene expression was assayed by edgeR (version 3.16.5), using a false discovery rate (FDR) of 0.05, and genes with threefold or higher were considered significant. GO term enrichment on differentially expressed genes was performed using Panther (Robinson et al., 2010; Mi et al., 2017). Raw and processed data for UAS-Dcr2;nosGAL4>bam RNAi and UAS-Dcr2;nosGAL4>Nipped-A RNAi RNAseq is deposited in the Gene Expression Omnibus (GEO) databank under accession number GSE119328.
ChIPseq analysis
To analyze Tip60 ChIPseq data, we aligned Tip60 reads to the Mus musculus genome (UCSCmm9) (Input [GSM798320]) [Karmodiya et al., 2012] and Experimental [GSE69671] [Ravens et al., 2015]) using Bowtie2. ChIPseq peaks were called by using MACS2 (narrow peaks), using default parameters, and then visualized using UCSC genome browser.
Quantification analysis
Statistical analysis.
The P values were determined by using Student’s t test, one-way analysis of variance (ANOVA), or chi-square analysis (see figure legends). All analyses were performed using Prism 7 software (GraphPad).
A.U. of protein levels.
To calculate intensities for H4K16ac UAS-Dcr2;nosGAL4, bam RNAi, Nipped-A RNAi, and UAS-Tip60DN, mutant germaria were taken under the same confocal settings with Z stacks. For quantification, nuclei of single cells approximately one cell diameter away from the niche were outlined, and the intensity of the selected region in the H4K16ac channel was analyzed using Fiji. Nuclei of a somatic cell in the same germarium was outlined, and the intensity of H4K16ac fluorescence in the selected region was also analyzed using Fiji. The ratio between mean intensity and area was measured for germ cells and somatic cells. Mean intensity of H4K16ac per area in germ cells were normalized to mean intensity of H4K16ac per area in somatic cells. The average of all ratios, per genotype was calculated and comparisons were made between UAS-Dcr2;nosGAL4 and bam RNAi controls and Nipped-A RNAi and UAS-Tip60DN experimentals. The P value was determined by Student’s t test by comparing the average mean H4K16ac intensities per area in germ cells normalized to average mean H4K16ac intensities per area in somatic cells of controls and experimentals. A minimum of five germaria were chosen for each quantification.
Materials and reagents
Fly food was made by using the procedures from the Ruth Lehmann lab at NYU (summer/winter mix) and filled narrow vials (Fisherbrand Drosophila Vials; Fischer Scientific) to approximately 12 ml.
Supplementary Material
Acknowledgments
We are particularly grateful to all members of the Rangan laboratory for discussion and comments on the manuscript, especially E.T.M. for Bioinformatic support. Additionally, we thank Felice Elefant, Ruth Lehmann, Gabriele Fuchs, the Bloomington Drosophila Stock Center, the Vienna Drosophila Resource Center, the Transgenic RNAi Project (National Institutes of Health/National Institute of General Medical Sciences [NIH/NIGMS] R01-GM084947), The BDGP Gene Disruption Project, and FlyBase for fly stocks and reagents. Furthermore, we thank the CFG Core Facility at the University at Albany for performing RNAseq analysis and Faraz Ahmed at UAlbany for discussion. P.R. acknowledges funding from NIH/NIGMS RO11119484-1-68857 and The Pew Biomedical Scholars Program.
Abbreviations used:
- ac
acetylation
- Act87E
Actin 87E
- Bam
bag of marbles
- Bgcn
benign gonial cell neoplasm
- CB
cystoblast
- chk1
grapes
- chk2
loki
- CycA
Cyclin A
- CycB
Cyclin B
- DDR
DNA damage response
- DN
dominant negative
- Dpp
Decapentaplegic
- DSB
double-stranded break
- ER
endoplasmic reticulum
- FC
fold change
- FDR
false discovery rate
- G2
gap phase 2
- GNAT
Gcn5 (general control of nuclear-5)-related N-acetyltransferases
- GO
Gene Ontology
- GSC
germline stem cell
- H2Av
histone H2A variant
- HAT
histone acetyltransferase
- hs
heat shock
- MYST
MOZ, Ybf2/Sas3, Sas2, and Tip60
- nos
nanos
- Pgc
polar granule component
- pH3
phospho-histone 3
- pH2Av
phosphorylated H2Av
- pMAD
Mothers-against-Dpp
- rept
reptin
- RNAseq
RNA sequencing
- SAGA
Spt-Ada-Gcn5-acetyltransferase
- Sax
Saxophone
- Tip60
Tat interactive protein 60-kDa
- tj
traffic jam
- TKV
Thick veins
- YTHDC2
YTH Domain Containing 2
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E18-06-0385) on September 19, 2018.
References
Boldface names denote co–first authors.
- Abdu U, Brodsky M, Schüpbach T. (2002). Activation of a meiotic checkpoint during Drosophila oogenesis regulates the translation of Gurken through Chk2/Mnk. Curr Biol , 1645–1651. [DOI] [PubMed] [Google Scholar]
- Acharya D, Hainer SJ, Yoon Y, Wang F, Bach I, Rivera-Pérez JA, Fazzio TG. (2017). KAT-independent gene regulation by Tip60 promotes ESC self-renewal but not pluripotency. Cell Rep , 671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard S, Utley RT, Savard J, Clarke A, Grant P, Brandl CJ, Pillus L, Workman JL, Côté J. (1999). NuA4, an essential transcription adaptor/histone H4 acetyltransferase complex containing Esa1p and the ATM-related cofactor Tra1p. Embo J , 5108–5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi A, Wu S, Ridderstrale K, Bierhoff H, Shiue C, Fatyol K, Fahlen S, Hydbring P, Soderberg O, Grummt I, et al. (2005). c-Myc associates with ribosomal DNA and activates RNA polymerase I transcription. Nat Cell Biol , 303–310. [DOI] [PubMed] [Google Scholar]
- Avvakumov N, Côté J. (2007a). Functions of myst family histone acetyltransferases and their link to disease. Subcell Biochem , 295–317. [PubMed] [Google Scholar]
- Avvakumov N, Côté J. (2007b). The MYST family of histone acetyltransferases and their intimate links to cancer. Oncogene , 5395–5407. [DOI] [PubMed] [Google Scholar]
- Bailey AS, Batista PJ, Gold RS, Chen YG, de Rooij DG, Chang HY, Fuller MT. (2017). The conserved RNA helicase YTHDC2 regulates the transition from proliferation to differentiation in the germline. Elife , 10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X, Srikanta D, Fanti L, Pimpinelli S, Badugu R, Kellum R, Rong YS. (2005). Drosophila ATM and ATR checkpoint kinases control partially redundant pathways for telomere maintenance. Proc Natl Acad Sci USA , 15167–15172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M, Wong C, Rocha M, Jones DL. (2007). Decline in self-renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell , 470–478. [DOI] [PubMed] [Google Scholar]
- Brodsky MH, Nordstrom W, Tsang G, Kwan E, Rubin GM, Abrams JM. (2000). Drosophila p53 binds a damage response element at the reaper locus. Cell , 103–113. [DOI] [PubMed] [Google Scholar]
- Brown CE, Howe L, Sousa K, Alley SC, Carrozza MJ, Tan S, Workman JL. (2001). Recruitment of HAT complexes by direct activator interactions with the ATM-related Tra1 subunit. Science , 2333–2337. [DOI] [PubMed] [Google Scholar]
- Brummel TJ, Twombly V, Marqués G, Wrana JL, Newfeld SJ, Attisano L, Massagué J, O’Connor MB, Gelbart WM. (1994). Characterization and relationship of Dpp receptors encoded by the saxophone and thick veins genes in Drosophila. Cell , 251–261. [DOI] [PubMed] [Google Scholar]
- Carrozza MJ, Utley RT, Workman JL, Côté J. (2003). The diverse functions of histone acetyltransferase complexes. Trends Genet , 321–329. [DOI] [PubMed] [Google Scholar]
- Casanueva MO. (2004). Germline stem cell number in the Drosophila ovary is regulated by redundant mechanisms that control Dpp signaling. Development , 1881–1890. [DOI] [PubMed] [Google Scholar]
- Chen D, McKearin D. (2003a). Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr Biol , 1786–1791. [DOI] [PubMed] [Google Scholar]
- Chen D, McKearin DM. (2003b). A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell. Development , 1159–1170. [DOI] [PubMed] [Google Scholar]
- Chen ES, Zhang K, Nicolas E, Cam HP, Zofall M, Grewal SIS. (2008). Cell cycle control of centromeric repeat transcription and heterochromatin assembly. Nature , 734–737. [DOI] [PubMed] [Google Scholar]
- Dansereau DA, Lasko P. (2008). The development of germline stem cells in Drosophila. Methods Mol Biol , 3–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cuevas M, Spradling AC. (1998). Morphogenesis of the Drosophila fusome and its implications for oocyte specification. Development , 2781–2789. [DOI] [PubMed] [Google Scholar]
- de Cuevas M, Lee JK, Spradling AC. (1996). alpha-spectrin is required for germline cell division and differentiation in the Drosophila ovary. Development , 3959–3968. [DOI] [PubMed] [Google Scholar]
- Deisenroth C, Zhang Y. (2010). Ribosome biogenesis surveillance: probing the ribosomal protein-Mdm2-p53 pathway. Oncogene , 4253–4260. [DOI] [PubMed] [Google Scholar]
- Falahati H, Pelham-Webb B, Blythe S, Wieschaus E. (2016). Nucleation by rRNA dictates the precision of nucleolus assembly. Curr Biol , 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzio TG, Huff JT, Panning B. (2008a). An RNAi screen of chromatin proteins identifies Tip60-p400 as a regulator of embryonic stem cell identity. Cell , 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzio TG, Huff JT, Panning B. (2008b). Chromatin regulation Tip(60)s the balance in embryonic stem cell self-renewal. Cell Cycle , 3302–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Shi Z, Chen X. (2017). Enhancer of polycomb coordinates multiple signaling pathways to promote both cyst and germline stem cell differentiation in the Drosophila adult testis. PLoS Genet , e1006571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Shi Z, Xie J, Ma B, Chen X. (2018). Enhancer of polycomb maintains germline activity and genome integrity in Drosophila testis. Cell Death Differ , 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegel K, Grushko O, Bolin K, Griggs E, Buttitta L. (2016). Roles for the histone modifying and exchange complex NuA4 in cell cycle progression in Drosophila melanogaster. Genetics , 1265–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora P, McCarthy A, Upadhyay M, Rangan P. (2017). Role of chromatin modifications in Drosophila germline stem cell differentiation. Signaling-Mediated Control of Cell Division: From Oogenesis to Oocyte-to-Embryo Development, Arur S., Cham, Switzerland: Springer International Publishing, 1–30. [DOI] [PubMed] [Google Scholar]
- Flora P, Schowalter S, Wong-Deyrup S, DeGennaro M, Nasrallah MA, Rangan P. (2018). Transient transcriptional silencing alters the cell cycle to promote germline stem cell differentiation in Drosophila. Dev Biol , 84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty P, Campbell SD, Abu-Shumays R, de Phalle BS, Yu KR, Uy GL, Goldberg ML, Sullivan W. (1997). The Drosophila grapes gene is related to checkpoint gene chk1/rad27 and is required for late syncytial division fidelity. Curr Biol , 418–426. [DOI] [PubMed] [Google Scholar]
- Gateff E. (1982). Gonial cell neoplasm of genetic origin affecting both sexes of drosophila melanogaster. Prog Clin Biol Res (Pt B), 621–632. [PubMed] [Google Scholar]
- Grant PA, Schieltz D, Pray-Grant MG, Yates JR, Workman JL. (1998). The ATM-related cofactor Tra1 is a component of the purified SAGA complex. Mol Cell , 863–867. [DOI] [PubMed] [Google Scholar]
- Huynh JR, St Johnston D. (2000). The role of BicD, Egl, Orb and the microtubules in the restriction of meiosis to the Drosophila oocyte. Development , 2785–2794. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Uno M, Honjoh S, Nishida E. (2017). The MYST family histone acetyltransferase complex regulates stress resistance and longevity through transcriptional control of DAF-16/FOXO transcription factors. EMBO Rep , 1716–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikura T, Ogryzko VV, Grigoriev M, Groisman R, Wang J, Horikoshi M, Scully R, Qin J, Nakatani Y. (2000). Involvement of the TIP60 histone acetylase complex in DNA repair and apoptosis. Cell , 463–473. [DOI] [PubMed] [Google Scholar]
- Jacquet K, Fradet-Turcotte A, Avvakumov N, Lambert JP, Roques C, Pandita RK, Paquet E, Herst P, Gingras AC, Pandita TK, et al. (2016). The TIP60 complex regulates bivalent chromatin recognition by 53BP1 through direct H4K20me binding and H2AK15 acetylation. Mol Cell , 409–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain D, Puno MR, Meydan C, Lailler N, Mason CE, Lima CD, Anderson KV, Keeney S. (2018). ketu mutant mice uncover an essential meiotic function for the ancient RNA helicase YTHDC2. Elife , 10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklevic BR, Su TT. (2004). Relative contribution of DNA repair, cell cycle checkpoints, and cell death to survival after DNA damage in Drosophila larvae. Curr Biol , 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James A, Cindass R, Mayer D, Terhoeve S, Mumphrey C, DiMario P. (2013). Nucleolar stress in Drosophila melanogaster: RNAi-mediated depletion of Nopp140. Nucleus , 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James A, Wang Y, Raje H, Rosby R, DiMario P. (2014). Nucleolar stress with and without p53. Nucleus , 402–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong KW, Kim K, Situ AJ, Ulmer TS, An W, Stallcup MR. (2011). Recognition of enhancer element-specific histone methylation by TIP60 in transcriptional activation. Nat Struct Mol Biol , 1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia D, Huang Y-C, Deng W-M. (2015). Analysis of cell cycle switches in Drosophila oogenesis. Methods Mol Biol , 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce EF, McKim KS. (2009). Drosophila PCH2 is required for a pachytene checkpoint that monitors double-strand-break-independent events leading to meiotic crossover formation. Genetics , 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce EF, Pedersen M, Tiong S, White-Brown SK, Paul A, Campbell SD, McKim KS. (2011). Drosophila ATM and ATR have distinct activities in the regulation of meiotic DNA damage and repair. J Cell Biol , 359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai T, Spradling A. (2003). An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proc Natl Acad Sci USA , 4633–4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidi A, Jackson SP. (2013). KAT5 tyrosine phosphorylation couples chromatin sensing to ATM signalling. Nature , 70–74. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Karmodiya K, Krebs AR, Oulad-Abdelghani M, Kimura H, Tora L. (2012). H3K9 and H3K14 acetylation co-occur at many gene regulatory elements, while H3K14ac marks a subset of inactive inducible promoters in mouse embryonic stem cells. BMC Genomics , 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Langmead B, Salzberg SL. (2015). HISAT: a fast spliced aligner with low memory requirements. Nat Methods , 357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Lee YC, Kim C. (2010a). Direct inhibition of Pumilo activity by Bam and Bgcn in Drosophila germ line stem cell differentiation. J Biol Chem , 4741–4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Woo AJ, Chu J, Snow JW, Fujiwara Y, Kim CG, Cantor AB, Orkin SH. (2010b). A Myc network accounts for similarities between embryonic stem and cancer cell transcription programs. Cell , 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klattenhoff C, Bratu DP, McGinnis-Schultz N, Koppetsch BS, Cook HA, Theurkauf WE. (2007). Drosophila rasiRNA pathway mutations disrupt embryonic axis specification through activation of an ATR/Chk2 DNA damage response. Dev Cell , 45–55. [DOI] [PubMed] [Google Scholar]
- Kusch T, Florens L, Macdonald WH, Swanson SK, Glaser RL, Yates JR, Abmayr SM, Washburn MP, Workman JL. (2004). Acetylation by Tip60 is required for selective histone variant exchange at DNA lesions. Science , 2084–2087. [DOI] [PubMed] [Google Scholar]
- Lau AC, Zhu KP, Brouhard EA, Davis MB, Csankovszki G. (2016). An H4K16 histone acetyltransferase mediates decondensation of the X chromosome in C. elegans males. Epigenetics Chromatin , 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurençon A, Purdy A, Sekelsky J, Hawley RS, Su TT. (2003). Phenotypic analysis of separation-of-function alleles of MEI-41, Drosophila ATM/ATR. Genetics , 589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie CA, Ohlstein B, McKearin DM. (1999). Localization and function of Bam protein require the benign gonial cell neoplasm gene product. Dev Biol , 405–413. [DOI] [PubMed] [Google Scholar]
- Lee KK, Workman JL. (2007). Histone acetyltransferase complexes: one size doesn’t fit all. Nat Rev Mol Cell Biol , 284–295. [DOI] [PubMed] [Google Scholar]
- Lehmann R. (2012). Germline stem cells: origin and destiny. Cell Stem Cell , 729–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letsou A, Arora K, Wrana JL, Simin K, Twombly V, Jamal J, Staehling-Hampton K, Hoffmann FM, Gelbart WM, Massagué J. (1995). Drosophila Dpp signaling is mediated by the punt gene product: a dual ligand-binding type II receptor of the TGF beta receptor family. Cell , 899–908. [DOI] [PubMed] [Google Scholar]
- Levine AJ. (1997). p53, the cellular gatekeeper for growth and division. Cell , 323–331. [DOI] [PubMed] [Google Scholar]
- Li X, Seidel CW, Szerszen LT, Lange JJ, Workman JL, Abmayr SM. (2017). Enzymatic modules of the SAGA chromatin-modifying complex play distinct roles in Drosophila gene expression and development. Genes Dev , 1588–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Minor NT, Park JK, McKearin DM, Maines JZ. (2009). Bam and Bgcn antagonize Nanos-dependent germ-line stem cell maintenance. Proc Natl Acad Sci USA , 9304–9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, Shi W. (2014). featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics , 923–930. [DOI] [PubMed] [Google Scholar]
- Lorbeck M, Pirooznia K, Sarthi J, Zhu X, Elefant F. (2011). Microarray analysis uncovers a role for Tip60 in nervous system function and general metabolism. PLoS One , e18412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W-J, Chapo J, Roig I, Abrams JM. (2010). Meiotic recombination provokes functional activation of the p53 regulatory network. Science , 1278–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKearin DM, Spradling AC. (1990). bag-of-marbles: a Drosophila gene required to initiate both male and female gametogenesis. Genes Dev , 2242–2251. [DOI] [PubMed] [Google Scholar]
- McKearin D, Ohlstein B. (1995). A role for the Drosophila bag-of-marbles protein in the differentiation of cystoblasts from germline stem cells. Development , 2937–2947. [DOI] [PubMed] [Google Scholar]
- Mi H, Huang X, Muruganujan A, Tang H, Mills C, Kang D, Thomas PD. (2017). PANTHER version 11: expanded annotation data from Gene Ontology and Reactome pathways, and data analysis tool enhancements. Nucleic Acids Res , D183–D189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Spradling AC. (2008). Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell , 598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narbonne-Reveau K, Besse F, Lamour-Isnard C, Busson D, Pret A-M. (2006). fused regulates germline cyst mitosis and differentiation during Drosophila oogenesis. Mech Dev , 197–209. [DOI] [PubMed] [Google Scholar]
- Navarro-Costa P, McCarthy A, Prudêncio P, Greer C, Guilgur LG, Becker JD, Secombe J, Rangan P, Martinho RG. (2016). Early programming of the oocyte epigenome temporally controls late prophase I transcription and chromatin remodelling. Nat Commun , 12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B, McKearin D. (1997). Ectopic expression of the Drosophila Bam protein eliminates oogenic germline stem cells. Development , 3651–3662. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Lavoie CA, Vef O, Gateff E, McKearin DM. (2000). The Drosophila cystoblast differentiation factor, benign gonial cell neoplasm, is related to DExH-box proteins and interacts genetically with bag-of-marbles. Genetics , 1809–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Chen S, Weng C, Call G, Zhu D, Tang H, Zhang N, Xie T. (2007). Stem cell aging is controlled both intrinsically and extrinsically in the Drosophila ovary. Cell Stem Cell , 458–469. [DOI] [PubMed] [Google Scholar]
- Patel JH, Du Y, Ard PG, Phillips C, Cardella B, Chen C, Rakowski C, Chatterjee C, Lieberman PM, Lane W, et al. (2004). The c-MYC oncoprotein is a substrate of the acetyltransferases hGCN5/PCAF and TIP60. Mol Cell Biol , 10826–10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penton A, Chen Y, Staehling-Hampton K, Wrana JL, Attisano L, Szidonya J, Cassill JA, Massagué J, Hoffmann FM. (1994). Identification of two bone morphogenetic protein type I receptors in Drosophila and evidence that Brk25D is a decapentaplegic receptor. Cell , 239–250. [DOI] [PubMed] [Google Scholar]
- Pestov DG, Strezoska Z, Lau LF. (2001). Evidence of p53-dependent cross-talk between ribosome biogenesis and the cell cycle: effects of nucleolar protein Bop1 on G(1)/S transition. Mol Cell Biol , 4246–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangan P, Malone CD, Navarro C, Newbold SP, Hayes PS, Sachidanandam R, Hannon GJ, Lehmann R. (2011). piRNA production requires heterochromatin formation in Drosophila. Curr Biol , 1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravens S, Yu C, Ye T, Stierle M, Tora L. (2015). Tip60 complex binds to active Pol II promoters and a subset of enhancers and co-regulates the c-Myc network in mouse embryonic stem cells. Epigenetics Chromatin , 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics , 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetto D, Truman AW, Kron SJ, Côté J. (2010). Epigenetic modifications in double-strand break DNA damage signaling and repair. Clin Cancer Res , 4543–4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth SY, Denu JM, Allis CD. (2001). Histone acetyltransferases. Annu Rev Biochem , 81–120. [DOI] [PubMed] [Google Scholar]
- Russo A, Russo G. (2017). Ribosomal proteins control or bypass p53 during nucleolar stress. Int J Mol Sci , 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh A, Schieltz D, Ting N, McMahon SB, Litchfield DW, Yates JR, Lees-Miller SP, Cole MD, Brandl CJ. (1998). Tra1p is a component of the yeast Ada.Spt transcriptional regulatory complexes. J Biol Chem , 26559–26565. [DOI] [PubMed] [Google Scholar]
- Sanchez CG, Teixeira FK, Czech B, Preall JB, Zamparini AL, Seifert JRK, Malone CD, Hannon GJ, Lehmann R. (2016). Regulation of ribosome biogenesis and protein synthesis controls germline stem cell differentiation. Cell Stem Cell , 276–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Molina S, Estarás C, Oliva JL, Akizu N, Asensio-Juan E, Rojas JM, Martínez-Balbás MA. (2014). Regulation of CBP and Tip60 coordinates histone acetylation at local and global levels during Ras-induced transformation. Carcinogenesis , 2194–2202. [DOI] [PubMed] [Google Scholar]
- Sekelsky JJ, Newfeld SJ, Raftery LA, Chartoff EH, Gelbart WM. (1995). Genetic characterization and cloning of mothers against dpp, a gene required for decapentaplegic function in Drosophila melanogaster. Genetics , 1347–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharov G, Voltz K, Durand A, Kolesnikova O, Papai G, Myasnikov AG, Dejaegere A, Ben Shem A, Schultz P. (2017). Structure of the transcription activator target Tra1 within the chromatin modifying complex SAGA. Nat Commun , 1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiloh Y. (2006). The ATM-mediated DNA-damage response: taking shape. Trends Biochem Sci , 402–410. [DOI] [PubMed] [Google Scholar]
- Sibon OC, Laurençon A, Hawley R, Theurkauf WE. (1999). The Drosophila ATM homologue Mei-41 has an essential checkpoint function at the midblastula transition. Curr Biol , 302–312. [DOI] [PubMed] [Google Scholar]
- Slaidina M, Lehmann R. (2014). Translational control in germline stem cell development. J Cell Biol , 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh YQS, Mikedis MM, Kojima M, Godfrey AK, de Rooij DG, Page DC. (2017). Meioc maintains an extended meiotic prophase I in mice. PLoS Genet , e1006704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Wong MD, Kawase E, Xi R, Ding BC, McCarthy JJ, Xie T. (2004a). Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development , 1353–1364. [DOI] [PubMed] [Google Scholar]
- Song Y-H, Mirey G, Betson M, Haber DA, Settleman J. (2004b). The Drosophila ATM ortholog, dATM, mediates the response to ionizing radiation and to spontaneous DNA damage during development. Curr Biol , 1354–1359. [DOI] [PubMed] [Google Scholar]
- Spradling AC, de Cuevas M, Drummond-Barbosa D, Keyes L, Lilly M, Pepling M, Xie T. (1997). The Drosophila germarium: stem cells, germ line cysts, and oocytes. Cold Spring Harb Symp Quant Biol , 25–34. [PubMed] [Google Scholar]
- Spradling AC, Nystul T, Lighthouse D, Morris L, Fox D, Cox R, Tootle T, Frederick R, Skora A. (2008). Stem cells and their niches: integrated units that maintain Drosophila tissues. Cold Spring Harb Symp Quant Biol , 49–57. [DOI] [PubMed] [Google Scholar]
- Spradling A, Fuller MT, Braun RE, Yoshida S. (2011). Germline stem cells. Cold Spring Harb Perspect Biol , a002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steunou A.-L., Rossetto D, Côté J. (2013). Regulating chromatin by histone acetylation. In: Fundamentals of Chromatin, New York: Springer New York, 147–212. [Google Scholar]
- Sun Y, Jiang X, Price BD. (2010). Tip60: connecting chromatin to DNA damage signaling. Cell Cycle , 930–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Luo J, Zhang W, Gu W. (2006). Tip60-dependent acetylation of p53 modulates the decision between cell-cycle arrest and apoptosis. Mol Cell , 827–839. [DOI] [PubMed] [Google Scholar]
- Tapias A, Zhou Z, Shi Y, Chong Z, Wang P, Groth M, Platzer M, Huttner W, Herceg Z, Yang Y, et al. (2014). Trrap-dependent histone acetylation specifically regulates cell-cycle gene transcription to control neural progenitor fate decisions. Cell Stem Cell , 632–643. [DOI] [PubMed] [Google Scholar]
- Tastan OY, Maines JZ, Li Y, McKearin DM, Buszczak M. (2010). Drosophila ataxin 2-binding protein 1 marks an intermediate step in the molecular differentiation of female germline cysts. Development , 3167–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauc HM, Tasdogan A, Meyer P, Pandur P. (2017). Nipped-A regulates intestinal stem cell proliferation in Drosophila. Development , 612–623. [DOI] [PubMed] [Google Scholar]
- Upadhyay M, Martino Cortez Y, Wong-Deyrup S, Tavares L, Schowalter S, Flora P, Hill C, Nasrallah MA, Chittur S, Rangan P. (2016). Transposon dysregulation modulates dWnt4 signaling to control germline stem cell differentiation in Drosophila. PLoS Genet , e1005918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev A, Yamauchi J, Kotani T, Prives C, Avantaggiati ML, Qin J, Nakatani Y. (1998). The 400 kDa subunit of the PCAF histone acetylase complex belongs to the ATM superfamily. Mol Cell , 869–875. [DOI] [PubMed] [Google Scholar]
- Voss AK, Thomas T. (2009). MYST family histone acetyltransferases take center stage in stem cells and development. Bioessays , 1050–1061. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Lu X. (2002). Live or let die: the cell’s response to p53. Nat Rev Cancer , 594–604. [DOI] [PubMed] [Google Scholar]
- Wylie A, Lu W-J, D’Brot A, Buszczak M, Abrams JM. (2014). p53 activity is selectively licensed in the Drosophila stem cell compartment. Elife , e01530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Jia S, Huang S, Wang H, Zhu Y, Mu Y, Kan L, Zheng W, Wu D, Li X, et al. (2010). The Fused/Smurf complex controls the fate of Drosophila germline stem cells by generating a gradient BMP response. Cell , 978–990. [DOI] [PubMed] [Google Scholar]
- Xie T, Spradling AC. (1998). Decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell , 251–260. [DOI] [PubMed] [Google Scholar]
- Xie T, Spradling AC. (2000). A niche maintaining germ line stem cells in the Drosophila ovary. Science , 328–330. [DOI] [PubMed] [Google Scholar]
- Xie T, Finelli AL, Padgett RW. (1994). The Drosophila saxophone gene: a serine-threonine kinase receptor of the TGF-beta superfamily. Science , 1756–1759. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.