Significance
Heteromannans are ancient plant cell wall polysaccharides, ubiquitous throughout the plant kingdom. In plants, these hemicellulosic polymers strengthen the cell wall and facilitate energy storage, and are involved in cell expansion and developmental signaling. Heteromannans are used extensively by humans, as healthy dietary fibers and as thickening agents in food, cosmetic, pharmaceutical, and mining industries. The fine structure of heteromannan is important for the polysaccharide’s biophysical properties, and thus its function and application. However, the biosynthesis of different heteromannan structures remains mechanistically unclear. We demonstrate that plant mannan and glucomannan can be synthesized heterologously in the yeast Pichia pastoris, and the polysaccharide’s structure can be modulated by specific plant cofactors, allowing for the synthesis of tailored mannan structures.
Keywords: plant cell wall, polysaccharide, glycosyltransferase, mannan, yeast
Abstract
Heteromannan (HM) is one of the most ancient cell wall polymers in the plant kingdom, consisting of β-(1–4)-linked backbones of glucose (Glc) and mannose (Man) units. Despite the widespread distribution of HM polysaccharides, their biosynthesis remains mechanistically unclear. HM is elongated by glycosyltransferases (GTs) from the cellulose synthase-like A (CSLA) family. MANNAN-SYNTHESIS RELATED (MSR) putative GTs have also been implicated in (gluco)mannan synthesis, but their roles have been difficult to decipher in planta and in vitro. To further characterize the products of the HM synthases and accessory proteins, we chose a synthetic biology approach to synthesize plant HM in yeast. The expression of a CSLA protein in Pichia pastoris led to the abundant production of plant HM: up to 30% of glycans in the yeast cell wall. Based on sequential chemical and enzymatic extractions, followed by detailed structural analyses, the newly produced HM polymers were unbranched and could be larger than 270 kDa. Using CSLAs from different species, we programmed yeast cells to produce an HM backbone composed exclusively of Man or also incorporating Glc. We demonstrate that specific MSR cofactors were indispensable for mannan synthase activity of a coffee CSLA or modulated a functional CSLA enzyme to produce glucomannan instead of mannan. Therefore, this powerful platform yields functional insight into the molecular machinery required for HM biosynthesis in plants.
Plants thrive in a wide range of aqueous and terrestrial environments, as their cells are shaped and reinforced by a carbohydrate-rich extracellular matrix. Plant cell wall polysaccharides make up the bulk of plant biomass, and thus represent the most abundant source of renewable material on the planet (1). One class of wall polysaccharides, the hemicelluloses, are a heterogenous group of polysaccharides consisting of β-(1–4)-linked backbones of glucose (Glc), mannose (Man), or xylose units (2). In addition to strengthening the wall, hemicelluloses facilitate cell expansion, store carbohydrates, and supply signal molecules in certain tissues (2). Heteromannan (HM) is regarded as the most ancient type of hemicellulose, and is found throughout the plant kingdom, including in red algae (3), early land plants (mosses, lycophytes), gymnosperms, and angiosperms (4). Along with these important biological functions, HM is important for human nutrition, and may play a role in the treatment of lifestyle diseases such as type 2 diabetes (5). In addition, HM polysaccharides are widespread ingredients used to stabilize foods and cosmetics, coat pharmaceutical drugs for the controlled release of active agents, and even waterproof dynamite sticks for the mining industry (6). The global demand for HM has increased substantially in recent years because of its utility as a fluid thickener in hydraulic fracturing for oil and gas (7). Despite the widespread distribution and important applications of HM, the roles of genetic factors that determine the polysaccharide’s fine structure remain to be characterized.
Changes in HM monosaccharide composition, length, or substitution modulate the polymer’s physical properties and determine, for instance, whether a polysaccharide forms a crystalline aggregate or is water-soluble. Two distinct HM backbones are found in plants (2): linear mannan consists almost exclusively (>90%) of Man subunits, whereas glucomannan also includes Glc residues. Mannan accumulates primarily in palm seeds, whereas glucomannan is more widespread and is, for example, highly enriched in konjac corms (8). The solubility of HM polysaccharides is enhanced by the substitution of Man units in (gluco)mannan with galactose side chains. For example, coffee seeds accumulate a galactosyl-substituted mannan (9), whereas galactoglucomannan is the most abundant hemicellulose in softwood (10).
Similar to other plant hemicelluloses, HM is synthesized by Golgi-localized glycosyltransferases (GTs), and the resulting polysaccharides are secreted to the plant cell wall via exocytosis. GTs from the Cellulose Synthase-Like A (CSLA) family have been demonstrated to use GDP-Man, and sometimes GDP-Glc, to elongate the HM backbone in vitro (11–14), but the polymers made by different CSLA isoforms have not been characterized in detail. In addition, MANNAN-SYNTHESIS RELATED (MSR) putative GTs are associated with (gluco)mannan synthesis (15, 16), but their roles have been difficult to decipher in planta and in vitro. Arabidopsis thaliana (At) MSRs have been hypothesized to synthesize a primer to initiate HM synthesis, glycosylate CSLA enzymes to enhance their activity, or promote CSLA stability or activity via glycosylation-independent interactions (15). Because these hypotheses have not been tested, the machinery that synthesizes different HM types remains unclear.
To further characterize the products of CSLA enzymes and the roles of accessory proteins such as MSR, we genetically engineered yeast to synthesize and secrete plant HM to their walls. Pichia pastoris (hereafter Pichia) was selected as a promising host because it only contains trace amounts of 4-linked Man in its wall (17), and active CSLA proteins have been expressed in this host for in vitro assays (8, 18). Related Cellulose Synthase-Like C enzymes that elongate the backbone of xyloglucan, another type of hemicellulose, were also functional in Pichia (17). We now show that CSLA expression in Pichia led to the abundant production of plant HM. Depending on the CSLA isoform used, yeast cells synthesized linear HM polymers built primarily of Man, or also incorporating Glc. Using this platform, we demonstrate that MSR proteins are important cofactors for the elongation and the composition of plant mannan and glucomannan.
Results
Engineering Pichia to Produce Plant HM in Its Cell Wall.
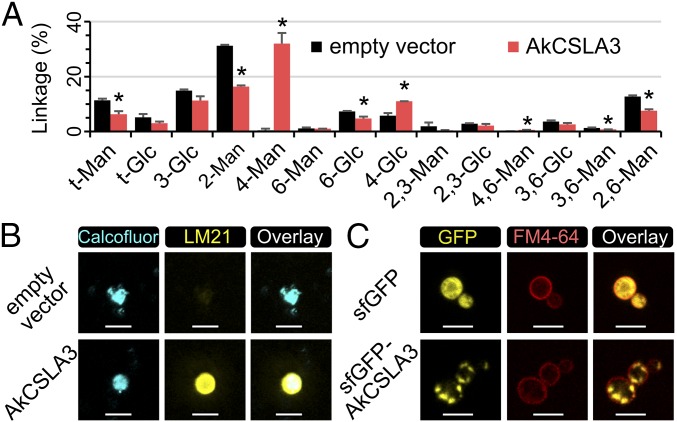
We engineered Pichia cells to produce plant HM to characterize the polysaccharides produced by CSLA enzymes and the roles of MSR proteins. As a proof of concept, we stably integrated the Amorphophallus konjac (Ak) CSLA3 coding sequence in the Pichia genome under the control of a methanol-inducible promoter. This gene was selected because Pichia microsomes expressing recombinant AkCSLA3 have glucomannan synthase activity in radiometric assays (8). Pichia cells containing the AkCSLA3 transgene were grown for biomass production before inducing recombinant protein expression. Pichia cell wall alcohol-insoluble residue (AIR) was isolated and subjected to glycosidic linkage analysis. The wall AIR of an empty vector control Pichia strain was rich in 2-Man and 3-Glc linkages, derived from native yeast α-mannoproteins and β-1,3-glucans, respectively, but only contained trace amounts of 4-Man (Fig. 1A and SI Appendix, Fig. S1A). The expression of AkCSLA3 led to the abundant production of 4-Man, characteristic of plant mannan, and also showed an increased 4-Glc content (Fig. 1A and SI Appendix, Fig. S1A). The abundance of 4-Man was elevated up to 30% of total carbohydrates in the AkCSLA3 cell wall AIR (Fig. 1A). To confirm the stability of HM production in yeast cells, an AkCSLA3 colony examined in Fig. 1A was reanalyzed alongside five additional AkCSLA3 colonies (SI Appendix, Fig. S1B), isolated from an independent transformation. Indeed, 4-Man was the dominant wall glycosidic linkage for all AkCSLA3 transformants (SI Appendix, Fig. S1B). In contrast to its effects on wall AIR, AkCSLA3 did not alter the composition of ethanol-precipitable glycans secreted in the culture media (SI Appendix, Fig. S1C).
Fig. 1.
Yeast cells expressing AkCSLA3 accumulate plant HM in their walls. (A) Glycosidic linkage analysis of Pichia wall AIR. Values represent molar percentage of total carbohydrates detected. Data show mean + SD of two independent transformants for each strain. Asterisks indicate significant changes (two-tailed t test, P < 0.05) between the strains. (B) Yeast wall polysaccharides with stained with calcofluor, a general β-glucan dye, and immunolabeled with the HM-binding LM21 antibody. (C) Subcellular localization of AkCSLA3 enzymes in Pichia cells. The plasma membrane was stained with the FM4-64 dye. The signal intensity of all channels for the fusion protein sfGFP-AkCSLA3 sample was increased postacquisition, relative to sfGFP control. (Scale bars, 5 µm.)
To investigate whether the products of AkCSLA3 were deposited in the yeast cell wall, Pichia spheroplasts were immunolabeled with LM21 and LM22, two HM-specific monoclonal antibodies (19) that cannot cross the plasma membrane. The AkCSLA3 cells were labeled by LM21 or, to a lesser extent, LM22 antibodies (SI Appendix, Fig. S2 A–C). Although LM21 epitopes were only detected in AkCSLA3-expressing cells, native yeast β-glucans were stained in a similar fashion by the calcofluor dye in both the AkCSLA3 and empty vector Pichia strains (SI Appendix, Fig. S2 D–G). Confocal microscopy confirmed that the LM21 signals detected in the AkCSLA3-expressing cells colocalized with the yeast wall counterstained with calcofluor (Fig. 1B). The subcellular localization of the AkCSLA3 enzyme was investigated using a superfolder green fluorescent protein (sfGFP) N-terminal tag (20). Although the free sfGFP tag was distributed throughout the cytosol (SI Appendix, Fig. S2H), the sfGFP-AkCSLA3 fusion proteins were localized in small punctae (SI Appendix, Fig. S2I), interior to the plasma membrane stained by FM4-64 (Fig. 1C). The sfGFP-AkCSLA3 protein was functional, but AkCSLA3 yielded more HM sugars (SI Appendix, Fig. S2J), so only untagged proteins were expressed in further experiments. Therefore, as observed in plants, Pichia cells expressed CSLA recombinant proteins in intracellular compartments, but deposited their HM products in the extracellular matrix.
Product Specificity of CSLA Enzymes from Different Species.
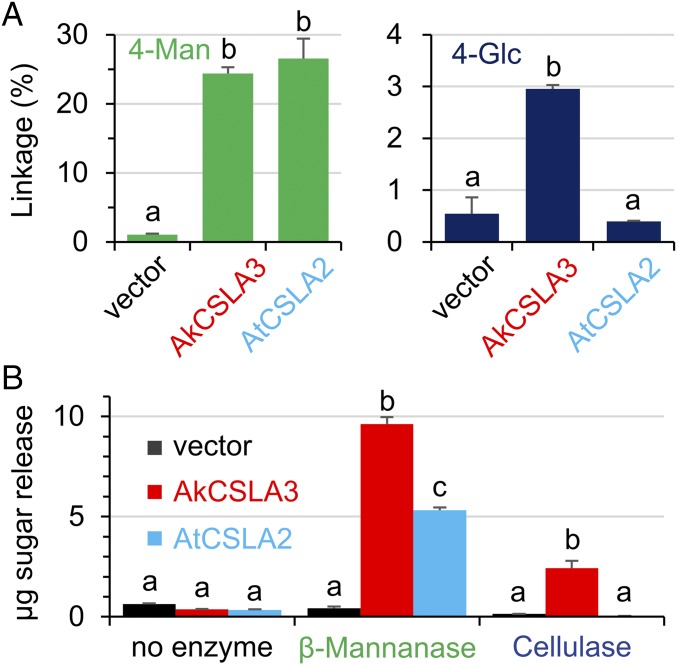
The products of AkCSLA3 and the At CSLA2 protein were compared to investigate whether different CSLA isoforms yield distinct HM structures, When expressed in Pichia, the two enzymes produced equally high amounts of 4-Man in the wall AIR, but only AkCSLA3 significantly increased the content of 4-Glc relative to the empty vector control (Fig. 2A). This suggests that the AkCSLA3 enzyme produces glucomannan, but AtCSLA2 synthesizes a relatively pure mannan in yeast. We enriched the novel HM polysaccharides by washing the cell wall AIR with hot 1 M sodium hydroxide (SI Appendix, Fig. S3A), which removes yeast α-mannoproteins (21). The resulting alkaline-insoluble (AKI) material was then treated with Zymolyase, which digests yeast β-1,3-glucans proteins (21), to yield an enriched HM (EM) fraction (SI Appendix, Fig. S3A). These sequential extractions removed most of the native yeast polymers, and thus enriched the CSLA product (SI Appendix, Fig. S3B). The EM polysaccharides were enzymatically characterized using purified β-mannanase and cellulase recombinant enzymes, which cleave β-1,4-Man and β-1,4-Glc bonds, respectively. Relative to the empty vector and undigested samples, the AkCSLA3 protein produced HM polymers that could be digested by β-mannanase and, to a lesser extent, cellulase treatment (Fig. 2B). In contrast, the AtCSLA2 product was only digested by β-mannanase (Fig. 2B), indicating a lack of β-1,4-Glc bonds. To further investigate their structure, EM polysaccharides were subjected to proton NMR (1H-NMR) spectroscopy (Fig. 3C), alongside standards for plant HM polymers and yeast glucans (SI Appendix, Table S2). No carbohydrate peaks were observed in the 1H-NMR spectrum of the empty vector control (Fig. 3C). The 1H-NMR spectrum of the EM polymers made by AkCSLA3 contained the anomeric peaks characteristic of β-1,4-glucan and β-1,4-mannan (Fig. 3C), as well as other aliphatic signals also found in konjac glucomannan (SI Appendix, Table S2). In contrast, the AtCSLA2 product lacked the β-1,4-glucan anomeric peak (Fig. 3C), but had signals with the identical chemical shifts as pure mannan extracted from ivory nut (SI Appendix, Table S2). Therefore, Pichia cells expressing AkCSLA3 and AtCSLA2 produced glucomannan and mannan, respectively.
Fig. 2.
Products after AkCSLA3 and AtCSLA2 expression. (A) Content of 4-Man and 4-Glc glycosidic linkages in the Pichia wall AIR. Values represent percentage of total carbohydrate area detected and show mean + SD of two biological replicates per Pichia strain. Vector, empty vector control. (B) Hexose sugars enzymatically released from enriched HM polysaccharides. Data show mean + SD of three technical replicates. For each linkage or digestion, different letters indicate significant changes based on one-way ANOVA with post hoc Tukey HSD Test (P < 0.01).
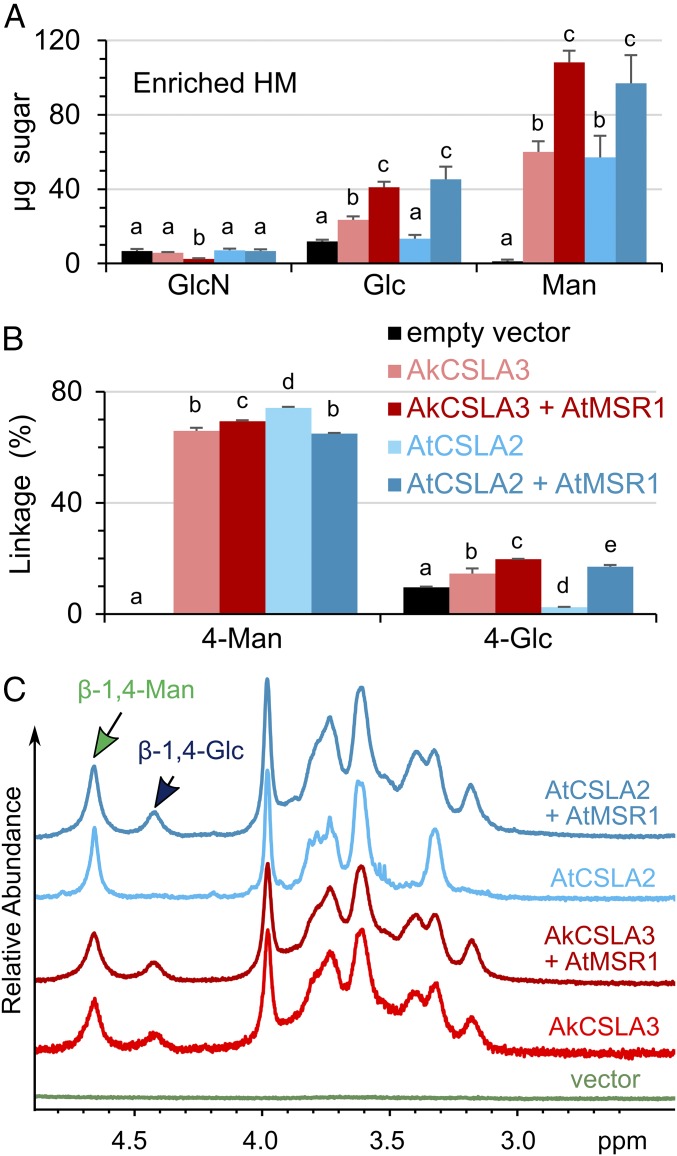
Fig. 3.
AtMSR1 is a cofactor for glucomannan synthesis. (A) Absolute composition of monosaccharides in EM polymers, enriched from Pichia wall AIR. (B) Relative content of 4-Man and 4-Glc linkages in the material analyzed in A. Values represent molar percentage of total carbohydrates detected. Data show mean + SD of at least three technical replicates. For each sugar or linkage, different letters denote significant changes based on one-way ANOVA with post hoc Tukey HSD Test (P < 0.05 for A; P < 0.01 for B). (C) 1H-NMR analysis of EM polymers. Arrows indicate diagnostic anomeric peaks.
The AtMSR1 Protein Is an Optional Cofactor for Glucomannan Synthesis.
We designed an in vitro multimer assembly strategy to efficiently integrate two or more transgenes in the Pichia genome (SI Appendix, Fig. S4 A and B). We domesticated (removed) the PmeI site in the methanol-inducible promoter of the pPICZ B vector to create a novel pPICZ X vector that was used to clone additional transcriptional units, which can be subsequently fused (SI Appendix, Fig. S4B). The Pichia wall AIR composition was not significantly altered by the expression of AtMSR1 alone (SI Appendix, Fig. S4 C and E), indicating that this gene is not sufficient for HM production. Interestingly, no AtMSR1 homolog was identified in Ak transcriptome datasets (8, 22). We thus evaluated the function of AtMSR1 in combination with the AkCSLA3 enzyme. Based on monosaccharide and glycosidic linkage analyses, coexpression of AtMSR1 with AkCSLA3 increased the amount of glucomannan in the Pichia EM fraction by at least 70% relative to AkCSLA3 alone (Fig. 3 A and B). The elevated content of EM polysaccharides in the two-gene construct (Fig. 3A) did not simply result from changes in CSLA gene expression (SI Appendix, Fig. S4E), nor from changes in HM solubility, as the addition of AtMSR1 also boosted the ability of AkCSLA3 to incorporate 4-Man and 4-Glc into cell wall AIR (SI Appendix, Fig. S4C). It is noteworthy that AtMSR1 only boosted the content of glucomannan produced by konjac AkCSLA3 without significantly altering its Man:Glc ratio (Fig. 3 and SI Appendix, Fig. S4C).
AtMSR1 Enables AtCSLA2 to Produce Glucomannan Instead of Mannan.
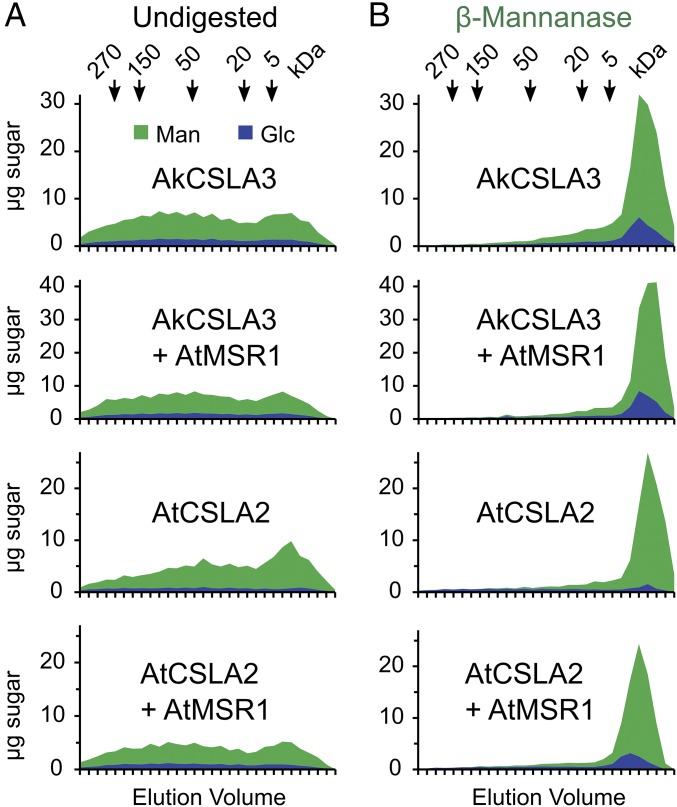
Interestingly, the AtMSR1 cofactor significantly altered the product of AtCSLA2, which is required for glucomannan elongation in Arabidopsis (23–25). Glycosidic linkage analysis of wall AIR as well as EM polymers indicated that AtCSLA2 alone produced mannan, but the AtCSLA2 + AtMSR1 Pichia strain synthesized glucomannan (Fig. 3B and SI Appendix, Fig. S4C and Table S1). To confirm this structural change, EM polymers were examined with 1H-NMR. The presence of AtMSR1 enabled the AtCSLA2 enzyme to produce both β-1,4-glucosyl- and β-1,4-mannosyl residues instead of pure mannan (Fig. 3C). On the basis of the 1H-NMR anomeric signals, the EM polymers contained a 4-Glc to 4-Man ratio of 1:3.2 (Fig. 3C), consistent with the 1:3.5–4.5 ratio in the glycosidic linkage analysis (Fig. 3B). We performed size-exclusion chromatography to confirm that these glycosyl units are part of the same polymer (i.e., glucomannan), and to investigate its size. EM polymers produced by AkCSLA3 or AtCSLA2, with or without AtMSR1, could only be solubilized by overnight incubation in 10% (wt/vol) sodium hydroxide. Polysaccharides solubilized in this manner were heterodisperse, ranging in size from below 5 kDa to above 270 kDa (Fig. 4A). EM polysaccharides from three Pichia strains (AkCSLA3, AkCSLA3 + AtMSR1, and AtCSLA2 + AtMSR1) were cleaved into fragments smaller than 5 kDa by β-mannanase digestion and contained around 20% Glc (Fig. 4B). In contrast, AtCSLA2 alone produced EM polysaccharides that were proportionally smaller (Fig. 4A) and contained more than 95% Man (Fig. 4B). Only 34% of AtCSLA2 solubilized products were above 50 kDa compared with around 50% of carbohydrates for the three glucomannan-producing strains. In summary, AtMSR1 modulated glucomannan production by AtCSLA2, which increased the content (Fig. 3A) and relative size (Fig. 4A) of EM polymers isolated from Pichia walls.
Fig. 4.
Size-exclusion chromatography of enriched HM. (A) EM polysaccharides were solubilized in 10% NaOH overnight, neutralized, and analyzed by size-exclusion chromatography. Arrows indicate the elution of Dextran standards of known sizes. Data show the Glc and Man content (stacked). (B) An equal amount of the EM material used in A was digested with β-mannanase before SEC analysis.
Influence of MSR1 Proteins on Other Mannan Synthases.
We also investigated how AtMSR1 modulates the activity of other mannan synthases such as AtCSLA7, which belongs to a phylogenetic clade distinct from AtCSLA2 (12) and only elongates mannan in vitro (13). Indeed, Pichia expressing AtCSLA7 incorporated significantly more 4-Man, but not 4-Glc, linkages into AKI polysaccharides (SI Appendix, Fig. S4D). In contrast to the AtCSLA2 + AtMSR1 combination (Fig. 3), the coexpression of the AtMSR1 gene with AtCSLA7 (SI Appendix, Fig. S4F) decreased 4-Man production in Pichia relative to the single gene controls, without altering the content of 4-Glc in the AKI fraction (SI Appendix, Fig. S4D).
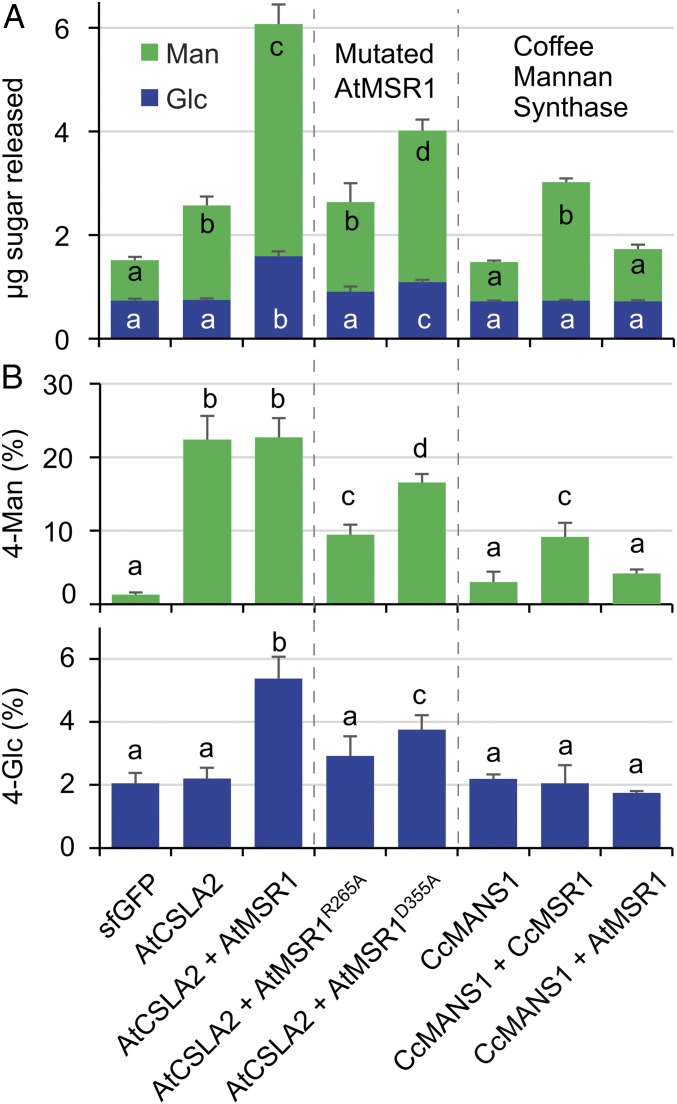
The Coffea canephora (Cc) MANS1 mannan synthase represents a prime candidate for the production of mannan in coffee seeds (26), but was inactive when expressed in Pichia microsomal membrane fractions and assayed in vitro (27). Unlike AtCSLA2 and AkCSLA3, expression of CcMANS1 alone did not produce any significant amounts of β-1,4-mannan in Pichia walls (Fig. 5). Also, coexpression of the Arabidopsis AtMSR1 with the coffee CcMANS1 did not produce HM polysaccharides that could be detected via β-mannanase digestion (Fig. 5A) or glycosidic linkage analysis of the AKI fraction (Fig. 5B). However, coexpression of CcMANS1 with CcMSR1, the cofactor found natively in coffee seeds (26), produced β-1,4-mannan in Pichia cells (Fig. 5A) without increasing the 4-Glc content of AKI polymers (Fig. 5B). Therefore, AtMSR1 enhanced glucomannan synthesis by AtCSLA2 and AkCSLA3, but did not enhance the CcMANS1 and AtCSLA7 mannan synthases. Unlike an auxiliary role of AtMSR1 in glucomannan production by AtCSLA2, we demonstrated that the CcMSR1 protein is an essential cofactor for the synthesis of mannan by the coffee CcMANS1 enzyme.
Fig. 5.
Importance of MSR1 sugar-binding motif and protein specificity. (A) HM sugars released from AKI material digested with β-mannanase. Two point mutations (R265A) and (D355A) were introduced into AtMSR1. CcMANS1, coffee mannan synthase; CcMSR1, coffee MSR1. (B) Abundance of 4-Man and 4-Glc in AKI polymers. In all panels, data show mean + SD of three biological replicates. For each glycosyl residue or linkage, different letters denote significant changes based on one-way ANOVA with post hoc Tukey HSD Test (P < 0.05).
Functional Characterization of AtMSR1 Conserved Motifs.
The AtMSR1 protein has an N-terminal transmembrane (TM) domain and a large PF10250 motif (SI Appendix, Fig. S5A), which defines a large family of putative GTs in land plants, and has structural similarity to the catalytic domain of protein O-fucosyltransferases (PoFUT1) from the animal kingdom (15). Multiple protein alignment pointed to three residues that are conserved in the PF10250 domain of the human (Homo sapiens) HsPoFUT1 and MSR-related proteins from Arabidopsis (SI Appendix, Fig. S5B). For example, the AtMSR1 R265 and D355 amino acids correspond to key residues of the HsPoFUT1 sugar-binding motif (SI Appendix, Fig. S5B). There, the R240 residue is indispensable for HsPoFUT1 catalytic activity, and D340 is within hydrogen-bonding distance of the guanine ring on GDP-fucose (28). Moreover, disulfide bridges contribute to the formation of the PoFUT1 sugar-binding site (29), with the C274 amino acid of AtMSR1 putatively involved in this process (SI Appendix, Fig. S5 A and B). To functionally characterize these motifs, we evaluated how AtMSR1 variants without the TM domain (Δ28) or with missense mutations in three conserved amino acids (R265A, C274A, and D355A) influence HM production by AtCSLA2. Relative to the coexpression of AtCSLA2 with wild-type AtMSR1, the AtMSR1Δ28 and AtMSR1C274A mutant variants decreased glucomannan production (SI Appendix, Fig. S5C). The coexpression of AtCSLA2 with mutated AtMSR1 proteins yielded 4-Glc levels similar to the sfGFP control and significantly reduced the 4-Man content relative to the AtCSLA2 + AtMSR1 strain (SI Appendix, Fig. S5D). Two mutations in AtMSR1 sugar-binding residues impaired glucomannan synthesis by AtCSLA2 to different degrees (SI Appendix, Fig. S5 C and D), and were thus characterized in more detail. Coexpression of AtCSLA2 + AtMSR1R265A not only failed to produce glucomannan but also reduced 4-Man production by more than 50% compared with the AtCSLA2 enzyme alone (Fig. 5). Interestingly, the AtCSLA2 + AtMSR1D355A strain produced HM containing both 4-Man and 4-Glc, albeit at significantly lower levels than the wild-type multimeric construct (Fig. 5). Although less severe than the AtMSR1R265A variant, the addition of AtMSR1D355A still reduced 4-Man accumulation compared with AtCSLA2 expressed alone (Fig. 5B). In summary, the AtMSR1 TM domain and sugar-binding motif were important modulators of glucomannan synthesis, and mutations in these regions reduced β-1,4-mannan production by AtCSLA2.
Discussion
Elucidating the mechanisms involved in the synthesis of cell wall polysaccharides remains a challenge in plant biology. Although the genomics era ushered in the identification of multiple genes involved in hemicellulose biosynthesis, many of them remain to be functionally characterized (30). We now demonstrate that yeast synthetic biology tools can be exploited to gain mechanistic insight into HM biosynthesis, which has been difficult to study in planta and in vitro. For example, as Arabidopsis csla7 mutants are embryo-lethal (25), it has previously not been possible to investigate whether AtCSLA7 functions as a mannan synthase in vivo. In addition to CSLA enzymes, other putative GTs such as MSR proteins were implicated in HM elongation based on plant mutant chemotypes and in vitro assays using plant extracts (15). However, even in heterologous plant hosts, GT activity assays are subject to pleiotropic effects because of the presence of endogenous carbohydrate-related substrates and enzymes. For instance, expression of Arabidopsis Cellulose Synthase-Like D 5 (AtCSLD5) or coexpression of AtCSLD2 + AtCSLD3 enhanced mannan synthase activity in tobacco leaves (31, 32). However, AtCSLD3 and AtCSLD5 have also been implicated in cellulose (33) and callose synthesis (34), respectively. GTs required for cell wall biosynthesis have at least one TM domain span, are typically expressed in very low abundance, and are difficult to purify in active form (35). Although in vitro activity assays were previously performed on Pichia microsomes containing CSLA proteins (8, 16, 18), these experiments only revealed that the enzymes incorporated exogenous GDP-Man and, to a lesser extent, GDP-Glc into alcohol-insoluble compounds. Because the in vitro assays had limited yields, they relied on [14C]-labeled nucleotide sugar donors. In contrast, we discovered that using only methanol as a carbon source, CSLA-expressing Pichia cells produce large amounts of plant HM (Figs. 1A and 2 and SI Appendix, Fig. S2D), which can be structurally characterized in detail using a variety of analytical tools. Because Pichia has a fast growth cycle and efficient homologous recombination, it also enables high-throughput studies of genetic permutations of these proteins that are not feasible in planta or in vitro.
In this study, we comprehensively analyzed the structures of HM polysaccharides produced by CSLA isoforms from different species. AkCSLA3 expression alone is sufficient for glucomannan production in Pichia cells (Fig. 1A), which can be enriched up to more than 70% purity in only two steps (Fig. 3C and SI Appendix, Fig. S3). The enriched HM from the AkCSLA3 yeast strain resembled the structure of konjac glucomannan, its native product (SI Appendix, Table S2). Consistent with hemicellulose synthesis in plants (2), AkCSLA3 enzymes were localized in small intracellular punctae in Pichia, and their HM products were deposited in the yeast cell wall (Fig. 1 B and C and SI Appendix, Fig. S1 I and J). Indeed, another (gluco)mannan synthase (AtCSLA9) was previously shown to be Golgi-localized in Pichia and had an active site facing the Golgi lumen (18). Thus, Pichia likely has endogenous proteins capable of producing and supplying activated GDP-Man and GDP-Glc sugar donors to the CSLA active site. In contrast to AkCSLA3, the expression of two Arabidopsis CSLAs yielded relatively pure mannan in Pichia. Recombinant AtCSLA2 and AtCSLA7 proteins produced in insect cells were known to have (gluco)mannan and pure mannan synthase activities in vitro, respectively (13). Pointing to diverged roles, AtCSLA2 and AtCSLA9 form a phylogenetic clade distinct from that of the remaining seven CSLA isoforms in Arabidopsis, which includes AtCSLA7 (12). Consistent with its lower activity in vitro, AtCSLA7 (SI Appendix, Fig. S4D) showed reduced mannan production compared with AtCSLA2 in yeast cells (Figs. 2, 3, and 5). However, in contrast to the in vitro study, the AtCSLA2 protein alone did not incorporate significant amounts of 4-Glc in Pichia. These observations could be a result of distinct posttranslational modifications of the AtCSLA2 protein and/or the presence of other accessory proteins in Drosophila cells that are not present in yeast.
Heterologous expression in yeast enabled us to investigate the roles of MSR accessory proteins in HM biosynthesis, which were previously unclear (15). Although MSR1 proteins alone did not alter Pichia wall composition (SI Appendix, Figs. S4C and S5C), they modulated HM synthesis by CSLA enzymes. Potentially, the yeast wall was unchanged after AtMSR1 expression (SI Appendix, Fig. S4 C and E) because the encoded protein was inactive or unstable in the absence of a CSLA interaction partner. The coffee CcMSR1 was an indispensable cofactor for mannan production by CcMANS1 (Fig. 5). In contrast, AtMSR1 did not enhance the AtCSLA7 (SI Appendix, Fig. S4D) or the CcMANS1 (Fig. 5) mannan synthases, but was an optional enhancer of glucomannan synthases. The expression of AtMSR1 increased the content of glucomannan produced by AkCSLA3 in Pichia (Fig. 3A and SI Appendix, Fig. S4C), without altering its Glc:Man ratio. Furthermore, AtMSR1 enabled AtCSLA2 to produce glucomannan, its expected product in Arabidopsis (23–25), instead of mannan (Figs. 3 and 4). Because a single CSLA was sufficient for either glucomannan or mannan synthesis in yeast, MSR proteins are not required to initiate HM elongation. Indeed, Arabidopsis msr1 msr2 double-mutant plants still contained mannan (15). Therefore, specific MSR cofactors are likely involved in the production of distinct types of HM by modulating CSLA activity. Plant MSR cofactors have sequence homology to mammalian PoFUT1 enzymes, which decorate proteins with O-glycan groups that are essential for cell development (28). Mutagenesis of three AtMSR1 conserved amino acids, which are involved in nucleotide sugar binding by PoFUT1 enzymes (28, 29), significantly reduced 4-Glc as well as 4-Man incorporation by AtCSLA2 into AKI polymers (Fig. 5 and SI Appendix, Fig. S5). Furthermore, the reduced accumulation of HM in the AtCSLA2 + AtMSR1Δ28 strain (SI Appendix, Fig. S5) highlighted the importance of the AtMSR1 membrane-anchoring domain. Although AtMSR1 was an optional modulator of HM synthesis in Pichia, the mutated forms tested were detrimental even for the basal activity of the AtCSLA2 enzyme. Because the PoFUT1 sugar-binding motif was structurally conserved and important for the function of AtMSR1 in yeast, we hypothesize that plant MSR proteins may affect CSLA activity via protein glycosylation. Future studies should test this hypothesis by investigating whether CSLA proteins are glycosylated and/or whether they physically interact with the MSR cofactors.
In summary, we established Pichia as a convenient biofactory for plant HM production and gained mechanistic insights into mannan and glucomannan production and modulation. This study advances our knowledge of hemicellulose synthesis and also points to other poorly understood aspects that could be resolved using the powerful yeast platform. Furthermore, our approach can be extended to the study of other plant GTs, provided that the appropriate repertoire of substrates is present in yeast, and to investigate how sugar units can be assembled into designer polymers with tailored properties for specific applications.
Methods
Plant genes were cloned and expressed in yeast cells, as recommended by the EasySelect Pichia Expression Kit (Invitrogen). After accumulating biomass using glycerol as a carbon source in culture tubes or in multiwell plates (Fig. 5 and SI Appendix, Figs. S2, S4, and S5), Pichia cells were induced to express recombinant proteins for 24 h, using methanol. For each genotype, at least three independent Pichia transformants showed a similar cell wall composition. Polysaccharides isolated from yeast were characterized via glycosidic linkage (36, 37) and monosaccharide analyses (23, 38), as previously described. After enzymatic digestion, solubilized carbohydrates were subjected to monosaccharide analysis, or the anthrone assay (39). Pichia cell walls were labeled with specific antibodies according to a protocol previously used for plant seeds (40, 41). Additional details on the protocols used to clone genes, transform and grow Pichia, quantify transgene expression, and extract and structurally characterize polysaccharides are presented in the SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank Katharina Lufen, Felix Roth, Mara Artz, Caroline Janke, and Ulrike Klauß for technical assistance. We gratefully acknowledge the Center for Advanced Imaging at Heinrich-Heine University for access to the Leica SP8 confocal microscope. The sfGFP vector was kindly provided by Tim Niedzwetzki. This research was supported by Cluster of Excellence on Plant Sciences–Deutsche Forschungsgemeinschaft Grant EXC1028.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1814003116/-/DCSupplemental.
References
- 1.Pauly M, Keegstra K. Cell-wall carbohydrates and their modification as a resource for biofuels. Plant J. 2008;54:559–568. doi: 10.1111/j.1365-313X.2008.03463.x. [DOI] [PubMed] [Google Scholar]
- 2.Scheller HV, Ulvskov P. Hemicelluloses. Annu Rev Plant Biol. 2010;61:263–289. doi: 10.1146/annurev-arplant-042809-112315. [DOI] [PubMed] [Google Scholar]
- 3.Lechat H, Amat M, Mazoyer J, Buleon A, Lahaye M. Structure and distribution of glucomannan and sulfated glucan in the cell walls of the red alga Kappaphycus alvarezii (Gigartinales, Rhodophyta) J Phycol. 2000;36:891–902. [Google Scholar]
- 4.Pauly M, et al. Hemicellulose biosynthesis. Planta. 2013;238:627–642. doi: 10.1007/s00425-013-1921-1. [DOI] [PubMed] [Google Scholar]
- 5.Behera SS, Ray RC. Konjac glucomannan, a promising polysaccharide of Amorphophallus konjac K. Koch in health care. Int J Biol Macromol. 2016;92:942–956. doi: 10.1016/j.ijbiomac.2016.07.098. [DOI] [PubMed] [Google Scholar]
- 6.Bewley JD, Bradford KJ, Hilhorst HWM, Nonogaki H. Seeds. Springer; New York: 2013. Structure and composition; pp. 1–25. [Google Scholar]
- 7.Kuravadi NA, et al. In: Guar: An Industrial Crop from Marginal Farms. Agricultural Sustainability. Bhullar GS, Bhullar NK, editors. Elsevier; London: 2013. pp. 47–60. [Google Scholar]
- 8.Gille S, et al. Deep sequencing of voodoo lily (Amorphophallus konjac): An approach to identify relevant genes involved in the synthesis of the hemicellulose glucomannan. Planta. 2011;234:515–526. doi: 10.1007/s00425-011-1422-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckeridge MS. Seed cell wall storage polysaccharides: Models to understand cell wall biosynthesis and degradation. Plant Physiol. 2010;154:1017–1023. doi: 10.1104/pp.110.158642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willför S, Sundberg A, Hemming J, Holmbom B. Polysaccharides in some industrially important softwood species. Wood Sci Technol. 2005;39:245–258. [Google Scholar]
- 11.Suzuki S, Li L, Sun Y-H, Chiang VL. The cellulose synthase gene superfamily and biochemical functions of xylem-specific cellulose synthase-like genes in Populus trichocarpa. Plant Physiol. 2006;142:1233–1245. doi: 10.1104/pp.106.086678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liepman AH, et al. Functional genomic analysis supports conservation of function among cellulose synthase-like a gene family members and suggests diverse roles of mannans in plants. Plant Physiol. 2007;143:1881–1893. doi: 10.1104/pp.106.093989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liepman AH, Wilkerson CG, Keegstra K. Expression of cellulose synthase-like (Csl) genes in insect cells reveals that CslA family members encode mannan synthases. Proc Natl Acad Sci USA. 2005;102:2221–2226. doi: 10.1073/pnas.0409179102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhugga KS, et al. Guar seed beta-mannan synthase is a member of the cellulose synthase super gene family. Science. 2004;303:363–366. doi: 10.1126/science.1090908. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Mortimer JC, Davis J, Dupree P, Keegstra K. Identification of an additional protein involved in mannan biosynthesis. Plant J. 2013;73:105–117. doi: 10.1111/tpj.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Alonso AP, Wilkerson CG, Keegstra K. Deep EST profiling of developing fenugreek endosperm to investigate galactomannan biosynthesis and its regulation. Plant Mol Biol. 2012;79:243–258. doi: 10.1007/s11103-012-9909-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cocuron J-C, et al. A gene from the cellulose synthase-like C family encodes a beta-1,4 glucan synthase. Proc Natl Acad Sci USA. 2007;104:8550–8555. doi: 10.1073/pnas.0703133104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis J, Brandizzi F, Liepman AH, Keegstra K. Arabidopsis mannan synthase CSLA9 and glucan synthase CSLC4 have opposite orientations in the Golgi membrane. Plant J. 2010;64:1028–1037. doi: 10.1111/j.1365-313X.2010.04392.x. [DOI] [PubMed] [Google Scholar]
- 19.Marcus SE, et al. Restricted access of proteins to mannan polysaccharides in intact plant cell walls. Plant J. 2010;64:191–203. doi: 10.1111/j.1365-313X.2010.04319.x. [DOI] [PubMed] [Google Scholar]
- 20.Pédelacq JD, Cabantous S, Tran T, Terwilliger TC, Waldo GS. Engineering and characterization of a superfolder green fluorescent protein. Nat Biotechnol. 2006;24:79–88. doi: 10.1038/nbt1172. [DOI] [PubMed] [Google Scholar]
- 21.Magnelli P, Cipollo JF, Abeijon C. A refined method for the determination of Saccharomyces cerevisiae cell wall composition and beta-1,6-glucan fine structure. Anal Biochem. 2002;301:136–150. doi: 10.1006/abio.2001.5473. [DOI] [PubMed] [Google Scholar]
- 22.Diao Y, et al. De novo transcriptome and small RNA analyses of two amorphophallus species. PLoS One. 2014;9:e95428. doi: 10.1371/journal.pone.0095428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voiniciuc C, et al. MUCILAGE-RELATED10 produces galactoglucomannan that maintains pectin and cellulose architecture in Arabidopsis seed mucilage. Plant Physiol. 2015;169:403–420. doi: 10.1104/pp.15.00851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu L, et al. CELLULOSE SYNTHASE-LIKE A2, a glucomannan synthase, is involved in maintaining adherent mucilage structure in Arabidopsis seed. Plant Physiol. 2014;164:1842–1856. doi: 10.1104/pp.114.236596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goubet F, et al. Cell wall glucomannan in Arabidopsis is synthesised by CSLA glycosyltransferases, and influences the progression of embryogenesis. Plant J. 2009;60:527–538. doi: 10.1111/j.1365-313X.2009.03977.x. [DOI] [PubMed] [Google Scholar]
- 26.Joët T, et al. Regulation of galactomannan biosynthesis in coffee seeds. J Exp Bot. 2014;65:323–337. doi: 10.1093/jxb/ert380. [DOI] [PubMed] [Google Scholar]
- 27.Couperthwaite JA. 2017 Characterization of CcMANS1, a putative mannan synthase from Coffea canephora. Senior Honors thesis (Eastern Michigan University, Ypsilanti, MI). Available at https://commons.emich.edu/honors/565/. Accessed January 12, 2018.
- 28.McMillan BJ, et al. Structure of human POFUT1, its requirement in ligand-independent oncogenic notch signaling, and functional effects of Dowling-Degos mutations. Glycobiology. 2017;27:777–786. doi: 10.1093/glycob/cwx020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lira-Navarrete E, et al. Structural insights into the mechanism of protein O-fucosylation. PLoS One. 2011;6:e25365. doi: 10.1371/journal.pone.0025365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liepman AH, Wightman R, Geshi N, Turner SR, Scheller HV. Arabidopsis–A powerful model system for plant cell wall research. Plant J. 2010;61:1107–1121. doi: 10.1111/j.1365-313X.2010.04161.x. [DOI] [PubMed] [Google Scholar]
- 31.Verhertbruggen Y, Yin L, Oikawa A, Scheller HV. Mannan synthase activity in the CSLD family. Plant Signal Behav. 2011;6:1620–1623. doi: 10.4161/psb.6.10.17989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin L, et al. The cooperative activities of CSLD2, CSLD3, and CSLD5 are required for normal Arabidopsis development. Mol Plant. 2011;4:1024–1037. doi: 10.1093/mp/ssr026. [DOI] [PubMed] [Google Scholar]
- 33.Park S, Szumlanski AL, Gu F, Guo F, Nielsen E. A role for CSLD3 during cell-wall synthesis in apical plasma membranes of tip-growing root-hair cells. Nat Cell Biol. 2011;13:973–980. doi: 10.1038/ncb2294. [DOI] [PubMed] [Google Scholar]
- 34.Gu F, et al. Arabidopsis CSLD5 functions in cell plate formation in a cell cycle-dependent manner. Plant Cell. 2016;28:1722–1737. doi: 10.1105/tpc.16.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandhu APS, Randhawa GS, Dhugga KS. Plant cell wall matrix polysaccharide biosynthesis. Mol Plant. 2009;2:840–850. doi: 10.1093/mp/ssp056. [DOI] [PubMed] [Google Scholar]
- 36.Ciucanu I, Kerek F. A simple and rapid method for the permethylation of carbohydrates. Carbohydr Res. 1984;131:209–217. [Google Scholar]
- 37.Ciucanu I. Per-O-methylation reaction for structural analysis of carbohydrates by mass spectrometry. Anal Chim Acta. 2006;576:147–155. doi: 10.1016/j.aca.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 38.Voiniciuc C, Günl M. Analysis of monosaccharides in total mucilage extractable from Arabidopsis seeds. Bio Protoc. 2016;6:e1801. [Google Scholar]
- 39.Foster CE, Martin TM, Pauly M. Comprehensive compositional analysis of plant cell walls (lignocellulosic biomass) part II: Carbohydrates. J Vis Exp. 2010;37:e1745. doi: 10.3791/1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voiniciuc C. Whole-seed immunolabeling of Arabidopsis mucilage polysaccharides. Bio Protoc. 2017;7:e2323. doi: 10.21769/BioProtoc.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voiniciuc C, Günl M, Schmidt MH-W, Usadel B. Highly branched xylan made by IRREGULAR XYLEM14 and MUCILAGE-RELATED21 links mucilage to Arabidopsis seeds. Plant Physiol. 2015;169:2481–2495. doi: 10.1104/pp.15.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.