Implantable cardiac pacemakers have been employed for the treatment of various arrhythmias beginning in the 1950s. Throughout the years, developments in microfabrication technologies, as well as advances in surgical procedures and the understanding of electrophysiology, have brought forth next-generation cardiac pacemakers. These are much smaller, capable of feedback regulation, and endowed with longer-lived batteries, which decrease the need for frequent surgery and battery replacement (1). In addition, the advent of antiinflammatory drug-eluting leads has significantly reduced the risk of inflammation and rejection after cardiac pacemaker implantation. However, these devices do not completely eliminate the immune response (2) and still require battery replacements every several years. Furthermore, implantation of foreign bodies on the heart still poses the risk of fouling in the chest cavity, with resultant unwanted electrochemical reactions. In PNAS, Parameswaran et al. (3) describe the development of an approach for cardiac cell and whole-heart pacing.
Traditional cardiac pacemakers rely on delivering an electrical pulse to the cardiac tissue to elicit a response in the form of cell contraction. This method is based on the depolarization of the membrane potential of the cardiomyocytes, which leads to intracellular calcium release and activation of the cell contraction machinery. Developments in the field of optogenetics have previously shown that by infecting cardiac cells with light-gated ion channels, it is possible to elicit cardiac cell contraction by light illumination (4). While this method is not invasive, it involves infection of the cardiac tissue with a virus expressing the light-activated ion channel. Other noninvasive methods based on light illumination have shown that cardiac cells can be paced without the need to apply an electric field. Gentemann et al. (5) described the use of gold nanoparticles irradiated with a 532-nm laser to induce heating of the cardiac cells, which leads to calcium oscillations and cell contraction when placed in a calcium-containing buffer. In a different approach, Savchenko et al. (6) used graphene’s ability to convert light into electricity to pace cardiac cells and tissues in vivo. Other works have described the use of direct laser illumination to induce cardiac contraction in embryonic quail hearts (7) and even adult rabbit hearts (8). While these methods show great promise, they rely on continual laser irradiation to achieve contraction at the same frequency of the applied pulse. Furthermore, the required laser radiant exposure required to achieve stimulation in these methods is very high and may result in a high percentage of cell death.
In their work, Parameswaran et al. (3) use photolithography to define a mesh composed of the polymer SU-8 that serves as a substrate for a high-density array of p-type/intrinsic/n-type silicon nanowires (PIN-SiNWs). Such wires were specifically chosen as they have been shown to elicit action potentials when illuminated due to their innate photoelectric capabilities (9). This is in contrast to other types of SiNWs, such as silicon core shell nanowires that lack a photoelectric response, and so were used as a negative control. The PIN-SiNWs were dispersed in high numbers onto the SU-8 substrate before photolithographic definition. Areas that were blocked from irradiation by the photomask were supposed to be washed away in development. However, the polymer directly underneath the SiNWs showed polymerization as well. This resulted in a network of SiNWs supported by thin films of polymerized SU-8. The final structure was composed of a polymer mesh covered with nanowires. The nanowires also penetrated into the holes in the mesh, creating a continuous, less organized network of polymer and nanowires. This could be advantageous in the case of cell seeding because it would aid in creating a continuous cell sheet with better cell–cell interactions.
Parameswaran et al. (3) first describe the development of a protocol for the pacing of cardiac cells grown on their polymer nanowire mesh. The cells were seeded and grown on top of the composite mesh. Due to the photoelectrochemical properties of the SiNWs, when they were illuminated with light, a faradaic current was produced that could then induce an action potential at the cell membrane. The protocol utilized scanning laser illumination at a frequency that is much higher than the possible contraction rate for cardiomyocytes and so was not used for direct pacing but rather for training. With their protocol, every pixel scanned by the laser (∼0.09 μm2 pixel area) was exposed to six primary incident fluxes per stimulation frame due to the diameter of the stimulating laser. This exposure was further increased by light propagation through the SiNWs. The combination of the scanning protocol and the light propagation caused by the SiNWs allowed the authors to keep the laser radiant exposure very low and thus minimize any damage to the illuminated cells. This protocol was put into practice when cardiac cells were seeded onto the composite mesh devices. The seeded cells were stimulated using cycles of laser irradiation with intervening off periods between them that allowed the researchers to monitor the result of the training cycles. Calcium imaging was used to monitor spontaneous beating frequencies and responses to the laser irradiation before and after training cycles. The authors demonstrate that the postirradiation contraction frequency increases compared with the preirradiation frequency and that this continues over three to four cycles until the target contraction frequency is achieved. They note that the path toward the desired contraction frequency varies between experiments, meaning that it is not always a clear linear increase, and they estimate that the stimulation radiant exposure used in this protocol is 2 to 3 orders of magnitude lower than that described in previous works in which cardiac tissues were paced using laser irradiation.
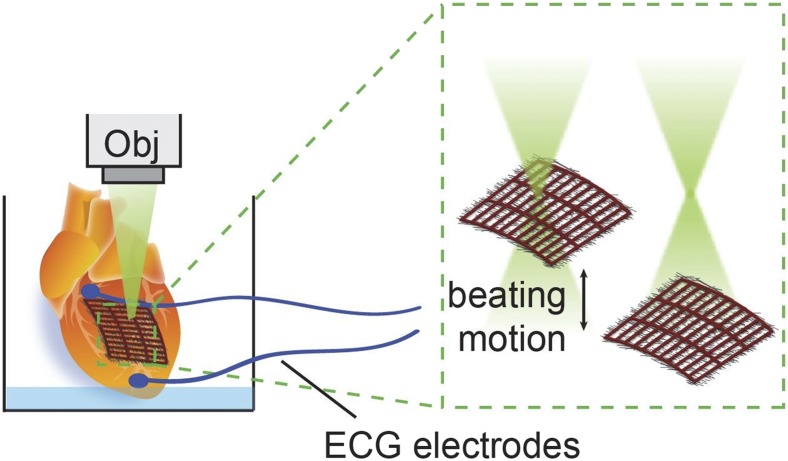
Lastly, the authors demonstrate ex vivo optical stimulation of isolated adult rat hearts. The mesh device was placed on the exposed left ventricle, and laser stimulation was used to pace the entire heart. The nanoscale structure of the mesh allowed it to conform to the exposed surface of the heart via capillary forces, without any further modifications. This time, however, the protocol did not consist of line scanning, as was done with cultured cells, but relied on the movements of the contracting heart in and out of focus (Fig. 1). In addition, no off periods were needed because contraction rates were measured using ECG electrodes conveniently placed on the heart. Due to the variation in laser spot location and intensity due to the contractile movements of the heart and the light-dispersing effect of the SiNWs, a stimulating effect was achieved similar to that on cultured cells. This protocol achieved the desired stimulation frequency after 5 min of light training. Despite the long irradiation periods, cytotoxicity assays showed no increase in cardiac cell death compared with untrained hearts.
Fig. 1.
Schematic illustration depicting the stimulation of a whole heart using the polymer–SiNW mesh. The mesh is placed onto the heart, and a microscope objective (Obj) is used to irradiate it. The beating motions of the heart move it in and out of focus, thus exposing the cells only to intermittent laser radiation each time, similar to the effect achieved with the scanning laser used on cultured cells. ECG electrodes were placed on the heart to monitor its response to the laser-induced pacing.
In PNAS, Parameswaran et al. describe the development of an approach for cardiac cell and whole-heart pacing.
Although the work by Parameswaran et al. (3) is not the first to demonstrate control of cardiac cell function by light, it shows the development of a much more efficient protocol to do so, while minimizing the damage to the stimulated tissue. This is due to the added effect of the SiNWs. In recent works, SiNWs have been widely employed as tools to interrogate excitable tissues; for example, by integrating them into field effect transistors in mesh devices, which can be used for studying specimens ranging from single-cell cultures to whole organs (10–13). However, these methods could not support pacing of electrogenic tissues without the integration of larger, noble metal electrodes similar to those commonly used in pacemaker leads (14). The work described by Parameswaran et al. shows a significant step toward the development of alternative pacing methods that could possibly replace those commonly used today. It is easy to imagine how these could be integrated into existing tissue engineering technologies involving the integration of electronic meshes inside engineered tissues (14, 15). Although the authors state that there is no need for large bulky electrode leads to be placed onto the heart, unless a method to inject this device is devised, a thoracotomy will still be needed to place the nanowire mesh onto the surface of the heart. Recent works have described methods to inject ultrathin, electronic meshes containing SiNW-based field-effect transistors into the brain (16–18). These methods could possibly be employed in the same manner to access the heart. Another challenge is that even though capillary forces can hold the mesh in place for the duration of the experiment, the mesh could easily dislodge if implanted for extended time periods. Therefore, additional steps will be needed to secure it to its location and minimize damage caused during implantation. Recent work by Malki and colleagues (19) has described a suture-free method to solder an engineered cardiac tissue onto the surface of the heart using laser irradiation and gold nanorod heating. Other suture-free methods similar to this one could be employed to prevent delamination from the dynamic surface of the heart.
One other possible issue that should be addressed is that, similar to cardiac pacemakers currently in use, an energy source will still be required to supply radiation to the nanowire mesh. This could possibly be overcome by the use of an on-board piezoelectric energy source that will harvest energy from the movements of the heart (20). Additionally, as Parameswaran et al. (3) surmise, methods to overcome the inability of light to penetrate the chest cavity will be needed. One could imagine the integration of micro-LEDs into the mesh network; however, in this context, it has yet to be demonstrated.
Overall, the field of cell and tissue stimulation using light irradiation is an exciting one, which could be envisioned as a tool in the laboratory to study fundamental cellular processes, or in the clinic for cardiac or neuronal therapeutics. The work presented by Parameswaran et al. has taken this a step closer to being a reality—a stimulating prospect that could help improve existing devices in the field of tissue–electronics interfaces.
Footnotes
The authors declare no conflict of interest.
See companion article on page 413.
References
- 1.Feiner R, Dvir T. Tissue–electronics interfaces: From implantable devices to engineered tissues. Nat Rev Mater. 2017;3:17076. [Google Scholar]
- 2.Berthelsen WA. 1990. Screw-in drug eluting lead. US Patent 4,953,564.
- 3.Parameswaran R, et al. Optical stimulation of cardiac cells with a polymer-supported silicon nanowire matrix. Proc Natl Acad Sci USA. 2019;116:413–421. doi: 10.1073/pnas.1816428115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nussinovitch U, Gepstein L. Optogenetics for in vivo cardiac pacing and resynchronization therapies. Nat Biotechnol. 2015;33:750–754. doi: 10.1038/nbt.3268. [DOI] [PubMed] [Google Scholar]
- 5.Gentemann L, et al. Modulation of cardiomyocyte activity using pulsed laser irradiated gold nanoparticles. Biomed Opt Express. 2016;8:177–192. doi: 10.1364/BOE.8.000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savchenko A, et al. Graphene biointerfaces for optical stimulation of cells. Sci Adv. 2018;4:t0351. doi: 10.1126/sciadv.aat0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenkins MW, et al. Optical pacing of the embryonic heart. Nat Photonics. 2010;4:623–626. doi: 10.1038/nphoton.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenkins MW, et al. Optical pacing of the adult rabbit heart. Biomed Opt Express. 2013;4:1626–1635. doi: 10.1364/BOE.4.001626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parameswaran R, et al. Photoelectrochemical modulation of neuronal activity with free-standing coaxial silicon nanowires. Nat Nanotechnol. 2018;13:260–266. doi: 10.1038/s41565-017-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian B, et al. Three-dimensional, flexible nanoscale field-effect transistors as localized bioprobes. Science. 2010;329:830–834. doi: 10.1126/science.1192033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian B, et al. Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nat Mater. 2012;11:986–994. doi: 10.1038/nmat3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie C, et al. Three-dimensional macroporous nanoelectronic networks as minimally invasive brain probes. Nat Mater. 2015;14:1286–1292. doi: 10.1038/nmat4427. [DOI] [PubMed] [Google Scholar]
- 13.Dai X, Zhou W, Gao T, Liu J, Lieber CM. Three-dimensional mapping and regulation of action potential propagation in nanoelectronics-innervated tissues. Nat Nanotechnol. 2016;11:776–782. doi: 10.1038/nnano.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feiner R, et al. Engineered hybrid cardiac patches with multifunctional electronics for online monitoring and regulation of tissue function. Nat Mater. 2016;15:679–685. doi: 10.1038/nmat4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feiner R, Fleischer S, Shapira A, Kalish O, Dvir T. Multifunctional degradable electronic scaffolds for cardiac tissue engineering. J Control Release. 2018;281:189–195. doi: 10.1016/j.jconrel.2018.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong G, et al. Syringe injectable electronics: Precise targeted delivery with quantitative input/output connectivity. Nano Lett. 2015;15:6979–6984. doi: 10.1021/acs.nanolett.5b02987. [DOI] [PubMed] [Google Scholar]
- 17.Zhou T, et al. Syringe-injectable mesh electronics integrate seamlessly with minimal chronic immune response in the brain. Proc Natl Acad Sci USA. 2017;114:5894–5899. doi: 10.1073/pnas.1705509114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J. 2018. Syringe injectable electronics. Biomimetics Through Nanoelectronics:Development of Three Dimensional Macroporous Nanoelectronics for Building Smart Materials, Cyborg Tissues and Injectable Biomedical Electronics. Springer Theses (Springer, Cham, Switzerland), pp 65–93.
- 19.Malki M, Fleischer S, Shapira A, Dvir T. Gold nanorod-based engineered cardiac patch for suture-free engraftment by near IR. Nano Lett. 2018;18:4069–4073. doi: 10.1021/acs.nanolett.7b04924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dagdeviren C, et al. Conformal piezoelectric energy harvesting and storage from motions of the heart, lung, and diaphragm. Proc Natl Acad Sci USA. 2014;111:1927–1932. doi: 10.1073/pnas.1317233111. [DOI] [PMC free article] [PubMed] [Google Scholar]