Significance
Phytohormone pathways provide plants with a powerful regulatory potential to prioritize their defense strategies to different attackers. However, some pathogens and herbivores can manipulate plant defenses for their own benefits by leveraging these pathways. Identification of effectors that modulate host defenses could provide new insights into pathogens/herbivores-plant interaction. Here we report that a newly discovered insect salivary effector, injected into plant tissues during feeding, facilitates insect performance by activation of plant salicylic acid-signaling pathway. The insect salivary effector achieves this function via interacting with a plant KNOTTED 1-like homeobox transcription factor. These findings extend the existing pathogen effector paradigm to herbivores and give valuable clues for developing novel strategies for pest management.
Keywords: phloem-feeding insects, plant-insect interaction, feeding behaviors, SA elicitor, KNOTTED 1-like homeobox protein
Abstract
Phloem-feeding insects feed on plant phloem using their stylets. While ingesting phloem sap, these insects secrete saliva to circumvent plant defenses. Previous studies have shown that, to facilitate their feeding, many phloem-feeding insects can elicit the salicylic acid- (SA-) signaling pathway and thus suppress effective jasmonic acid defenses. However, the molecular basis for the regulation of the plant's defense by phloem-feeding insects remains largely unknown. Here, we show that Bt56, a whitefly-secreted low molecular weight salivary protein, is highly expressed in the whitefly primary salivary gland and is delivered into host plants during feeding. Overexpression of the Bt56 gene in planta promotes susceptibility of tobacco to the whitefly and elicits the SA-signaling pathway. In contrast, silencing the whitefly Bt56 gene significantly decreases whitefly performance on host plants and interrupts whitefly phloem feeding with whiteflies losing the ability to activate the SA pathway. Protein-protein interaction assays show that the Bt56 protein directly interacts with a tobacco KNOTTED 1-like homeobox transcription factor that decreases whitefly performance and suppresses whitefly-induced SA accumulation. The Bt56 orthologous genes are highly conserved but differentially expressed in different species of whiteflies. In conclusion, Bt56 is a key salivary effector that promotes whitefly performance by eliciting salicylic acid-signaling pathway.
In nature, a wide range of attackers with different feeding mechanisms continuously assail plants. Plants first recognize attackers and then initiate sophisticated defense strategies through a complex phytohormone system (1). The crosstalk between salicylic acid (SA) and jasmonic acid (JA) pathways plays a crucial role in helping plants to initiate defenses according to the type of attacker (2, 3). Generally, plants produce JA to activate defenses against necrotrophic microbes, some phloem-feeding insects, and chewing herbivores (4, 5), whereas SA-mediated plant defenses are induced by some phloem-feeding insects and biotrophic pathogens (2, 6). Plant pathogens have evolved to suppress plant defenses they induce in hosts by leveraging SA-JA crosstalk using chemical toxins and virulent effector proteins they secrete (7, 8). Similarly, herbivore manipulation of plant defenses has been suggested to be associated with the saliva they secrete (1, 9, 10).
Many salivary chemical compounds and proteins have been identified in chewing herbivores and phloem-feeding insects (1, 11). The caterpillar Helicoverpa zea salivary component glucose oxidase suppresses the production of nicotine induced by the caterpillar feeding on tobacco (12). Calcium-binding proteins in aphid saliva enable continuous feeding on plants by undermining the occlusion of sieve tubes triggered by mechanical injury (9). In recent years, a number of salivary effector proteins have been identified in aphids, planthoppers, and spider mites, such as C002, Armet, Mp1, Mp2, Mp10, Me47, GroEL, and migration inhibitory factor (13–21). These studies demonstrate that salivary effectors from phloem feeders play important roles in their feeding and plant defense regulation. A recent study showed that the effector Mp1 from Myzus persicae associates with the host protein VPS52 to promote virulence (22). However, the molecular mechanisms underlying the regulation of plant-defense-signaling pathways by many salivary proteins remain unclear (23).
The whitefly Bemisia tabaci is a small phloem-feeding insect now known to be a species complex including over 30 cryptic species (24, 25). Among them, Middle East-Asia Minor 1 (MEAM1) and Mediterranean 1 (MED1) are two widely distributed invasive pests that have been recorded to occur on many plant species (26, 27). As a phloem-feeding insect, a whitefly gains access to nutrients from living cells (phloem sieve elements) for hours to days (28, 29) and thus must repress special plant defenses, such as clogging of phloem sieve elements (9). On Arabidopsis thaliana, infestations of whitefly and aphid induce SA-signaling pathway genes but reduce or only modestly induce effectual JA defenses that deter insect performance (30–32). Activation of the SA pathway in tobacco improves the performance of the whitefly via reduction of JA signaling (33), whereas exogenous MeJA-treated tobacco suppresses the performance of the whitefly significantly (34). Therefore, it has been hypothesized that phloem-feeding insects can leverage the crosstalk between SA- and JA-signaling networks to manipulate plant defenses for their own benefit (35, 36).
Here, we identify a salivary protein that regulates plant defenses. We show that Bt56, a whitefly salivary protein, enhances whitefly performance on tobacco, induces the SA-signaling pathway in tobacco, and promotes whitefly phloem-feeding on host plants. In addition, we performed yeast-two-hybrid (Y2H) screens against a tobacco library and identified host targets of the Bt56 protein.
Results
Whitefly Infestation Promotes the Susceptibility of Tobacco.
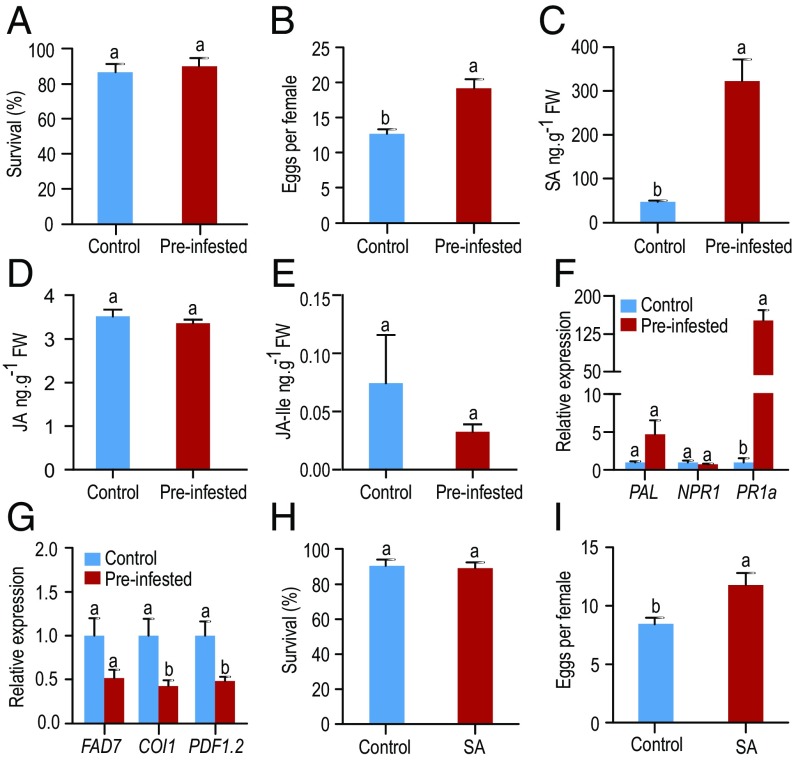
To assess whether whitefly preinfestation can regulate plant defenses and thus affect the susceptibility of tobacco to subsequent whitefly performance, we released MED1 adults on MED1 preinfested and control plants for 3 d and then observed whitefly survival and fecundity. Compared with control tobacco, MED1 whitefly-preinfested tobacco plants did not significantly affect the survival of subsequent whiteflies (Fig. 1A; F1,12 = 0.314, P = 0.585); however, the fecundity of whiteflies on the preinfested tobacco was significantly higher than that on control tobacco plants (Fig. 1B; F1,12 = 19.286, P < 0.001). To examine the effect of MED1 infestation on tobacco defenses, we measured the levels of SA, JA, JA-Ile, abscisic acid (ABA), and indoleacetic acid (IAA) in whitefly-infested tobacco plants. After infestation for 3 d, the level of SA in whitefly-infested tobacco was significantly higher than that in control tobacco (Fig. 1C; F1,10 = 30.477, P < 0.001), whereas the levels of JA, JA-Ile, ABA, and IAA were not significantly changed (Fig. 1 D and E; F1,10 = 0.805–0.994, P = 0.342–0.391, SI Appendix, Fig. S1 A and B; F1,10 = 0.076–0.745, P = 0.408–0.789). The expression of the SA-response gene (PR1a) was significantly induced in preinfested tobacco (F1,10 = 58.808, P < 0.001) but not PAL (F1,8 = 3.863, P = 0.085) and NPRI (F1,10 = 0.836, P = 0.382) (Fig. 1F); in contrast, the expression of COI1 and PDF1.2 genes in the JA-signaling pathway (34, 37) were significantly depressed (F1,9 = 6.739, P = 0.029 for COI1 and F1,8 = 9.265, P = 0.016 for PDF1.2) but not FAD7 (F1,10 = 4.808, P = 0.053) (Fig. 1G).
Fig. 1.
Whitefly preinfestation improves the susceptibility of tobacco. (A and B) Whiteflies were allowed to feed on whitefly-preinfested and -uninfested control tobacco plants for 3 d. The survival (A) and fecundity (B) of whiteflies were determined to assess host plant susceptibility. Each treatment included six plants, and each plant had two clip cages (n = 12). (C–E) The mean levels of SA (C), JA (D), and jasmonoyl-isoleucine (JA-Ile) (E) in the control and preinfested tobacco plants were measured (n = 6). (F and G) Expression levels of marker genes in SA- (F) and JA- (G) signaling pathways of control and preinfested tobacco plants: phenylalanine ammonia lyase (PAL), nonexpresser of PR genes 1 (NPR1), pathogenesis-related protein 1a (PR1a); x3 fatty acid desaturase 7 (FAD7), coronatine-insensitive 1 (COI1), and plant defensin (PDF1.2) (n = 5 to 6). (H and I) The effect of exogenous SA application on whitefly survival (H) and fecundity (I). Eight plants were used for each treatment, and each plant included two clip cages (n = 16). The data shown are mean ± standard error (SE). The letters above the bars indicate significant differences among different treatments at P < 0.05 [nested analysis of variance (ANOVA) for whitefly bioassays and one-way ANOVA for other experiments].
To examine whether the increased SA in tobacco affects the performance of the whitefly, preinfestation tests were performed on SA suppressing NahG transgenic tobacco. MED1 preinfestation did not affect whitefly fecundity and longevity at 3 d on NahG transgenic tobacco (SI Appendix, Fig. S1 C and D; F1,12 = 1.238–2.667, P = 0.128–0.288). Finally, we examined the effect of exogenous SA treatment on whitefly survival and fecundity. Following SA treatment, the expression levels of FAD7 and PDF1.2 in the JA-signaling pathway were down-regulated significantly (SI Appendix, Fig. S1E; F1,10 = 9.346–17.552, P = 0.002–0.012). Again, the survival was not significantly affected (Fig. 1H; F1,16 = 0.119, P = 0.735), but MED1 laid more eggs on SA-treated tobacco than on control plants (Fig. 1I; F1,16 = 10.982, P = 0.004). Collectively, these data suggest that MED1 infestation increases the susceptibility of tobacco probably by activating the SA-signaling pathway and repressing effective JA-based defenses independent of JA and JA-Ile levels.
B. tabaci Salivary Protein Bt56 Facilitates Whitefly Performance on Host Plants.
To identify salivary proteins that influence whitefly performance, we selected candidate effector genes from the transcriptome of MED1 salivary glands (38). Among them, we identified a salivary effector Bt56 (GenBank no.: KY986869). The nucleotide sequence of Bt56 from MED1 is 1,015 bp encoding a protein of 112 amino acids with a predicted signal peptide at its N terminus (SI Appendix, Fig. S2). The molecular weight of the predicted mature protein is 9.8 kDa. Blasting the Bt56 protein sequence against the National Center for Biotechnology Information GenBank did not reveal any sequence similar to this protein.
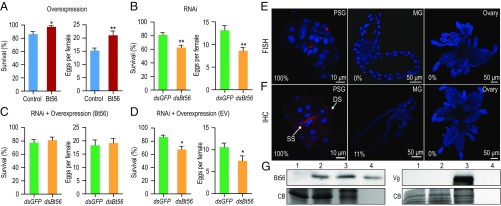
Overexpression of Bt56 in tobacco did not induce chlorosis or cell death in the infiltrated leaf areas 2 and 6 d post infiltration (SI Appendix, Fig. S3A), and the Bt56 transcript could be detected in tobacco from 24 h post infiltration onwards and declined dramatically by day 9 (SI Appendix, Fig. S3B). Both survival and fecundity of whiteflies fed on Bt56-overexpressed leaf areas were significantly higher than those on empty vector (control) infiltrated areas (Fig. 2A; F1,16 = 7.021–10.163, P = 0.006–0.017; SI Appendix, Fig. S3C; F1,12 = 0.667–13.859, P = 0.003–0.43). Knockdown of the Bt56 gene by RNA interference (RNAi) (SI Appendix, Fig. S3D; F1,4 = 47.545, P = 0.002) resulted in a significant decrease in survival and fecundity of the whitefly on tobacco (Fig. 2B; F1,16 = 12.227–15.705, P = 0.001–0.003; SI Appendix, Fig. S3E; F1,12 = 0.781–12.655, P = 0.004–0.413). Complete recovery of survival and fecundity was obtained when Bt56-down-regulated (RNAi) whiteflies were fed on tobacco expressing Bt56 (Fig. 2C; F1,16 = 0.160–0.206, P = 0.656–0.694); however, similar results were not observed on control plants (Fig. 2D; F1,16 = 7.884–8.333, P = 0.011–0.013).
Fig. 2.
Effects of salivary protein Bt56 on the performance of the whitefly on host plants. The Bt56 gene was transiently overexpressed in Nicotiana tabacum by agroinfiltration and empty vector (EV) as the control. Two days after infiltration, whiteflies were transferred to feed on tobacco expressing Bt56 and control tobacco (A); double-stranded RNA- (dsRNA-) fed whiteflies were transferred to noninfiltrated tobacco (B), tobacco expressing Bt56 (C) and tobacco expressing EV (D). dsGFP, dsRNA targeting the gene coding for the green fluorescent protein; dsBt56, dsRNA targeting the gene coding for Bt56 protein. The survival and number of progeny produced per female were examined after 3 d. Each treatment included eight plants, and each plant had two clip cages (n = 16). The data shown are mean ± SE. Nested ANOVA, *P < 0.05, **P < 0.01. (E) Fluorescence in situ hybridization (FISH) to detect Bt56 transcripts. Tissues were hybridized with a Cy3-labeled Bt56 probe (red), and nuclei were stained with 4′, 6-diamidino-2-phenylindole (blue). (F) Immunohistochemical (IHC) localization of the Bt56 protein in different tissues of the whitefly using the rabbit polyclonal anti-Bt56 antibody. Nuclei are shown in blue, red is the positive signal for anti-Bt56. Secretory section of the primary salivary gland (PSG) and ductal section of the PSG. The percentages represent the ratios of PSGs, midguts (MGs), and ovaries with positive signals. The data were surveyed separately from 18 dissected PSGs, MGs, and ovaries in three independent experiments. (G) Western blot analysis of Bt56 (Left) and Vg protein (Right). Lane 1, protein extracted from a cotton leaf without whitefly feeding; lane 2, protein extracted from a cotton leaf fed upon by whiteflies; lane 3, protein extracted from whitefly adults; lane 4, the saliva collected from a 0.4% resorcinol diet fed by whiteflies. An anti-Vg antibody was used to detect whitefly residual contamination on the whitefly-infested leaves. Coomassie blue (CB) staining showed the equal loading of lane 1 and lane 2. The CB staining regions were cut around 10 kDa for Bt56 and 100 kDa for Vg.
Next, we investigated whether Bt56 influences the performance of the whitefly on a more suitable host plant cotton Gossypium hirsutum. Bt56 transcript knockdown significantly shortened the life span of B. tabaci on cotton from day 3 to day 7 (SI Appendix, Fig. S3F; F1,12 = 5.785–9.792, P = 0.009–0.033) but had no impact on MED1 performance when they fed on an artificial diet (SI Appendix, Fig. S3G; F1,12 = 0.399–1.918, P = 0.191–0.54). These data suggest that the shortened life span of Bt56-silenced whiteflies on host plants was not due to the negative effects of Bt56 silencing on the whitefly itself but likely due to the loss of capability to regulate host plant defenses.
To determine if Bt56 is a salivary protein, we examined the gene expression and protein location of Bt56. FISH revealed the presence of transcript Bt56 in a few cells only around the center of the secretory section of MED1 PSGs but not the MG and ovary (Fig. 2E). Analysis by the quantitative reverse transcription–polymerase chain reaction (qRT-PCR) showed that Bt56 was expressed extremely high in whitefly PSGs; the expression level of Bt56 in nymphs and adults was much higher than that in eggs (SI Appendix, Fig. S4A). Using the polyclonal anti-Bt56 antibody, the Bt56 protein can be specifically recognized in the MED1 head and thorax but not in other parts of the body (SI Appendix, Fig. S4B). Further IHC staining revealed that Bt56 was specifically located in the PSGs especially in the secretory section and ductal section (Fig. 2F). To investigate whether Bt56 could be secreted into host plants, Western blot was used to detect the Bt56 protein in whitefly saliva and whitefly-infested plants. The protein was detected in MED1-infested plant leaves (lane 2), MED1 whole bodies (lane 3), and saliva (lane 4) but was not found in leaves from the uninfected plant (lane 1) (Fig. 2G and SI Appendix, Fig. S4 C and D). To further demonstrate that the Bt56 protein detected in whitefly-infested plant leaves was not from residual contamination of whitefly adults or eggs on plant leaves, we used a monoclonal antibody to detect whitefly Vg, an abundant protein in whitefly adults and eggs (39). The results showed that no Vg was detected on whitefly-infested plant leaves (Fig. 2G, Right). Together, these results indicate that Bt56 is a secretory salivary effector protein highly expressed in whitefly PSGs.
Bt56 Promotes Whitefly Feeding.
We used an electrical penetration graph to monitor the feeding behaviors of Bt56-silenced whiteflies on cotton (40, 41) (SI Appendix, Fig. S5 and Table S1). Compared with the control group [double-stranded green fluorescent protein (dsGFP)] (SI Appendix, Fig. S5A), phloem feeding was interrupted in Bt56-silenced whiteflies [double-stranded Bt56 (dsBt56)] (SI Appendix, Fig. S5B). There was no significant variation in nonphloem phase feeding and salivation behaviors between dsGFP- and dsBt56-fed whiteflies (SI Appendix, Fig. S5 C and D; F1,30 = 0.001–2.902, P = 0.099–0.98, SI Appendix, Table S1). By contrast, Bt56-silenced whiteflies had significantly reduced averages and total durations of phloem ingestion (SI Appendix, Fig. S5D; F1,30 = 9.512–9.743, P = 0.004, SI Appendix, Table S1). Compared with dsGFP-fed whiteflies, the percentage of whiteflies having, at least, one phloem ingestion was much lower in Bt56-silenced whiteflies (SI Appendix, Table S1). These results demonstrate that Bt56 promotes whiteflies to feed on the phloem.
Bt56 Gene Induces SA-Signaling Pathway.
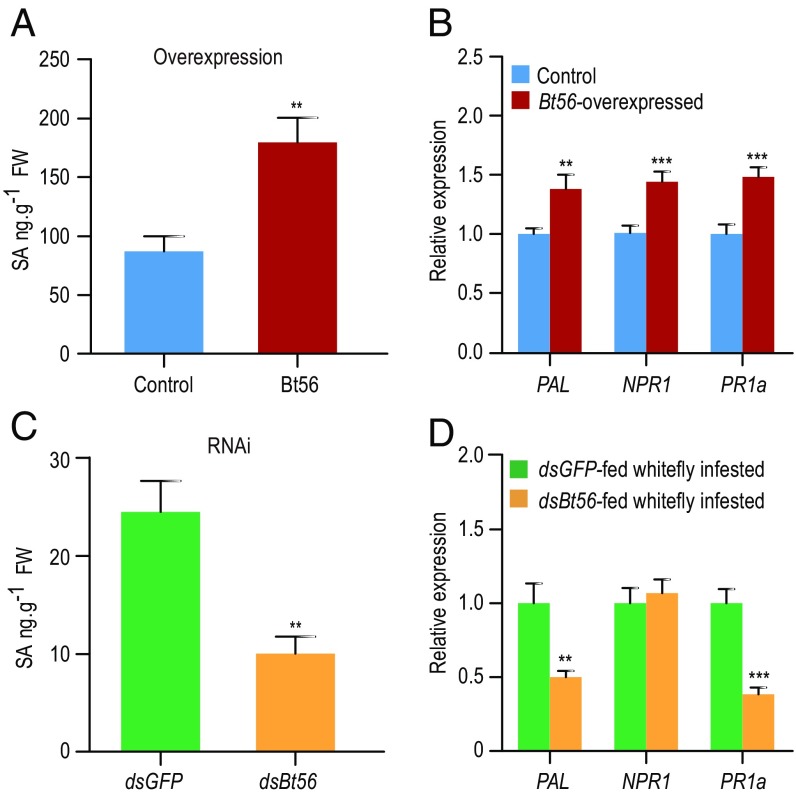
To explore whether Bt56 affects SA-JA-signaling pathways and thus modulates plant defenses, we investigated the level of SA, JA, and the expression levels of marker genes in both pathways. Overexpression of Bt56 in tobacco significantly increased the production of SA (Fig. 3A; F1,10 = 14.623, P = 0.003) but did not change the levels of JA and JA-Ile (SI Appendix, Fig. S6 A and B; F1,10 = 1.834–4.561, P = 0.058–0.205). The transcript levels of PAL, NPR1, and PR1a in the SA-signaling pathway were significantly induced in Bt56-overexpressed tobacco (Fig. 3B; F1,22 = 8.233–18.282, P = 0.000308–0.009), whereas the marker genes in the JA-signaling pathway were not significantly changed (SI Appendix, Fig. S6C; F1,22 = 0.052–1.883, P = 0.184–0.821). To confirm the role of Bt56 in regulating SA-JA-signaling pathways, we used Bt56-silenced whiteflies to infest tobacco plants. After 3 d of infestation, the production of SA in tobacco infested by Bt56-silenced whiteflies was significantly lower than in tobacco infested by dsGFP-fed whiteflies (Fig. 3C; F1,10 = 15.889, P = 0.003). In tobacco plants infested by Bt56-silenced whiteflies, a significant reduction was observed in the expression levels of PAL and PR1a genes in the SA pathway but not in NPR1 (Fig. 3D; F1,14 = 12.667–33.98, P = 0.000044–0.003 for PAL and PR1a and F1,14 = 0.236, P = 0.635 for NPR1), whereas the JA response gene PDF1.2 was up-regulated significantly (SI Appendix, Fig. S6D; F1,14 = 0.412–0.65, P = 0.433–0.531 for FAD7 and COI1 and F1,14 = 20.3, P < 0.001 for PDF1.2). Together, these data suggest that the whitefly Bt56 protein plays an important role in the activation of the SA-signaling pathway in tobacco.
Fig. 3.
Bt56 induces a tobacco SA-signaling pathway. (A and B) The effect of overexpressing Bt56 on the tobacco SA pathway. The level of SA (A) and the expression levels of SA-marker genes (B) were measured 48 h after agroinfiltration. (C and D) The effect of infestation by Bt56-silenced whiteflies on the tobacco SA pathway. Three days after infestation by dsGFP- and dsBt56-fed whiteflies, the level of SA (C), and the expression levels of SA-marker genes (D) were measured. The data shown are mean ± SE, n = 6 for the phytohormone measurement, n = 12 for the gene expression on Bt56-overexpressed tobacco, and n = 8 for dsRNA-fed whitefly-infested tobacco. One-way ANOVA, the least significant difference (LSD) test, **P < 0.01, ***P < 0.001.
NTH202 Interacts with Bt56 and Represses Whitefly-Induced SA Accumulation.
To identify host plant targets of the Bt56 protein, we performed Y2H to screen a tobacco complementary DNA (cDNA) library. In preliminary experiments, we obtained a few putative positive targets (SI Appendix, Table S2), including a tobacco class II KNOTTED 1-like homeobox (KNOX) transcription factor gene (GenBank: KY986874). This gene was named NTH202 following the naming rule for tobacco KNOX proteins (SI Appendix, Fig. S7A) (42). Previous studies show KNOX proteins regulate multiple transcript factors and phytohormone pathways including SA and JA (43–45).
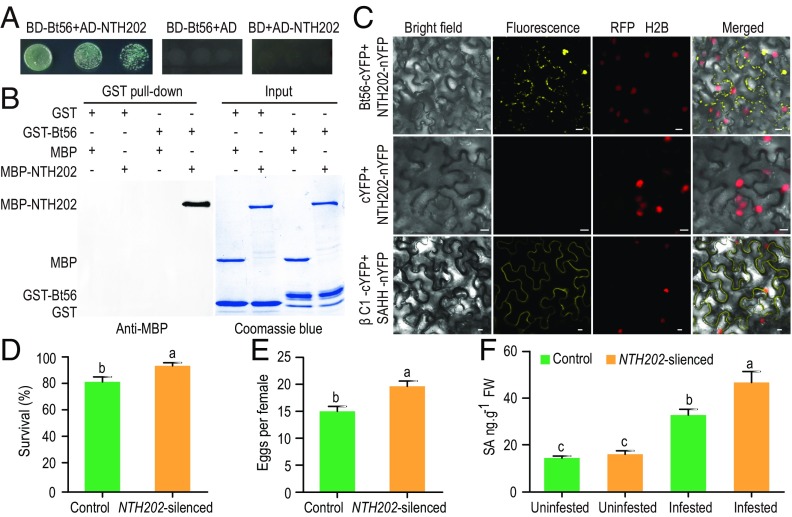
DNA-binding domain- (BD-) Bt56 and activating domain- (AD-) NTH202 yeast transformants were able to grow on quadruple dropout media, whereas yeast transformants carrying two control constructs were unable to do so (Fig. 4A). NTH202 was pulled down by glutation GST-Bt56 but not by GST (Fig. 4B), indicating a specific interaction between NTH202 and Bt56 in vitro. To further confirm the interaction, we overexpressed Bt56-green fluorescent protein (Bt56-GFP) and NTH202-cyan fluorescent protein (NTH202-CFP) fusion proteins in Nicotiana benthamiana and found that Bt56-GFP and NTH202-CFP were colocalized mainly in the cytosol (SI Appendix, Fig. S8). Finally, we performed a bimolecular fluorescence complementation (BiFC) assay to confirm the protein interaction in planta. We found that coexpression of Bt56–C-terminal part of yellow fluorescent protein (Bt56-cYFP) with NTH202–N-terminal part of yellow fluorescent protein (NTH202-nYFP) in N. benthamiana leaves produced yellow fluorescent protein (YFP) fluorescence mainly in the cytosol, whereas no YFP signal was observed in negative controls (Fig. 4C). We further showed that NTH202 is expressed in the phloem (SI Appendix, Fig. S7 B–D). As whitefly salivary proteins are expected to be delivered into the phloem and apoplast during feeding, these data suggest that Bt56 and NTH202 have the potential to interact in the phloem.
Fig. 4.
Interaction between Bt56 and plant NTH202 proteins. (A) Interaction between Bt56 and NTH202 in the Y2H. Yeast strain Y2H gold cotransformed with the indicated plasmids was spotted on the quadruple dropout media. The EVs pGBKT7 (BD) and pGADT7 (AD) were used as negative controls. (B) GST pull-down assays. Glutathione S-transferase (GST) or GST-Bt56 fusion proteins were used to pull down maltose-binding protein (MBP) or MBP-NTH202 fusion proteins. Western blot was performed using an anti-MBP antibody to detect the associated proteins. Sodium dodecyl sulfate polyacrylamide gel electrophoresis gel was stained with CB to monitor the input protein amount. (C) BiFC analysis of Bt56 interaction with NTH202 in vivo. Fluorescence was observed as complementation of the Bt56-cYFP fusion protein and the NTH202-nYFP fusion protein. Red fluorescent protein- (RFP-) histone 2B (RFP-H2B) was used as a marker for the nucleus. Unfused cYFP was coexpressed with NTH202-nYFP as a negative control, and βC1 was expressed with S-adenosyl homocysteine hydrolase (SAHH) as a positive control (55). The BiFC assays were repeated three times, and a total of 24 images were analyzed. Bars = 10 μm. The survival (D) and fecundity (E) of whiteflies feeding on the control and NTH202-silenced tobacco. Each treatment included nine plants, and each plant had two clip cages (n = 18). (F) Mean level of SA in the control, NTH202-silenced, whitefly-infested, and NTH202-silenced plus whitefly-infested tobacco plants (n = 7 to 8). Fresh weight. Values shown are means ± SE. The letters above the bars indicate significant differences among different treatments at P < 0.05 (nested ANOVA for whitefly bioassays; one-way ANOVA followed by the LSD test for other experiments).
The interaction between Bt56 and NTH202 raises the question of whether NTH202 is involved in SA-mediated whitefly resistance. We found that the expression levels of NTH202 in Bt56-overexpressed and whitefly-infested tobacco were significantly lower than that in uninfested control plants (SI Appendix, Fig. S9A; F3,24 = 5.425, P < 0.01). So we used virus-induced gene silencing (VIGS) to silence tobacco NTH202 (SI Appendix, Fig. S9 B and C; F1,10 = 25.658, P < 0.001) and examined whitefly performance on NTH202-silenced tobacco. The survival and fecundity of whiteflies were significantly increased on NTH202-silenced plants (Fig. 4 D and E; F1,18 = 6.365–11.881, P = 0.003–0.021). Compared with the uninfested control tobacco, the production of SA did not change significantly in uninfested NTH202-silenced tobacco; however, when tobacco was infested by whiteflies for 3 d, the levels of SA in both NTH202-silenced and control tobacco increased significantly, and the level of SA in NTH202-silenced tobacco was significantly higher than that in control tobacco (Fig. 4F; F3,27 = 30.051, P < 0.0001). These data indicate that the host plant protein NTH202 does not directly affect SA in noninfested plants but it represses whitefly-induced SA accumulation.
Conservation of Bt56 in Different Whitefly Species.
To investigate Bt56 orthologous genes, we cloned the Bt56 genes from MEAM1, Asia II 3, Asia II 1, and China 2 whitefly species (GenBank: KY986870, KY986871, KY986872, and KY986873). All of the putative Bt56 protein sequences have predicted signal peptides, and sequence alignment showed high similarities (SI Appendix, Fig. S10A). FISH analyses showed that Bt56 could be detected in salivary glands of MEAM1, Asia II 3, and China 2 whiteflies (SI Appendix, Fig. S10 B and C). All five Bt56 proteins can interact with host NTH202 in the Y2H system (SI Appendix, Fig. S10D). Analysis by qRT-PCR showed that the expression levels of Bt56 in MED1 on cotton and tobacco were both much higher than those in Asia II 3 and that the Bt56 gene in MED1 was significantly up-regulated 24 h after transfer from cotton to tobacco but not in Asia II 3 (SI Appendix, Fig. S11A; F3,8 = 154.224, P < 0.0001). To detect whether the high level of Asia II 3 Bt56 in plants is beneficial to Asia II 3, we overexpressed the Asia II 3 Bt56 gene in tobacco and observed the performance of Asia II 3. The survival and fecundity of Asia II 3 were significantly higher on tobacco overexpressing Bt56 (SI Appendix, Fig. S11 B and C; F1,16 = 11.447–13.324, P = 0.003–0.004). Unlike MED1, when Asia II 3 infested tobacco for 3 d, the level of SA did not change significantly (SI Appendix, Fig. S11D; F1,9 = 1.633, P = 0.233). Together, these data indicate that Bt56 orthologous genes are highly conserved in different whitefly species and the expression level of Bt56 may affect whitefly performance on host plants.
Discussion
Crosstalk between SA-JA-signaling pathways is thought to help a plant choose an efficient defense strategy, depending on the attackers it is encountering (3). However, plant attackers have evolved to leverage the crosstalk to deceive plant defenses. Although there are exceptions to the deception of plant defenses (inducing SA and repressing JA) by insects (32, 46, 47), the induced SA pathways in tobacco and Arabidopsis were advantageous to whitefly performance in our and previous studies (30, 33). Previous research has shown that the low concentration of SA can neutralize the repellent effect of high concentration JA application on herbivore behavior (48); however, SA accumulation can activate the early induction of JA-responsive genes and de novo JA synthesis (49). Therefore, we propose that the effect of SA defenses on phloem feeders may have a close relationship with SA concentration and whiteflies may regulate the level of SA by secreting Bt56 and possibly other salivary proteins into plants.
Previous studies found that some salivary effectors in phloem-feeding insects can positively (13–15, 18, 19) or negatively (16, 50) affect insect performance on host plants by regulating plant defense responses. Like those positive effectors, Bt56 can facilitate whitefly performance and promote whitefly phloem feeding on host plants. Interestingly, Bt56 promotion of whitefly performance on tobacco could be due to its function as a SA elicitor. However, compared with Bt56-overexpressed tobacco (Fig. 3 A and B), whitefly infestation induced much higher levels of SA production (Fig. 1C) and different expression patterns of SA-marker genes (Fig. 1F) in tobacco. A previous study showed whitefly honeydew contained SA and significantly increased endogenous free SA in plants (51), so we suspect that, apart from the salivary protein Bt56, some other components of saliva or honeydew might also affect plant defenses and then further affect whitefly performance. In addition, the 35S promoter directs the nonphysiological level of Bt56 expression in a multitude of cell types, and this misexpression may differ from the actual function of Bt56 in plants.
BiFC showed that Bt56 can interact with NTH202 mainly at some punctated structures in the cytosol, but the specific subcellular localization of Bt56-NTH202 interaction needs further investigation. We suspect that, except acting as a transcription factor in the nucleus, NTH202 may have other functions in cytosol that are influenced by Bt56. In a previous chromatin immunoprecipitation-sequencing study, functional categories of NTH202 targets showed that some SA- and JA-hormonal pathway genes are regulated by KNOX1 in maize (45). Here, we found silencing NTH202 in tobacco improved the performance of the whitefly, possibly due to the negative effect of NTH202 on whitefly-infestation-induced SA accumulation. However, without whitefly infestation, the levels of SA in NTH202-silenced tobacco did not change significantly (Fig. 4F), which is different from the change in the SA level in Bt56-overexpressed tobacco (Fig. 3 A and B). These data suggest that NTH202-silenced plants may be only a partial phenocopy of Bt56-overexpressed tobacco and NTH202 may have no direct effect on SA signaling in tobacco. In addition, the SA level in the whitefly-preinfested tobacco in Fig. 1C was much higher than that in the whitefly-infested control and NTH202-silenced plants in Fig. 4F, which was probably because the plants used for Fig. 4F were VIGS treated. The detailed mechanisms of how Bt56-NTH202 interaction regulates plant defenses warrant further investigation.
In conclusion, this paper showed that whiteflies regulate plant defenses using a salivary effector Bt56 that activates the SA-signaling pathway. We identified a Bt56 host target protein NTH202 in tobacco that is involved in plant defenses against whiteflies. These findings reveal an important molecular mechanism of how phloem feeders regulate plant defenses for their own benefit.
Methods
Host Plant Susceptibility Bioassays.
Clip cages were installed on the abaxial surface of a tobacco leaf (third leaf from the top, two cages per plant) before whitefly preinfestation. Six tobacco plants were preinfested by MED1 (about 1000 adults per plant) for 3 d with six uninfested plants as controls. Then, the MED1s (five female adults per cage) were released into the preinstalled clip cages on infested and control plants. Three days after the release of the whiteflies, the number of live adults and eggs deposited on the leaf within the clip cages were counted to assess host plant suitability. Each clip cage was treated as a biological replicate; 12 replicates for each treatment.
Analysis of SA, JA, JA-Ile, ABA, and IAA.
The phytohormones were analyzed as described previously (52). The details are in SI Appendix, Materials and Methods.
Overexpression of the Bt56 Gene in Tobacco.
The open reading frame encoding sequence of the mature protein of Bt56 (after the signal peptide encoding sequences, 35S:mBt56) was overexpressed in N. tabacum. Whitefly bioassays, phytohormones, and defense-marker gene expression analysis were performed on the infiltrated area. The primers are listed in SI Appendix, Table S3. The details are in SI Appendix, Materials and Methods.
RNAi Experiments.
We silenced the Bt56 gene in whiteflies by feeding them with an artificial diet containing dsRNA (15% sucrose solution, 200 ng/μL dsRNA) using an artificial diet-feeding device as previously described (39). The details are in SI Appendix, Materials and Methods.
FISH, IHC, and Western Blot.
To localize the transcript of Bt56 in individual whitefly organs, FISH was performed as described previously (53). The locations of the Bt56 protein in PSGs, MGs, and ovaries were detected by IHC staining as previously described (54). Western blot was performed to detect the Bt56 protein in the whitefly body, saliva, and whitefly-infested plant leaves. The details are in SI Appendix, Materials and Methods.
Data Analysis.
All of the data analyses were performed using SPSS 20.0 Statistics. For whitefly bioassays, the survival and fecundity were analyzed using nested ANOVA at P < 0.05 to decipher the subgroup effect of plants as well as that of treatment. Proportion survival was first transformed by the arcsine square root before analysis. For other experiments, datasets were first examined for normality and then compared using one-way ANOVA followed by LSD tests.
Supplementary Material
Acknowledgments
We thank Professor Qiao-Mei Wang, Professor Yong-Gen Lou, and Dr. Ran Li for conducting the phytohormones analysis and Professor Qi-Yi Tang (Zhejiang University, China) for help with the statistical analyses of whitefly survival and fecundity datasets. We also thank Professor Myron Zalucki (The University of Queensland, Australia) for critical reading of this paper. Financial support for this paper was provided by the National Key Research and Development Program (Grant 2016YFC1200601), the National Natural Science Foundation of China (Grant 31672029), and the Zhejiang Provincial Natural Science Foundation of China (Grant LR15C140001).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. KY986869–KY986874).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1714990116/-/DCSupplemental.
References
- 1.Wu J, Baldwin IT. New insights into plant responses to the attack from insect herbivores. Annu Rev Genet. 2010;44:1–24. doi: 10.1146/annurev-genet-102209-163500. [DOI] [PubMed] [Google Scholar]
- 2.Thaler JS, Humphrey PT, Whiteman NK. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 2012;17:260–270. doi: 10.1016/j.tplants.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Koornneef A, Pieterse CM. Cross talk in defense signaling. Plant Physiol. 2008;146:839–844. doi: 10.1104/pp.107.112029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomma BP, et al. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA. 1998;95:15107–15111. doi: 10.1073/pnas.95.25.15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howe GA, Jander G. Plant immunity to insect herbivores. Annu Rev Plant Biol. 2008;59:41–66. doi: 10.1146/annurev.arplant.59.032607.092825. [DOI] [PubMed] [Google Scholar]
- 6.Spoel SH, Johnson JS, Dong X. Regulation of tradeoffs between plant defenses against pathogens with different lifestyles. Proc Natl Acad Sci USA. 2007;104:18842–18847. doi: 10.1073/pnas.0708139104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xin XF, He SY. Pseudomonas syringae pv. tomato DC3000: A model pathogen for probing disease susceptibility and hormone signaling in plants. Annu Rev Phytopathol. 2013;51:473–498. doi: 10.1146/annurev-phyto-082712-102321. [DOI] [PubMed] [Google Scholar]
- 8.Kaloshian I, Walling LL. Hemipteran and dipteran pests: Effectors and plant host immune regulators. J Integr Plant Biol. 2016;58:350–361. doi: 10.1111/jipb.12438. [DOI] [PubMed] [Google Scholar]
- 9.Will T, Tjallingii WF, Thönnessen A, van Bel AJ. Molecular sabotage of plant defense by aphid saliva. Proc Natl Acad Sci USA. 2007;104:10536–10541. doi: 10.1073/pnas.0703535104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaloshian I, Walling LL. Hemipterans as plant pathogens. Annu Rev Phytopathol. 2005;43:491–521. doi: 10.1146/annurev.phyto.43.040204.135944. [DOI] [PubMed] [Google Scholar]
- 11.Sharma A, et al. Salivary proteins of plant-feeding hemipteroids–Implication in phytophagy. Bull Entomol Res. 2014;104:117–136. doi: 10.1017/S0007485313000618. [DOI] [PubMed] [Google Scholar]
- 12.Musser RO, et al. Herbivory: Caterpillar saliva beats plant defences. Nature. 2002;416:599–600. doi: 10.1038/416599a. [DOI] [PubMed] [Google Scholar]
- 13.Mutti NS, et al. A protein from the salivary glands of the pea aphid, Acyrthosiphon pisum, is essential in feeding on a host plant. Proc Natl Acad Sci USA. 2008;105:9965–9969. doi: 10.1073/pnas.0708958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, et al. Armet is an effector protein mediating aphid-plant interactions. FASEB J. 2015;29:2032–2045. doi: 10.1096/fj.14-266023. [DOI] [PubMed] [Google Scholar]
- 15.Naessens E, et al. A secreted MIF cytokine enables aphid feeding and represses plant immune responses. Curr Biol. 2015;25:1898–1903. doi: 10.1016/j.cub.2015.05.047. [DOI] [PubMed] [Google Scholar]
- 16.Bos JI, et al. A functional genomics approach identifies candidate effectors from the aphid species Myzus persicae (green peach aphid) PLoS Genet. 2010;6:e1001216. doi: 10.1371/journal.pgen.1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elzinga DA, De Vos M, Jander G. Suppression of plant defenses by a Myzus persicae (green peach aphid) salivary effector protein. Mol Plant Microbe Interact. 2014;27:747–756. doi: 10.1094/MPMI-01-14-0018-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji R, et al. A salivary endo-β-1,4-glucanase acts as an effector that enables the brown planthopper to feed on rice. Plant Physiol. 2017;173:1920–1932. doi: 10.1104/pp.16.01493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kettles GJ, Kaloshian I. The potato aphid salivary effector Me47 is a glutathione-S-transferase involved in modifying plant responses to aphid infestation. Front Plant Sci. 2016;7:1142. doi: 10.3389/fpls.2016.01142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villarroel CA, et al. Salivary proteins of spider mites suppress defenses in Nicotiana benthamiana and promote mite reproduction. Plant J. 2016;86:119–131. doi: 10.1111/tpj.13152. [DOI] [PubMed] [Google Scholar]
- 21.Pitino M, Hogenhout SA. Aphid protein effectors promote aphid colonization in a plant species-specific manner. Mol Plant Microbe Interact. 2013;26:130–139. doi: 10.1094/MPMI-07-12-0172-FI. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez PA, Escudero-Martinez C, Bos JIB. An aphid effector targets trafficking protein VPS52 in a host-specific manner to promote virulence. Plant Physiol. 2017;173:1892–1903. doi: 10.1104/pp.16.01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elzinga DA, Jander G. The role of protein effectors in plant-aphid interactions. Curr Opin Plant Biol. 2013;16:451–456. doi: 10.1016/j.pbi.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 24.De Barro PJ, Liu S-S, Boykin LM, Dinsdale AB. Bemisia tabaci: A statement of species status. Annu Rev Entomol. 2011;56:1–19. doi: 10.1146/annurev-ento-112408-085504. [DOI] [PubMed] [Google Scholar]
- 25.Liu SS, et al. Asymmetric mating interactions drive widespread invasion and displacement in a whitefly. Science. 2007;318:1769–1772. doi: 10.1126/science.1149887. [DOI] [PubMed] [Google Scholar]
- 26.Xu J, Lin KK, Liu SS. Performance on different host plants of an alien and an indigenous Bemisia tabaci from China. J Appl Entomol. 2011;135:771–779. [Google Scholar]
- 27.Liu SS, Colvin J, De Barro PJ. Species concepts as applied to the whitefly Bemisia tabaci systematics: How many species are there? J Integr Agric. 2012;11:176–186. [Google Scholar]
- 28.Walker GP, Perring TM. Feeding and oviposition behavior of whiteflies (Homoptera: Aleyrodidae) interpreted from AC electronic feeding monitor waveforms. Ann Entomol Soc Am. 1994;87:363–374. [Google Scholar]
- 29.Jiang Y, et al. Probing and feeding behavior of two distinct biotypes of Bemisia tabaci (Homoptera: Aleyrodidae) on tomato plants. J Econ Entomol. 1999;92:357–366. [Google Scholar]
- 30.Zarate SI, Kempema LA, Walling LL. Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol. 2007;143:866–875. doi: 10.1104/pp.106.090035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang PJ, et al. Feeding by whiteflies suppresses downstream jasmonic acid signaling by eliciting salicylic acid signaling. J Chem Ecol. 2013;39:612–619. doi: 10.1007/s10886-013-0283-2. [DOI] [PubMed] [Google Scholar]
- 32.Moran PJ, Thompson GA. Molecular responses to aphid feeding in Arabidopsis in relation to plant defense pathways. Plant Physiol. 2001;125:1074–1085. doi: 10.1104/pp.125.2.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alon M, et al. Activation of the Phenylpropanoid pathway in Nicotiana tabacum improves the performance of the whitefly Bemisia tabaci via reduced jasmonate signaling. PLoS One. 2013;8:e76619. doi: 10.1371/journal.pone.0076619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang T, et al. Begomovirus-whitefly mutualism is achieved through repression of plant defences by a virus pathogenicity factor. Mol Ecol. 2012;21:1294–1304. doi: 10.1111/j.1365-294X.2012.05457.x. [DOI] [PubMed] [Google Scholar]
- 35.Walling LL. Avoiding effective defenses: Strategies employed by phloem-feeding insects. Plant Physiol. 2008;146:859–866. doi: 10.1104/pp.107.113142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pieterse CM, Dicke M. Plant interactions with microbes and insects: From molecular mechanisms to ecology. Trends Plant Sci. 2007;12:564–569. doi: 10.1016/j.tplants.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Paschold A, Bonaventure G, Kant MR, Baldwin IT. Jasmonate perception regulates jasmonate biosynthesis and JA-Ile metabolism: The case of COI1 in Nicotiana attenuata. Plant Cell Physiol. 2008;49:1165–1175. doi: 10.1093/pcp/pcn091. [DOI] [PubMed] [Google Scholar]
- 38.Su YL, et al. Transcriptomic analysis of the salivary glands of an invasive whitefly. PLoS One. 2012;7:e39303. doi: 10.1371/journal.pone.0039303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei J, et al. Vector development and vitellogenin determine the transovarial transmission of begomoviruses. Proc Natl Acad Sci USA. 2017;114:6746–6751. doi: 10.1073/pnas.1701720114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson D, Walker G. Intracellular punctures by the adult whitefly Bemisia argentifolii on DC and AC electronic feeding monitors. Entomol Exp Appl. 1999;92:257–270. [Google Scholar]
- 41.Jiang Y, Nombela G, Muñiz M. Analysis by DC-EPG of the resistance to Bemisia tabaci on an Mi‐tomato line. Entomol Exp Appl. 2001;99:295–302. [Google Scholar]
- 42.Yoshii A, et al. NTH201, a novel class II KNOTTED1-like protein, facilitates the cell-to-cell movement of Tobacco mosaic virus in tobacco. Mol Plant Microbe Interact. 2008;21:586–596. doi: 10.1094/MPMI-21-5-0586. [DOI] [PubMed] [Google Scholar]
- 43.Tsuda K, Kurata N, Ohyanagi H, Hake S. Genome-wide study of KNOX regulatory network reveals brassinosteroid catabolic genes important for shoot meristem function in rice. Plant Cell. 2014;26:3488–3500. doi: 10.1105/tpc.114.129122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hake S, et al. The role of knox genes in plant development. Annu Rev Cell Dev Biol. 2004;20:125–151. doi: 10.1146/annurev.cellbio.20.031803.093824. [DOI] [PubMed] [Google Scholar]
- 45.Bolduc N, et al. Unraveling the KNOTTED1 regulatory network in maize meristems. Genes Dev. 2012;26:1685–1690. doi: 10.1101/gad.193433.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhattarai KK, Xie QG, Pourshalimi D, Younglove T, Kaloshian I. Coil-dependent signaling pathway is not required for Mi-1-mediated potato aphid resistance. Mol Plant Microbe Interact. 2007;20:276–282. doi: 10.1094/MPMI-20-3-0276. [DOI] [PubMed] [Google Scholar]
- 47.Zhang PJ, et al. Whiteflies interfere with indirect plant defense against spider mites in lima bean. Proc Natl Acad Sci USA. 2009;106:21202–21207. doi: 10.1073/pnas.0907890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei J, et al. Reciprocal crosstalk between jasmonate and salicylate defence-signalling pathways modulates plant volatile emission and herbivore host-selection behaviour. J Exp Bot. 2014;65:3289–3298. doi: 10.1093/jxb/eru181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu L, et al. Salicylic acid receptors activate jasmonic acid signalling through a non-canonical pathway to promote effector-triggered immunity. Nat Commun. 2016;7:13099. doi: 10.1038/ncomms13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chaudhary R, Atamian HS, Shen Z, Briggs SP, Kaloshian I. GroEL from the endosymbiont Buchnera aphidicola betrays the aphid by triggering plant defense. Proc Natl Acad Sci USA. 2014;111:8919–8924. doi: 10.1073/pnas.1407687111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.VanDoorn A, de Vries M, Kant MR, Schuurink RC. Whiteflies glycosylate salicylic acid and secrete the conjugate via their honeydew. J Chem Ecol. 2015;41:52–58. doi: 10.1007/s10886-014-0543-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu J, et al. Induced jasmonate signaling leads to contrasting effects on root damage and herbivore performance. Plant Physiol. 2015;167:1100–1116. doi: 10.1104/pp.114.252700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kliot A, Ghanim M. Fluorescent in situ hybridization for the localization of viruses, bacteria and other microorganisms in insect and plant tissues. Methods. 2016;98:74–81. doi: 10.1016/j.ymeth.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Wei J, et al. Specific cells in the primary salivary glands of the whitefly Bemisia tabaci control retention and transmission of begomoviruses. J Virol. 2014;88:13460–13468. doi: 10.1128/JVI.02179-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang X, et al. Suppression of methylation-mediated transcriptional gene silencing by βC1-SAHH protein interaction during geminivirus-betasatellite infection. PLoS Pathog. 2011;7:e1002329. doi: 10.1371/journal.ppat.1002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.