Abstract
Discovered 40 years ago, the Lec5 glycosylation mutant cell line has a complex recessive genotype and is characterized by accumulation of lipid-linked oligosaccharide assembly intermediates, reduced conversion of polyprenols to dolichols, and an unusual phenotypic dependence upon cell culture conditions such as temperature, plating density and medium quality. The heritable defect in Lec5 is unknown. Here we demonstrate an unexpected epigenetic basis for Lec5, with a surprising linkage to increased expression of homeobox genes, which in turn is associated with increased transcription of cholesterol biosynthesis genes. These results suggest testable hypotheses for the biochemical abnormalities of the Lec5 mutant.
Keywords: cholesterol, dolichol, homeobox, lipid-linked oligosaccharide, polyprenol
Glycosylation-defective mutant cell lines have provided seminal information about oligosaccharide biosynthetic pathways, their enzymes, and their genes (Esko and Stanley 2017). Numerous mutants with recessive or dominant genotypes of Chinese Hamster Ovary (CHO) cells have been obtained following chemical mutagenesis. However, several CHO mutants have been selected without prior mutagenesis. An example of the latter is Lec35, a recessive mutant with a glycosylation-defective, lectin-resistant phenotype (Lehrman and Zeng 1989) caused by mutation of the MPDU1 gene required for mannose-P-dolichol and glucose-P-dolichol utilization. Lec35 mutants may arise spontaneously due to deletion of unstable stretches of MPDU1 intronic repetitive elements (Anand et al., 2001). Another example is Lec5, a recessive genotype exemplified by the B211 CHO line (Krag et al., 1977; Cifone et al., 1979). Lec5 mutant cells have a lectin-resistance phenotype and exhibit defective maturation of Glucose3Mannose9N-acetylglucosamine2-P-P-dolichol (G3M9-LLO) lipid-linked oligosaccharide (LLO) (Krag 1979).
The gene defect in Lec5 is unknown, but Lec5 mutants lack activity for the enzyme polyprenol reductase, consistent with their dolichol deficiency (Kaiden et al., 1998) and LLO abnormality. The Lec5 genotype studied in somatic cell hybrid complementation experiments is unusually complex. Lec5 appeared to be in the same complementation group as Lec9, a genotype also associated with a polyprenol reductase deficiency, when hybrids were tested directly for that enzyme’s products (Kaiden et al., 1998). However, Lec5 and Lec9 acted as different complementation groups in cellular lectin toxicity tests (Stanley 1983). This complexity appears consistent with more than one genetic abnormality rather than a single gene mutation.
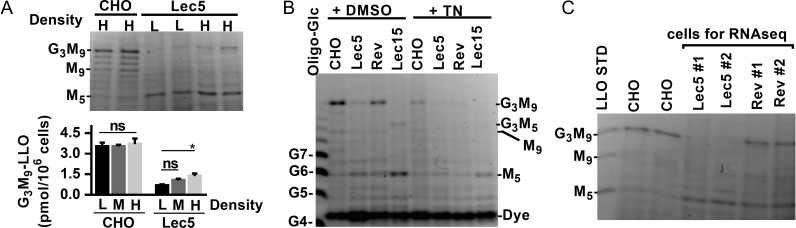
We reassessed LLO synthesis in validated Lec5 (B211) cells (Stanley 1983) in comparison with the CHO wild-type line designated CHO-K1 (ATTC CCL-61; which is distinct from the CHO wild-type line designated Pro-5, ATCC-1781 (Stanley 1983)), using the non-radioactive method “Fluorophore-Assisted Carbohydrate Electrophoresis” (Gao and Lehrman 2006; Gao et al., 2013) (Figure 1). Our results closely recapitulated those reported with radioisotopic methods (Krag et al., 1977; Krag 1979), including loss of G3M9-LLO, accumulation of the LLO intermediate M5-LLO, and suppression of these phenotypes at high cell density.
Fig. 1.
Properties of Lec5 and Lec5Rev cells. LLOs were analyzed by fluorophore-assisted carbohydrate electrophoresis (Gao and Lehrman 2006; Gao et al., 2013). (A) G3M9-LLO in Lec5 cells increases with cell density (L, low; M, medium; H, high). A representative gel, and a graph of G3M9-LLO quantity is shown. (B) G3M9-LLO is elevated in Lec5Rev cells (Rev). For comparison, LLOs in Lec5 (M5-LLO) and Lec15 (M5-LLO, G3M5-LLO) mutants are shown. All LLOs were sensitive to 2 h treatments with 5 μg/mL tunicamycin (TN). (C) LLOs of Lec5 subclones and revertants used for RNA-Seq.
To identify the genetic defect in Lec5, we considered comparison of Lec5 with its wild-type parental line by DNA sequencing. However, neither the actual parental CHO line “WTB” nor the closely related “WTC” (Krag et al., 1977; Cifone et al., 1979) are still available. Moreover, CHO-K1 was an inappropriate substitute. CHO-K1 and WTB diverged from a common Chinese hamster source (Tjio and Puck 1958), with the provenance of the WTB line eventually used to obtain B211 (Cifone et al., 1979) involving transfers between several investigators over many years (Tobey et al., 1966; Leeper et al., 1972; Thompson and Baker 1973). During this period, many random point mutations distinguishing CHO-K1 and WTB were probably acquired.
As an alternative, we isolated spontaneous temperature-resistant Lec5 revertants (Lec5Rev), as done previously (Krag et al., 1977; Cifone et al., 1979). In our hands, LLO biosynthesis in Lec5Rev was greatly improved, though not to the extent in CHO-K1 (Figure 1). The <10−4 Lec5 reversion frequency (Cifone et al., 1979) was sufficient for us to isolate numerous, independent revertants. This, coupled with the original spontaneous isolation, suggested that the Lec5 defect was epigenetic rather than genetic, and that an underlying abnormality might be detected by transcriptional profiling rather than DNA sequencing. To test this assumption, a transcriptional comparison of Lec5 and Lec5Rev was performed by the “RNA-Seq” method.
Table I shows the results of RNA-Seq analysis with two independent Lec5 subclones and two Lec5Rev isolates of each. This analysis had three main findings. (i) There were no significant increases or decreases of transcripts for genes having a direct association with dolichol synthesis, LLO synthesis, LLO precursor metabolism, or N-glycosylation (Supplementary Information A), including the proposed polyprenol reductase SRD5A3 (Cantagrel et al., 2010). (ii) Pathway analysis by IPA® (Krämer et al., 2014) identified the Superpathway Of Cholesterol (CHL) Biosynthesis as the most significantly induced (P = 3.00 × 10−10) pathway or process in Lec5 compared with Lec5Rev (Table I). Each of the three IPA-defined subsections of the superpathway was also strongly associated with Lec5 (Supplementary Information B). The most highly induced CHL pathway-related gene in Lec5 relative to Lec5Rev (3-hydroxy-3-methylglutaryl-CoA synthase 1; Hmgcs1) was increased only 1.7-fold. However, the pathway analysis showed that approximately 75% of CHL biosynthesis genes were coordinately induced in Lec5 (Table I, Supplementary Information B), consistent with an aggregate effect. (iii) Seven of the top nine transcript hits were genes encoding homeobox transcription factors, each induced ~ 500–1000 fold in Lec5 compared with Lec5Rev. Some were undetectable in Lec5Rev (Table II, Supplementary Information A). In mammals, homeobox genes have critical transcriptional roles during embryonic body planning and development of axial structures (Mallo et al., 2010). Six of the top nine hits were HOXA genes, a homeobox subgroup linked to development of craniofacial and upper thoracic structures (Maconochie et al., 1996).
Table I.
Top canonical pathways by IPA® (Lec5 VS Lec5Rev)a
| Pathways | −log10 (P-value) | Upregulated in Lec5 | Downregulated in Lec5 |
|---|---|---|---|
| Superpathway of cholesterol biosynthesis | 9.52 | 21/28 (75%) | 3/28 (11%) |
| Axonal guidance signaling | 7.08 | 174/452 (38%) | 147/452 (33%) |
| GP6 signaling pathway | 6.77 | 53/134 (40%) | 42/134 (31%) |
| Leukocyte extravasation signaling | 6.75 | 82/211 (39%) | 66/211 (31%) |
| Hepatic fibrosis/hepatic stellate cell activation | 6.44 | 49/187 (26%) | 66/187 (35%) |
| PPARα/RXRα activation | 6.09 | 88/180 (49%) | 52/180 (29%) |
| Cholesterol biosynthesis | 5.92 | 10/13 (77%) | 1/13 (8%) |
| Role of macrophages, fibroblasts and endothelial cells in rheumatoid arthritis | 5.30 | 113/312 (36%) | 106/312 (34%) |
| Unfolded protein response | 5.21 | 35/55 (64%) | 13/55 (24%) |
a1.2 × 106 Lec5 or Lec5Rev cells were seeded in 15 cm dishes with Ham’s F-12 medium (ThermoFisher, #11765054) supplemented with 10% FBS. After incubation for 48 h at 34°C, resulting in ~5 × 106 cells, cells were harvested by trypsin/EDTA treatment, washed once with ice-cold PBS, and pellets were stored at −80°C for later RNA extraction using an RNeasy Mini Kit (Qiagen, #74104) with on-column DNase digestion with RNase-Free DNase (Qiagen, #79254) to eliminate genomic DNA contamination. RNA purity and integrity were verified by 260 nm/280 nm absorbance ratio and gel electrophoresis, respectively, before processing for RNA-Seq at the McDermott Center for Human Genetics sequencing facility, UT Southwestern Medical Center.
Table II.
Top transcript hits and fold-increases in Lec5
| Gene name | Fold change |
|---|---|
| (log2) | |
| Lec5/Lec5Rev | |
| Hoxa10 | 10.30 |
| Hoxa6 | 9.78 |
| Hoxa7 | 9.58 |
| Znf605 | 9.55 |
| Hoxa5 | 9.39 |
| Hoxa9 | 9.33 |
| LOC107978730 | 8.75 |
| Meis1 | 7.76 |
| Hoxa3 | 7.19 |
| Adra1d | 6.82 |
The CHL and dolichol biosynthetic pathways share metabolic precursors through their common intermediate, farnesyl pyrophosphate. Competition between the two pathways was implicated to explain apparently enhanced LLO production by inhibitors of lanosterol synthesis acting in the CHL pathway downstream of farnesyl pyrophosphate (Harding et al., 2005; Haeuptle et al., 2011). Our results raise the possibility that the LLO abnormality in Lec5 may be similarly related to dysregulation of CHL biosynthesis due to consumption of shared precursors, especially if this imbalance in some way suppresses polyprenol reductase activity.
We are unaware of any associations (genetic or otherwise) between homeobox genes and either CHL or LLO metabolism, and whether such associations explain the Lec5 phenotype remains a point of speculation. Nonetheless, since mammalian homeobox genes can be coordinated and controlled epigenetically (Mallo et al., 2010), our results provide a conceptional framework for the spontaneity of Lec5, its facile reversion to Lec5Rev, and its complexity in somatic cell complementation studies. Similarly, the sensitivities of the Lec5 LLO defect to cell density, serum quality, and medium formulation (Krag et al., 1977; Krag 1979) seem consistent with CHL dysregulation. It is also noteworthy that mice with various HOXA gene defects (Krumlauf 1994), and patients with Congenital Disorder of Glycosylation (CDG)-related LLO abnormalities (Jaeken and Matthijs 2001), are both frequently characterized with developmental abnormalities, including dysmorphia in the thoracic region. This implies that testing of individual homeobox genes, especially HOXA genes, may reveal new transcriptional controls for LLO and CHL synthesis, as well as novel causes of CDG-like disorders and hypercholesterolemia.
Supplementary Material
Acknowledgments
We are very grateful to Drs. Sharon Krag and Pamela Stanley for cell samples and highly valuable suggestions on the manuscript.
Abbreviations
- CHL
Cholesterol
- CHO
Chinese Hamster Ovary
- G3M9-LLO
Glucose3Mannose9N-acetylglucosamine2-P-P-dolichol
- Lec5Rev
Lec5 revertant
- LLO
lipid-linked oligosaccharide.
Funding
This work was supported by National Institutes of Health [R01-GM038545 to M.A.L.; UL1TR001105 partially supporting C.X.].
References
- Anand M, Rush JS, Ray S, Doucey MA, Weik J, Ware FE, Hofsteenge J, Waechter CJ, Lehrman MA. 2001. Requirement of the Lec35 gene for all known classes of monosaccharide-P-dolichol-dependent glycosyltransferase reactions in mammals. Mol Biol Cell. 12:487–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantagrel V, Lefeber DJ, Ng BG, Guan Z, Silhavy JL, Bielas SL, Lehle L, Hombauer H, Adamowicz M, Swiezewska E et al. . 2010. SRD5A3 is required for converting polyprenol to dolichol and is mutated in a congenital glycosylation disorder. Cell. 142:203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifone MA, Hynes RO, Baker RM. 1979. Characteristics of concanavalin A-resistant Chinese hamster ovary cells and certain revertants. J Cell Physiol. 100:39–54. [DOI] [PubMed] [Google Scholar]
- Esko JD, Stanley P. 2017. Glycosylation mutants of cultured mammalian cells In: Varki A, Cummings RD, Esko J D, Stanley P, Hart G W, Aebi M, Darvill A G, Kinoshita T, Packer N H, Prestegard J H, Schnaar R L, Seeberger P H, editors. Essentials of Glycobiology, 3rd ed. N.Y: Cold Spring Harbor Press. [Google Scholar]
- Gao N, Holmes J, Lehrman MA. 2013. Letter to the Glycoforum: Improved protocols for preparing lipid-linked and related saccharides for fluorophore-assisted carbohydrate electrophoresis (FACE). Glycobiology. 23:1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N, Lehrman MA. 2006. Non-radioactive analysis of lipid-linked oligosaccharide compositions by fluorophore-assisted carbohydrate electrophoresis (FACE). Methods Enzymol. 415:3–20. [DOI] [PubMed] [Google Scholar]
- Haeuptle MA, Welti M, Troxler H, Hülsmeier AJ, Imbach T, Hennet T. 2011. Improvement of dolichol-linked oligosaccharide biosynthesis by the squalene synthase inhibitor zaragozic acid. J Biol Chem. 286:6085–6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Khersonsky S, Marciniak S, Scheuner D, Kaufman RJ, Javitt N, Chang YT, Ron D. 2005. Bioactive small molecules reveal antagonism between the integrated stress response and sterol-regulated gene expression. Cell Metab. 2:361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeken J, Matthijs G. 2001. Congenital disorders of glycosylation. Annu Rev Genomics Hum Genet. 2:129–151. [DOI] [PubMed] [Google Scholar]
- Kaiden A, Rosenwald AG, Cacan R, Verbert A, Krag SS. 1998. Transfer of two oligosaccharides to protein in a Chinese hamster ovary cell B211 which utilizes polyprenol for its N-linked glycosylation intermediates. Arch Biochem Biophys. 358:303–312. [DOI] [PubMed] [Google Scholar]
- Krag SS. 1979. A concanavalin A-resistant chinese hamster ovary cell line is deficient in the synthesis of [3 H] glucosyl oligosaccharide- lipid. J Biol Chem. 254:9167–9177. [PubMed] [Google Scholar]
- Krag SS, Cifone M, Robbins PW, Baker RM. 1977. Reduced synthesis of [14C]mannosyl oligosaccharide-lipid by membranes prepared from concanavalin A-resistant Chinese hamster ovary cells. J Biol Chem. 252:3561–3564. [PubMed] [Google Scholar]
- Krumlauf R. 1994. Hox genes in vertebrate development. Cell. 78:191–201. [DOI] [PubMed] [Google Scholar]
- Krämer A, Green J, Pollard JJ, Tugendreich S. 2014. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 30:523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeper DB, Schneiderman MH, Dewey WC. 1972. Radiation-induced division delay in synchronized Chinese hamster ovary cells in monolayer culture. Radiat Res. 50:401–417. [PubMed] [Google Scholar]
- Lehrman MA, Zeng Y. 1989. Pleiotropic resistance to glycoprotein processing inhibitors in Chinese hamster ovary cells. The role of a novel mutation in the asparagine-linked glycosylation pathway. J Biol Che. 264:1584–1593. [PubMed] [Google Scholar]
- Maconochie M, Nonchev S, Morrison A, Krumlauf R. 1996. Paralogous HOX genes: function and regulation. Annu Rev Genet. 30:529–556. [DOI] [PubMed] [Google Scholar]
- Mallo M, Wellik DM, Deschamps J. 2010. Hox genes and regional patterning of the vertebrate body plan. Dev Biol. 344:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P. 1983. Lectin-resistant CHO cells: selection of new mutant phenotypes. Somatic Cell Genet. 9:593–608. [DOI] [PubMed] [Google Scholar]
- Thompson LH, Baker RM. 1973. Isolation of mutants of cultured mammalian cells In: Prescott DM, editor. Methods in Cell Biology. Cambridge, Massachusetts: Academic Press; p. 209–281. [DOI] [PubMed] [Google Scholar]
- Tjio JH, Puck TT. 1958. Genetics of somatic mammalian cells. II. Chromosomal constitution of cells in tissue culture. J Exp Med. 108:259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobey RA, Petersen DF, Anderson EC, Puck TT. 1966. Life cycle analysis of mammalian cells. 3. The inhibition of division in Chinese hamster cells by puromycin and actinomycin. Biophys J. 6:567–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.