Abstract
Biofertilizer is a good substitute for chemical fertilizer in sustainable agriculture, but its effects are often hindered by drought stress. Super absorbent polymer (SAP), showing good capacity of water absorption and retention, can increase soil moisture. However, limited information is available about the efficiency of biofertilizer amended with SAP. This study was conducted to investigate the effects of synergistic application of SAP and biofertilizers (Paenibacillus beijingensis BJ-18 and Bacillus sp. L-56) on plant growth, including wheat and cucumber. Potted soil was treated with different fertilizer combinations (SAP, BJ-18 biofertilizer, L-56 biofertilizer, BJ-18 + SAP, L-56 + SAP), and pot experiment was carried out to explore its effects on viability of inoculants, seed germination rate, plant physiological and biochemical parameters, and expression pattern of stress-related genes under drought condition. At day 29 after sowing, the highest viability of strain P. beijingensis BJ-18 (264 copies ng−1 gDNA) was observed in BJ-18 + SAP treatment group of wheat rhizosphere soil, while that of strain Bacillus sp. L-56 (331 copies ng−1 gDNA) was observed in the L-56 + SAP treatment group of cucumber rhizosphere soil. In addition, both biofertilizers amended with SAP could promote germination rate of seeds (wheat and cucumber), plant growth, soil fertility (urease, sucrose, and dehydrogenase activities). Quantitative real-time PCR analysis showed that biofertilizer + SAP significantly down-regulated the expression levels of genes involved in ROS scavenging (TaCAT, CsCAT, TaAPX, and CsAPX2), ethylene biosynthesis (TaACO2, CsACO1, and CsACS1), stress response (TaDHN3, TaLEA, and CsLEA11), salicylic acid (TaPR1-1a and CsPR1-1a), and transcription activation (TaNAC2D and CsNAC35) in plants under drought stress. These results suggest that SAP addition in biofertilizer is a good tactic for enhancing the efficiency of biofertilizer, which is beneficial for plants in response to drought stress. To the best of our knowledge, this is the first report about the effect of synergistic use of biofertilizer and SAP on plant growth under drought stress.
Keywords: Paenibacillus, Drought stress, Super absorbent polymer, Wheat, Bacillus, Biofertilizer, Cucumber
Introduction
Traditional agricultural practices have negative influences on continental ecosystem due to huge inputs of chemical fertilizer (Simonsen et al., 2015), which has given rise to serious problems such as soil degradation, genetic diversity loss, nitrous oxide emission, and nitrate leaching (Webb, Harrison & Ellis, 2000; Kaur, Brar & Dhillon, 2007). Biofertilizer containing plant growth-promoting rhizobacteria (PGPR) is an ideal candidate for reducing application of chemical fertilizer in sustainable agriculture (Rajkumar et al., 2010; Choudhury, Kecskes & Kennedy, 2014). Numerous studies have been reported with respect to growth promotion and biocontrol (Vrieze et al., 2015) and colonization of PGPR in different plants. Some PGPR could promote plant growth by enhancing phosphorus (P) solubilization, nitrogen (N) fixation, zinc solubilization, potassium solubilization, and indole-3-acetic acid (IAA) production (Calvo et al., 2010; Khan et al., 2010; Islam et al., 2013; Gontia-Mishra et al., 2016). Previous studies have confirmed the roles of Pseudomonas, Azospirillum, Bacillus, Burkholderia, and Paenibacillus on the growth of pea, strawberry, rice, rape, and maize, respectively (Chen et al., 2013b; Guerrero-Molina et al., 2015; Oteino et al., 2015; Shakeel et al., 2015; Li et al., 2017). PGPR, such as Lysinibacillus sphaericus ZA9, showed great potential in plant growth promotion and biocontrol (Naureen et al., 2017). The inoculation with endophytic P. aeruginosa PW09 ameliorated both biotic (Sclerotium rolfsii) and abiotic (NaCl stress) stresses in cucumber seedlings (Pandey et al., 2012). Similarly, the tolerance of chickpea to biotic (Sclerotinia sclerotiorum) and abiotic (NaCl stress) stresses was also significantly enhanced after inoculation with P. putida S1 or P. aeruginosa Cgr (Ankita et al., 2014). The application of endophytic Burkholderia phytofirmans PsJN mitigated drought stress, thereby improving the growth and development of wheat (Naveed et al., 2013). PGPR could also successfully colonize the tissues of plants (Yang et al., 2013; Gontia-Mishra et al., 2016; Hao & Chen, 2017; Wang et al., 2017). However, the application of some biofertilizers still remains limitation (Zhang et al., 2012; Grady et al., 2016). The reason might be that the reproduction of PGPR was affected by adverse climatic conditions (Zhang et al., 2012; Bashan et al., 2013) such as limited precipitation. Therefore, in arid and semi-arid regions of northern China (Islam et al., 2010), drought is a crucial limiting factor, and water shortage hindered the application of biofertilizer in practices.
Drought stress is one of the most destructive environmental factors affecting agricultural production worldwide (Gontia-Mishra et al., 2016). Wheat often suffered from periodic drought stress in the growth cycle (Naveed et al., 2013; Naresh, Pramod & Sandeep, 2014). Super absorbent polymer (SAP), a macromolecular cross-linked and environmentally-friendly polymer, had high capacity of water adsorption and retention (Johnson & Veltkamp, 1985), which is conductive to improving seed germination and seedling survival. SAP could be degraded through physical or chemical processes (Mikkelsen, 1994). Hence, increasing number of SAP commercial products have been developed to raise water use efficiency, alleviate drought stress, improve soil physical properties and increase crop yield (Terry, Richard & Nelson, 1986; Mikkelsen, 1994; Gray, 2011). Hydrogel supply enhanced soil moisture and seed germination rate (Rehman, Ahmad & Safdar, 2011). The application of a hydrophilic polymer (Superab A200) increased dry matter accumulation and water use efficiency of maize (Dorraji, Golchin & Ahmadi, 2010). Most studies only focused on the application effect of SAP alone or mixed with a chemical fertilizer, however, to the authors’ knowledge, studies on synergistic use of SAP with biofertilizer are rarely reported, particularly under water deficit conditions.
It is of great significance to investigate whether the application of SAP can retain more water and create a suitable environment for reproduction of inoculants.
Soil enzyme activity, as a susceptive indicator of soil health, has potential of comprehensively evaluating soil microbial functional diversity and its functional changes (Hestrin & Goldblum, 1953; Lebrun et al., 2012). Some soil enzymes participate in N, P, C cycling (Hestrin & Goldblum, 1953) and microbial activity. N cycling is mainly governed by urease, protease, and asparaginase; P cycling by alkaline and acidity phosphatase; C cycling by cellulase, sucrase, and β-glucosidase; and microbial activity is regulated by catalase (Kandeler, Kampichler & Horak, 1996). Fusarium could produce and secrete extracellular enzymes such as phosphatase (Meyer, Garber & Shaeffer, 1964). SAP application had little effect on soil microbial metabolism (Sojka, Entry & Fuhrmann, 2006), but it still needs more understanding regarding the effect of synergistic use of biofertilizer and SAP on soil enzymes involved in C, N, P cycling, and microbial activity.
In this paper, two important but different crops (wheat: monocotyledon; cucumber: dicotyledon) were selected to comparatively investigate the effects of synergistic use of biofertilizer and SAP on the following aspects: (i) viability of inoculants and soil enzyme activity; (ii) seed germination and plant growth promotion; (iii) plant biochemical indexes and expression of drought stress-related genes under severe drought condition (40% relative soil moisture). Our results are expected to popularize the synergistic use of biofertilizer and SAP, especially in the areas suffered from long-term drought stress.
Materials & Methods
Bacterial strains and potted plant soil
Table 1 details the bacteria used in this study.
Table 1. Bacterial cultures and genomes used in this study.
| Strain No. | Original source | ID | References/Accession No. |
|---|---|---|---|
| BJ-18 | Wheat (rhizosphere isolate), China. | Paenibacillus beijingensis | Wang et al. (2013)JN873136 |
| L-56 | Maize (rhizosphere isolate), China | Bacillus sp. | MF988362 |
The topsoil (0–20 cm depth) was collected from Shangzhuang Experimental Station of China Agricultural University, Beijing, China (40°08′12.15″N, 116°10′44.83″E, 50.21 m above sea level). After air-dried at room temperature, the soil was screened by a 10-mesh sieve to remove plant residues and reduce soil heterogeneity. Then the soil was sterilized at 121 °C for 30 min. The experimental soil were low N-content sandy loam (pH: 7.7, Olsen-P: 7.3 mg kg−1, Nmin: 7.8 mg kg−1, NH4OAc-K: 115.8 mg kg−1, organic matter, 7.2 g kg−1).
Preparation of biofertilizer and super absorbent polymer (SAP)
Two PGPR, P.beijingensis BJ-18 and Bacillus sp. L-56, were inoculated in Erlenmeyer flasks (250 mL) containing 100 mL of Luria Bertani (LB) broth and cultured at 30 °C for 36 h at 180 rpm, respectively. The culture was centrifuged at 6,000 rpm for 5 min and adjusted to 5 ×108 cells mL−1 with sterile normal saline (0.89% w/v NaCl in water). Rice hull was used as carrier, which was ground and screened by a 200-mesh sieve. Then the powder was sterilized at 121 °C for 20 min. The above bacterial suspension was mixed aseptically with sterile rice hull powder (1:1, v/w), and then the mixture was air dried separately in shade as biofertilizer.
The SAP used in this study is polyacrylamide (Dongying Huaye New Material Co., Ltd), which is developed for agriculture and forestry use only. It was sterilized by ultraviolet (UV) light for 1 h in super clean bench (SW-CJ-1F(D)/2F(D), AIRTECH, China), and then mixed with biofertilizer (1:100, w/w) according to the instructions of SAP.
Plant culture and collection
The experiment was performed to evaluate the role of SAP + biofertilizer under drought condition. Plastic pots (35 cm in diameter; 25 cm in height), which were sterilized by soaking in 0.5 N nitric acid for 24 h, were filled with the above sterilized soil (5 kg). The treatments were listed in Table 2. The mixture (rice hull powder, biofertilizer, and SAP) of each treatment was applied as base manure (9 g/per pot). Before sowing, the pots were watered to 40% relative soil moisture by weighing method. Then, the plants were watered (200 mL each pot) every 5 days till harvest.
Table 2. Description of treatments used for pot experiment.
| Treatments | Description |
|---|---|
| Control | Rice hull powder without biofertilizer and SAP |
| SAP | Rice hull powder + SAP (100:1, w/w) |
| BJ-18 | Biofertilizer of P. beijingensis BJ-18 |
| L-56 | Biofertilizer of Bacillus sp. L-56 |
| BJ-18 +SAP | Biofertilizer of P. beijingensis BJ-18 + SAP (100:1, w/w) |
| L-56+SAP | Biofertilizer of Bacillus sp. L-56 + SAP (100:1, w/w) |
Seed germination assay
For assessment of seed germination, plump seeds of wheat and cucumber were surface sterilized with sodium hypochlorite (10% v/v) for 10 min, followed by rinsing with sterile deionized water. One hundred seeds of each plant were sown in each plastic pot. The experiment design was completely randomized with three replicates for each treatment. The pots were arranged to the greenhouse under optimum condition (15 h day/25 °C–30 °C/day temperature and 9 h night/15 °C–20 °C/night temperature).
The numbers of germinated seeds were recorded daily from 6 days to 14 days after sowing, and the germination rate was calculated according to the reported method (Sudisha et al., 2010).
Viability assessment of inoculants in rhizosphere soil
Two weeks after sowing, the seedlings (wheat and cucumber) were thinned to about 10 cm apart, with only nine uniform and healthy seedlings were left in each pot. At the same time, the mixture (rice hull powder, biofertilizer, and SAP) was applied again according to the description of Table 2 (9 g / per pot, ignoring the weight of SAP). To analyze the viability of inoculants in the rhizosphere soil, the population density was measured by quantitative PCR (qPCR) (Savazzini et al., 2008) at day 14, 19, 24, 29, 34, 39, and 44 after sowing. In order to collect the rhizosphere soil, the seedling was uprooted and shaken gently to remove the loosely adhering soil, and the tightly adhering soil regarded as rhizosphere soil. For the qPCR counting, DNA was extracted form rhizosphere soil using an TIANamp Soil DNA Kit (Tiangen Biotech CO., LTD., Beijing, China) according to the manufacturer’s protocol. Suitable primers for qPCR were designed based on nifB of P. beijingensis BJ-18 and amyE of Bacillus sp. L-56 with the AlleleID 6.01 (PREMIER Biosoft International, Palo Alto CA, USA; Table 3). The 107 bp (nifB) and 138 bp (amyE) fragments were amplified by conventional PCR and then gel-purified. The PCR products were ligated to PMD 19-T Vector (Takara, Otsu, Japan) and transformed into Escherichia coli JM109 competent cells by electrotransformation. The successfully transformed E. coli JM109 was cultured in LB liquid medium, afterwards, the plasmids were extracted and purified using a TIANprep Mini Plasmid Kit (TIANGEN BIOTECH(BEIJING) CO., LTD). A standard curve was generated for each run with a dilution range of the recombinant plasmids from 2 × 101 to 2 × 107 copies. The above gDNA isolated from different treatments was mixed with the SYBR® Premix Ex Taq™ (Takara, Kyoto, Japan), primer pairs and ddH2O to a total volume of 20 uL for qPCR. Target DNA was quantified based on the above standard curve.
Table 3. Primers sequence and accession number in NCBI.
| Primer | Primer sequence 5′–3′ | NCBI Accession No. | Reference |
|---|---|---|---|
| nifB | GAAGGTGAGAGTGAGGATGGTTGCTTCAGGCTCATCTCC | MH202771 | This study |
| amyE | CTTCTCGTTCAGGCAGTACTATTGACCGCAGTGATAGC | MH202772 | This study |
| TaCAT | CCATCTGGCTCTCCTACTGGAGAACTTGGACGACGGCCCTGA | E16461 | Moloudi et al. (2013) |
| CsCAT | TTCGGCGTACAAGCATATTCCGTGGCGTGACTGTGATTCG | AY274258 | This study |
| TaAPX | CACCACATCTAAGGGACATCTTCAGAGGGTCACGAGTC | EF555121 | This study |
| CsAPX | ATGATTGTTGACTTGTTATGTGGATGAGGCAGAACGAACC | EU798448 | This study |
| TaNAC2D | ACCTCAGCTACGACGACATCCAGGCGGCGAAGAAGTCATCCGTTCC | GQ231954.1 | Huang & Wang (2016) |
| CsNAC22 | GACCTCGGTCTCGTCTGAAG CTACAATGTTGTTGGACTTCGG | — | Zhang et al. (2017) |
| TaLEA | AGATCGACGGTGACGTGAAGGTCCATGATCTTGCCCAGTAG | — | Li et al. (2018) |
| CsLEA11 | CGAGCAGTTCCAGCTCTACTTCCGGTTAACTTCTCCTT | — | Zhou et al. (2017) |
| TaACO2 | GAGGAACGAGGGCGAGGAG TCAGTTATCAGGCGGTGGC | — | Zhu et al. (2014) |
| CsACO1 | ACCTTCTTCTTACGCCATCGCCACCTACCTTGTCATC | AB006806 | Wei et al. (2015) |
| CsACS1 | CTTCAGGCGTTATTCAGATGGTGCTCGTGTTCTCCATT | U59813.1 | Wei et al. (2015) |
| TaDHN3 | TGGGACGGGCTCAGTGCTATGGGCGGGAGGAGGAAG | — | Zhu et al. (2014) |
| TaPR1-1a | TTCATCATCTGCAGCTACAACC CGGTACATATATACAGCCGGTCTAA | — | Qi et al. (2012) |
| CsPR1-1a | AACTCTGGCGGACCTTACTCAATATGGCCTTTGGTATAAG | DQ641122 | Dong et al. (2014) |
| TaACTIN | GTCGGTGAAGGGGACTTACATTCATACAGCAGGCAAGCAC | AB181991.1 | Huang & Wang (2016) |
| CsACTIN | AGAGATGGCTGGAATAGAACCTGGTGATGGTGTGAGTC | DQ115883 | Wan et al. (2010) |
Sample collection and preparation
The plant and rhizosphere soil samples of wheat and cucumber were harvested at 44 days after sowing. The growth parameters, i.e., root length, shoot length and fresh weight (FW) of each plant, were immediately determined. Four plants per pot were oven dried at 105 °C for 30 min, followed by 65 °C for 72 h. The dry weight (DW) was recorded. Chlorophyll content of seedlings was determined at 8:30–9:30 am on the harvest day using the SPAD-502 chlorophyll meter (Minolta Camera Co. Ltd., Tokyo, Japan). The remaining plant samples were rapidly frozen in liquid N and then stored at −80 °C for further use. Rhizosphere soil samples were screened by a 10-mesh sieve, and then stored in plastic bags for soil enzyme activity determination.
Determination of free proline content and total soluble sugars (TSS) in plant leaves
Under drought stress, accumulation of osmolytes (free proline content and total soluble sugars) can promote cell growth under adverse osmotic conditions under adverse osmotic conditions. (Sandhya et al., 2010). Free proline content in leaves was measured using acid ninhydrin (Sharma et al., 2010), and total soluble sugars (TSS) was determined using anthrone reagent (Shukla, Agarwal & Jha, 2012).
Soil enzyme activity determination
A total of 5 g soil was taken to measure activities of enzymes, which was related to N cycling: urease (EC 3.5.1.5); P cycling: alkaline phosphatase (EC 3.1.3.1) and acid phosphatase (EC 3.1.3.2); C cycling: invertase (EC 3.2.1.26) as well as microbial activity: dehydrogenase (EC 1.1.1). Urease activity was determined with urea as a substrate (Kaur, Brar & Dhillon, 2007) and the released N-NH4+ amount (mg N-NH4+g soil−1 h−1) was determined spectrophotometrically at OD578. Alkaline phosphatase and acid phosphatase were assessed with p-nitrophenyl phosphate as a substrate (Tabatabai & Bremner, 1969) and the released p-nitrophenol (pNP) amount (mg pNP g soil−1 h−1) was measured spectrophotometrically at OD660. Sucrase activity was measured with sucrose as a substrate (Chen et al., 2013a) and the released glucose amount (mg glucose g soil−1 h−1) was determined spectrophotometrically at OD508. Dehydrogenase activity was determined with 2,3,5-triphenyltetrazolium chloride (TTC) as a substrate (Schinner et al., 1996) and the reduced triphenylformazan (TPF) amount (mg TPF g soil−1 h−1) was determined spectrophotometrically at OD485. All assays were carried out in triplicate.
Quantitative real-time (qRT) PCR analysis of drought stress-related genes
Total RNA was extracted from leaf samples of wheat and cucumber using Trizol reagent according to the manufacturer’s protocol. RNA concentration was determined at 260 nm using a spectrophotometer (Nanodrop 1000; Thermo Scientific, Waltham, MA, USA). Strand cDNA was synthesized using PrimeScript™ RT reagent kit (Takara, Kyoto, Japan) following the instructions of the manufacturer. Then, cDNA was used as template to determine gene expression levels. qRT-PCR was performed by using the SYBR® Premix Ex Taq™ (Takara, Kyoto, Japan). The specific primers of ACTIN were used as reference. The specific primers used for qRT-PCR were designed by using the AlleleID 6.01 (PREMIER Biosoft International, Palo Alto, CA, USA) or from previous literature (Table 3). The relative expression levels were calculated according to the standard comparative C(t) method (Livak & Schmittgen, 2001). Each treatment had three biological replicates, with three technical replicates for each biological replicate.
Statistical analysis
All data was statistically analyzed using SPSS software version 20 (SPSS Inc., Chicago, IL, USA). The significant differences among treatments were analyzed using one-way analysis of variance (ANOVA) followed by least significant difference (LSD) at 0.05 level of probability. Graphs were prepared with SigmaPlot software version 12.5 (Systat Software, Inc, San Jose, CA, USA).
Results
Effects of biofertilizer and SAP on seed germination
The number of germination seeds was dynamically observed from day 5 to day 14 after sowing (Table 4). The synergistic use of biofertilizer and SAP had significant effects on germination rate and germination time of wheat and cucumber seeds. The germination time of wheat seeds treated with biofertilizer + SAP shortened by 4 days compared with that of the control, while 2 days compared with that of SAP treatment group. The germination rate of wheat seeds was 22.70% in the control; while 70.0% in BJ-18 + SAP treatment group and 62.0% in L-56 + SAP treatment group. The results indicated that adding SAP to the biofertilizer of P. beijingensis BJ-18 and Bacillus sp. L-56 increased the seed germination rate by 47.3% and 39.3%, respectively, compared with the control.
Table 4. Effects of super absorbent polymer supply on germination of wheat and cucumber seeds.
| Seed germination (%) at different day | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant | Treatments | 5 D | 6 D | 7 D | 8 D | 9 D | 10 D | 11 D | 12 D | 13 D | 14 D |
| Wheat | Control | 0a | 0a | 0b | 0c | 0d | 0c | 6.3 ± 1.2d | 9.7 ± 0.3d | 18.0 ± 1.0e | 22.7 ± 1.3e |
| SAP | 0a | 0a | 0b | 0c | 5.7 ± 1.2c | 12.3 ± 0.9b | 24.3 ± 1.5b | 37.7 ± 1.8b | 48.0 ± 2.3c | 52.3 ± 3.0c | |
| BJ-18 | 0a | 0a | 0b | 0c | 0d | 1.0 ± 1.0c | 7. 7 ± 0.7c | 17.7 ± 1.2c | 29.0 ± 1.2d | 33.0 ± 0.6d | |
| L-56 | 0a | 0a | 0b | 0c | 0 d | 1.0 ± 1.0c | 7. 7 ± 0.7cd | 13.0 ± 1.7cd | 22.7 ± 1.4de | 29.7 ± 1.3d | |
| BJ-18 +SAP | 0a | 0a | 8.7 ± 1.3a | 14.7 ± 2.1a | 20.3 ± 2.0b | 46.0 ± 2.5a | 50.7 ± 1.8a | 56.3 ± 2.3a | 67.7 ± 1.8a | 70.0 ± 1.0a | |
| L-56+SAP | 0a | 0a | 6.7 ± 2.0a | 17.3 ± 2.5b | 24.7 ± 1.2a | 44.3 ± 2.4a | 52.3 ± 2.2a | 57.7 ± 7.8a | 60.7 ± 1.4b | 62.0 ± 2.1b | |
| Cucumber | Control | 0a | 0a | 0a | 0b | 0c | 0c | 0d | 4.3 ± 0.8c | 6.3 ± 0.7d | 14.3 ± 0.9c |
| SAP | 0a | 0a | 0a | 0b | 0c | 13.3 ± 2.0b | 17.3 ± 2.2b | 21.7 ± 3.9b | 31.3 ± 4.1b | 39.3 ± 3.3b | |
| BJ-18 | 0a | 0a | 0a | 0b | 0c | 1.0 ± 1.0c | 5.3 ± 1.2c | 8.7 ± 1.2c | 12.0 ± 1.5 cd | 19.3 ± 1.2c | |
| L-56 | 0a | 0a | 0a | 0b | 0c | 0c | 4.3 ± 0.9 cd | 7.3 ± 2.0c | 13.7 ± 1.2c | 20.3 ± 1.3c | |
| BJ-18 +SAP | 0a | 0a | 0a | 8.3 ± 2.1a | 10.0 ± 1.0b | 27.7 ± 3.8a | 35.0 ± 1.5a | 37.7 ± 2.2a | 44.7 ± 2.0a | 51.3 ± 1.8a | |
| L-56+SAP | 0a | 0a | 0a | 8.3 ± 3.1a | 13.0 ± 1.0a | 24.3 ± 2.3a | 32.7 ± 2.0a | 41.7 ± 2.6a | 49.7 ± 2.8a | 56.0 ± 3.2a | |
Notes.
Values are given as mean ± SE of three independent biological replicates, and bearing different letters (a, b, c) at one specific time (e.g., 5 or 6 days) are significantly different from each other according to the least significant difference (LSD) test (p < 0.05).
- D
- day
- SAP
- Super Absorbent Polymer
- BJ-18
- P. beijingensis strain BJ-18
- L-56
- Bacillus sp. strain L-56
Similarly, biofertilizer + SAP treatment group shorten the germination time of cucumber seed by 2–4 days compared with other treatment groups. Among all the treatments of cucumber, L-56 + SAP treatment group had the highest germination rate, with a 35.7% increase compared to the L-56 treatment group.
Effect of biofertilizer and SAP on viability of inoculant
The qPCR method was used to determine the inoculant population density in the rhizosphere soil, and the viability of inoculants was significantly enhanced when biofertilizer was applied with SAP as compared to other treatments (Table 5). The inoculant populations increased at first and then declined. In wheat rhizosphere, the strain of BJ-18 + SAP treatment group showed the highest bacterial population (264 copies ng−1 gDNA) at day 29 after sowing. After reaching the highest population, both strains of P. beijingensis BJ-18 and Bacillus sp. L-56 showed a continuous declining trend. In cucumber rhizosphere, the L-56 + SAP treatment group showed the highest bacterial population (331.7 copies ng−1 gDNA) at day 29; and retained a higher level (75.0 copies ng−1 gDNA) till day 44 than others. No such inoculants (P. beijingensis BJ-18 and Bacillus sp. L-56) populations were detected in the rhizosphere soil of un-inoculated seedlings (wheat and cucumber).
Table 5. Survival of different bacterial strains inoculated to wheat and cucumber and population dynamics in the rhizosphere after thinning.
| Inoculant concentration in the rhizospher (copies ng−1 gDNA) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Plant | Treatments | 14 D | 19 D | 24 D | 29 D | 34 D | 39 D | 44 D |
| Wheat | BJ-18 | 123.9 ± 8.1b | 142.0 ± 4.0d | 140.0 ± 1.0d | 124.2 ± 3.9d | 96.1 ± 3.2c | 37.1 ± 1.6c | 19.3 ± 0.2b |
| L-56 | 198.9 ± 20.0a | 214.5 ± 0.9b | 200.2 ± 1.4b | 180.7 ± 5.8b | 130.3 ± 1.4b | 48.6 ± 3.7c | 17.2 ± 0.2b | |
| BJ-18 +SAP | 121.2 ± 1.6b | 157.7 ± 3.7c | 173.1 ± 3.2c | 264.4 ± 14.7c | 184.2 ± 4.6a | 109.8 ± 7.9a | 68.5 ± 4.3a | |
| L-56+SAP | 210.2 ± 1.9a | 236.6 ± 2.4a | 254.4 ± 1.0a | 237.7 ± 2.0a | 182.9 ± 2.1a | 140.3 ± 1.4a | 61.9 ± 6.7a | |
| Cucumber | BJ-18 | 110.7 ± 3.3c | 129.1 ± 1.5d | 127.2 ± 4.9c | 116.3 ± 12.1d | 83.6 ± 5.4c | 53.8 ± 3.9c | 9.7 ± 0.6d |
| L-56 | 184.0 ± 12.9b | 195.2 ± 1.8b | 156.8 ± 5.2b | 144.5 ± 5.7c | 99.8 ± 10.4c | 45.1 ± 2.9c | 18.4 ± 0.3c | |
| BJ-18 +SAP | 116.4 ± 2.6c | 154.8 ± 8.4c | 161.7 ± 3.2b | 188.0 ± 6.5b | 158.7 ± 10.6b | 94.5 ± 2.6b | 54.0 ± 2.6b | |
| L-56+SAP | 213.0 ± 3.9a | 240.9 ± 3.2a | 317.2 ± 2.1a | 331.7 ± 2.5a | 240.9 ± 11.8a | 113.5 ± 6.0a | 75.0 ± 2.8a | |
Notes.
Values are given as mean ± SE of three independent biological replicates, and bearing different letters (a, b, c) at one specific time (e.g., 14 or 19 days) are significantly different from each other according to the least significant difference (LSD) test (p < 0.05).
- D
- day
- SAP
- Super Absorbent Polymer
- BJ-18
- P. beijingensis strain BJ-18
- L-56
- Bacillus sp. strain L-56
Effect of biofertilizer and SAP on plant growth parameters
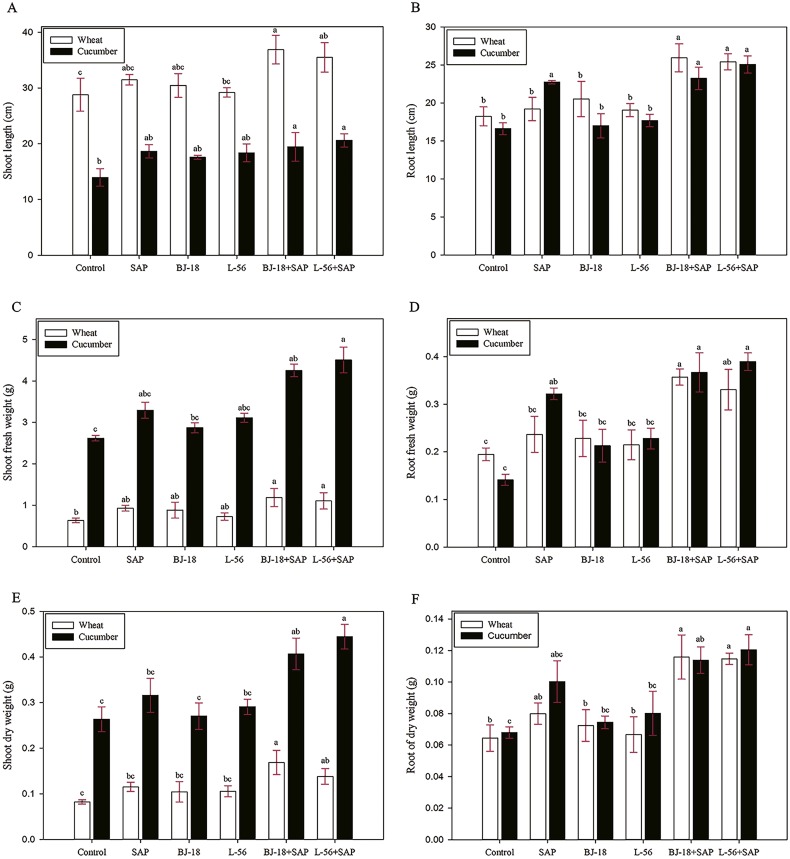
The treatments of adding SAP to biofertilizer significantly increased the growth parameters of both wheat and cucumber seedlings, including plant length, FW and DW (Fig. 1). The BJ-18 + SAP treatment group showed maximum increase over control in shoot length (28.2%), root length (42.3%), shoot FW (86.9%), root FW (83.4%), shoot DW (104.9%) and root DW (79.7%) of wheat seedlings. However, no significant effect was found in BJ-18 treatment group and L-56 treatment group in comparison to control.
Figure 1. Effects of super absorbent polymer supply on shoot length (A), root length (B), shoot fresh weight (C), root fresh weight (D), shoot dry weight (E) and root dry weight (F) of wheat and cucumber seedlings.
Values are given as mean of three independent biological replicates, and bearing different letters (a, b, c) are significantly different from each other according to the least significant difference (LSD) test (p < 0.05). The bars represent the standard error. SAP: Super Absorbent Polymer, BJ-18: P. beijingensis strain BJ-18, L-56: Bacillus sp. strain L-56.
In case of cucumber plants, the treatment group of L-56 + SAP showed maximum increase over control in shoot length (47.8%), root length (50.8%), shoot FW (71.9%), root FW (175.7%), shoot DW (68.9%) and root DW (76.9%). All the biometric growth parameters showed an order of L-56 + SAP >BJ-18 + SAP >L-56 >BJ-18 >Control. Similarly, cucumber treated with biofertilizer alone had no significant difference in all the biometric growth parameters as compared to control.
Effect of biofertilizer and SAP on biochemical parameters of seedlings
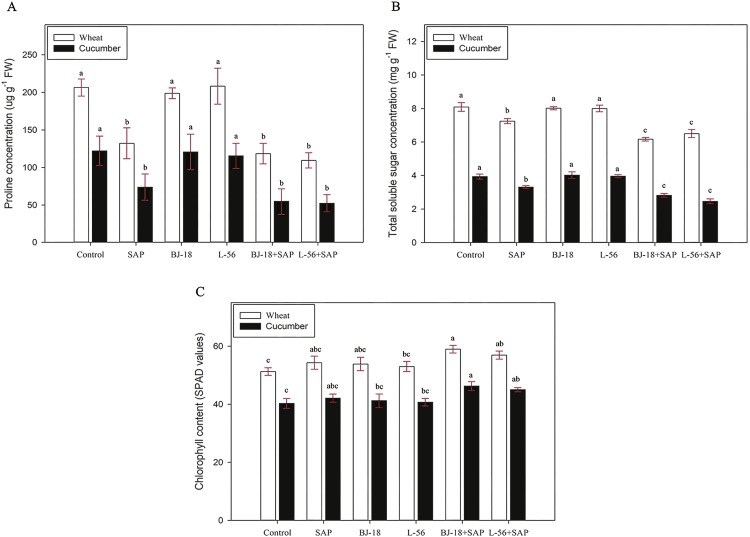
For both wheat and cucumber, the biochemical parameters (proline content, TSS content, and chlorophyll content) of leaves were quantified (Fig. 2). The results indicated that L-56 + SAP treatment group showed the lowest proline content, followed by BJ-18 + SAP group. The seedlings also showed a significant decline in total soluble sugar content in contrast to corresponding control. The chlorophyll content was expressed as SPAD value. A significant increase in chlorophyll content of both wheat and cucumber seedlings was observed in the BJ-18 + SAP treatment group compared to corresponding control (SPAD value of 59.0 and 46.3, respectively).
Figure 2. Effects of super absorbent polymer supply on proline concentration (A), total soluble sugar concentration (B), chlorophyll content (C) of wheat and cucumber seedlings.
Values are given as mean of three independent biological replicates, and bearing different letters (a, b, c) are significantly different from each other according to the least significant difference (LSD) test (p < 0.05). The bars represent the standard error. SAP: Super Absorbent Polymer, BJ-18: P. beijingensis strain BJ-18, L-56: Bacillus sp. strain L-56.
Effect of biofertilizer and SAP on soil extracellular enzyme activities
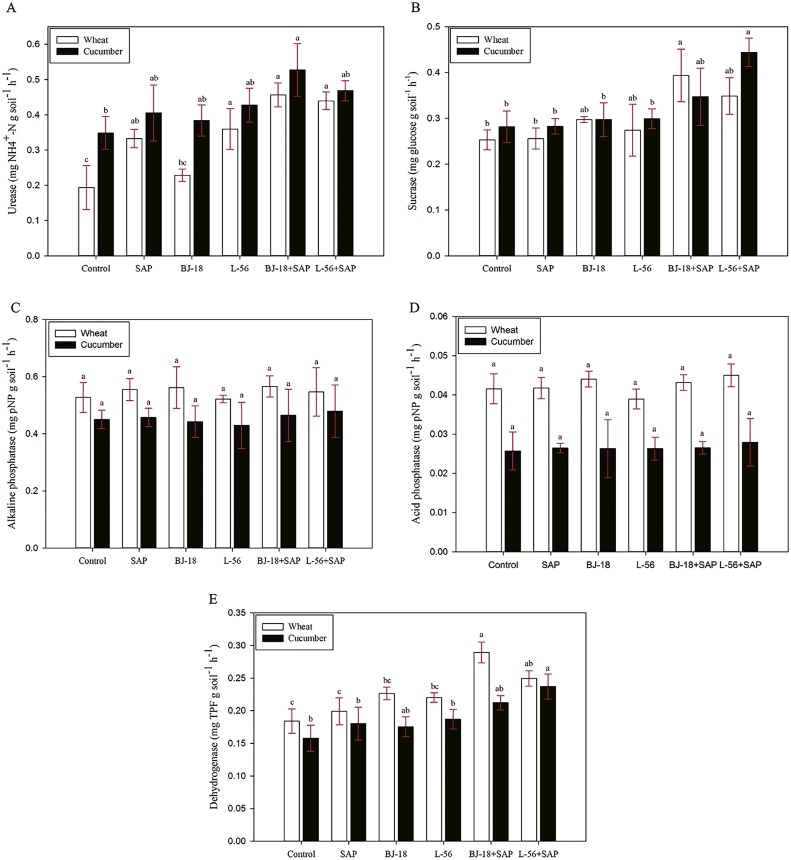
The activities of extracellular enzymes (urease, sucrose, acid phosphate, alkaline phosphate, and catalase) in rhizosphere soil were determined (Fig. 3). In the wheat and cucumber rhizosphere soil, urease activity in the BJ-18 + SAP group was greater than others (Fig. 3A). The BJ-18 + SAP treatment had significant positive effects on sucrose activity of the wheat rhizosphere soil as compared to control (Fig. 3B). However, the L-56 + SAP group showed the lowest sucrose activity in the cucumber rhizosphere soil (Fig. 3B). It was observed that the activity of alkaline phosphatase in different treatments was higher than that of acid phosphatase, and all treatment groups showed no significant difference in both acid and alkaline phosphatase activities in comparison to corresponding control (Figs. 3C and 3D). In the Fig. 3E, the dehydrogenase activity was the lowest in BJ-18 + SAP group of wheat rhizosphere soil, while it was the lowest in the L-56 + SAP group of cucumber rhizosphere soil.
Figure 3. Effects of super absorbent polymer supply on enzyme activities of urease (A), sucrose (B), alkaline phosphatase (C), acid phosphatase (D) and dehydrogenase (E) of rhizosphere soil.
The values are given as mean of three independent biological replicates, and bearing different letters (a, b, c) are significantly different from each other according to the LSD test (p < 0.05). The bars represent the standard error. SAP: Super Absorbent Polymer, BJ-18: P. beijingensis strain BJ-18, L-56: Bacillus sp. strain L-56, pNP: p-Nitrophenol, TPF: Triphenylformazan.
Effect of PGPR and SAP on expression of stress-responsive genes
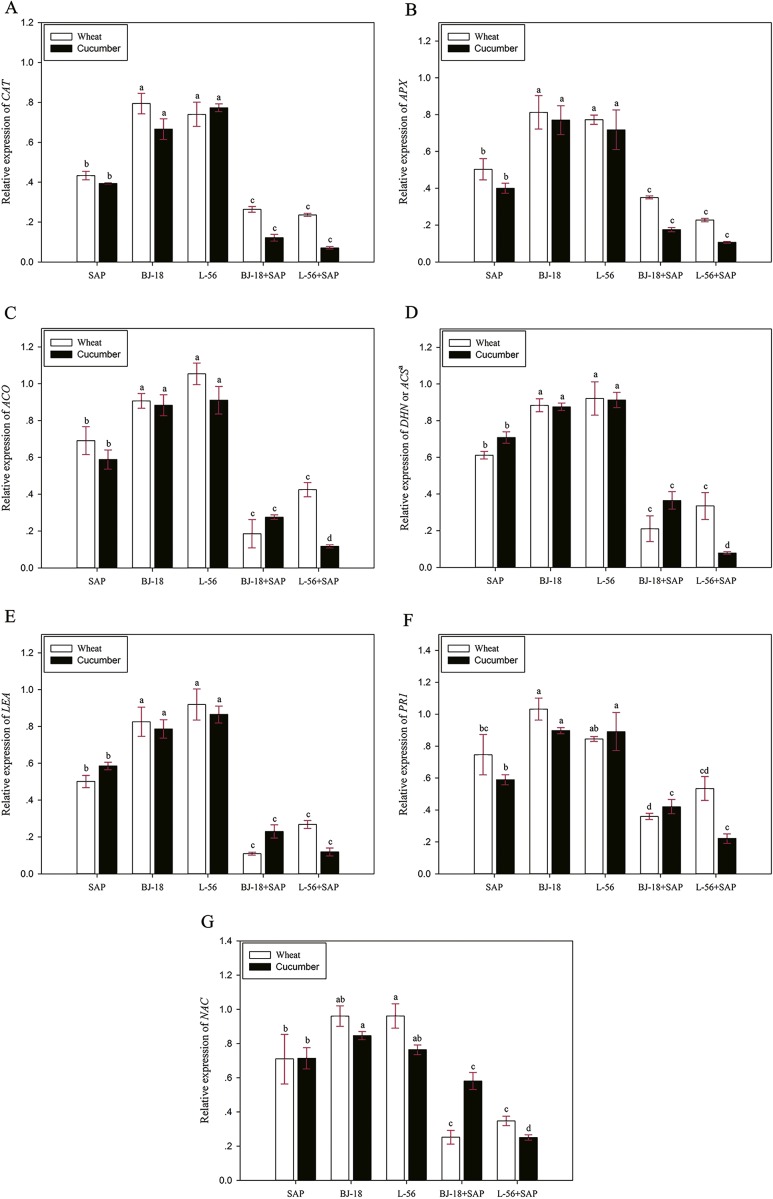
The qRT-PCR results showed the drought stress-related genes had different expression patterns under drought stress in biofertilizer + SAP treatment group and SAP treatment group (Fig. 4). The transcript levels of TaCAT, TaAPX, TaACO, TaDHN, TaLEA, TaPR1, and TaNAC were down-regulated in wheat treated with BJ-18+SAP and L-56 + SAP as compared to the control.
Figure 4. Effects of super absorbent polymer supply on relative gene expression level of stress-genes in wheat and cucumber seedlings.
The values are given as mean of three independent biological replicates, and bearing different letters (a, b, c) are significantly different from each other according to the least significant difference (LSD) test (p < 0.05). The bars represent the standard error. SAP: Super Absorbent Polymer, BJ-18: P. beijingensis strain BJ-18, L-56: Bacillus sp. strain L-56.. a: DHN for wheat, ACS for cucumber.
Variability in expression was also observed in the cucumber seedlings. The transcript levels of CsCAT, CsAPX, CsACO, CsACS, CsLEA, CsPR1, and CsNAC were down-regulated in cucumber treated with BJ-18+SAP and L-56 + SAP as compared to the control
Disscussion
Water deficiency suppresses PGPR reproduction of biofertilizer in the arid and semiarid areas of North China, and the application of biofertilizer was limited because no positive effect on crop growth and yield was observed. This study was aimed at minimizing the negative effect of water deficiency on biofertilizer by adding SAP to biofertilizer. The results revealed that synergistic use of biofertilizer and SAP decreased adverse effect of water deficiency on PGPR, and improved the efficiency of biofertilizer.
Germination is one of the most vital stages in a crop life cycle, which is significantly impacted by drought stress (Sleimi et al., 2013). Both the SAP treatment group and the PGPR + SAP treatment group significantly enhanced the germination rate of wheat and cucumber seeds. The use of SAP could improve the water-holding capacity of soil (Johnson, 1984), which is beneficial to seed germination. The application of SAP improved Caragana korshinskii seed germination rate (Su et al., 2017). Inoculation with PGPR (P. fluorescens, Enterobacter hormaechei, and P. migulae) could promote foxtail millet seed germination and seedling growth under drought condition (Niu et al., 2018). PGPR of Burkholderia sp. L2 and Bacillus sp. A30 could increase tomato seed germination (Tripti et al., 2017). In addition, the inoculation of Pseudomonas, Azospirillum, and Azotobacter significantly improve maize seed germination (Gholami, Shahsavani & Nezarat, 2009). In this study, the SAP application created an optimal environment for PGPR reproduction and seed germination by increasing soil moisture. Therefore, both BJ-18 + SAP and L-56 + SAP groups presented the maximum germination rate of wheat and cucumber.
The survival and persistence of PGPR introduced into rhizospher soil is a most vital factor for successful application of biofertilizer (Naqqash et al., 2016). Here, the survival and persistence of inoculants (P. beijingensis BJ-18 and Bacillus sp. L-56) in the rhizosphere soil was dynamically observed during the period of day 14–44 using qPCR. All groups except control exhibited consistent trend in populations of inoculants, which were increased firstly and declined afterwards. Similar trend has been observed in the strain of Phomopsis liquidambari (Wang et al., 2014). In general, the combination use of biofertilizer and SAP significantly increased the bacterial populations compared with corresponding biofertilizer use alone, in both wheat and cucumber rhizosphere soil. Even at day 44, the largest bacterial population was observed in BJ-18 + SAP group of wheat rhizosphere (68.5 copies ng−1 gDNA), and in L-56 + SAP group of cucumber rhizosphere (75.0 copies ng−1 gDNA). Numerous studies have reported the populations of introduced microorganisms were significantly reduced in the natural soil (Pujol et al., 2006; Kumar, Trivedi & Pandey, 2007; Longa et al., 2009) due to the hostility of adverse biotic factor (competition with native organisms for finite nurture) and abiotic factors (temperature, humidity, pH values) within the soil (Avrahami & Bohannan, 2007). Our results indicated that adding SAP to biofertilizer could minimize the adverse effect of water deficiency, thus improving the inoculant viability and prolonging persistence. It has been reported that the population density of Pseudome fluorescents in biochar and/or compost treated soil significantly higher than that in un-treated soil, which is achieved by increasing soil moisture (Nadeem et al., 2017).
In wheat and cucumber, both BJ-18 group and L-56 group did not significantly improve plant growth parameters (length, FW, DW) as compared to the control, which was not consistent with the results of previous studies, where Klebsiella sp. MBE02, Paenibacillus sp. NSY50, Bacillus, and Pseudomonas sp. MS16 enhanced the growth of peanut, cucumber, rice, wheat and respectively (Sharma, Kulkarni & Jha, 2016; Du et al., 2016; Feng et al., 2017; Muhammad et al., 2018). However, with the combination use of SAP (BJ-18 + SAP group and L-56 + SAP group), the above parameters (length, FW, DW) were significantly elevated in both wheat and cucumber seedlings as compared to the corresponding control. Particularly, BJ-18 + SAP treatment significantly promoted the root length, root FW, shoot DW and root DW compared with the SAP treatment in wheat, and also increased shoot FW, shoot FW and shoot DW in cucumber; L-56 + SAP treatment significantly promoted the shoot FW, root FW and shoot DW in wheat compared with the SAP treatment, and also increased shoot FW and shoot DW in cucumber. Considering the water-holding capacity of SAP, it was hypothesized that SAP is conductive to creating a more suitable environment for the reproduction of introduced inoculants in soil under drought stress. Such hypothesis was proven by the fact that survival of P. beijingensis BJ-18 and Bacillus sp. L-56 was greater in SAP treated soil as compared to the un-amended soil.
After application of biofertilizer amended with SAP in wheat and cucumber, the activities of urease, sucrose and dehydrogenase were significantly increased as compared the control. Urease increased utilization of N fertilizer by catalysing urea hydrolysis into ammonia (Bowles et al., 2014). No matter for wheat or cucumber, the BJ-18 + SAP group showed the strongest urease activity in all treatments, which might be related to the N-fixing ability of P. beijingensis BJ-18 (Wang et al., 2013). The activity of dehydrogenase, a vital indicator of microorganism activity, was increased after the application of inoculation to soil (Siddikee et al., 2016). Here, the highest activity of dehydrogenase and sucrose was detected in the BJ-18 + SAP group of wheat rhizosphere soil and L-56 + SAP group of cucumber rhizosphere soil, which was consistent with the survival of P. beijingensis BJ-18 and Bacillus sp. L-56 in the wheat or cucumber rhizosphere soil. These findings were also consistent with previous report (Xun et al., 2015), which demonstrates that inoculation with PGPR could significantly enhance the activities of soil enzymes (urease, dehydrogenase, and sucrose). However, acid phosphatase (alkaline phosphatase) activity showed no significant difference among different treatments, indicating that these two inoculants have little effects on this enzyme. In addition, the activity of alkaline phosphatase in different treatments was higher than that of acid phosphatase, which might be related to the influence of alkaline soil. Similar results have been reported in rice inoculated with Phomopsis liquidambari under LN level (Siddikee et al., 2016).
Under drought stress, the accumulation of proline and total soluble sugar can protect plant cells from osmotic damage (Delauney & Verma, 1993; Gontia-Mishra et al., 2016; Tiwari et al., 2016). Accordingly, a reduction in proline and total soluble sugar content was observed in both wheat and cucumber seedlings in the SAP treatment group compared with control, BJ-18 and L-56 treatments. However, wheat and cucumber showed a further decrease in proline and total soluble sugar in the BJ-18 + SAP and L-56 + SAP treatments as compared with the SAP treatment group. It was obvious that SAP amendment could help the biofertilizer to perform better by providing a suitable habitat for inoculants in soil, and therefore, less proline and total soluble sugar were accumulated by synergistic use of biofertilizer and SAP. Under drought stress, less proline accumulation of Caragana korshinskii grown in SAP amended soil was also reported by (Su et al., 2017). Chlorophyll content is also significant biochemical indicator of stress tolerance in plants (Percival, Fraser & Oxenham, 2003). In this study, the BJ-18 + SAP and L-56 + SAP treatments significantly improved the chlorophyll content in the wheat and cucumber seedlings. The application of biochar could also increase the chlorophyll content of Chenopodium quinoa by improving soil moisture content (Gorim & Asch, 2017). PGPR-inoculated plants also showed a significant increase in chlorophyll content (Kakar et al., 2016; Abdelkrim et al., 2018; El-Esawi et al., 2018).
To better understand how the plants (wheat and cucumber) respond to the different treatments at the transcriptional level, some representative drought-responsive genes were selected. Both CAT and APX genes were involved in reactive oxygen species (ROS) scavenging, and ROS overproduction resulted in a negative oxidative stress on plant growth. (Lata et al., 2011; Tiwari et al., 2016). qRT-PCR analysis showed that under drought stress, the expression levels of APX and CAT in seedlings of the biofertilizer + SAP groups were lower than those of other treatment groups, suggesting that biofertilizer + SAP treatments could relieve drought stress and ensure normal growth of seedlings. It was also reported chickpea inoculated with Pseudomonas showed significantly lower expression of CAT and APX during the stress period (Tiwari et al., 2016; Saif & Khan, 2018). Ethylene has been reported to regulate some different aspects of plant growth and development, particularly abiotic stressed such as drought stress (Yang, Kloepper & Ryu, 2009; Habben et al., 2014). Both ACO and ACS are involved in ethylene metabolism. In this study, the biofertilizer + SAP treatments significantly repressed the expression levels of CAT and APX, indicating less ethylene accumulation in plants treated with biofertilizer + SAP. Dehydrin and late embryogenesis abundant protein encoded by DHN and LEA genes play great roles in adaptive responses of plants to drought stress (Gao et al., 2008). The biofertilizer + SAP groups showed relatively low expression level of DHN and LEA. There were plenty of evidence for the involvement of salicylic acid in plant drought stress (Fujita et al., 2006). PR1 participate in salicylic acid metabolism. In this study, the biofertilizer + SAP treatments significantly inhibited the expression of PR1. NAC transcription factors have been reported to participate in biotic and abiotic stress tolerance in plants (Delessert et al., 2005; Nakashima, Ito & Yamaguchi-Shinozaki, 2009; Wu et al., 2009). TaNAC2D was induced by abiotic stress (Huang & Wang, 2016) and CsNAC22 responded to drought stress (Zhang et al., 2017). In this study, the biofertilizer + SAP groups inhibited the expression of NAC. Taken together, our results suggest that the biofertilizer + SAP treatments significantly ameliorate drought stress.
Conclusions
This study showed that the addition of SAP significantly enhanced survival rate of inoculants (P. beijingensis BJ-18 and Bacillus sp. L-56), and then promoted seed germination of wheat and cucumber, plant growth, soil fertility (urease, sucrose and dehydrogenase). qRT-PCR analysis also showed that the transcript levels of some stress-related genes were down-regulated in wheat and cucumber treated with biofertilizer + SAP, respectively, which imply that the biofertilizer + SAP treatments contribute to drought tolerance of wheat and cucumber. Our results indicate that SAP addition in biofertilizer is a good strategy for improving the efficiency of biofertilizer, especially in the areas suffering from long-term drought stress. This study was carried out in the greenhouse; therefore, further experiments are still needed to confirm the effect of biofertilizer amended with SAP in the field.
Supplemental Information
Acknowledgments
We would like to thank Caixia Wang for helping to improve manuscript.
Funding Statement
This work was supported by the National Key Research and Development Program of China (No. 2017YFD0200807). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Yongbin Li conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Haowen Shi performed the experiments, analyzed the data.
Haowei Zhang performed the experiments.
Sanfeng Chen conceived and designed the experiments, contributed reagents/materials/analysis tools.
DNA Deposition
Data Availability
The following information was supplied regarding data availability:
The raw measurements are provided in Data S1.
References
- Abdelkrim et al. (2018).Abdelkrim S, Jebara SH, Saadani O, Jebara M. Potential of efficient and resistant plant growth-promoting rhizobacteria in lead uptake and plant defence stimulation in Lathyrus sativus under lead stress. Plant Biology. 2018;20:857–869. doi: 10.1111/plb.12863. [DOI] [PubMed] [Google Scholar]
- Ankita et al. (2014).Ankita S, Singh PJ, Sudheer Y, Sarma BK, Singh SJ, Singh HB. Studies on rhizosphere-bacteria mediated biotic and abiotic stress tolerance in chickpea (Cicer arietinum L.) Vegetos. 2014;27(1):158–169. doi: 10.5958/j.2229-4473.27.1.025. [DOI] [Google Scholar]
- Avrahami & Bohannan (2007).Avrahami S, Bohannan BJ. Response of Nitrosospira sp. strain AF-like ammonia oxidizers to changes in temperature, soil moisture content, and fertilizer concentration. Applied and Environmental Microbiology. 2007;73(4):1166–1173. doi: 10.1128/AEM.01803-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashan et al. (2013).Bashan Y, De-Bashan LE, Prabhu SR, Hernandez J-P. Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998–2013) Plant and Soil. 2013;378(1–2):1–33. doi: 10.1007/s11104-013-1956-x. [DOI] [Google Scholar]
- Bowles et al. (2014).Bowles TM, Acosta-Martínez V, Calderón F, Jackson LE. Soil enzyme activities, microbial communities, and carbon and nitrogen availability in organic agroecosystems across an intensively-managed agricultural landscape. Soil Biology and Biochemistry. 2014;68:252–262. doi: 10.1016/j.soilbio.2013.10.004. [DOI] [Google Scholar]
- Calvo et al. (2010).Calvo P, Ormeno-Orrillo E, Martinez-Romero E, Zuniga D. Characterization of Bacillus isolates of potato rhizosphere from andean soils of peru and their potential PGPR characteristics. Brazilian Journal of Microbiology. 2010;41:899–906. doi: 10.1590/S1517-83822010000400008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2013a).Chen J, Liu X, Zheng J, Zhang B, Lu H, Chi Z, Pan G, Li L, Zheng J, Zhang X, Wang J, Yu X. Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from Southwest China. Applied Soil Ecology. 2013a;71:33–44. doi: 10.1016/j.apsoil.2013.05.003. [DOI] [Google Scholar]
- Chen et al. (2013b).Chen ZJ, Sheng XF, He LY, Huang Z, Zhang WH. Effects of root inoculation with bacteria on the growth, Cd uptake and bacterial communities associated with rape grown in Cd-contaminated soil. Journal of Hazardous Materials. 2013b;244–245:709–717. doi: 10.1016/j.jhazmat.2012.10.063. [DOI] [PubMed] [Google Scholar]
- Choudhury, Kecskes & Kennedy (2014).Choudhury ATMA, Kecskes ML, Kennedy LR. Utilization of BNF technology supplementing urea N for sustainable rice production. Journal of Plant Nutrition. 2014;37:1627–1647. doi: 10.1080/01904167.2014.888746. [DOI] [Google Scholar]
- Delauney & Verma (1993).Delauney A, Verma D. Proline biosynthesis and osmoregulation in plants. Plant Journal. 1993;4(2):215–223. doi: 10.1046/j.1365-313X.1993.04020215.x. [DOI] [Google Scholar]
- Delessert et al. (2005).Delessert C, Kazan K, Wilson IW, Van Der Straeten D, Manners J, Dennis ES, Dolferus R. The transcription factor ATAF2 represses the expression of pathogenesis-related genes in Arabidopsis. Plant Journal. 2005;43(5):745–757. doi: 10.1111/j.1365-313X.2005.02488.x. [DOI] [PubMed] [Google Scholar]
- Dong et al. (2014).Dong C-J, Li L, Shang Q-M, Liu X-Y, Zhang Z-G. Endogenous salicylic acid accumulation is required for chilling tolerance in cucumber (Cucumis sativus L.) seedlings. Planta. 2014;240(4):687–700. doi: 10.1007/s00425-014-2115-1. [DOI] [PubMed] [Google Scholar]
- Dorraji, Golchin & Ahmadi (2010).Dorraji SS, Golchin A, Ahmadi S. The effects of hydrophilic polymer and soil salinity on corn growth in sandy and loamy soils. CLEAN—Soil, Air, Water. 2010;38(7):584–591. doi: 10.1002/clen.201000017. [DOI] [Google Scholar]
- Du et al. (2016).Du N, Shi L, Yuan Y, Li B, Shu S, Sun J, Guo S. Proteomic analysis reveals the positive roles of the plant-growth-promoting Rhizobacterium NSY50 in the response of cucumber roots to Fusarium oxysporum f. sp. cucumerinum inoculation. Frontiers in Plant Science. 2016;7 doi: 10.3389/fpls.2016.01859. Article 1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Esawi et al. (2018).El-Esawi MA, Alaraidh IA, Alsahli AA, Alamri SA, Ali HM, Alayafi AA. Bacillus firmus (SW5) augments salt tolerance in soybean (Glycine max L.) by modulating root system architecture, antioxidant defense systems and stress-responsive genes expression. Plant Physiology and Biochemistry. 2018;132:375–384. doi: 10.1016/j.plaphy.2018.09.026. [DOI] [PubMed] [Google Scholar]
- Feng et al. (2017).Feng F, Ge J, Li Y, He S, Zhong J, Liu X, Yu X. Enhanced degradation of chlorpyrifos in rice (Oryza sativa L.) by five strains of endophytic bacteria and their plant growth promotional ability. Chemosphere. 2017;184:505–513. doi: 10.1016/j.chemosphere.2017.05.178. [DOI] [PubMed] [Google Scholar]
- Fujita et al. (2006).Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K. Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Current Opinion in Plant Biology. 2006;9(4):436–442. doi: 10.1016/j.pbi.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Gao et al. (2008).Gao WR, Wang XS, Liu QY, Peng H, Chen C, Li JG, Zhang JS, Hu SN, Ma H. Comparative analysis of ESTs in response to drought stress in chickpea (C. arietinum L.) Biochemical and Biophysical Research Communications. 2008;376(3):578–583. doi: 10.1016/j.bbrc.2008.09.030. [DOI] [PubMed] [Google Scholar]
- Gholami, Shahsavani & Nezarat (2009).Gholami A, Shahsavani S, Nezarat S. The effect of plant growth promoting rhizobacteria (PGPR) on germination, seedling growth and yield of maize. Proceedings of World Academy of Science Engineering & Technology. 2009;49:19–24. [Google Scholar]
- Gontia-Mishra et al. (2016).Gontia-Mishra I, Sapre S, Sharma A, Tiwari S. Amelioration of drought tolerance in wheat by the interaction of plant growth-promoting rhizobacteria. Plant Biology. 2016;18(6):992–1000. doi: 10.1111/plb.12505. [DOI] [PubMed] [Google Scholar]
- Gorim & Asch (2017).Gorim L, Asch F. Seed coating increases seed moisture uptake and restricts embryonic oxygen availability in germinating cereal seeds. Biology. 2017;6(2) doi: 10.3390/biology6020031. Article 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady et al. (2016).Grady EN, MacDonald J, Liu L, Richman A, Yuan ZC. Current knowledge and perspectives of Paenibacillus: a review. Microbial Cell Factories. 2016;15(1) doi: 10.1186/s12934-016-0603-7. Article 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray (2011).Gray D. Fluid drilling of vegetable seeds. Horticultural Reviews. 2011;3:1–27. doi: 10.1002/9781118060766.ch1. [DOI] [Google Scholar]
- Guerrero-Molina et al. (2015).Guerrero-Molina MF, Lovaisa NC, Salazar SM, Martinez-Zamora MG, Diaz-Ricci JC, Pedraza RO. Physiological, structural and molecular traits activated in strawberry plants after inoculation with the plant growth-promoting bacterium Azospirillum brasilense REC3. Plant Biology. 2015;17(3):766–773. doi: 10.1111/plb.12270. [DOI] [PubMed] [Google Scholar]
- Habben et al. (2014).Habben JE, Bao X, Bate NJ, DeBruin JL, Dolan D, Hasegawa D, Helentjaris TG, Lafitte RH, Lovan N, Mo H, Reimann K, Schussler JR. Transgenic alteration of ethylene biosynthesis increases grain yield in maize under field drought-stress conditions. Plant Biotechnology Journal. 2014;12:685–693. doi: 10.1111/pbi.12172. [DOI] [PubMed] [Google Scholar]
- Hao & Chen (2017).Hao T, Chen S. Colonization of wheat, maize and cucumber by Paenibacillus polymyxa WLY78. PLOS ONE. 2017;12(1):e0169980. doi: 10.1371/journal.pone.0169980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestrin & Goldblum (1953).Hestrin S, Goldblum J. Lævanpolyas. Nature. 1953;172:1046–1047. doi: 10.1038/1721046b0. [DOI] [PubMed] [Google Scholar]
- Huang & Wang (2016).Huang Q, Wang Y. Overexpression of TaNAC2D displays opposite responses to abiotic stresses between seedling and mature stage of transgenic Arabidopsis. Frontiers in Plant Science. 2016;7 doi: 10.3389/fpls.2016.01754. Article 1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam et al. (2010).Islam MR, Eneji AE, Changzhong R, Yuegao H, Gong C, Xuzhang X. Oat-basedcropping system for sustainable agricultural development in aridregions of northern China. Journal of Agriculture, Biotechnology & Ecology. 2010;3(3):1–8. [Google Scholar]
- Islam et al. (2013).Islam MR, Sultana T, Joe MM, Yim W, Cho JC, Sa T. Nitrogen-fixing bacteria with multiple plant growth-promoting activities enhance growth of tomato and red pepper. Journal of Basic Microbiology. 2013;53(12):1004–1015. doi: 10.1002/jobm.201200141. [DOI] [PubMed] [Google Scholar]
- Johnson (1984).Johnson MS. The effects of gel-forming polyacrylamides on moisture storage in sandy soils. Journal Science Food Agriculture. 1984;35(11):1196–1200. doi: 10.1002/jsfa.2740351110. [DOI] [Google Scholar]
- Johnson & Veltkamp (1985).Johnson MS, Veltkamp CJ. Structure and functioning of water-storing agricultural polyacrylamides. Journal Science Food Agriculture. 1985;36:789–793. doi: 10.1002/jsfa.2740360905. [DOI] [Google Scholar]
- Kakar et al. (2016).Kakar KU, Ren XL, Nawaz Z, Cui ZQ, Li B, Xie GL, Hassan MA, Ali E, Sun GC. A consortium of rhizobacterial strains and biochemical growth elicitors improve cold and drought stress tolerance in rice (Oryza sativa L.) Plant Biology. 2016;18(3):471–483. doi: 10.1111/plb.12427. [DOI] [PubMed] [Google Scholar]
- Kandeler, Kampichler & Horak (1996).Kandeler E, Kampichler C, Horak O. Influence of heavy metals on the functional diversity of soil microbial communities. Biology and Fertility of Soils. 1996;23(3):299–306. doi: 10.1007/BF00335958. [DOI] [Google Scholar]
- Kaur, Brar & Dhillon (2007).Kaur T, Brar BS, Dhillon NS. Soil organic matter dynamics as affected by long-term use of organic and inorganic fertilizers under maize–wheat cropping system. Nutrient Cycling in Agroecosystems. 2007;81(1):59–69. doi: 10.1007/s10705-007-9152-0. [DOI] [Google Scholar]
- Khan et al. (2010).Khan MS, Zaidi A, Ahemad M, Oves M, Wani PA. Plant growth promotion by phosphate solubilizing fungi-current perspective. Archives of Agronomy and Soil Science. 2010;56(1):73–98. doi: 10.1080/03650340902806469. [DOI] [Google Scholar]
- Kumar, Trivedi & Pandey (2007).Kumar B, Trivedi P, Pandey A. Pseudomonas corrugata: a suitable bacterial inoculant for maize grown under rainfed conditions of Himalayan region. Soil Biology and Biochemistry. 2007;39(12):3093–3100. doi: 10.1016/j.soilbio.2007.07.003. [DOI] [Google Scholar]
- Lata et al. (2011).Lata C, Jha S, Dixit V, Sreenivasulu N, Prasad M. Differential antioxidative responses to dehydration-induced oxidative stress in core set of foxtail millet cultivars (Setaria italica (L.)) Protoplasma. 2011;248:817–828. doi: 10.1007/s00709-010-0257-y. [DOI] [PubMed] [Google Scholar]
- Lebrun et al. (2012).Lebrun JD, Trinsoutrot-Gattin I, Vinceslas-Akpa M, Bailleul C, Brault A, Mougin C, Laval K. Assessing impacts of copper on soil enzyme activities in regard to their natural spatiotemporal variation under long-term different land uses. Soil Biology and Biochemistry. 2012;49:150–156. doi: 10.1016/j.soilbio.2012.02.027. [DOI] [Google Scholar]
- Li et al. (2018).Li N, Zhang S, Liang YJ, Qi YH, Chen J, Zhu WN, Zhang LS. Label-free quantitative proteomic analysis of drought stress-responsive late embryogenesis abundant proteins in the seedling leaves of two wheat (Triticum aestivum L.) genotypes. Journal of Proteomics. 2018;172:122–142. doi: 10.1016/j.jprot.2017.09.016. [DOI] [PubMed] [Google Scholar]
- Li et al. (2017).Li Y, Liu X, Hao T, Chen S. Colonization and maize growth promotion induced by phosphate solubilizing bacterial isolates. International Journal of Molecular Sciences. 2017;18(7) doi: 10.3390/ijms18071253. Article 1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak & Schmittgen (2001).Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−DeltaDeltaC(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Longa et al. (2009).Longa CM, Savazzini F, Tosi S, Elad Y, Pertot I. Evaluating the survival and environmental fate of the biocontrol agent Trichoderma atroviride SC1 in vineyards in northern Italy. Journal of Applied Microbiology. 2009;106(5):1549–1557. doi: 10.1111/j.1365-2672.2008.04117.x. [DOI] [PubMed] [Google Scholar]
- Meyer, Garber & Shaeffer (1964).Meyer JA, Garber ED, Shaeffer SG. Genetics of phytopathogenic fungi. XII. Detection of esterases and phosphatases in culture filtrates of Fusarium oxysporum and F. xylarioides by starch-gel zone electrophoresis. International Journal of Plant Sciences. 1964;125(4):298–300. [Google Scholar]
- Mikkelsen (1994).Mikkelsen RL. Using hydrophilic polymers to control nutrient release. Nutrient Cycling in Agroecosystems. 1994;38(1):53–59. [Google Scholar]
- Moloudi et al. (2013).Moloudi F, Navabpour S, Soltanloo H, Ramazanpour SS, Sadeghipour H. Catalase and Metallothionein genes expression analysis in wheat cultivars under drought stress condition. Journal of Plant Molecular Breeding. 2013;1:54–68. doi: 10.22058/JPMB.2013.3262. [DOI] [Google Scholar]
- Muhammad et al. (2018).Muhammad S, Sumera Y, Maria R, Mahreen Y, Babar MA, Muhammad SM. Phosphate solubilizing bacteria with glucose dehydrogenase gene for phosphorus uptake and beneficial effects on wheat. PLOS ONE. 2018;13(9):e0204408. doi: 10.1371/journal.pone.0204408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeem et al. (2017).Nadeem SM, Imran M, Naveed M, Khan MY, Ahmad M, Zahir ZA, Crowley DE. Synergistic use of biochar, compost and plant growth-promoting rhizobacteria for enhancing cucumber growth under water deficit conditions. Journal of the Science of Food and Agriculture. 2017;97(15):5139–5145. doi: 10.1002/jsfa.8393. [DOI] [PubMed] [Google Scholar]
- Nakashima, Ito & Yamaguchi-Shinozaki (2009).Nakashima K, Ito Y, Yamaguchi-Shinozaki K. Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiology. 2009;149(1):88–95. doi: 10.1104/pp.108.129791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqqash et al. (2016).Naqqash T, Hameed S, Imran A, Hanif MK, Majeed A, Van Elsas JD. Differential response of potato toward inoculation with taxonomically diverse plant growth promoting rhizobacteria. Frontiers of Plant Science. 2016;7 doi: 10.3389/fpls.2016.00144. Article 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naresh, Pramod & Sandeep (2014).Naresh PS, Pramod KP, Sandeep KV. Morpho-physiological characterization of Indian wheat genotypes and their evaluation under drought condition. African Journal of Biotechnology. 2014;13(20):2022–2027. doi: 10.5897/ajb2013.13486. [DOI] [Google Scholar]
- Naureen et al. (2017).Naureen Z, Rehman NU, Hussain H, Hussain J, Gilani SA, Al Housni SK, Mabood F, Khan AL, Farooq S, Abbas G, Harrasi AA. Exploring the potentials of Lysinibacillus sphaericus ZA9 for plant growth promotion and biocontrol activities against phytopathogenic fungi. Frontiers in Microbiology. 2017;8 doi: 10.3389/fmicb.2017.01477. Article 1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveed et al. (2013).Naveed M, Hussain MB, Zahir ZA, Mitter B, Sessitsch A. Drought stress amelioration in wheat through inoculation with Burkholderia phytofirmans strain PsJN. Plant Growth Regulation. 2013;73(2):121–131. doi: 10.1007/s10725-013-9874-8. [DOI] [Google Scholar]
- Niu et al. (2018).Niu XG, Song LC, Xiao YN, Ge WD. Drought-tolerant plant growth-promoting rhizobacteria associated with foxtail millet in a semi-arid and their potential in alleviating drought stress. Frontiers in Microbiology. 2018;8 doi: 10.3389/fmicb.2017.02580. Article 2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oteino et al. (2015).Oteino N, Lally RD, Kiwanuka S, Lloyd A, Ryan D, Germaine KJ, Dowling DN. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Frontiers in Microbiology. 2015;6 doi: 10.3389/fmicb.2015.00745. Article 745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey et al. (2012).Pandey PK, Yadav SK, Singh A, Sarma BK, Mishra A, Singh HB. Cross-species alleviation of biotic and abiotic stresses by the endophyte Pseudomonas aeruginosa PW09. Journal of Phytopathology. 2012;160(10):532–539. doi: 10.1111/j.1439-0434.2012.01941.x. [DOI] [Google Scholar]
- Percival, Fraser & Oxenham (2003).Percival GC, Fraser GA, Oxenham G. Foliar salt tolerance of Acer genotypes using chlorophyll fluorescence. Journal of Arboriculture. 2003;29:61–65. [Google Scholar]
- Pujol et al. (2006).Pujol M, Badosa E, Manceau C, Montesinos E. Assessment of the environmental fate of the biological control agent of fire blight, Pseudomonas fluorescens EPS62e, on apple by culture and real-time PCR methods. Applied and Environmental Microbiology. 2006;72(4):2421–2427. doi: 10.1128/AEM.72.4.2421-2427.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi et al. (2012).Qi PF, Johnston A, Balcerzak M, Rocheleau H, Harris LJ, Long XY, Wei YM, Zheng YL, Ouellet T. Effect of salicylic acid on Fusarium graminearum, the major causal agent of fusarium head blight in wheat. Fungal Biology. 2012;116(3):413–426. doi: 10.1016/j.funbio.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Rajkumar et al. (2010).Rajkumar M, Ae N, Prasad MNV, Freitas H. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends in Biotechnology. 2010;28:142–149. doi: 10.1016/j.tibtech.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Rehman, Ahmad & Safdar (2011).Rehman A, Ahmad R, Safdar M. Effect of hydrogel on the performance of aerobic rice sown under different techniques. Plant Soil and Environment. 2011;57(7):321–325. doi: 10.17221/81/2011-PSE. [DOI] [Google Scholar]
- Saif & Khan (2018).Saif S, Khan MS. Assessment of toxic impact of metals on proline, antioxidant enzymes, and biological characteristics of Pseudomonas aeruginosa inoculated Cicer arietinum grown in chromium and nickel-stressed sandy clay loam soils. Environmental Monitoring and Assessment. 2018;190(5):290. doi: 10.1007/s10661-018-6652-0. [DOI] [PubMed] [Google Scholar]
- Sandhya et al. (2010).Sandhya V, Ali SZ, Venkateswarlu B, Reddy G, Grover M. Effect of osmotic stress on plant growth promoting Pseudomonas spp. Archives of Microbiology. 2010;192:867–876. doi: 10.1007/s00203-010-0613-5. [DOI] [PubMed] [Google Scholar]
- Savazzini et al. (2008).Savazzini F, Longa CM, Pertot I, Gessler C. Real-time PCR for detection and quantification of the biocontrol agent Trichoderma atroviride strain SC1 in soil. Journal of Microbiological Methods. 2008;73(2):185–194. doi: 10.1016/j.mimet.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Schinner et al. (1996).Schinner F, Ohlinger R, Kandeler E, Margesin R. Mtthods in soil biology. Springer-Verlag; Berlin: 1996. [Google Scholar]
- Shakeel et al. (2015).Shakeel M, Rais A, Hassan MN, Hafeez FY. Root associated Bacillus sp. improves growth, yield and zinc translocation for basmati rice (Oryza sativa) varieties. Frontiers in Microbiology. 2015;6 doi: 10.3389/fmicb.2015.01286. Article 1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma et al. (2010).Sharma A, Gontia I, Agarwal PK, Jha B. Accumulation of heavy metals and its biochemical responses in Salicornia brachiata, an extreme halophyte. Marine Biology Research. 2010;6(5):511–518. doi: 10.1080/17451000903434064. [DOI] [Google Scholar]
- Sharma, Kulkarni & Jha (2016).Sharma S, Kulkarni J, Jha B. Halotolerant rhizobacteria promote growth and enhance salinity tolerance in peanut. Frontiers in Microbiology. 2016;7 doi: 10.3389/fmicb.2016.01600. Article 1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla, Agarwal & Jha (2012).Shukla PS, Agarwal PK, Jha B. Improved salinity tolerance of Arachis hypogaea (L.) by the interaction of halotolerant plant-growth-promoting rhizobacteria. Journal of Plant Growth Regulation. 2012;31(2):195–206. doi: 10.1007/s00344-011-9231-y. [DOI] [Google Scholar]
- Siddikee et al. (2016).Siddikee MA, Zereen MI, LI C, Dai C. Endophytic fungus Phomopsis liquidambari and different doses of N-fertilizer alter microbial community structure and function in rhizosphere of rice. Scientific Reports. 2016;6:32270. doi: 10.1038/srep32270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen et al. (2015).Simonsen AK, Han S, Rekret P, Rentschler CS, Heath KD, Stinchcombe JR. Short-term fertilizer application alters phenotypic traits of symbiotic nitrogen fixing bacteria. PeerJ. 2015;3:e1291. doi: 10.7717/peerj.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleimi et al. (2013).Sleimi N, Bankaji I, Touchan H, Corbineau F. Effects of temperature and water stresses on germination of some varieties of chickpea (Cicer arietinum) African Journal of Biotechnology. 2013;12:2201–2206. doi: 10.5897/AJB12.2323. [DOI] [Google Scholar]
- Sojka, Entry & Fuhrmann (2006).Sojka RE, Entry JA, Fuhrmann JJ. The influence of high application rates of polyacrylamide on microbial metabolic potential in an agricultural soil. Applied Soil Ecology. 2006;32(2):243–252. doi: 10.1016/j.apsoil.2005.06.007. [DOI] [Google Scholar]
- Su et al. (2017).Su LQ, Li JG, Xue H, Wang XF. Super absorbent polymer seed coatings promote seed germination and seedling growth of Caragana korshinskii in drought. Journal of Zhejiang University Science B (Biomedicine & Biotechnology) 2017;18(8):696–706. doi: 10.1631/jzus.B1600350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudisha et al. (2010).Sudisha J, Niranjana SR, Sukanya SL, Girijamba R, Lakshmi Devi N, Shekar Shetty H. Relative efficacy of strobilurin formulations in the control of downy mildew of sunflower. Journal of Pest Science. 2010;83(4):461–470. doi: 10.1007/s10340-010-0316-3. [DOI] [Google Scholar]
- Tabatabai & Bremner (1969).Tabatabai M, Bremner J. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biology and Biochemistry. 1969;1(4):201–207. doi: 10.1016/0038-0717(69)90012-1. [DOI] [Google Scholar]
- Terry, Richard & Nelson (1986).Terry Richard E, Nelson SD. Efficts of polyacrylamide and irrigation method on soil physical properties. Soil Science. 1986;141(5):317–320. doi: 10.1097/00010694-198605000-00003. [DOI] [Google Scholar]
- Tiwari et al. (2016).Tiwari S, Lata C, Chauhan PS, Nautiyal CS. Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. during drought stress and recovery. Plant Physiology and Biochemistry. 2016;99:108–117. doi: 10.1016/j.plaphy.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Tripti et al. (2017).Tripti, Kumar A, Usmani Z, Kumar V, Anshumali P. Biochar and flyash inoculated with plant growth promoting rhizobacteria act as potential biofertilizer for luxuriant growth and yield of tomato plant. Journal of Environmental Management. 2017;190:20–27. doi: 10.1016/j.jenvman.2016.11.060. [DOI] [PubMed] [Google Scholar]
- Vrieze et al. (2015).Vrieze MD, Pandey P, Bucheli TD, Varadarajan AR, Ahrens CH, Weisskopf L, Bailly A. Volatile organic compounds from native potato-associated Pseudomonasas potential anti-oomycete agents. Frontiers in Microbiology. 2015;6:e1003311. doi: 10.3389/fmicb.2015.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan et al. (2010).Wan H, Zhao Z, Qian C, Sui Y, Malik AA, Chen J. Selection of appropriate reference genes for gene expression studies by quantitative real-time polymerase chain reaction in cucumber. Analytical Biochemistry. 2010;399(2):257–261. doi: 10.1016/j.ab.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2014).Wang HW, Dai CC, Zhu H, Wang XX. Survival of a novel endophytic fungus Phomopsis liquidambari B3 in the indole-contaminated soil detected by real-time PCR and its effects on the indigenous microbial community. Microbiology Research. 2014;169(12):881–887. doi: 10.1016/j.micres.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2013).Wang LY, Li J, Li QX, Chen SF. Paenibacillus beijingensis sp. nov., a nitrogen-fixing species isolated from wheat rhizosphere soil. Antonie Van Leeuwenhoek. 2013;104(5):675–683. doi: 10.1007/s10482-013-9974-5. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2017).Wang Z, Xu G, Ma P, Lin Y, Yang X, Cao C. Isolation and characterization of a phosphorus-solubilizing bacterium from rhizosphere soils and its colonization of chinese cabbage (Brassica campestris ssp. chinensis) Frontiers in Microbiology. 2017;8 doi: 10.3389/fmicb.2017.01270. Article 1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb, Harrison & Ellis (2000).Webb J, Harrison R, Ellis S. Nitrogen fluxes in three arablesoils in the UK. European Journal of Agronomy. 2000;13:207–223. doi: 10.1016/S1161-0301(00)00075-7. [DOI] [Google Scholar]
- Wei et al. (2015).Wei L, Deng X-G, Zhu T, Zheng T, Li P-X, Wu J-Q, Zhang D-W, Lin H-H. Ethylene is involved in brassinosteroids induced alternative respiratory pathway in cucumber (Cucumis sativus L.) seedlings response to abiotic stress. Frontiers in Plant Science. 2015;6:982. doi: 10.3389/fpls.2015.00982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu et al. (2009).Wu YR, Deng ZY, Lai JB, Zhang YY, Yang CP, Yin BJ, Zhao QZ, Zhang L, Li Y, Yang CW, Xie Q. Dual function of Arabidopsis ATAF1 in abiotic and biotic stress responses. Cell Research. 2009;19(11):1279–1290. doi: 10.1038/cr.2009.108. [DOI] [PubMed] [Google Scholar]
- Xun et al. (2015).Xun F, Xie B, Liu S, Guo C. Effect of plant growth-promoting bacteria (PGPR) and arbuscular mycorrhizal fungi (AMF) inoculation on oats in saline-alkali soil contaminated by petroleum to enhance phytoremediation. Environmental Science & Pollution Research. 2015;22(1):598–608. doi: 10.1007/s11356-014-3396-4. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2013).Yang B, Wang X-M, Ma H-Y, Jia Y, Li X, Dai C-C. Effects of the fungal endophyte Phomopsis liquidambari on nitrogen uptake and metabolism in rice. Plant Growth Regulation. 2013;73(2):165–179. doi: 10.1007/s10725-013-9878-4. [DOI] [Google Scholar]
- Yang, Kloepper & Ryu (2009).Yang J, Kloepper JW, Ryu CM. Rhizosphere bacteria help plants tolerate abiotic stress. Trends in Plant Science. 2009;14(1):1–4. doi: 10.1016/j.tplants.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2012).Zhang J, Liu J, Meng L, Ma Z, Tang X, Cao Y, Sun L. Isolation and characterization of plant growth-promoting rhizobacteria from wheat roots by wheat germ agglutinin labeled with fluorescein isothiocyanate. Journal of Microbiology. 2012;50(2):191–198. doi: 10.1007/s12275-012-1472-3. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2017).Zhang XM, Yu HJ, Sun C, Deng J, Zhang X, Liu P, Li YY, Li Q, Jiang WJ. Genome-wide characterization and expression profiling of the NAC genes under abiotic stresses in Cucumis sativus. Plant Physiology and Biochemistry. 2017;113:98–109. doi: 10.1016/j.plaphy.2017.01.023. [DOI] [PubMed] [Google Scholar]
- Zhou et al. (2017).Zhou Y, He P, Xu Y, Liu Q, Yang Y, Liu S. Overexpression of CsLEA11, a Y3SK2-type dehydrin gene from cucumber (Cucumis sativus), enhances tolerance to heat and cold in Escherichia coli. Amb Express. 2017;7 doi: 10.1186/s13568-017-0483-1. Article 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu et al. (2014).Zhu X, Qi L, Liu X, Cai S, Xu H, Huang R, Li J, Wei X, Zhang Z. The wheat ethylene response factor transcription factor pathogen-induced ERF1 mediates host responses to both the necrotrophic pathogen rhizoctonia cerealis and freezing stresses. Plant Physiology. 2014;164(3):1499–1514. doi: 10.1104/pp.113.229575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements are provided in Data S1.