Abstract
Bowel cancer risk is strongly influenced by lifestyle factors including diet and physical activity. Several studies have investigated the effects of adherence to the WCRF/AICR cancer prevention recommendations on outcomes such as all-cause and cancer-specific mortality, but the relationships with molecular mechanisms that underlie the effects on bowel cancer risk are unknown. This study aimed to investigate the relationships between adherence to the WCRF/AICR cancer prevention recommendations and WNT pathway-related markers of bowel cancer risk, including the expression of WNT pathway genes and regulatory miRNAs, SFRP1 methylation and colonic crypt proliferative state in colorectal mucosal biopsies. Dietary and lifestyle data from 75 healthy participants recruited as part of the DISC Study were used. A scoring system was devised including seven of the cancer prevention recommendations and smoking status. The effects of total adherence score and scores for individual recommendations on the measured outcomes were assessed using Spearman’s rank correlation analysis and unpaired t-tests, respectively. Total adherence score correlated negatively with expression of c-MYC (p=0.039) and WNT11 (p=0.025) and high-adherers had significantly reduced expression of CCND1 (p=0.042), WNT11 (p=0.012) and c-MYC (p=0.048). Expression of AXIN2, GSK3β, CTNNB1 and WNT11 and of the oncogenic miRNA miR-17 and colonic crypt kinetics correlated significantly with scores for individual recommendations, including body fatness, red meat intake, plant food intake and smoking status. The findings from this study provide evidence for positive effects of adherence to the WCRF/AICR cancer prevention recommendations on WNT pathway-related markers of bowel cancer risk.
Keywords: WCRF/AICR cancer prevention recommendations, bowel cancer, WNT signalling pathway, bowel cancer risk
Introduction
Bowel cancer risk is strongly modulated by lifestyle and environmental factors. Higher incidence rates are associated with Western dietary and lifestyle factors, including diets rich in red and processed meats, high in fat content and low in fibre, and by sedentary behaviours and excess adiposity(1). The World Cancer Research Fund (WCRF)/ American Institute for Cancer Research (AICR) published ‘10 recommendations for Cancer Prevention’(2)(Supplementary Table 1) which include maintaining a BMI within the normal range(2) and performing at least 30 minutes of moderate physical activity daily. Six of the recommendations are based on dietary factors, including eating mainly foods of plant origin and limiting the consumption of red and processed meats.
Previous studies have examined the effects of adherence to the recommendations on several health outcomes including all-cause mortality, disease-specific mortality and bowel cancer risk in both healthy populations and cancer survivors(3; 4; 5). Participants in the European Prospective Investigation of Cancer and Nutrition (EPIC) Study with higher adherence scores (scores ≥4 in males and ≥5 in females) had 27% lower bowel cancer risk than those with the lowest scores (scores 0-2 in males and 0-3 in females)(6) and pre-diagnostic adherence to the recommendations was associated with lower risk of bowel cancer-related death and all-cause mortality(7). In the VITamins And Lifestyle (VITAL) Study, individuals meeting 4-6 of the recommendations compared with none had 58% lower bowel cancer incidence(8). For each one-point increment in overall adherence score based on six of the nutrition-related cancer prevention recommendations, there was a 25% reduction in bowel cancer risk in a Spanish cohort with 1,781 bowel cancer cases(9).
Fewer studies have investigated the effects of adhering to individual recommendations. Using pooled data from two Italian case-control studies (10), greater adherence to the recommendations for body fatness, physical activity and consumption of plant foods was associated with reduced bowel cancer risk. In a study in 2,983 individuals from the Offspring generation of The Framingham Study, significant links between adherence to the recommendations on plant foods, sodium and alcohol intake and bowel cancer risk were identified(11). Among 35,000 middle-aged participants in the UK Women’s Cohort Study, adherence to the recommendations for body fatness and animal foods were associated with a lower risk of proximal colon, distal colon and rectal cancers (12).
The stage(s) at which the lifestyle factors included in the WCRF/AICR cancer prevention recommendations impact on bowel cancer development is not well understood. We hypothesised that these lifestyle factors act early in tumorigenesis and that effects of adherence to the recommendations would be apparent on biomarkers measured in the apparently-normal mucosa. We investigated the links between adherence to the recommendations on WNT signalling in the large bowel mucosa. WNT signalling is central to regulation of homeostasis and normal function in colonocytes(13) and dysregulation of this pathway is a key driver of bowel cancer development(14). Indeed, WNT signalling is hyperactive in approximately 90% of sporadic colorectal cancer cases(15) and disruption of normal WNT signalling is an early event (if not the initiating event) in many such cancers. Specifically, we investigated links between adherence to the recommendations and (i) the expression of 12 WNT pathway components as indicators of WNT pathway activity, (ii) the methylation state of SFRP1 (a WNT antagonist frequently downregulated in large bowel cancer)(16), (iii) the expression of microRNAs that may regulate SFRP1 (including miR-17 and miR-19a, miRNAs belonging to the miR-17-92 oncogenic cluster which are upregulated in colorectal carcinogenesis(17; 18)) and (iv) colonic crypt cell proliferation, a key functional outcome of WNT signalling that is dysregulated in bowel cancer (19).
Experimental Methods
Study participants
The participants in this study were recruited to The DISC Study, a dietary intervention investigating the effects of non-digestible carbohydrates on large bowel health and bowel cancer risk (ClinicalTrials.gov Identifier: NCT01214681)(20). For the current analysis, pre-intervention (baseline) data were utilised. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Newcastle and North Tyneside Research Ethics Committee (REC No. 09/H0907/77). Written informed consent was obtained from all subjects. Healthy participants were recruited from gastroenterology out-patients departments at North Tyneside General Hospital, North Shields, UK and Wansbeck General Hospital, Ashington, UK between May 2010 and July 2011. The exclusion criteria are described in the Supplementary Materials. Rectal mucosal biopsies were collected at endoscopy (colonoscopy or flexible sigmoidoscopy) using Medical Innovations Biobite Biopsy forceps (diameter 2.3mm, length 230cm with spike, coated, REF BF23230-S/C) from the mid-rectum (10cm from the anorectal verge). Anthropometric measurements (weight, height, and waist, hip and thigh circumferences) were made by trained staff and participants completed a food frequency questionnaire (FFQ) and lifestyle questionnaire, providing information on physical activity and smoking status. The FFQ was adapted from that used in the EPIC Study(21; 22). Further details of the study have been published (20).
WNT pathway-related outcome measures
All measurements were performed in rectal mucosal biopsies. The expression of 12 WNT pathway-related genes and eight miRNAs were quantified by reverse transcriptase-quantitative PCR (RT-qPCR). The methylation state of SFRP1 was quantified by Pyrosequencing. Colorectal crypt proliferative state was assessed following whole crypt microdissection as described by Mills and colleagues(19). Further details of the laboratory methods are provided in the Supplementary Materials.
The WCRF/AICR Adherence Score
A scoring system was devised from the general recommendations for cancer prevention featured in Chapter 12 of the Second Expert Report(2). The recommendation to limit the consumption of energy-dense foods and avoid sugary drinks was excluded because of limited data to operationalise this recommendation. In addition, we incorporated smoking status as a lifestyle component based on the evidence that tobacco smoking causes colorectal cancer(23), with increased risk in both former and current smokers(24). Although this is not included in the WCRF/AICR Cancer Prevention recommendations, the Panel suggests not to smoke tobacco and to avoid exposure to tobacco smoke(2).
Our adherence scoring system had eight components (Table 1). Using baseline data (FFQ, Lifestyle Questionnaire and anthropometrical data), participants were allocated 1 point for adherence to each recommendation and 0 points if they did not meet the criterion, with the exception of the score for the recommendation on ‘plant foods’. The plant foods recommendation was divided into two sub-components (a) the recommendation on fruit and vegetable intake and (b) the recommendation on dietary fibre intake, allowing participants to score 0.5 for adherence to each part and scoring a maximum score of 1 for the plant foods recommendation. The individual scores for each recommendation were summed to produce a total WCRF/AICR recommendations adherence score (range 0 – 8).
Table 1. Adherence score assignment for each WCRF/AICR recommendation.
| WCRF/AICR recommendation | Data available within the DISC Study | Operationalisation of recommendation | Score |
|---|---|---|---|
| 1. Body fatness Maintain body weight within the normal range from age 21. |
BMI | 18.5 - ≤ 24.9 ≥25.0 |
1 0 |
| 2. Physical activity Be moderately physically active, equivalent to brisk walking, for at least 30 minutes every day. |
Physical activity level (Lifestyle Questionnaire) | ≥30mins moderate PA/d <30mins moderate PA/d |
1 0 |
3. Plant foods
|
Total daily fruit and vegetable intake (FFQ) Total dietary fibre intake (FFQ) |
≥5 servings/d <5 servings/d ≥25g per day <25g per day |
0.5 0 0.5 0 |
| 4. Animal foods People who eat red meat to consume less than 500g a week, very little if any to be processed. |
Total weekly red meat and processed meat intake (FFQ) | <500g/week ≥500g/week |
1 0 |
| 5. Alcoholic drinks If alcoholic drinks are consumed, limit consumption to no more than two drinks a day for men and one drink a day for women. |
Daily alcohol intake (FFQ) | ≤2 (♂) or ≤1 (♀) standard drink/d >2 (♂) or >1 (♀) standard drink/d |
1 0 |
| 6. Preservation, processing, preparation Limit consumption of processed foods with added salt to ensure an intake of less than 6g (2.4g sodium) a day. |
Daily salt intake (FFQ) | <2.4g sodium/day ≥2.4g sodium/day |
1 0 |
| 7. Dietary supplements Dietary supplements are not recommended for cancer prevention. |
Dietary supplement use | No dietary supplements Any dietary supplement |
1 0 |
| 8. Smoking Avoid tobacco in any form (2). |
Smoking status (Lifestyle Questionnaire) | Never smoked Current or former smoker |
1 0 |
BMI, body mass index; FFQ, food frequency questionnaire; NSP, non-starch polysaccharide; PA, physical activity
For red and processed meat intake, FFQ responses expressed as medium servings were translated into weight (g) using standard portion sizes(25). For cooked dishes, the proportion of red and processed meat was calculated using standard recipes described in McCance and Widdowson’s The Composition of Foods(26; 27), and the total weight of red and processed meat determined using standard portion sizes(25). For food items such as ham and bacon, a serving size of two items (e.g. two slices of ham or two rashers of bacon) was applied. A standard drink of alcohol was defined in the FFQ as ½ pint of beer, cider or lager, 1 glass of wine, martini or cinzano, 1 small glass of sherry or port or 1 measure of spirits or liqueurs. Participants reported whether they took dietary supplements as (i) vitamins, (ii) minerals, (iii) fish oils or (iv) other. Participants taking at least one dietary supplement were allocated a score of 0 and a score of 1 if they did not take any supplements.
Statistical analyses
Spearman’s rank correlation analysis was used to investigate relationships between total adherence score and each outcome. To investigate the relationships between the score for each recommendation individually and the measured outcomes, unpaired t-tests were used following the assessment of the distribution of data using the Kolmogorov-Smirnov test. Where data were not normally distributed, data were transformed appropriately. In addition, for continuous variables (BMI, moderate physical activity levels and the intakes of fruit and vegetables, red meat, sodium and alcohol), Spearman’s rank correlation analysis was applied to investigate relationships between these factors and biomarker outcomes. To test for differences in our measured outcomes between low and high adherence, participants were divided by dichotomising at the median score (3). Low adherers were those scoring a total adherence score of ≤3, and high adherers were those scoring >3. The ANOVA General Linear Model (adjusting for age, gender, BMI, smoking and endoscopy procedure as covariates) was used to investigate differences between low and high adherence scores.
Results
Participant demographics
Seventy-five healthy participants were recruited to The DISC Study (Table 2). Comparable numbers of male and female participants were recruited and all but two participants were Caucasian. The mean age of participants was 52 years (range 30 – 80 years). Only 17% of the study participants had a normal BMI, with 26% being overweight and 47% classed as obese. Half of the participants had never smoked, approximately a quarter were former smokers and the remainder were current smokers.
Table 2. Characteristics of the DISC Study participants.
| Demographics | |
| Total n | 75 |
| Female n (%) | 40 (53) |
| Age (years) mean (SD) | 52.4 (12.2) |
| Ethnicity | |
| Caucasian n (%) | 73 (97.3) |
| Black African n (%) | 1 (1.3) |
| Mixed race n (%) | 1 (1.3) |
| Endoscopy procedure | |
| Flexible sigmoidoscopy n (%) | 52 (69.3) |
| Colonoscopy n (%) | 23 (30.7) |
| Anthropometrics | |
| Height (m) mean (SD) | 1.7 (0.1) |
| Weight (kg) mean (SD) | 83.0 (16.1) |
| BMI (kg/m2) mean (SD) | 30.0 (5.3) |
| Waist circumference (cm) mean (SD Females Males |
99.6 (13.0) 96.2 (13.8) 103.4 (11.1) |
| Hip circumference (cm) mean (SD) Females Males |
107.0 (11.7) 108.8 (14.3) 105.1 (7.5) |
| Thigh circumference (cm) mean (SD) | 60.0 (6.6) |
| Smoking status | |
| Never n (%) | 38 (51) |
| Former n (%) | 21 (28) |
| Current n (%) | 16 (21) |
Information on participants’ habitual diet with respect to the WCRF/AICR cancer prevention recommendations is summarised in Table 3. The intake of fruits and vegetables was low, with a mean total intake of 3.2 medium servings per day but mean dietary fibre intake was just 10% less than the recommended 25g NSP per day. The mean intake of alcoholic drinks was 1.1 standard drinks per day. On average, this was lower for females (0.92 standard drinks/d) but 14 female participants consumed more than the recommended one standard drink per day. Mean alcohol intake in males was almost 50% greater than in females, but only seven male participants consumed more than the recommended upper limit of two standard drinks per day. Mean intake of red and processed meat was approximately 20% greater than the recommended weekly intake of 500g. Sodium intake amongst the DISC Study participants was more than 50% greater than the recommended daily intake. Fifty-five percent of participants did not take any form of dietary supplements. The remainder reported consuming at least one of multivitamins, minerals, fish oils or other supplements. Approximately 40% of participants undertook a minimum of 30 minutes of moderate physical activity per day whereas 22 participants reported that they did not undertake any moderately-active physical activity.
Table 3. Lifestyle characteristics of DISC Study participants.
| Food category | Mean intake (SD) |
|---|---|
|
Plant foods Fruit (medium servings/d) Vegetables/salad (medium servings/d) Dietary fibre (g/d) |
1.48 (1.37) 1.75 (1.19) 22.6 (10.8) |
|
Alcoholic drinks (standard drinks/d) All participants Females Male |
1.10 (1.17) 0.92 (0.89) 1.31 (1.41) |
|
Animal foods Red and processed meat (g/week) |
594 (396) |
| Sodium (g/d) | 3.90 (2.07) |
| Dietary supplements | Number of participants (%)ǂ |
| None | 41 (55) |
| Multivitamins | 26 (35) |
| Minerals | 13 (17) |
| Fish oils | 16 (21) |
| Other | 9 (12) |
| Physical activity level | Mean min/d (SD) |
| Mild | 90 (81) |
| Moderate | 43 (61) |
| Vigorous | 6 (14) |
Some participants consumed supplements from more than one category.
Relationships between total adherence score and biomarker outcomes
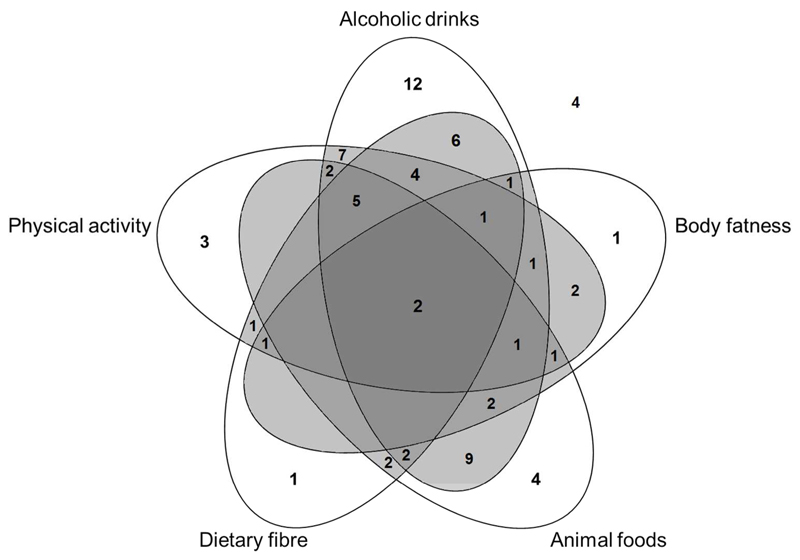
The total adherence score was calculated by adding the scores for each individual recommendation (0 or 1) to yield a maximum total score of 8. The distribution of total scores is summarised in Supplementary Table 2. No participant scored the maximum or minimum adherence score (8 and 0, respectively), the mean total adherence score was 3.2 and the majority of participants scored between 2 and 4. Figure 1 illustrates the clustering patterns of adherence to five of the cancer prevention recommendations specific to bowel cancer. In particular, adherence to the recommendations for alcoholic drinks, dietary fibre, physical activity and animal foods appeared to cluster. Interestingly, only nine participants adhered to both the recommendation for physical activity and body fatness. Among the 75 participants, four participants did not adhere to any of the five recommendations specific to bowel cancer.
Figure 1. Clustering of adherence to WCRF/AICR cancer prevention recommendations specific to bowel cancer.
Data refer to the numbers of participants adhering to each specific recommendation.
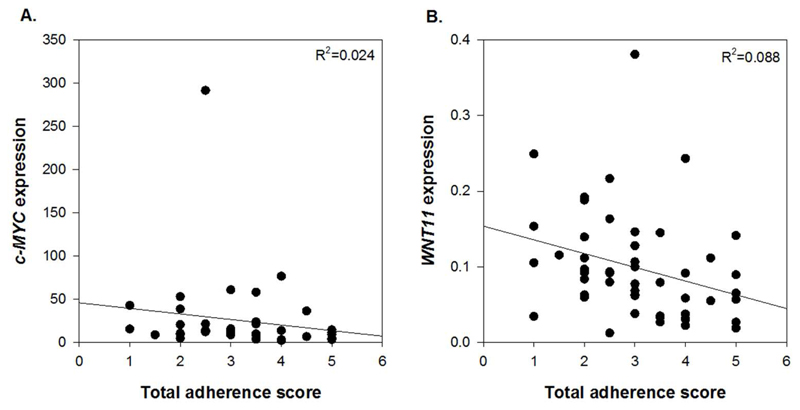
Since age is a strong risk factor for bowel cancer(28), Supplementary Table 2 includes mean participant age and shows that this was comparable for each total adherence score. Further, there was no relationship between participant age and total adherence score (Spearman’s rank correlation coefficient (ρ)=0.156, p=0.182). There were significant negative correlations between total adherence score and the expression of c-MYC (ρ=-0.328, p=0.039) and WNT11 (ρ=-0.407, p=0.004) (Figure 2A and 2B). In addition, there were trends (p<0.1) for inverse relationships between total adherence score and expression of CCND1 (ρ=-0.293, p=0.067) and c-JUN (ρ=-0.216, p=0.086) as well as colonic crypt width (ρ=-0.216, p=0.073). A summary of all analyses is provided in Supplementary Table 3.
Figure 2. Correlation between total score for adherence to the WCRF/AICR recommendations and (A) c-MYC expression and (B) WNT11 expression.
Expression of C-MYC and WNT11 is presented as adjusted copies (2-ΔCt x 10,000) relative to 18S and β2M genes.
We used the ANOVA General Linear model to investigate differences in our measured outcomes between low (total score ≤3) and high adherers (total score >3) and observed significantly reduced expression of CCND1 (LS mean 37.6 vs. 77.7, p=0.042), WNT11 (LS mean 0.05 vs. 0.1, p=0.012) and c-MYC (LS mean 8.7 vs. 16.6, p=0.048) in the high adherence group vs. low adherers (data not shown).
Relationships between scores for adherence to each of the individual WCRF/AICR recommendations and biomarker outcomes
Table 4 describes the number of participants who were awarded scores 0 and 1, respectively, and reports the mean values for each of the operationalised WCRF/ AICR recommendations. For five of the eight recommendations (body fatness, physical activity, plant foods, animal foods and preparation, preservation and cooking), the majority of participants scored 0 (Table 4). The mean BMI of participants who did not adhere to the recommendation on body fatness was approximately a third greater than those with a normal BMI. The mean time spent in moderate physical activity reported by those who adhered to the recommendation on physical activity was >11 times greater than that reported by those who were non-adherent. Participants who adhered to the recommendations on plant foods consumed three-times more fruit and vegetables and double the amount of dietary fibre compared with those who did not meet this recommendation. Mean salt intake by participants who adhered to the recommendation was less than half that consumed by non-adherents. The consumption of red and processed meat and the intake of alcohol were approximately four-fold greater in non-adherents compared with those who adhered to the recommendations on animal foods and alcoholic drinks. Most participants were adherents with respect to alcoholic drinks, and comparable numbers were allocated scores of 0 and 1 for the recommendations on dietary supplements and smoking.
Table 4. Adherence scores by DISC Study Participants for each individual WCRF/AICR recommendation for cancer prevention.
| WCRF/AICR recommendation | Score | n | % | Mean (SD) |
|---|---|---|---|---|
| 1. Body fatness BMI (kg/m2) | 1 0 |
13 62 |
17 83 |
23.7 (0.5) 31.3 (4.9) |
| 2. Physical activity Moderate PA (mins/d) | 1 0 |
31 44 |
41 59 |
92.4 (69.3) 7.9 (9.3) |
| 3. Plant foods Fruit and vegetable intake (medium servings/d) |
0.5 0 |
15 60 |
20 80 |
6.9 (1.3) 2.3 (1.2) |
| NSP intake (g/d) | 0.5 0 |
26 49 |
35 65 |
34.4 (8.0) 16.1 (5.5) |
| 4. Animal foods Red and processed meat intake (g/week) |
1 0 |
30 45 |
40 60 |
220 (160) 843 (297) |
| 5. Alcoholic drinks Alcohol intake (standard drinks/d) |
1 0 |
55 20 |
73 27 |
0.6 (0.5) 2.6 (1.2) |
| 6. Preservation, processing, preparation Sodium intake (g/d) | 1 0 |
14 61 |
19 81 |
1.9 (0.3) 4.4 (2.0) |
| 7. Dietary supplements | 1 0 |
41 34 |
55 45 |
|
| 8. Smoking | 1 0 |
38 37 |
51 49 |
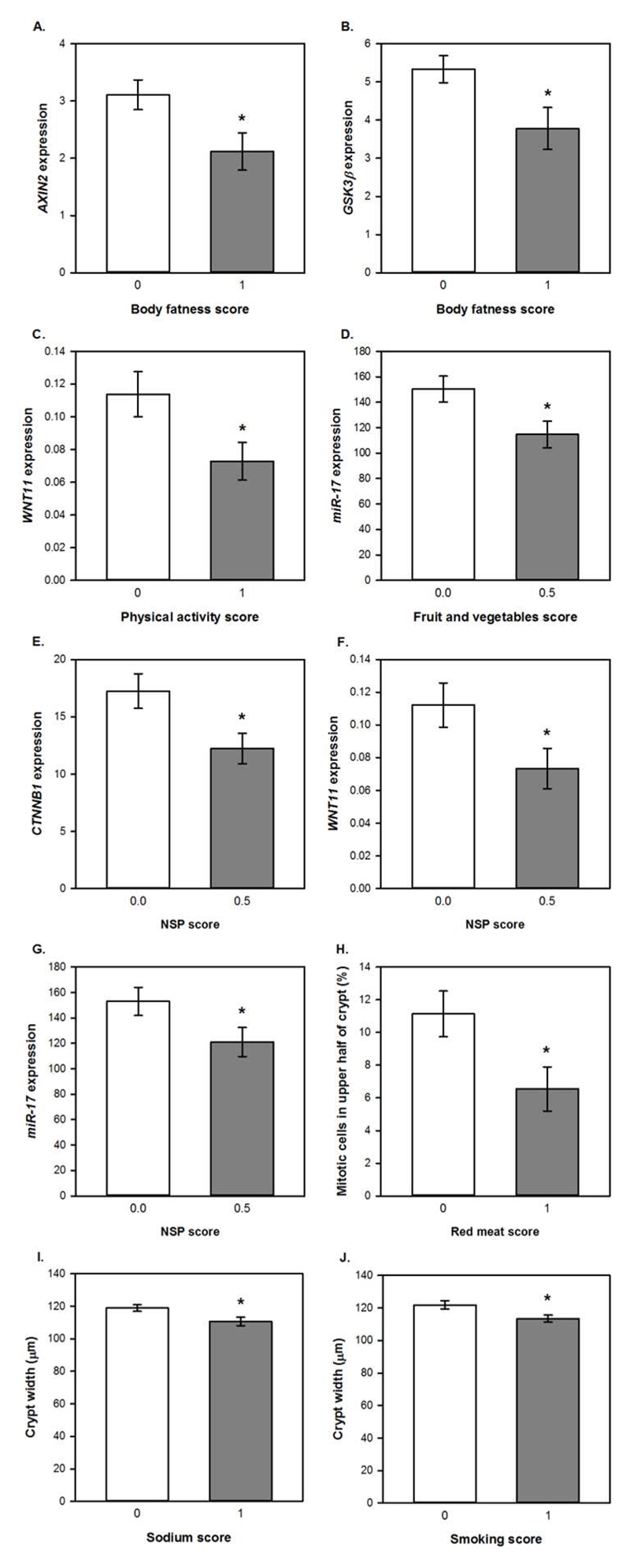
Unpaired t-tests were used to compare colorectal mucosal marker values for those who scored 0 and 1 for adherence to each of the individual recommendations and one-way ANOVA was used for the recommendation on plant foods, where a score of 0, 0.5 or 1 was possible. In addition, each component of the plant foods recommendation (intakes of dietary fibre and of fruits and vegetables) was investigated individually using unpaired t-tests. Adherence to the WCRF/AICR recommendation on body fatness was associated with significantly reduced expression of AXIN2 (p=0.025) and GSK3β (p=0.027) compared with non-adherence (Figure 3A and 2B). For continuous data, such as body fatness, Spearman’s correlation analysis was used to investigate relationships between individual participant values for these recommendations and the biomarker outcomes (Supplementary Table 4). A significant negative correlation was observed between BMI and miR-19b expression (ρ=-0.285, p= 0.034) whilst BMI correlated positively with crypt length (ρ=0.525, p=0.035).
Figure 3. Relationships between adherence scores for individual WCRF/ AICR recommendations and colorectal mucosal biomarkers.
Data are presented as means and error bars represent standard error of the mean. Gene expression data are adjusted copies (2-ΔCt x 10,000) relative to 18S and β2M housekeeping genes. miRNA expression data are presented as adjusted copies (2-ΔCt x 10,000) relative to RNU-6 and SNORD68. * p<0.05 for differences between scores analysed using unpaired t-tests.
Participants who met the recommendation for physical activity had significantly reduced expression of WNT11 (p=0.033) (Figure 3C) and this correlated negatively with moderate physical activity levels (ρ=-0.334, p=0.018). Adherence to the recommendation for fruit and vegetable intake was associated with significantly lower expression of miR-17 (p=0.035) (Figure 3D) and fruit and vegetable intake correlated negatively with WNT11 expression (ρ=-0.384, p= 0.006).
Dietary fibre intake score was significantly associated with three outcomes. Expression of two WNT pathway genes, CTNNB1 (p=0.046) and WNT11 (p=0.034), was approximately a third lower in participants consuming at least 25g NSP per day (Figure 3E and 3F). Daily NSP intake was also inversely correlated with expression of AXIN2 (p= 0.032), CTNNB1 (p= 0.002) and GSK3β (p= 0.028). Expression of the oncogenic miR-17 was 20% lower (p=0.030) in these adherent participants (Figure 3G) and expression levels of this miRNA were negatively correlated with NSP intake (ρ=-0.271, p= 0.044). There was no significant association between the overall plant foods score and any of the measured outcomes.
Lastly, colonic crypt proliferative state was associated with intakes of red meat and sodium as well as smoking status. Participants who consumed ≥500g red meat/week, had a 70% greater proportion of mitotic cells in the upper half of the mucosal crypts (p=0.015) (Figure 3H). Crypts were significantly wider in participants who did not meet the recommendation for sodium intake (≥2.4g sodium/day) (p=0.020) and for smoking (current or former smokers) (p=0.015) (Figure 3I and 3J). Daily sodium intake also correlated negatively with CTNNB1 expression (p= 0.034).
Discussion
This study aimed to investigate the relationships between adherence to AICR/WCRF cancer prevention recommendations and early WNT pathway-related markers of bowel cancer risk in apparently-healthy participants. We devised a scoring system that included seven of the recommendations plus smoking status. Overall, adherence to these recommendations correlated positively with reductions in markers of bowel cancer risk.
Higher total adherence was associated with reduced expression of c-MYC, CCND1 and WNT11. Since c-MYC and CCND1 are target genes of the WNT signalling pathway, reduced expression suggests reduced WNT pathway activity. CCND1 and c-MYC are oncogenes which control many aspects of cell growth and metabolism and which are frequently overexpressed in bowel cancers(29; 30; 31). WNT11 is a WNT ligand that activates both the canonical and non-canonical WNT signalling pathways and stimulates proliferation, migration and invasion of cancer-derived cells(32). WNT11 upregulation occurs in colorectal adenocarcinomas(33) and may contribute to cancer progression(34). Therefore, reduced expression of c-MYC, CCND1 and WNT11 is consistent with a protective effect of adherence to the WCRF/AICR cancer prevention recommendations.
Adherence to individual recommendations also correlated with mucosal biomarkers. Meeting the recommendation for body fatness was associated with significantly reduced expression of AXIN2 and GSK3β, two negative regulators of the WNT signalling implicated in colorectal carcinogenesis(35; 36; 37). A negative correlation between NSP intake and the expression of these two oncogenes suggests a mechanistic basis for the observation that higher intakes of dietary fibre are associated with lower bowel cancer risk(38). Adherence to the recommendations on body fatness, plant foods and physical activity has been associated with reduced bowel cancer risk(10). In the Framingham Offspring cohort, each increment in the score for the NSP subcomponent was associated with a 66% reduction in bowel cancer risk(11). In the VITAL Study, bowel cancer risk was 15% lower in those meeting the recommendation for body fatness(8).
WNT11 expression was approximately a third lower in participants who consumed at least 25g NSP/d or undertook at least 30 minutes of moderate physical activity per day, and correlated negatively with levels of moderate physical activity and with intakes of fruits and vegetables. Adherence to the plant foods recommendation was associated with significantly lower expression of the oncogenic miR-17 and miR-17 expression correlated inversely with daily NSP intake. A 30% lower expression of CTNNB1, encoding β–catenin, was observed in participants adhering to the dietary fibre recommendation. β–catenin is a the key player in the canonical WNT pathway that leads to activation of WNT target gene transcription and effects on cellular processes such as cell proliferation(39).
Colonic crypt cell proliferation is a major cellular process regulated by WNT signalling and is dysregulated in bowel cancer. An upward expansion of the proliferative zone within the crypt is one of the earliest detectable changes in the mucosa pre-malignancy and in those at increased bowel cancer risk(40; 41; 42). Participants who did not meet the recommendation for animal foods had a significantly greater proportion of mitotic cells in the top half of the colonic crypts, in line with the convincing evidence that high intakes of red and processed meat increase bowel cancer risk(43). Meeting the recommendation for animal foods was associated with a 19% lower bowel cancer risk in the VITAL Study(8) and a 32% reduction in colon cancer incidence in women in the UK Women’s Cohort Study(12). In the Framingham Offspring cohort, a 58% reduction in bowel cancer risk was observed for every increment in the subcomponent score for animal foods(11). A high red meat diet (300g red meat per day) increased cell proliferation in the human rectal mucosa (44) possibly because the haem represses inhibitors of cell proliferation, including Wif1 (a WNT pathway antagonist), Ihh and IL-15(45).
A limitation of this study is the relatively small sample size, however this is one of the largest studies with robust data on multiple molecular and functional markers of bowel health and bowel cancer risk measured in colorectal mucosal biopsies from apparently-healthy individuals. This provides a significant resource for investigating possible molecular mechanisms linking healthier diets and lifestyle with bowel cancer risk which act at an early stage in the carcinogenesis pathway.
Participants were recruited from a gastroenterology outpatient department and had been referred for endoscopy for a range of often non-specific symptoms ranging from suspected blood in stool to change in bowel habit. At endoscopy, any patients with diagnoses of disease such as colonic inflammation or adenomatous polyps were excluded. Whilst these participants may not be entirely representative of the healthy adult population, it is very difficult to recruit healthy participants from the general population who are willing to undergo endoscopic procedures for the collection of colorectal mucosal biopsies. We have used this recruitment method successfully in other studies of nutritional effects on colorectal mucosal biology(46). Overweight and obesity are common in our society – 68% of the population in the North East of England are overweight or obese and ii) smoking remains common – in the NE of England, 17% and 34% of adults are smokers and ex-smokers, respectively. From that perspective, our study cohort is similar to the population from which they were drawn. Equally important, both excess adiposity and smoking were included in our panel of 8 factors contributing to the WCRF/ AICR recommendations for cancer prevention (see Table 1).
We implemented clear and objective criteria to derive adherence scores. The addition of smoking status as a factor strengthened the utility of our scoring system as a measure of adherence to a healthier lifestyle. Smoking raises the risk of several cancers including bowel cancer(24) and the WCRF/AICR Panel recommend the avoidance of tobacco in any form(2). A limitation of our scoring system is that, because of lack of detailed information on sugar-sweetened beverages, we excluded the recommendation on foods and drinks that promote weight gain. However, we included BMI (used to assess body fatness) and further investigation will be necessary to determine whether consumption of “foods and drinks that promote weight gain” per se rather than adiposity drives bowel cancer risk. Our scoring system gave each recommendation the same weighting. It is possible that not all of these recommendations apply to bowel cancer specifically, so that a more specific sub-set of recommendations could be derived using effect sizes from meta-analyses to estimate bowel cancer-specific weightings.
In conclusion, our evidence suggests that adherence to the WCRF/AICR cancer prevention recommendations has positive effects on WNT pathway-related markers of bowel cancer risk. Whilst additional, larger studies are required to confirm these findings, this suggests that these WNT pathway-related biomarkers have potential as surrogate outcomes for intervention studies aiming to reduce bowel cancer risk by implementing the WCRF/AICR recommendations or other healthy eating guidelines. In addition, our observations may stimulate further research to identify the individual food components responsible for these effects and to investigate the mechanisms through which these effects are mediated.
Supplementary Material
Financial Support
This work was supported by a grant from the Biotechnology and Biological Sciences Research Council (BBSRC) Diet and Health Research Industry Club (DRINC) (grant number BB/H005013/1) to JCM, ITJ, NJB and SK. IM was funded by a Fellowship from Northumbria NHS Foundation Trust. We acknowledge further support from the Newcastle University Centre for Ageing and Vitality which is funded by the Medical Research Council (MRC) and BBSRC as part of the cross-council Lifelong Health and Wellbeing Initiative (grant number: MR/L016354/1).
Footnotes
Conflict of Interest: None
Authorship
FCM, NDW, IM, NJB, SK, DMB, ITJ and JCM designed research; FCM, NDW, IM and LX conducted research; FCM analyzed data; FCM and JCM wrote the manuscript and ITJ, NJB, NDW and SK edited the manuscript. All authors read and approved the final manuscript.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. Globocan 2012 v1.0 cancer incidence and mortality worldwide: IARC CancerBase No. 11. [accessed 2nd September 2014];2013 http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
- 2.WCRF/AICR. World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective. Washington DC: 2007. [Google Scholar]
- 3.Vergnaud AC, Romaguera D, Peeters PH, et al. Adherence to the World Cancer Research Fund/American Institute for Cancer Research guidelines and risk of death in Europe: results from the European Prospective Investigation into Nutrition and Cancer cohort study1,4. The American journal of clinical nutrition. 2013;97:1107–1120. doi: 10.3945/ajcn.112.049569. [DOI] [PubMed] [Google Scholar]
- 4.Lohse T, Faeh D, Bopp M, et al. Adherence to the cancer prevention recommendations of the World Cancer Research Fund/American Institute for Cancer Research and mortality: a census-linked cohort. The American journal of clinical nutrition. 2016;104:678–685. doi: 10.3945/ajcn.116.135020. [DOI] [PubMed] [Google Scholar]
- 5.Hastert TA, Beresford SA, Sheppard L, et al. Adherence to the WCRF/AICR cancer prevention recommendations and cancer-specific mortality: results from the Vitamins and Lifestyle (VITAL) Study. Cancer causes & control : CCC. 2014;25:541–552. doi: 10.1007/s10552-014-0358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romaguera D, Vergnaud AC, Peeters PH, et al. Is concordance with World Cancer Research Fund/American Institute for Cancer Research guidelines for cancer prevention related to subsequent risk of cancer? Results from the EPIC study. The American journal of clinical nutrition. 2012;96:150–163. doi: 10.3945/ajcn.111.031674. [DOI] [PubMed] [Google Scholar]
- 7.Romaguera D, Ward H, Wark PA, et al. Pre-diagnostic concordance with the WCRF/AICR guidelines and survival in European colorectal cancer patients: a cohort study. BMC medicine. 2015;13:107. doi: 10.1186/s12916-015-0332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hastert TA, White E. Association between meeting the WCRF/AICR cancer prevention recommendations and colorectal cancer incidence: results from the VITAL cohort. Cancer causes & control : CCC. 2016;27:1347–1359. doi: 10.1007/s10552-016-0814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romaguera D, Gracia-Lavedan E, Molinuevo A, et al. Adherence to nutrition-based cancer prevention guidelines and breast, prostate and colorectal cancer risk in the MCC-Spain case-control study. International journal of cancer Journal international du cancer. 2017;141:83–93. doi: 10.1002/ijc.30722. [DOI] [PubMed] [Google Scholar]
- 10.Turati F, Bravi F, Di Maso M, et al. Adherence to the World Cancer Research Fund/American Institute for Cancer Research recommendations and colorectal cancer risk. European journal of cancer. 2017;85:86–94. doi: 10.1016/j.ejca.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Makarem N, Lin Y, Bandera EV, et al. Concordance with World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) guidelines for cancer prevention and obesity-related cancer risk in the Framingham Offspring cohort (1991-2008) Cancer causes & control : CCC. 2015;26:277–286. doi: 10.1007/s10552-014-0509-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones P, Cade JE, Evans CEL, et al. Does adherence to the World Cancer Research Fund/American Institute of Cancer Research cancer prevention guidelines reduce risk of colorectal cancer in the UK Women's Cohort Study? The British journal of nutrition. 2018;119:340–348. doi: 10.1017/S0007114517003622. [DOI] [PubMed] [Google Scholar]
- 13.Gregorieff A, Pinto D, Begthel H, et al. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–638. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 14.White BD, Chien AJ, Dawson DW. Dysregulation of Wnt/beta-catenin signaling in gastrointestinal cancers. Gastroenterology. 2012;142:219–232. doi: 10.1053/j.gastro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nature reviews Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 16.Qi J, Zhu YQ, Luo J, et al. Hypermethylation and expression regulation of secreted frizzled-related protein genes in colorectal tumor. World journal of gastroenterology : WJG. 2006;12:7113–7117. doi: 10.3748/wjg.v12.i44.7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falzone L, Scola L, Zanghi A, et al. Integrated analysis of colorectal cancer microRNA datasets: identification of microRNAs associated with tumor development. Aging (Albany NY) 2018;10:1000–1014. doi: 10.18632/aging.101444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diosdado B, van de Wiel MA, Terhaar Sive Droste JS, et al. MiR-17-92 cluster is associated with 13q gain and c-myc expression during colorectal adenoma to adenocarcinoma progression. British journal of cancer. 2009;101:707–714. doi: 10.1038/sj.bjc.6605037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mills SJ, Mathers JC, Chapman PD, et al. Colonic crypt cell proliferation state assessed by whole crypt microdissection in sporadic neoplasia and familial adenomatous polyposis. Gut. 2001;48:41–46. doi: 10.1136/gut.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malcomson FC, Willis ND, McCallum I, et al. Effects of supplementation with nondigestible carbohydrates on fecal calprotectin and on epigenetic regulation of SFRP1 expression in the large-bowel mucosa of healthy individuals. Am J Clin Nutr. 2017;105:400–410. doi: 10.3945/ajcn.116.135657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bingham SA, Gill C, Welch A, et al. Validation of dietary assessment methods in the UK arm of EPIC using weighed records, and 24-hour urinary nitrogen and potassium and serum vitamin C and carotenoids as biomarkers. Int J Epidemiol. 1997;26(Suppl 1):S137–151. doi: 10.1093/ije/26.suppl_1.s137. [DOI] [PubMed] [Google Scholar]
- 22.Kroke A, Klipstein-Grobusch K, Voss S, et al. Validation of a self-administered food-frequency questionnaire administered in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: comparison of energy, protein, and macronutrient intakes estimated with the doubly labeled water, urinary nitrogen, and repeated 24-h dietary recall methods. The American Journal of Clinical Nutrition. 1999;70:439–447. doi: 10.1093/ajcn/70.4.439. [DOI] [PubMed] [Google Scholar]
- 23.WCRF/AICR. World Cancer Research Fund / American Institute for Cancer Research. Continuous update project report. Food, nutrition, physical activity, and the prevention of colorectal cancer 2011 [Google Scholar]
- 24.Liang PS, Chen TY, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. International journal of cancer Journal international du cancer. 2009;124:2406–2415. doi: 10.1002/ijc.24191. [DOI] [PubMed] [Google Scholar]
- 25.Crawley H, Mills A, Patel S, et al. Food portion sizes. 3rd ed. TSO; London: 2002. [Google Scholar]
- 26.Finglas PM, Roe MA, Pinchen HM, et al. McCance and Widdowson's The Composition of Foods. Seventh Summary Edition. The Royal Society of Chemistry; 2012. [Google Scholar]
- 27.Paul AA, Southgate DAT. McCance and Widdowson's The Composition of Foods. Fourth revised and extended edition of MRC Special Report No 297. London: Her Majesty's Stationery Office; 1978. [Google Scholar]
- 28.Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22:191–197. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller DM, Thomas SD, Islam A, et al. c-Myc and cancer metabolism. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:5546–5553. doi: 10.1158/1078-0432.CCR-12-0977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart J, Evan G, Watson J, et al. Detection of the c-myc oncogene product in colonic polyps and carcinomas. British journal of cancer. 1986;53:1–6. doi: 10.1038/bjc.1986.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Wei J, Xu C, et al. Prognostic significance of cyclin d1 expression in colorectal cancer: a meta-analysis of observational studies. PloS one. 2014;9:e94508. doi: 10.1371/journal.pone.0094508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mori H, Yao Y, Learman BS, et al. Induction of WNT11 by hypoxia and hypoxia-inducible factor-1alpha regulates cell proliferation, migration and invasion. Sci Rep. 2016;6 doi: 10.1038/srep21520. 21520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirikoshi H, Sekihara H, Katoh M. Molecular cloning and characterization of human WNT11. International journal of molecular medicine. 2001;8:651–656. doi: 10.3892/ijmm.8.6.651. [DOI] [PubMed] [Google Scholar]
- 34.Nishioka M, Ueno K, Hazama S, et al. Possible involvement of Wnt11 in colorectal cancer progression. Molecular carcinogenesis. 2013;52:207–217. doi: 10.1002/mc.21845. [DOI] [PubMed] [Google Scholar]
- 35.Lustig B, Jerchow B, Sachs M, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Molecular and cellular biology. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan D, Wiesmann M, Rohan M, et al. Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/beta -catenin signaling is activated in human colon tumors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:14973–14978. doi: 10.1073/pnas.261574498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shakoori A, Ougolkov A, Yu ZW, et al. Deregulated GSK3beta activity in colorectal cancer: its association with tumor cell survival and proliferation. Biochemical and biophysical research communications. 2005;334:1365–1373. doi: 10.1016/j.bbrc.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 38.Aune D, Chan DS, Lau R, et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;343:d6617. doi: 10.1136/bmj.d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong NA, Pignatelli M. Beta-catenin--a linchpin in colorectal carcinogenesis? The American journal of pathology. 2002;160:389–401. doi: 10.1016/s0002-9440(10)64856-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dronamraju SS, Coxhead JM, Kelly SB, et al. Cell kinetics and gene expression changes in colorectal cancer patients given resistant starch: a randomised controlled trial. Gut. 2009;58:413–420. doi: 10.1136/gut.2008.162933. [DOI] [PubMed] [Google Scholar]
- 41.Roncucci L, Scalmati A, Ponz de Leon M. Pattern of cell kinetics in colorectal mucosa of patients with different types of adenomatous polyps of the large bowel. Cancer. 1991;68:873–878. doi: 10.1002/1097-0142(19910815)68:4<873::aid-cncr2820680433>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 42.Anti M, Marra G, Armelao F, et al. Rectal epithelial cell proliferation patterns as predictors of adenomatous colorectal polyp recurrence. Gut. 1993;34:525–530. doi: 10.1136/gut.34.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.WCRF/AICR. World Cancer Research Fund / American Institute for Cancer Research. Continuous update project report. Food, nutrition, physical activity, and the prevention of colorectal cancer 2011. 2011 [Google Scholar]
- 44.Humphreys KJ, Conlon MA, Young GP, et al. Dietary manipulation of oncogenic microRNA expression in human rectal mucosa: a randomized trial. Cancer Prev Res (Phila) 2014;7:786–795. doi: 10.1158/1940-6207.CAPR-14-0053. [DOI] [PubMed] [Google Scholar]
- 45.IJ N, Rijnierse A, de Wit N, et al. Dietary haem stimulates epithelial cell turnover by downregulating feedback inhibitors of proliferation in murine colon. Gut. 2012;61:1041–1049. doi: 10.1136/gutjnl-2011-300239. [DOI] [PubMed] [Google Scholar]
- 46.Tapp HS, Commane DM, Bradburn DM, et al. Nutritional factors and gender influence age-related DNA methylation in the human rectal mucosa. Aging Cell. 2013;12:148–155. doi: 10.1111/acel.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.