Abstract
Objective
To examine temporal trends in overall and stage-specific incidence of melanoma.
Methods
Using population-based data on patients diagnosed with melanoma in East Anglia, England, 1996-2015, we estimated age-standardised time-trends in annual incidence rates for each stage at diagnosis. Negative binomial regression was used to model trends over time adjusted for sex, age group, and deprivation; and to subsequently examine variation in stage-specific trends by sex and age group.
Results
The age-standardised incidence increased from 14 to 29 cases/100,000 persons (i.e. 4% annually). Increasing incidence was apparent across stages but was steepest for stage I (adjusted annual increase: 5% [95% CI: 5%-6%] and more gradual for stage II-IV disease [stage II: 3% [95% CI 2%-4%]; stage III/IV: 2% [95% CI: 1%-3%]). Stage II-IV increase was apparent in men across age groups and in women aged 50+. Increases in incidence were steeper in 70+ year olds, and in men.
Conclusion
The findings suggest both a degree of overdiagnosis and a genuine increase in the incidence of consequential illness may be responsible for the observed increasing incidence trends in melanoma in our population during the study period. They also suggest potentially lower effectiveness of public health awareness campaigns in men and older people.
Keywords: Cancer, Melanoma, Early Diagnosis, Longitudinal Studies, Stage, Time-trends, Overdiagnosis
Introduction
The incidence of melanoma has increased dramatically in countries with predominantly Caucasian populations in recent decades and is projected to continue to increase for several countries.(1–4)
Two key potential drivers of increasing incidence are increased population exposure to ultraviolet radiation (the principal known environmental risk factor for melanoma),(5) and overdiagnosis of tumours that would not spread during that patient’s lifetime.(6, 7) The former hypothesis (actual increase in incidence of consequential illness) concords with increasing recreational exposure to sunlight since the 1950’s.(8, 9) On the other hand, stable trends in melanoma mortality despite increasing incidence have been observed, which may reflect either earlier diagnosis and more effective treatment of increasing consequential illness, or increasing overdiagnosis of relatively indolent lesions.(10–14)
Analyses of stage-specific time-trends of melanoma incidence could help to provide insights into mechanisms underlying increasing incidence: genuine increases in ultraviolet radiation exposure could be assumed to result in increasing incidence of each stage equally, while overdiagnosis may lead to disproportionately greater increase in early stage disease. Levell et al reported increasing incidence trends for stage I-III melanoma in 1991-2004, with a steeper increase of stage I disease.(13) However, it is important to examine more recent patient cohorts, and potential differences in stage-specific variation over time by socio-demographic group.(15)
Methods
Data
We analysed anonymised data on cases of primary invasive malignant melanoma (International Classification of Diseases for Oncology-3 code C43) diagnosed 1996-2015 in East Anglia, UK, for patients aged 15 years and older. These data were collected initially by the population-based cancer registry for the Anglia sub-region of the East of England, and successor organisations who took over responsibility (currently the Public Health England National Cancer Registration and Analysis Service).
Patients who were first diagnosed with melanoma in the fifteen years before the study period (1981–1995) (n = 309/10,199) were a priori excluded from analyses, while for those with multiple tumours diagnosed during the study period (1996-2015), the earliest diagnosis was selected.(16, 17) For patients with more than one tumour in a given year, the tumour with the more advanced stage at diagnosis was included.
Information was available on year of diagnosis, stage at diagnosis, sex, age group (15-49y and five-year age groups thereafter), deprivation group, method of diagnosis (e.g. histological or other basis), and survival status to death or censoring. Stage was assigned by registry staff based on clinical, imaging, and pathological information according to the 4th edition of the TNM classification (i.e. unified AJCC/UICC classifications).(18) We combined stage III and IV cases into a single ‘advanced stage’ category, because of the small number of stage IV cases (n=155/9,890). The deprivation measure used was the Index of Multiple Deprivation [IMD] quintiles (fifths) of the income domain scores for the Lower Super Output Area of the patient’s residence at diagnosis: IMD 2004 for diagnoses in 1996–2002; IMD 2007 for 2003–06; IMD 2010 for 2007–11; IMD 2015 for 2012-15. As we used all available data for the period 1996-2015, a sample size calculation was not carried out.
Statistical analysis
Our general approach was to calculate incidence rates by treating the number of melanoma cases as the numerator and the estimated mid-year population residing within the boundaries of East Anglia as the denominator. Population estimates were stratified by year of diagnosis, sex, age group, and deprivation.(19) All analyses were performed in Stata v.13 (StataCorp, College Station, TX, USA).
We first summarised the numbers of melanoma cases by sex, age group, deprivation, and stage, overall and by five-year diagnosis periods. As 5.0% of patients (493/9,890) had no recorded stage at diagnosis, we used multiple imputation (MI) prior to the main analyses – see below.(20, 21) In sensitivity analysis, we carried out a ‘complete case analysis’ (CCA; i.e., on those with non-missing stage, n = 9,397).
Incidence trends, by stage
We presented overall incidence trends partitioned into stage-specific incidence trends, by plotting sex-specific age-standardised rates (using the European Standard Population 2013) by year,(22) for all cases and by stage at diagnosis. We plotted these trends on the log-scale, to allow a fair representation of relative changes over time between stage groups with different baseline incidence.
We initially considered a Poisson model for assessing temporal trends while adjusting for stage, sex, age group, deprivation, and the interaction term stage*year, but variability in incidence rates was larger than assumed by a Poisson model (p<0.0001). Hence, we adopted negative binomial models which include an additional parameter for variance over-dispersion (‘Model 1’, Supplementary Box S1).(23)
Negative binomial models provided estimates of adjusted Incidence Rate Ratios (IRRs). The IRR for the independent variable year (hereafter referred to as the ’annual IRR’) represents the percentage increase in incidence per year as (IRR-1)×100 (e.g. an annual IRR of 1.05 is equivalent to a 5% increase per year).
Stage-specific incidence trends, by patient group
We explored whether stage-specific incidence trends varied by patient group, by investigating whether including three- and four-way interaction terms between the two demographic variables (age and sex) and the stage*year interaction improved the fit of the original negative binomial (which included main effects variables for stage, sex, age group, deprivation, year and the interaction stage*year). The investigation of interaction terms was performed using a step-wise process. We included interactions with demographic variables in descending order of strength of association for the main effects in the original model, and used the likelihood ratio test at each step to assess the improvement of fit by including the variable at that step.
The final model included the same main effect variables (i.e. stage, sex, age group, deprivation, and year), as well as a four-way interaction term sex*age group*stage*year (and its two-way and three-way components; ‘Model 2’; Supplementary Box S1). As this updated model required a substantially greater number of degrees of freedom, these variables were more broadly categorised using two stage groups (I, II-IV); three age groups (15-49, 50-69, 70+ years old); and three deprivation groups (least deprived, 2nd-4th quintiles, most deprived). Finally, we calculated the adjusted annual IRRs for each patient group (all combinations of stage, sex and age group), predicted from this updated model.
Multiple imputation
We created five datasets (following the generic rule that number of imputations should correspond to percentage of missing data),(24) where missing stage was imputed. We used multinomial logistic regression, based on sex, age group, deprivation group, survival status, and the Nelson-Aalen estimator of the cumulative hazard,(25) censoring survival information at one year post-diagnosis, to minimise bias from deaths unrelated to the melanoma. We carried out analyses as described above on each of these five datasets, using Rubin’s Rules to combine resulting estimates.(26) In sensitivity analysis, we carried out a ‘complete case analysis’ by restricting the analysis sample to cases with completely observed stage, and compared the two approaches as the percentage change in the model coefficients (equivalent to percentage change in IRRs on the log scale).
Results
There were 9,890 cases of melanoma diagnosed in East Anglia during 1996–2015 in an approximate population of 2.5 million during the study period. Table 1 shows the patients’ characteristics by five-year periods. Just over half (51%) of cases were women, and just over half (53%) of cases were under 65. Stage I disease accounted for the majority of cases (62%) during the entire study period. The proportion of younger patients (15-49) decreased over time (from 31% in 1996-2000 to 20% in 2011-2015) while the proportion of stage I cases increased (57% to 64%).
Table 1. Demographic and clinical characteristics of primary melanoma cases diagnosed in East Anglia during 1996–2015 (split into five– year periods).
| 1996-2000 | 2001-05 | 2006–10 | 2011-15 | 1996–2015 (total study period) |
|
|---|---|---|---|---|---|
| Total | 1516 (100.0) | 2033 (100.0) | 2,955 (100.0) | 3,386 (100.0) | 9,890 (100.0) |
| Sex | |||||
| Female | 844 (55.7) | 1,095 (53.9) | 1,465 (49.6) | 1675 (49.5) | 5,079 (51.4) |
| Male | 672 (44.3) | 938 (46.1) | 1,490 (50.4) | 1711 (50.5) | 4,811 (48.6) |
| Age group (years) | |||||
| 15–49 | 467 (30.8) | 553 (27.2) | 654 (22.1) | 662 (19.6) | 2,336 (23.6) |
| 50–54 | 172 (11.3) | 175 (8.6) | 210 (7.1) | 253 (7.5) | 810 (8.2) |
| 55–59 | 150 (9.9) | 229 (11.3) | 278 (9.4) | 264 (7.8) | 921 (9.3) |
| 60–64 | 148 (9.8) | 198 (9.7) | 391 (13.2) | 366 (10.8) | 1,103 (11.2) |
| 65–69 | 141 (9.3) | 201 (9.9) | 324 (11.0) | 449 (13.3) | 1,115 (11.3) |
| 70–74 | 152 (10.0) | 209 (10.3) | 353 (11.9) | 396 (11.7) | 1,110 (11.2) |
| 75–79 | 105 (6.9) | 211 (10.4) | 305 (10.3) | 392 (11.6) | 1,013 (10.2) |
| 80–84 | 100 (6.6) | 142 (7.0) | 233 (7.9) | 298 (8.8) | 773 (7.8) |
| 85+ | 81 (5.3) | 115 (5.7) | 207 (7.0) | 306 (9.0) | 709 (7.2) |
| Deprivation (IMD quintile) | |||||
| 1 (Least) | 404 (26.6) | 481 (23.7) | 690 (23.4) | 828 (24.5) | 2,403 (24.3) |
| 2 | 406 (26.8) | 608 (29.9) | 880 (29.8) | 1022 (30.2) | 2,916 (29.5) |
| 3 | 383 (25.3) | 532 (26.2) | 865 (29.3) | 921 (27.2) | 2,701 (27.3) |
| 4 | 236 (15.6) | 294 (14.5) | 385 (13.0) | 419 (12.4) | 1,334 (13.5) |
| 5 (Most) | 87 (5.7) | 118 (5.8) | 135 (4.6) | 196 (5.8) | 536 (5.4) |
| Stage at diagnosis | |||||
| I | 863 (56.9) | 1,192 (58.6) | 1,935 (65.5) | 2150 (63.5) | 6,140 (62.1) |
| II | 349 (23.0) | 449 (22.1) | 572 (19.4) | 652 (19.3) | 2,022 (20.4) |
| III-IV | 210 (13.9) | 287 (14.1) | 382 (12.9) | 356 (10.5) | 1,235 (12.5) |
| Missing | 94 (6.2) | 105 (5.2) | 66 (2.2) | 228 (6.7) | 493 (5.0) |
Incidence trends, by stage
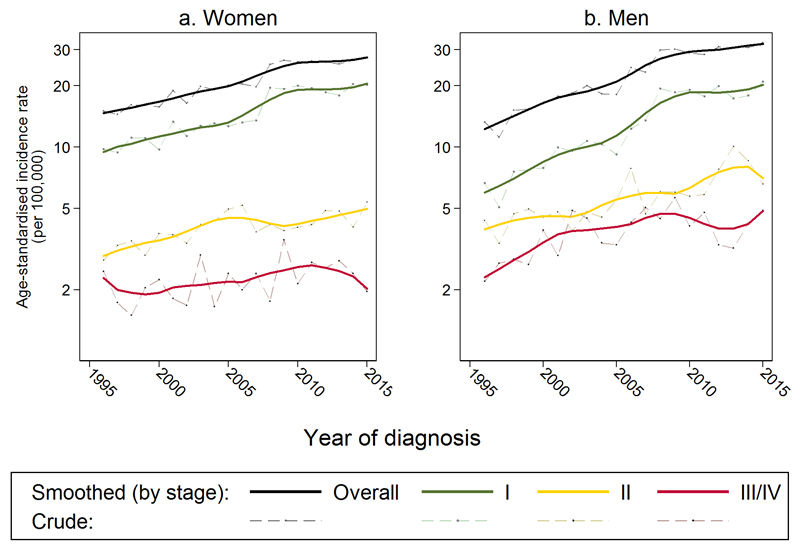
During the study period, the age-standardised incidence of melanoma increased substantially from 14.0 per 100,000 in 1996 to 29.4 in 2015 (i.e. by +4% annually) (Figure 1 and Supplementary Table S1). Overall (all-stage) incidence rates (black lines) increased with time with a slight acceleration around 2005 (women: 15.0 per 100,000 in 1996 to 27.6, men: 13.3 to 32.5, Figure 1). This overall time-trend was mirrored by that for stage I cases (green lines), where the age-standardised incidence increased from 8.2 per 100,000 in 1996 to 20.3 in 2015 (+5% annually). Age-standardised incidence of stage II and stage III/IV cases (yellow and red lines) also increased, but less steeply than for stage I cases (+2.8% and +1.9% annually).
Figure 1.
Age-standardised incidence of melanoma (smoothed and observed) by stage at diagnosis, for a) women and b) men
Smoothed rates are ‘lowess’ (locally weighted scatterplot smoothed) (i.e., the average of each observed yearly rate and rates in nearby years; in this case the bandwidth was set at 0.2). For 493 patients (5% of entire dataset) with missing values on stage, these values were imputed as described under ‘Multiple imputation’.
After adjustment for sex, age group and deprivation, there was strong statistical evidence of increasing incidence in stage I, II, and III/IV disease (p<0.0001 for all adjusted annual IRRs), but a steeper increase for stage I disease (adjusted annual IRR for stage I disease: 1.05, i.e. a 5% increase per year [95% CI: 1.05-1.06]; stage II: 1.03, i.e. +3% increase per year [1.02-1.04]; stage III/IV: 1.02, i.e. +2% increase per year [1.01-1.03] [Table 2]).
Table 2. Adjusted Incidence Rate Ratios (IRRs) of melanoma*.
| Variables | IRR | (95% CI) | p-value† | % change from IRR in complete case analysis‡ | ||
|---|---|---|---|---|---|---|
| Sex (ref Female) | ||||||
| Male | 1.12 | (1.07, 1.18) | <0.0001 | 6.8 | ||
| Age-group (ref 65-59) | ||||||
| 15-49 | 0.22 | (0.20, 0.24) | <0.0001 | 0.0 | ||
| 50-54 | 0.56 | (0.50, 0.62) | <0.0001 | -0.4 | ||
| 55-59 | 0.67 | (0.60, 0.74) | <0.0001 | 0.4 | ||
| 60-64 | 0.82 | (0.74, 0.91) | 0.0001 | 2.5 | ||
| 65-69 | - | - | - | - | ||
| 70-74 | 1.18 | (1.07, 1.31) | 0.0013 | -2.3 | ||
| 75-79 | 1.36 | (1.23, 1.51) | <0.001 | 11.1 | ||
| 80-84 | 1.45 | (1.30, 1.62) | <0.001 | 7.3 | ||
| 85+ | 1.55 | (1.39, 1.74) | <0.001 | 42.3 | ||
| Deprivation (ref least deprived) | ||||||
| 2 | 0.94 | (0.88, 1.00) | 0.064 | -21.3 | ||
| 3 | 0.84 | (0.78, 0.90) | <0.0001 | 4.3 | ||
| 4 | 0.70 | (0.65, 0.76) | <0.0001 | -0.9 | ||
| Most deprived | 0.53 | (0.48, 0.59) | <0.0001 | -0.7 | ||
| Stage at diagnosis (ref 1) | ||||||
| 2 | 0.44 | (0.39, 0.50) | <0.0001 | 1.0 | ||
| 3 & 4 | 0.30 | (0.26, 0.35) | <0.0001 | -3.6 | ||
| Year of diagnosis (by stage at diagnosis)‖ | Increase in incidence per year§ (%) | Increase in incidence across the study period 1996-2015§§ (%) | ||||
| Stage I | 1.05 | (1.05, 1.06) | <0.0001 | 1.2 | 5 | 153 |
| Stage II | 1.03 | (1.02, 1.04) | <0.0001 | -8.2 | 3 | 75 |
| Stage III/IV | 1.02 | (1.01, 1.03) | <0.0001 | 5.4 | 2 | 46 |
CI = Confidence Interval; IRR = Incidence Rate Ratio; ref = reference category
Pseudo-R2 of Model 1 = 17%, AIC = 16,094
Estimated from negative binomial model including main effect variables for year, stage, sex, age group, deprivation, and interaction stage*year. Exact model form provided as ‘Model 1’ in Supplementary Box S1. For 493 patients with missing values on stage (5% of entire dataset), these values were imputed as described under ‘Multiple imputation’.
Null hypothesis: IRR = 1. Presented up to 4 decimal points and up to two significant figures.
On the log scale (i.e. % change in beta coefficients). Median change in model coefficients: 2.1%, inter-quartile range: -0.9% to 8.3%.
Derived as (IRR-1) ×100
Derived as IRR = exp(β5 year + β6k[stagek × year]) – see Supplementary Box S1, Model 1.
Stage-specific incidence trends, by sex-age subgroups
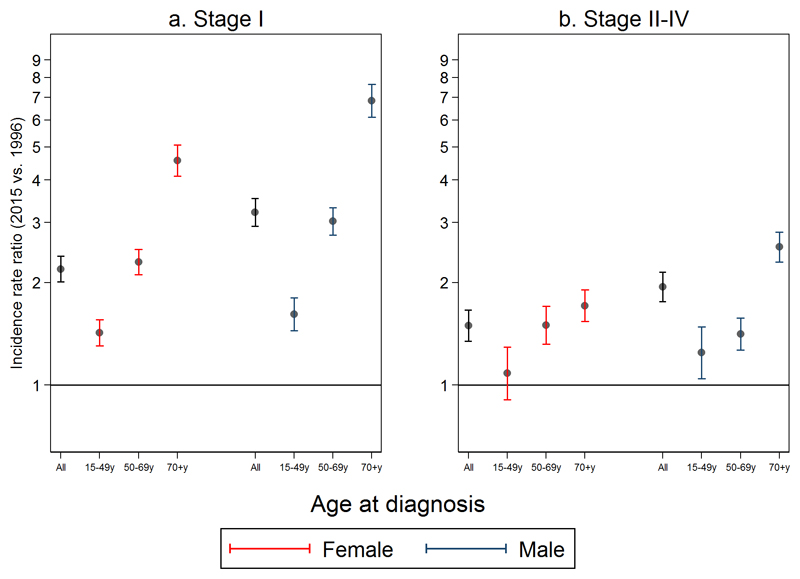
There was increasing incidence of stage I disease for all age-sex strata, with adjusted annual IRR estimates significantly greater than 1 for all groups (lower bounds of 95% CIs for IRRs ranged from 1.01 to 1.10 [Figure 2; Table 3]). Larger increases in the incidence of stage 1 disease over time were seen with increasing age (e.g. adjusted annual IRR for females, 70+ year olds: 1.08 [95%CI 1.04-1.05], females overall: 1.04 [1.04-1.05]. Within each age group, IRRs were larger for men than women.
Figure 2.
Estimated incidence rate ratios (IRRs) of a) Stage 1 and b) Stages II-IV melanoma for 2015 (vs. 1996), according to sex and age-group
Displayed incidence rate ratios for stage-specific disease per year estimated from Model 2, to the power 19 (model form provided in Supplementary Box S1). For 493 patients with missing values on stage, these values were imputed as described under ‘Multiple imputation’.
Table 3. Adjusted incidence rate ratios (IRRs) of melanoma (both annual and across the entire period 1996-2015) for different patient subgroups*.
| Stage 1 | Stages 2-4 | ||||||
|---|---|---|---|---|---|---|---|
| Sex | Age | Adjusted annual IRR (95% CI) | p-value † | Adjusted IRR for entire period ‡ (95% CI) | Adjusted annual IRR (95% CI) ‡ | p-value ‖ | Adjusted IRR for entire period ‡ (95% CI) |
| Women | 15-49 | 1.02 (1.01, 1.02) | <0.0001 | 1.42 (1.30, 1.55) | 1.00 (0.99, 1.01) | 0.38 | 1.08 (0.91, 1.29) |
| 50-69 | 1.04 (1.04, 1.05) | <0.0001 | 2.30 (2.11, 2.50) | 1.02 (1.01, 1.03) | <0.0001 | 1.50 (1.32, 1.70) | |
| 70+ | 1.08 (1.08, 1.09) | <0.0001 | 4.57 (4.11, 5.07) | 1.03 (1.02, 1.03) | <0.0001 | 1.71 (1.54, 1.90) | |
| Overall | 1.04 (1.04, 1.05) | <0.0001 | 2.19 (2.01, 2.39) | 1.02 (1.02, 1.03) | <0.0001 | 1.50 (1.35, 1.66) | |
| Men | 15-49 | 1.03 (1.02, 1.03) | <0.0001 | 1.62 (1.45, 1.81) | 1.01 (1.00, 1.02) | 0.014 | 1.24 (1.04, 1.48) |
| 50-69 | 1.06 (1.05, 1.07) | <0.0001 | 3.02 (2.76, 3.32) | 1.02 (1.01, 1.02) | <0.0001 | 1.41 (1.27, 1.58) | |
| 70+ | 1.11 (1.10, 1.11) | <0.0001 | 6.84 (6.12, 7.64) | 1.05 (1.04, 1.06) | <0.0001 | 2.54 (2.30, 2.82) | |
| Overall | 1.06 (1.06, 1.07) | <0.0001 | 3.21 (2.93, 3.53) | 1.04 (1.03, 1.04) | <0.0001 | 1.94 (1.76, 2.15) | |
CI = Confidence Interval; IRR = Incidence Rate Ratio;
Sex-age-stage-specific IRRs were estimated from the updated negative binomial model, which included main effects year, stage, sex, age group, deprivation, and four-way interaction sex*age group*stage*year. Exact model form provided as ‘Model 2’in Supplementary Box S1). Overall IRRs were estimated according to Model 1 (form provided in Supplementary Box S1). For 493 patients with missing values on stage (5% of the entire sample), these values were imputed as described under ‘Multiple imputation’.
Null hypothesis: IRR = 1. Presented up to 4 decimal points and up to two significant figures.
Calculated as adjusted annual IRR to the power 19.
Note that in 15-49 year old men the 95% CI of the adjusted annual IRR for stages 2-4 contains 1.00 but the p-value is 0.014, due to rounding to two decimal places
Considering the respective adjusted annual IRRs for stage II-IV melanoma, increasing temporal trends in incidence were observed both in older women (e.g. IRR for 70+ year olds: 1.03 [95% CI: 1.02-1.03]) and, more so, in older men (e.g. 70+ year olds: 1.05 [95% CIs: 1.04 to 1.06]) (Figure 2 and Table 3). In younger people (aged 15-49) the increase in the incidence of stage II-IV melanoma was minimal, and not significant for women (adjusted annual IRR for females: 1.00 [95%CI 0.99-1.01], p = 0.38; males: 1.01 [1.00-1.02], p = 0.01). Increases in rates of stage II-IV disease were greater in older age, but this gradient was not as steep as that for stage I disease.
Sex-age-stage-specific IRRs estimates from Model 2 are provided in Supplementary Table S2. The complete-case sensitivity analyses produced similar results to those obtained in the main analyses, where missing stage was imputed (see also Table 2 and Supplementary Figure S1).
Discussion
Summary of main findings
The age-standardised incidence of melanoma increased substantially in the East Anglia region during 1996–2015. This increase predominantly reflected increasing incidence of stage I cases, while the incidence of stage II-IV disease increased less steeply. In women aged under 50, though there was an increased incidence of stage I cases, this was not accompanied by an increase in the incidence of late stage disease, For either sex, the increase in incidence was steeper in the oldest patients (70+ year olds); and across age groups, increases in incidence were steeper in men than women.
Strengths and limitations
The principal strengths of this analysis are the use of population-based data covering an extensive period, using high quality and highly complete information on stage at diagnosis. In order to account for potential biases in estimates arising from missing stage data, we used multiple imputation. Nonetheless, as shown in sensitivity analyses, most estimates obtained from complete case analysis were similar to those obtained using multiply imputed data.
The main limitation is that we cannot confidently infer the causes of the observed stage-specific incidence trends. Further analyses that consider changes in case-mix over time of other disease factors, for example, in tumour sub-site (trunk, limbs, face, etc.) or diagnostic route (e.g. identification through healthcare encounters triggered by unrelated reasons), or in the rate of investigations for suspected melanoma in the general population, could help elucidate potential mechanisms. We examined data from the former East Anglia cancer registry, a geographically-defined population of South England whose residents are relatively older and more affluent, and more likely to be of White ethnic origin, compared with the rest of the English population.(27) These considerations might limit the generalizability of the findings, though not their internal validity. Analyses of England-wide data for the same 20-year era (to overcome these generalisability limitations) are not possible for our study period, due to historically poor nationwide completeness of stage information for melanoma, until recent years. We examined the stratification of stage-specific incidence by sex and age group, but larger studies could additionally enable stratification by socio-economic status.
Comparisons with the literature
Our study indicates a continuation (to 2015) of previously reported trends in our population (1991-2004).(13) The observed steeper increase in stage I incidence mirrors upwards incidence rates of thin melanoma tumours (< 1.5 mm), and incidence rates of melanoma in certain sub-sites (e.g. trunk, head and neck) in studies covering similar time-periods in Denmark,(28, 29) Finland,(30) Scotland,(31) and Northern England.(32)
Implications for policy, practice, and research
There are two main implications arising from the findings. First, the observed trends are compatible with a hypothesis of potential overdiagnosis of indolent cases, across age groups. However, it would be wrong to assign all of the increase in the incidence of melanoma to overdiagnosis. Had this been the case, no increase in advanced stage at diagnosis would have been observed in men (all age groups) and women (aged 50 and over). Secondly, the observed trends are also compatible with a hypothesis of increasing rates of genuinely consequential illness for older patients, particularly older men. Such a hypothesis is supported by evidence from South-Eastern Europe, where increasing incidence of melanoma has been accompanied by increasing mortality for men and women aged 50 and over,(33) and the US, where increasing incidence was not accompanied by any decrease in tumour thickness for stage III and IV patients.(34)
Quantifying the extent of overdiagnosis or increasing rates of consequential disease in a population is challenging. Nevertheless, our data suggest that both appear to be plausible, partial, explanations. These realisations emphasise the importance of shared decision making about investigating skin lesions, and increased communication of issues around overdiagnosis in conversations with patients about the management of those diagnosed with stage I disease.(35)
The 1-5% annual increase in advanced stage melanoma (II-IV) in women aged 50 and over and men in all age groups (Figure 2, Table 3), indicates a genuine increase in later stage melanoma, which accounts for a 50% increase in incidence in older women and a 94% increase in older men over the 19-year study period (Table 3). This may reflect different risk behaviour regarding sun exposure, or different biological behaviour of melanoma in different age groups. The observation of different patterns observed in the incidence of stage II-IV melanoma women in the under 50s and those aged 50 and over warrants further investigation.
The increase in melanoma incidence puts pressure on limited health service budgets, particularly regarding the use of novel, effective but expensive biological treatments for late stage disease, which have become available in recent years, offering life-extending management options. Our findings indicate an ongoing need for public health education campaigns, to increase awareness of melanoma risk factors and symptoms and achieve earlier diagnosis, particularly in the elderly, and in men, who experienced the steepest increases in stage II-IV disease.
Conclusions
The findings suggest both a degree of overdiagnosis and a genuine increase in the incidence of consequential illness are likely to be responsible for the observed increasing incidence trends in our population during the study period. They also may warrant targeting of men and older people in future public health awareness campaigns.
Supplementary Material
List of Supplemental Digital Content
A single Supplemental Digital Content (SDC) file is included, comprising a box, two supplementary tables and a supplementary figure.
Acknowledgements
GL and AH are supported by a Cancer Research UK Advanced Clinician Scientist Fellowship Award to GL (C18081/A18180). GL is an associate director (co-investigator) of the multi-institutional CanTest Research Collaborative funded by a Cancer Research UK Population Research Catalyst award (C8640/A23385). This project involves data derived from patient-level information collected by the NHS, as part of the care and support of cancer patients. The data are collated, maintained and quality assured by the National Cancer Registration and Analysis Service, which is part of Public Health England.
Funding sources: Cancer Research UK Advanced Clinician Scientist Fellowship award (No. C18081/A18180) to GL.
Footnotes
Conflicts of interest: None declared
References
- 1.de Vries E, Bray FI, Eggermont AM, Coebergh JW, European Network of Cancer R Monitoring stage-specific trends in melanoma incidence across Europe reveals the need for more complete information on diagnostic characteristics. Eur J Cancer Prev. 2004;13(5):387–95. doi: 10.1097/00008469-200410000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Garbe C, Leiter U. Melanoma epidemiology and trends. Clin Dermatol. 2009;27(1):3–9. doi: 10.1016/j.clindermatol.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Research UK. Skin cancer incidence statistics. 2016 [Google Scholar]
- 4.Whiteman DC, Green AC, Olsen CM. The Growing Burden of Invasive Melanoma: Projections of Incidence Rates and Numbers of New Cases in Six Susceptible Populations through 2031. J Invest Dermatol. 2016;136(6):1161–71. doi: 10.1016/j.jid.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 5.Parkin DM, Mesher D, Sasieni P. 13. Cancers attributable to solar (ultraviolet) radiation exposure in the UK in 2010. Br J Cancer. 2011;105(Suppl 2):S66–9. doi: 10.1038/bjc.2011.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605–13. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 7.Marcus PM, Prorok PC, Miller AB, DeVoto EJ, Kramer BS. Conceptualizing overdiagnosis in cancer screening. J Natl Cancer Inst. 2015;107(4) doi: 10.1093/jnci/djv014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leiter U, Garbe C. Epidemiology of melanoma and nonmelanoma skin cancer--the role of sunlight. Adv Exp Med Biol. 2008;624:89–103. doi: 10.1007/978-0-387-77574-6_8. [DOI] [PubMed] [Google Scholar]
- 9.Wallingford SC, Alston RD, Birch JM, Green AC. Regional melanoma incidence in England, 1996-2006: reversal of north-south latitude trends among the young female population. Br J Dermatol. 2013;169(4):880–8. doi: 10.1111/bjd.12460. [DOI] [PubMed] [Google Scholar]
- 10.Weyers W. The 'epidemic' of melanoma between under- and overdiagnosis. J Cutan Pathol. 2012;39(1):9–16. doi: 10.1111/j.1600-0560.2011.01831.x. [DOI] [PubMed] [Google Scholar]
- 11.Glusac EJ. The melanoma 'epidemic': lessons from prostate cancer. J Cutan Pathol. 2012;39(1):17–20. doi: 10.1111/j.1600-0560.2011.01848.x. [DOI] [PubMed] [Google Scholar]
- 12.De Giorgi V, Gori A, Grazzini M, Rossari S, Oranges T, Longo AS, et al. Epidemiology of melanoma: is it still epidemic? What is the role of the sun, sunbeds, Vit D, betablocks, and others? Dermatol Ther. 2012;25(5):392–6. doi: 10.1111/j.1529-8019.2012.01483.x. [DOI] [PubMed] [Google Scholar]
- 13.Levell NJ, Beattie CC, Shuster S, Greenberg DC. Melanoma epidemic: a midsummer night's dream? Br J Dermatol. 2009;161(3):630–4. doi: 10.1111/j.1365-2133.2009.09299.x. [DOI] [PubMed] [Google Scholar]
- 14.Welch HG, Woloshin S, Schwartz LM. Skin biopsy rates and incidence of melanoma: population based ecological study. BMJ. 2005;331(7515):481. doi: 10.1136/bmj.38516.649537.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NHS Digital. Indicator Portal: Record of Stage of Cancer at Diagnosis. 2015 [Google Scholar]
- 16.Rowe CJ, Law MH, Palmer JM, MacGregor S, Hayward NK, Khosrotehrani K. Survival outcomes in patients with multiple primary melanomas. J Eur Acad Dermatol Venereol. 2015;29(11):2120–7. doi: 10.1111/jdv.13144. [DOI] [PubMed] [Google Scholar]
- 17.Hwa C, Price LS, Belitskaya-Levy I, Ma MW, Shapiro RL, Berman RS, et al. Single versus multiple primary melanomas: old questions and new answers. Cancer. 2012;118(17):4184–92. doi: 10.1002/cncr.27407. [DOI] [PubMed] [Google Scholar]
- 18.Hermanek P, Sobin LH. In: TNM classification of malignant tumours. 4th fully rev. ed. Hermanek P, Sobin LH, editors. Berlin ; London: Springer; 1987. [Google Scholar]
- 19.Office for National Statistics. Lower super output area mid-year population estimates. 2015 [Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/lowersuperoutputareamidyearpopulationestimates.
- 20.Howlader N, Noone AM, Yu MD, Cronin KA. Use of Imputed Population-based Cancer Registry Data as a Method of Accounting for Missing Information: Application to Estrogen Receptor Status for Breast Cancer. Am J Epidemiol. 2012;176(4):347–56. doi: 10.1093/aje/kwr512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falcaro M, Carpenter JR. Correcting bias due to missing stage data in the non-parametric estimation of stage-specific net survival for colorectal cancer using multiple imputation. Cancer Epidemiol. 2017;48:16–21. doi: 10.1016/j.canep.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Pace M, Lanzieri G, Glickman M, Grande E, Zupanic T, Wojtyniak B, et al. Revision of the European Standard Population: Report of Eurostat’s task force. 2013 [Google Scholar]
- 23.Cameron AC, Trivedi PK. Regression analysis of count data. Cambridge: Cambridge University Press; 1998. [Google Scholar]
- 24.von Hippel PT. Regression with missing Y's: an improved strategy for analyzing multiply imputed data. 2007 [Google Scholar]
- 25.White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med. 2009;28(15):1982–98. doi: 10.1002/sim.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubin DB. Multiple imputation for nonresponse in surveys. New York ; Chichester: Wiley; 1987. [Google Scholar]
- 27.Office for National Statistics. Population Estimates for UK, England and Wales, Scotland and Northern Ireland, Mid-2011 and Mid-2012. 2013 [Google Scholar]
- 28.Fuglede NB, Brinck-Claussen UO, Deltour I, Boesen EH, Dalton SO, Johansen C. Incidence of cutaneous malignant melanoma in Denmark, 1978-2007. Br J Dermatol. 2011;165(2):349–53. doi: 10.1111/j.1365-2133.2011.10361.x. [DOI] [PubMed] [Google Scholar]
- 29.Bay C, Kejs AM, Storm HH, Engholm G. Incidence and survival in patients with cutaneous melanoma by morphology, anatomical site and TNM stage: a Danish Population-based Register Study 1989-2011. Cancer Epidemiol. 2015;39(1):1–7. doi: 10.1016/j.canep.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Stang A, Pukkala E, Sankila R, Soderman B, Hakulinen T. Time trend analysis of the skin melanoma incidence of Finland from 1953 through 2003 including 16,414 cases. Int J Cancer. 2006;119(2):380–4. doi: 10.1002/ijc.21836. [DOI] [PubMed] [Google Scholar]
- 31.MacKie RM, Bray C, Vestey J, Doherty V, Evans A, Thomson D, et al. Melanoma incidence and mortality in Scotland 1979-2003. Brit J Cancer. 2007;96(11):1772–7. doi: 10.1038/sj.bjc.6603801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Downing A, Newton-Bishop JA, Forman D. Recent trends in cutaneous malignant melanoma in the Yorkshire region of England; incidence, mortality and survival in relation to stage of disease, 1993-2003. Br J Cancer. 2006;95(1):91–5. doi: 10.1038/sj.bjc.6603216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbaric J, Sekerija M, Agius D, Coza D, Dimitrova N, Demetriou A, et al. Disparities in melanoma incidence and mortality in South-Eastern Europe: Increasing incidence and divergent mortality patterns. Is progress around the corner? Eur J Cancer. 2016;55:47–55. doi: 10.1016/j.ejca.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 34.Shaikh WR, Dusza SW, Weinstock MA, Oliveria SA, Geller AC, Halpern AC. Melanoma Thickness and Survival Trends in the United States, 1989 to 2009. J Natl Cancer Inst. 2016;108(1) doi: 10.1093/jnci/djv294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghanouni A, Renzi C, McBride E, Waller J. Comparing perceived clarity of information on overdiagnosis used for breast and prostate cancer screening in England: an experimental survey. BMJ Open. 2017;7(8):e015955. doi: 10.1136/bmjopen-2017-015955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.