Abstract
Dendrimers are versatile macromolecules with tremendous potential as magnetic resonance imaging (MRI) contrast agents. Dendrimer-based agents provide distinct advantages over low-molecular-weight gadolinium chelates, including enhanced r1 relaxivity due to slow rotational dynamics, tunable pharmacokinetics that can be adapted for blood pool, liver, kidney, and lymphatic imaging, the ability to be a drug carrier, and flexibility for labeling due to their inherent multivalency. Clinical applications are increasingly being developed, particularly in lymphatic imaging. Herein we present a broad overview of dendrimer-based MRI contrast agents with attention to the unique chemistry and physical properties as well as emerging clinical applications.
INTRODUCTION
Dendrimers represent a family of versatile nanoparticles with a useful role as macromolecular magnetic resonance imaging (MRI) contrast agents as well as optical and nuclear medicine agents. Dendrimer-based contrast agents provide numerous advantages over low-molecular-weight gadolinium chelates for MRI, including enhanced r1 relaxivity1,2 due to slow rotational dynamics, tunable pharmacokinetics that can be adapted for blood pool,3,4 liver,5,6 kidney,7,8 and lymphatic imaging,9,10 the ability to be a drug carrier, and flexibility for labeling due to their inherent multivalency.11,12 Herein, we discuss the structural, chemical, and pharmacokinetic characteristics related their use as contrast agents for magnetic resonance imaging as well as review recent advances in dendrimer MR imaging applications.
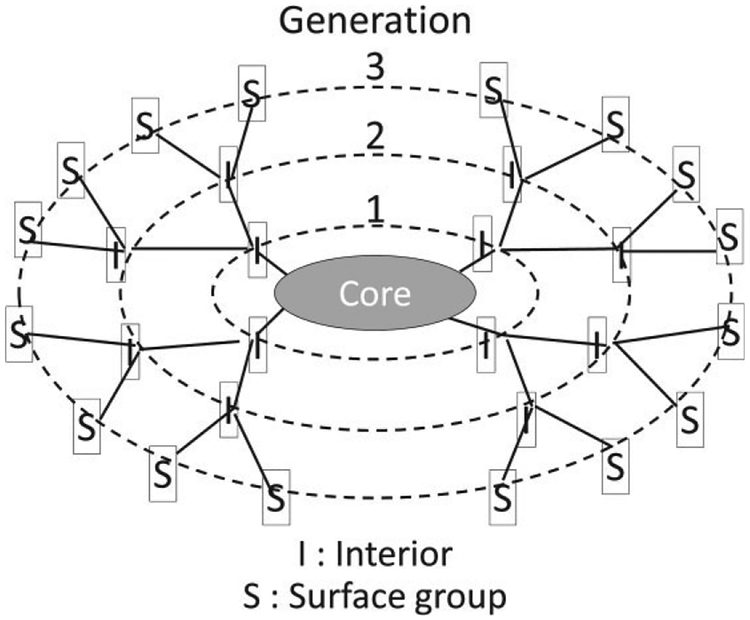
Dendrimers are unique nanoparticles characterized by branching polymerization resulting in a multivalent, three dimensional tree-like structure. Dendrimers’ precise nanoscale scaffolding, nanocontainer properties, and tunable variation in size, surface chemistry, and interior void space offer tremendous potential as drug-delivery molecules but, when properly labeled < can also act as molecular imaging biomarkers.13 The word ‘dendrimer’ originates from the Greek word, dendron, meaning tree and refers to the basic structure of the molecule: a central molecule from which numerous polymerized branches extend. Dendrimers can be divided into three main components: the core, the interior, and the surface shell (Figure 1). The interior core is surrounded concentrically by annular shells (or branches), which create an interior void space. The stepwise addition of these layers or shells to a given core creates monophasic nanoparticles that differ only by size with each successive layer referred to as a ‘generation’ (i.e., G1, G2, G3). The branches terminate peripherally to provide numerous functional groups comprising the surface of the dendrimer14 These properties have led to considerable interest in the development of dendrimers as contrast agents for MR imaging.
FIGURE 1 |.
Schema of the dendrimer structure.
Dendrimers are composed of combinations of core types, including ethylene diamine (EDA), diaminobutyl (DAB), polyamidoamine (PAMAM), and polypropylimine (PPI). Cores used as imaging agents, such as PAMAM or PPI dendrimers, are highly soluble in aqueous solution and generally have a primary amine rich surface that is uncharged at pH greater than 9. The abundance of primary amine functional groups on the exterior shell provides attachment sites for conjugating chelates that bind gadolinium to act as a signaling beacon or for conjugating targeting moieties such as antibodies for site-specific targeting. The dual-functioning surface of DAB/PPI and PAMAM dendrimers with simultaneous targeting and chelating capability has propelled the use of dendrimers as contrast agents for molecular imaging. Recently, newer families of dendrimers with cystamine (rather than amide) functional group core component have been developed. Cystamine residues enable maleimide and sulfohydryl coupling reactions, for the conjugation of peptides, monoclonal antibodies, biotin, or other targeting moieties.
RELAXIVITY
Compared to low-molecular-weight agents, macro-molecular agents, such as dendrimers, demonstrate increased relaxivity as a result of slower particle tumbling rates in solution,2,15 which reduces the injected dose of gadolinium ions required to achieve proton relaxation. For this reason, the molar relaxivity of conventional MR agents requires the use of large doses (0.1 mM/kg) of gadolinium ions compared to macromolecule based solutions.16,17 Approaches that use lower doses of gadolinium are of interest due to the concern for gadolinium-associated nephrogenic systemic fibrosis which occurs in patients with compromised renal function.
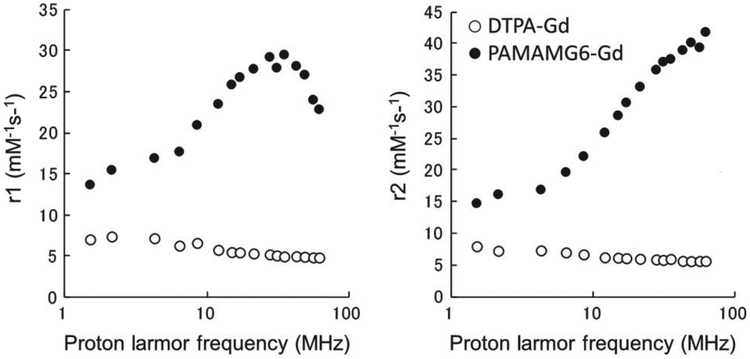
The enhancement of molar relaxivity relates to the strength of the magnetostatic field. The relaxivity of Gd-chelated PAMAM dendrimers and lysine-dendri-grafts varies greatly depending on the magnetic field strength. In magnetic fields ranging from 0.035 to 1.5 T (1.5–62 MHz)1,2 the r1 (1/T1) values increased along with frequency and peaked at around 1 T (35 MHz), then decreased dramatically at higher magnetic fields. The low-molecular-weight contrast agent Gd-BzDTPA exhibited almost constant r1 values in the same range of magnetic fields (Figure 2). Recently, MRI with higher magnetic field (3 T and higher) have become more common both for animal imaging and for human imaging. In such a high magnetic field, the enhancement of molar relaxivity of macromolecular agents compared to low molecular contrast agents was minimal. The relaxation effect is also related to the number of chelated Gd ions. Macromolecular MRI agents are attractive because they have numerous attachment sites for Gd-chelates and have higher T1 relaxivity per molecule compared with conventional small molecular weight contrast agents. Gd(III) chelates must be able to interact with the protons of the water molecules in the microenvironment to affect T1. The numerous attachment sites available on the dendrimer surface offer multiple (in some cases hundreds) of Gd(III) conjugation sites that are exposed to the external environment. Although there are two major classes of MR chelates, DTPA, and DOTA, utilized to chelate Gd(III), differences in r1 relaxivity are negligible (4.7 mM−1 s−1 at 20 MHz and 25°C).18–21 The physical and pharmocokinetic properties of dendrimers conjugated with Gd(III) DOTA and Gd(III)-1B4M-DTPA are also comparable.
FIGURE 2 |.
Relaxivity profiles of dendrimer-based MRI contrast agents compared with a small gadolinium chelate.
We compared the relaxivity of Gd-chelated lysine-dendri-grafts with PAMAM dendrimers.1 Gd-chelates are located on the surface of PAMAM dendrimer contrast agents, and they constantly interact with water. In contrast, lysine-dendri-grafts have many interior Gd-chelates. The r1 of the lysine-dendri-graft based agents was lower than that of PAMAM dendrimers with similar size especially at low temperature. This is likely because the more internally located chelated Gd ions are not as fully accessible by water. An increase in temperature results in a decrease in r1 in PAMAM dendrimers due to the decrease in the rotational correlation time, however, lysine-dendri-grafts showed higher r1 as the temperature increases. The increase in relaxivity with increasing temperature may be attributed to an increase in the accessibility of water to internal Gd-chelates in lysine-dendri-grafts. This and other research suggest the flexibility of the macromolecule can also affect its relaxivity.22,23
KINETICS
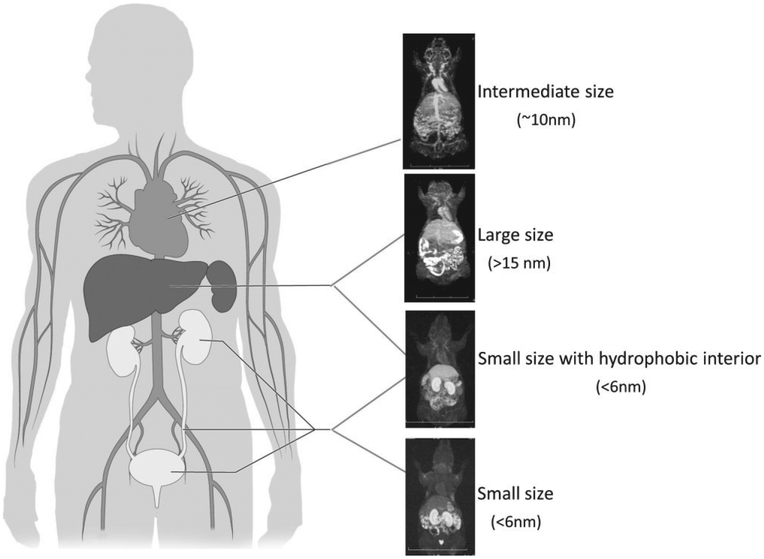
Dendrimer-based MR contrast agents possess tunable kinetic characteristics in the body after either systemic intravenous injection or locally via interstitial injection. Systemic applications were demonstrated by Wiener et al. with in vivo studies using dendrimer-based macromolecules as blood pool contrast agents for MR angiography.24 Later research in dendrimer-based MR contrast agents has revealed that modification of dendrimer properties such as size, cores and interiors, and exterior shell chemistry permit application-specific customization (Figure 3).7,15,19,25,26 Among the tunable properties, size has been shown to have a dominant effect on in vivo behavior, even when comparing molecules with similar chemical properties.7,19 Small changes in diameter of dendrimer-based MR contrast agents can greatly impact their pharmacokinetics, permeability across the vascular barrier, excretion rate and route, and recognition by the reticuloendothelial system.15 In vivo studies have demonstrated that, MR imaging with smaller generation agents (<5 nm diameter) demonstrates leakage of the agent from the vascular space similar to that observed with traditional Gd(III) chelates, but at a slightly slower rate. These smaller generations have potential in the assessment of renal function where the sieving function of the glomerulus can be studied in proteinuria and related disorders.8,19,25,27,28 Intermediate generation dendrimers (G4 or G7) between 5 and 8 nm in diameter leak through hyperpermeable tumor vasculature but are retained by normal vessels and therefore may serve as ideal tumor imaging agents.29 Larger generation dendrimers (G9 and G10), greater than 13 nm in diameter, provide enhancement of the reticuloendothelial system of the liver and spleen. These and larger generation dendrimers demonstrate blood pool retention within both normal and tumor vessels and are therefore excellent candidates for vascular imaging contrast agents.3,4,6
FIGURE 3 |.
Typical pharmacokinetics of represented dendrimer-based magnetic resonance imaging (MRI) contrast agents with various characteristics.
In addition to size, the chemical characteristics of dendrimers impact in vivo behavior. Among these characteristics, particle hydrophilicity greatly impacts agent pharmacokinetics. Increasing the hydrophilicity of G4-PAMAM dendrimers by PEGylation decreases the renal excretion, increasing particle half-life in the circulation and thus, improving imaging of the vasculature.30 Studies have shown that PEGylated dendrimers have minimal liver and kidney uptake and remained in circulation for at least 1 h.31 Interestingly, PEGylation also influences relaxivity. PAMAM dendrimers bearing gadolinium (Gd)-chelates with long PEG chains (5 k) on smaller (G4) dendrimers results in reduced relaxivity compared to non-PEGylated dendrimers; whereas short PEG chains (2 k) on larger (G5) dendrimers produce relaxivities comparable to non-PEGylated G4 dendrimers.31 It has also been demonstrated that the relaxivity of PEGylated or lysine-conjugated dendrimers increases at higher temperature, whereas that of intact G4 Gd-PAMAM dendrimer decreases.31 Therefore, surface-PEGylated Gd-PAMAM dendrimers show decreased plasma clearance and prolonged retention in the blood pool and shorter PEG, higher generation conjugates exhibit higher relaxivity.31 Conversely, hydrophobic dendrimers, such as those with DAB cores preferentially accumulate in the liver compared to similar sized PAMAM agents, making them promising hepatic MR imaging agents.5
Regional imaging of lymphatics with local interstitial injection is a second type of imaging application with different kinetic considerations. Despite the important role that the lymphatics play in health and disease, it has been difficult to directly image them largely due to challenges with accessing the lymphatic system, which requires either direct cannulation of these small fragile vessels which is technically very challenging or interstitial injection.32 Existing lymphatic imaging techniques include lymphoscintigraphy, in which a 99mTc-labeled sulfur colloid or a similar macromolecule is injected interstitially and serial imaging is performed while the agent accumulates in the lymphatics.33 The approach has been used in sentinel node detection and in lymphedema studies, however, it is limited by low spatial resolution and may not provide visualization of individual lymphatic channels.33 MRI has been widely used for other types of vascular imaging, yet it has not been utilized for lymphatic imaging. The need for lymphatic imaging extends beyond sentinel lymph node detection and includes numerous other potential applications such as mapping lymphatic malformations/anomalies and traumatic lymphatic interruptions during operative procedures, such as transaction of the thoracic duct during cardiothoracic surgeries. Interestingly, higher generation dendrimer-based contrast agents are potentially useful lymphatic imaging agents because their size is ideally suited to accumulation in the lymphatics. Larger dendrimers (>8 nm) preferentially drain from the injection site into the local lymphatic capillaries and thence to the major lymphatic vessels and nodes. In general, lymphatic imaging is improved by increasing dendrimer generation.34 Increasing hydrophilicity and reducing surface charge also enhances draining from subcutaneous injections sites, however, surface charge and hydrophobicity promote retention.34
APPLICATIONS
The versatility of dendrimer nanoparticle behavior has led to the development of numerous dendrimer-based MR contrast agent applications based on the physical relaxivity and pharmacological kinetics. As mentioned above, macromolecular MR contrast agents are useful for vascular imaging, specifically microvascular imaging and determining functional capillary density in tumor tissues.19 Among the dendrimer-based agents, generation G7 and G8 agents permit visualization of the blood vessels both within and outside tumors most clearly and PEG conjugation further improves image quality as well as excretion profiles.19 Dendrimers with hydrophobic properties have proven useful in hepatic imaging. Utilizing the predisposition of slightly less hydrophilic macromolecules to accumulate in the liver, a G5 DAB/PPI dendrimer with a hydrophobic 64 surface amine core unit (DAB-Am64-(1B4M-Gd(III))64, DABG5, was shown to homogenously enhance liver parenchyma.19 Furthermore, DABG5 applied to dynamic micro-MRIs enabled serial visualization of growing metastatic colon cancer foci as small as 0.3-mm in diameter within the livers of living mice.19
The utility of dendrimer-based MR contrast agents has also been demonstrated in the functional assessment of organs as well. Although small dendrimer-based agents are quickly excreted from the kidneys, Kobayashi et al. found that certain agents are initially retained in the blood vessels in the kidney with minimal leakage into the extravascular tissue and that after glomerular filtration, they are concentrated in the proximal tubule, forming a high intensity band within the kidney.19 The absence or delayed formation of this high intensity band correlated well with renal tubular dysfunction related to acute tubular necrosis (ATN) in a mouse model and the enhancement pattern was predictive of acute and/or chronic renal failure (e.g., heavy metal exposure, ischemia, obstruction, and sepsis).26,28 Of the dendrimer-based MR contrast agents, it was shown that a G2 DAB agent was the most effective for evaluation of renal function.19
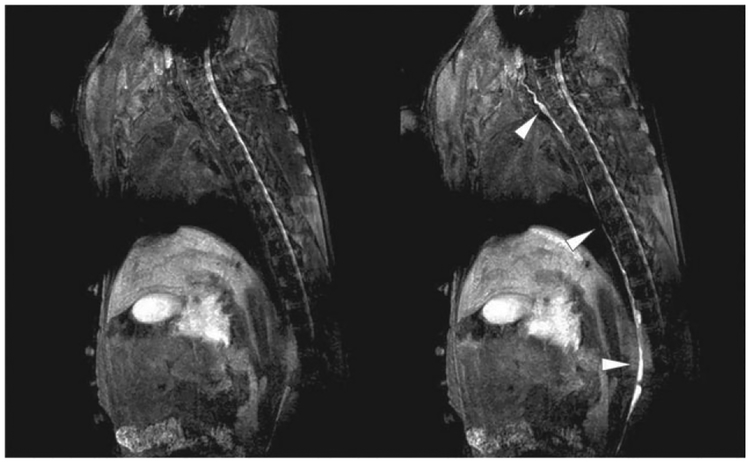
As previously discussed, there is a clinical need for reliable techniques for high resolution lymphatic imaging. Our group recently demonstrated the use of gadolinium-labeled dendrimers to image the lymphatics in small and large animal models during magnetic resonance lymphangiography. Herein, a polyamidoamine G6-Gd_1B4M_N-hydroxysuccinimide was synthesized and administered intradermally in the extremities of normal mice and pigs at several doses. The lymphatics were well demonstrated in both animal models and there was rapid uptake in the deep lymphatic system, including the thoracic duct. A significant dose reduction was achieved (1 μmol Gd/kg) in the 35-kg pig compared with mice, while still producing excellent results (Figure 4). No toxicity was observed and only minor inflammatory changes were observed at the injection site 30 days later. The agent provided excellent T1 relaxivity with threefold higher relaxivity than existing US FDA-approved low-molecular-weight Gdchelates at 3-T and even higher relaxivities at 1.5-T.32
FIGURE 4 |.
Lymphatic imaging application of a dendrimer-based magnetic resonance imaging (MRI) contrast agent in a pig. Thoracic ducts (arrowheads) are clearly visualized 10 min after injection of G6 dendrimer-based contrast agents (right panel) with a small dose (1 μmol Gd/kg) due to high relaxivity.
Recently, multimodal imaging using a dendrimer-based MRI contrast agent was used to visualize cancer cell migration to regional lymph nodes in a murine model.35 A sixth generation dendrimer-gadolinium chelate (Gd-G6) was used to simultaneously image the lymphatics and iron-oxide-labeled cancer cells.25,32,35,36 Gd-G6 is a positive contrast agent on T1w-FFE and b-FFE sequences whereas iron-oxide is a negative contrast agent, and as a result lymphatic basins were positively enhanced but cells were seen as defects. The larger size of the G6 dendrimer scaffold resulted in gains in R1 compared with small-molecule Gd chelates. Previously, cancer cells have been imaged with optical probes, including a combination of quantum dot-labeled cancer cells and fluorophore-conjugated dendrimers.10 This work demonstrated that MRI with a negative (iron-oxide) labeled cell and a positive (Gd-G6) labeled lymphatic system can directly image the formation of early micrometastastic deposits of cancer cells in lymph nodes in vivo.
SUMMARY/FUTURE PROSPECTIVE
Dendrimer-based contrast agents for MRI have tremendous potential due to the flexible nature of the particle and uniquely high relaxivity properties. Emerging clinical applications highlight the use of dendrimers particularly as lymphatic imaging agents. The high relaxivity of these agents minimizes the total dose needed without compromising image quality, thus reducing risks of toxicity. To date, no safety concerns have been raised regarding these particles and given the opportunity to reduce the dose of gadolinium, dendrimer-based agents may have improved safety profiles compared to low-molecular weight agents.37 In addition, to their role as effective imaging agents, dendrimers are potent drug-delivery platforms with numerous pre-clinical applications ranging from cancer therapeutics to antimicrobial drugs and analgesic drug delivery.37–40 Future exciting and emerging applications include combining breakthroughs in imaging with dendrimer-based therapeutics for so-called theranostic imaging.41–44 In summary, the unique physical and chemical properties make dendrimer-based contrast agents a powerful technology to advance imaging and medicine. We look forward to continued progress in research and clinical applications utilizing this important imaging technology.
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
This article is a U.S. Government work, and as such, is in the public domain in the United States of America.
Conflict of interest: The authors have declared no conflicts of interest for this article.
REFERENCES
- 1.Ogawa M, Regino CA, Marcelino B, Williams M, Kosaka N, Bryant LH, Choyke PL, Kobayashi H. New nanosized biocompatible MR contrast agents based on lysine-dendri-graft macromolecules. Bioconjug Chem 2010, 21:955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bryant LH Jr, Brechbiel MW, Wu C, Bulte JW, Herynek V, Frank JA. Synthesis and relaxometry of high-generation (G = 5, 7, 9, and 10) PAMAM dendrimer-DOTA-gadolinium chelates. J Magn Reson Imaging 1999, 9:348–352. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi H, Sato N, Hiraga A, Saga T, Nakamoto Y, Ueda H, Konishi J, Togashi K, Brechbiel MW. 3D-micro-MR angiography of mice using macro-molecular MR contrast agents with polyamidoamine dendrimer core with reference to their pharmacokinetic properties. Magn Reson Med 2001, 45: 454–460. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi H, Kawamoto S, Saga T, Sato N, Hiraga A, Konishi J, Togashi K, Brechbiel MW. Micro-MR angiography of normal and intratumoral vessels in mice using dedicated intravascular MR contrast agents with high generation of polyamidoamine dendrimer core: reference to pharmacokinetic properties of dendrimer-based MR contrast agents. J Magn Reson Imaging 2001, 14:705–713. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi H, Kawamoto S, Saga T, Sato N, Hiraga A, Ishimori T, Akita Y, Mamede MH, Konishi J, Togashi K, et al. Novel liver macromolecular MR contrast agent with a polypropylenimine diaminobutyl dendrimer core: comparison to the vascular MR contrast agent with the polyamidoamine dendrimer core. Magn Reson Med 2001, 46:795–802. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi H, Saga T, Kawamoto S, Sato N, Hiraga A, Ishimori T, Konishi J, Togashi K, Brechbiel MW. Dynamic micro-MRI of liver micrometastasis with a novel liver macromolecular MR contrast agent DAB-Am64-(1B4M-Gd)64. Acad Radiol 2002, 9(Suppl 2):S452–S454. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi H, Kawamoto S, Jo SK, Bryant HL Jr, Brechbiel MW, Star RA. Macromolecular MRI contrast agents with small dendrimers: pharmacokinetic differences between sizes and cores. Bioconjug Chem 2003, 14:388–394. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi H, Kawamoto S, Jo SK, Sato N, Saga T, Hiraga A, Konishi J, Hu S, Togashi K, Brechbiel MW, et al. Renal tubular damage detected by dynamic micro-MRI with a dendrimer-based magnetic resonance contrast agent. Kidney Int 2002, 61:1980–1985. [DOI] [PubMed] [Google Scholar]
- 9.Koyama Y, Talanov VS, Bernardo M, Hama Y, Regino CA, Brechbiel MW, Choyke PL, Kobayashi H. A dendrimer-based nanosized contrast agent dual-labeled for magnetic resonance and optical fluorescence imaging to localize the sentinel lymph node in mice. J Magn Reson Imaging 2007, 25:866–871. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi H, Ogawa M, Kosaka N, Choyke PL, Urano Y. Multicolor imaging of lymphatic function with two nanomaterials: quantum dot-labeled cancer cells and dendrimer-based optical agents. Nanomedicine (Lond) 2009, 4:411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi H, Koyama Y, Barrett T, Hama Y, Regino CA, Shin IS, Jang BS, Le N, Paik CH, Choyke PL, et al. Multimodal nanoprobes for radionuclide and five-color near-infrared optical lymphatic imaging. ACS Nano 2007, 1:258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi H, Longmire MR, Ogawa M, Choyke PL. Rational chemical design of the next generation of molecular imaging probes based on physics and biology: mixing modalities, colors and signals. Chem Soc Rev 2011, 40:4626–4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Svenson S, Tomalia DA. Dendrimers in biomedical applications--reflections on the field. Adv Drug Deliv Rev 2005, 57:2106–2129. [DOI] [PubMed] [Google Scholar]
- 14.Tomalia DA, Reyna LA, Svenson S. Dendrimers as multi-purpose nanodevices for oncology drug delivery and diagnostic imaging. Biochem Soc Trans 2007, 35(Pt 1):61–67. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi H, Brechbiel MW. Dendrimer-based nano-sized MRI contrast agents. Curr Pharm Biotechnol 2004, 5:539–549. [DOI] [PubMed] [Google Scholar]
- 16.Thomsen HS, Marckmann P, Logager VB. Nephrogenic systemic fibrosis (NSF): a late adverse reaction to some of the gadolinium based contrast agents. Cancer Imaging 2007, 7:130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prince MR, Zhang H, Morris M, MacGregor JL, Grossman ME, Silberzweig J, DeLapaz RL, Lee HJ, Magro CM, Valeri AM. Incidence of nephrogenic systemic fibrosis at two large medical centers. Radiology 2008, 248:807–816. [DOI] [PubMed] [Google Scholar]
- 18.Raymond KN, Pierre VC. Next generation, high relaxivity gadolinium MRI agents. Bioconjug Chem 2005, 16:3–8. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi H, Brechbiel MW. Nano-sized MRI contrast agents with dendrimer cores. Adv Drug Deliv Rev 2005, 57:2271–2286. [DOI] [PubMed] [Google Scholar]
- 20.Fu Y, Nitecki DE, Maltby D, Simon GH, Berejnoi K, Raatschen HJ, Yeh BM, Shames DM, Brasch RC. Dendritic iodinated contrast agents with PEG-cores for CT imaging: synthesis and preliminary characterization. Bioconjug Chem 2006, 17:1043–1056. [DOI] [PubMed] [Google Scholar]
- 21.Nwe K, Xu H, Regino CA, Bernardo M, Ileva L, Riffle L, Wong KJ, Brechbiel MW. A new approach in the preparation of dendrimer-based bifunctional diethylenetriaminepentaacetic acid MR contrast agent derivatives. Bioconjug Chem 2009, 20:1412–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicolle GM, Toth E, Schmitt-Willich H, Raduchel B, Merbach AE. The impact of rigidity and water exchange on the relaxivity of a dendritic MRI contrast agent. Chemistry 2002, 8:1040–1048. [DOI] [PubMed] [Google Scholar]
- 23.Laus S, Sour A, Ruloff R, Toth E, Merbach AE. Rotational dynamics account for pH-dependent relaxivities of PAMAM dendrimeric, Gd-based potential MRI contrast agents. Chemistry 2005, 11:3064–3076. [DOI] [PubMed] [Google Scholar]
- 24.Wiener EC, Brechbiel MW, Brothers H, Magin RL, Gansow OA, Tomalia DA. Dendrimer-based metal chelates: a new class of magnetic resonance imaging contrast agents. Magn Reson Med 1994, 31:1–8. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi H, Jo SK, Kawamoto S, Yasuda H, Hu X, Knopp MV, Brechbiel MW, Choyke PL, Star RA. Polyamine dendrimer-based MRI contrast agents for functional kidney imaging to diagnose acute renal failure. J Magn Reson Imaging 2004, 20:512–518. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi H, Kawamoto S, Brechbiel MW, Jo SK, Hu X, Yang T, Diwan BA, Waldmann TA, Schnermann J, Choyke PL, et al. Micro-MRI methods to detect renal cysts in mice. Kidney Int 2004, 65:1511–1516. [DOI] [PubMed] [Google Scholar]
- 27.Dear JW, Kobayashi H, Brechbiel MW, Star RA. Imaging acute renal failure with polyamine dendrimer-based MRI contrast agents. Nephron Clin Pract 2006, 103:c45–c49. [DOI] [PubMed] [Google Scholar]
- 28.Dear JW, Kobayashi H, Jo SK, Holly MK, Hu X, Yuen PS, Brechbiel MW, Star RA. Dendrimer-enhanced MRI as a diagnostic and prognostic biomarker of sepsis-induced acute renal failure in aged mice. Kidney Int 2005, 67:2159–2167. [DOI] [PubMed] [Google Scholar]
- 29.Barrett T, Ravizzini G, Choyke PL, Kobayashi H. Dendrimers in medical nanotechnology. IEEE Eng Med Biol Mag 2009, 28:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi H, Kawamoto S, Saga T, Sato N, Hiraga A, Ishimori T, Konishi J, Togashi K, Brechbiel MW. Positive effects of polyethylene glycol conjugation to generation-4 polyamidoamine dendrimers as macromolecular MR contrast agents. Magn Reson Med 2001, 46:781–788. [DOI] [PubMed] [Google Scholar]
- 31.Kojima C, Turkbey B, Ogawa M, Bernardo M, Regino CA, Bryant LH Jr., Choyke PL, Kono K, Kobayashi H. Dendrimer-based MRI contrast agents: the effects of PEGylation on relaxivity and pharmacokinetics. Nanomed Nanotechnol Biol Med 2011, 7:1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sena LM, Fishman SJ, Jenkins KJ, Xu H, Brechbiel MW, Regino CA, Kosaka N, Bernardo M, Choyke PL, Kobayashi H. Magnetic resonance lymphangiography with a nano-sized gadolinium-labeled dendrimer in small and large animal models. Nanomedicine (Lond) 2010, 5:1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barrett T, Choyke PL, Kobayashi H. Imaging of the lymphatic system: new horizons. Contrast Media Mol Imaging 2006, 1:230–245. [DOI] [PubMed] [Google Scholar]
- 34.Kaminskas LM, Porter CJ. Targeting the lymphatics using dendritic polymers (dendrimers). Adv Drug Deliv Rev 2011, 63(10–11):890–900. [DOI] [PubMed] [Google Scholar]
- 35.Kosaka N, Bernardo M, Mitsunaga M, Choyke PL, Kobayashi H. MR and optical imaging of early micrometastases in lymph nodes: triple labeling with nano-sized agents yielding distinct signals. Contrast Media Mol Imaging 2012, 7:247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi H, Kawamoto S, Sakai Y, hoyke PL, Star RA, Brechbiel MW, Sato N, Tagaya Y, Morris JC, Waldmann TA. Lymphatic drainage imaging of breast cancer in mice by micro-magnetic resonance lymphangiography using a nano-size paramagnetic contrast agent. J Natl Cancer Inst 2004, 96:703–708. [DOI] [PubMed] [Google Scholar]
- 37.Baker JR Jr. Dendrimer-based nanoparticles for cancer therapy. Am Soc Hematol Educ Program 2009:708–719. [DOI] [PubMed] [Google Scholar]
- 38.Choi SK, Myc A, Silpe JE, Sumit M, Wong PT, McCarthy K, Desai AM, Thomas TP, Kotlyar A, Holl MM, et al. Dendrimer-based multivalent vancomycin nanoplatform for targeting the drug-resistant bacterial surface. ACS Nano 2013, 7:214–228. [DOI] [PubMed] [Google Scholar]
- 39.Witte AB, Timmer CM, Gam JJ, Choi SK, Banaszak Holl MM, Orr BG, Baker JR, Sinniah K. Biophysical characterization of a riboflavin-conjugated dendrimer platform for targeted drug delivery. Biomacromolecules 2012, 13:507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas TP, Huang B, Choi SK, Silpe JE, Kotlyar A, Desai AM, Zong H, Gam J, Joice M, Baker JR. Polyvalent dendrimer-methotrexate as a folate receptor-targeted cancer therapeutic. Mol Pharm 2012, 9:2669–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lo ST, Kumar A, Hsieh JT, Sun X. Dendrimer nanoscaffolds for potential theranostics of prostate cancer with a focus on radiochemistry. Mol Pharm 2013, 10:793–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grunwald GK, Vetter A, Klutz K, Willhauck MJ, Schwenk N, Senekowitsch-Schmidtke R, Schwaiger M, Zach C, Wagner E, Goke B, et al. Systemic image-guided liver cancer radiovirotherapy using dendrimer-coated adenovirus encoding the sodium iodide symporter as theranostic gene. J Nucl Med 2013. [DOI] [PubMed] [Google Scholar]
- 43.Cancino J, Paino IM, Micocci KC, Selistre-de-Araujo HS, Zucolotto V. In vitro nanotoxicity of single-walled carbon nanotube-dendrimer nanocomplexes against murine myoblast cells. Toxicol Lett 2013, 219:18–25. [DOI] [PubMed] [Google Scholar]
- 44.Al-Jamal KT, Al-Jamal WT, Akerman S, Podesta JE, Yilmazer A, Turton JA, Bianco A, Vargesson N, Kanthou C, Florence AT, et al. Systemic antiangiogenic activity of cationic poly-L-lysine dendrimer delays tumor growth. Proc Natl Acad Sci USA 2010, 107:3966–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]