Abstract
Objective
To compare cancer detection rates and concordance between magnetic resonance imaging and ultrasound (MRI-US) fusion-guided prostate biopsy cores obtained from axial and sagittal approaches.
Patients and Methods
Institutional records of MRI-US fusion-guided biopsy were reviewed. Detection rates for all cancers, Gleason ≥3 + 4 cancers, and Gleason ≥4 + 3 cancers were computed. Agreement between axial and sagittal cores for cancer detection, and frequency where one was upgraded the other was computed on a per-target and per-patient basis.
Results
In all, 893 encounters from 791 patients that underwent MRI-US fusion-guided biopsy in 2007–2013 were reviewed, yielding 4688 biopsy cores from 2344 targets for analysis. The mean age and PSA level at each encounter was 61.8 years and9.7 ng/mL (median 6.45 ng/mL). Detection rates for all cancers, ≥3 + 4 cancers, and ≥4 + 3 cancers were 25.9%, 17.2%, and 8.1% for axial cores, and 26.1%, 17.6%, and 8.6% for sagittal cores. Per-target agreement was 88.6%, 93.0%, and 96.5%, respectively. On a per-target basis, the rates at which one core upgraded or detected a cancer missed on the other were 8.3% and 8.6% for axial and sagittal cores, respectively. Even with the inclusion of systematic biopsies, omission of axial or sagittal cores would have resulted in missed detection or under-characterisation of cancer in 4.7% or 5.2% of patients, respectively.
Conclusion
Cancer detection rates, Gleason scores, and core involvement from axial and sagittal cores are similar, but significant cancer may be missed if only one core is obtained for each target. Discordance between axial and sagittal cores is greatest in intermediate-risk scenarios, where obtaining multiple cores may improve tissue characterisation.
Keywords: multiparametric MRI, fusion biopsy, prostate cancer
Introduction
Prostate cancer is the most common non-cutaneous cancer in men in the USA with a lifetime risk of one in six, and is the second leading cause of cancer-related mortality [1]. The standard-of-care for diagnosing prostate cancer is the systematic TRUS-guided biopsy, where 10–14 cores are obtained from various anatomical sections of the prostate [2–5]. Smaller and iso-echoic lesions are not visualised on TRUS, and the overlap between benign and malignant appearances means that prostate cancer remains the only solid tumour where biopsy is not directed at particular lesions [6].
MRI is the most sensitive imaging method for prostate cancer because of its superior soft tissue resolution [7]. In addition, imaging parameters on multi-parametric MRI (mpMRI) can be used to estimate tumour aggressiveness and are correlated with detection rates [8–10]. Although MRI can be directly used to guide biopsy, it is time consuming, costly and impractical because the patient occupies the MR gantry for the whole duration of the procedure [11,12]. MRI-ultrasound (US) fusion guidance has thus been developed to address these issues [13]. It allows targeted biopsy of MRI-visible lesions in the office setting, increasing cancer detection rates [10,14,15]. MRI-US fusion guidance has also been shown to be of particular value in improving detection rates for men with enlarged prostate glands or a history of prior negative biopsies [16,17], where systematic biopsy historically has lower detection rates. Even if cancer is detected on systematic biopsy, MRI-US-fusion biopsy upgrades the Gleason score in 32% of patients, and thus, improves detection of clinically significant cancers [18].
MRI-US fusion-guided biopsy is a rapidly growing field, with at least three different companies offering competing products [19]. Image registration between the volumetric MRI and US imaging is achieved by semi-automated segmentation and manual intra-procedural adjustment is necessary to compensate for movement of the prostate. These fusion guidance platforms allow biopsy in any imaging plane, with the axial and sagittal approaches being the most common. MRI-US fusion has been shown to improve detection of clinically significant cancer with fewer cores compared with standard biopsy [20]. However, in terms of the number of fusion-guided cores to be obtained, as well as the imaging plane to be used, optimal usage of these fusion guidance platforms is not well-defined. In the present study, we investigated the cancer detection rates and Gleason grades obtained from biopsies from axial and sagittal approaches using an end-fire biopsy probe.
Patients and Methods
A review of patients enrolled in a prospective trial assessing MRI-US fusion-guided prostate biopsy with electromagnetic tracking at the National Cancer Institute between August 2007 and June 2013 was performed (ClinicalTrials.gov identifier: NCT00102544). All patients were enrolled in an Institutional Review Board-approved clinical study with written informed consent. Patients were referred to our institution for initial evaluation, for clinical suspicion of prostate cancer despite a history of prior negative prostate biopsies, or for diagnosed low-grade disease that was not concordant with their high PSA levels or PSA dynamics. All candidates underwent standardised mpMRI, where suspicious lesions were identified according to the previous National Institutes of Health (NIH) validated scoring system and selected as targets for MRI-US fusion-guided biopsy. This scoring system has been previously validated to correlate with D’Amico risk stratification and cancer detection rate [9,10]. Point targets were designated in accordance to the planned biopsy location of the MRI-visible lesions, in larger lesions where a single target was felt to be inadequate for coverage; more than one biopsy target was designated. In each patient, a combination of standard-of-care 12-core systematic biopsy and two MRI-US fusion-guided biopsies (Fig. 1, one axial, one sagittal [21]) were obtained for each MRI-visible lesion. The MRI-US fusion-guidance platforms used were research iterations of the now commercially available UroNav system (Invivo, Gainesville, FL, USA). Patients without MRI-visible lesions were not eligible for the study and advised to follow-up with their referring urologist for standard-of-care management.
Fig. 1.
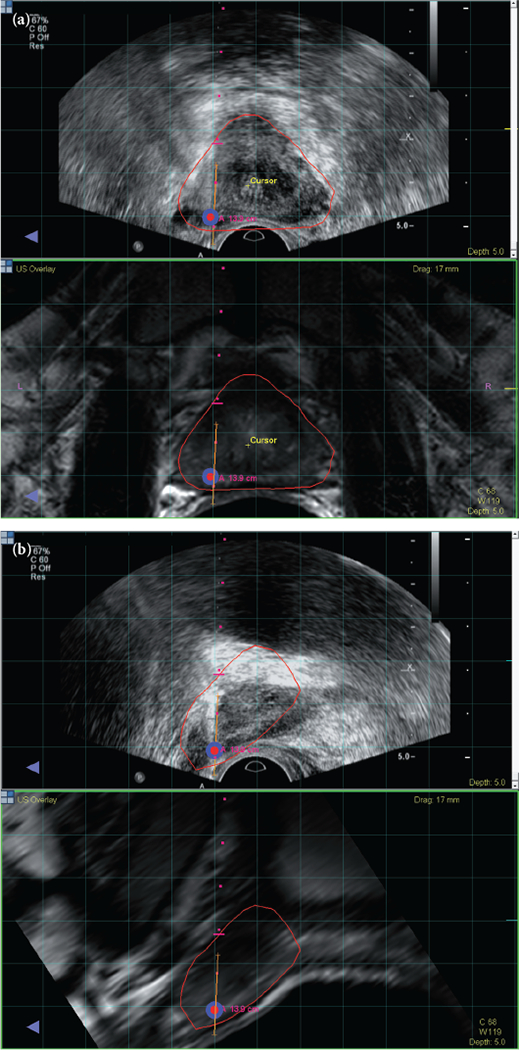
MRI registered with ultrasound visualized in the axial plane (a) and sagittal plane (b). A biopsy target is located in the right apical mid area of the prostate and visualized in red surrounded by blue. Needle trajectory is shown by the red dots, and the orange line maps the biopsy location for archiving and later use. Reproduced from Hong et al. 2014 [21].
MRI Acquisition and Interpretation
All images were acquired using a 3.0 T MRI scanner (Achieva, Philips Healthcare, Cleveland, OH, USA) with a six or 16-channel body coil (SENSE, Philips Healthcare, Cleveland, OH, USA) and an endorectal coil (BPX-30, Medrad, Pittsburgh, PA, USA). Routine pre-contrast axial T1-weighted imaging was done, and mpMRI consisted of triplanar T2-weighted (T2W) imaging, diffusion-weighted (DW) imaging generating apparent diffusion coefficient (ADC) maps, axial three-dimensional fast-field-echo dynamic contrast-enhanced MRI, and three-dimensional MRI spectroscopy. mpMRIs were interpreted by two experienced genitourinary radiologists (B.T. and P.L.C., with 6 and 13 years of experience in prostate MRI, respectively). The criterion for a suspicious area on T2W and DW imaging was a well-circumscribed, round-ellipsoid, low-signal intensity region within the prostate [22,23]. Each lesion found on MRI was assigned a suspicion level based on the positive sequences [9]. Biopsy targets designated in the seminal vesicles and based on prior biopsy locations were not assigned a suspicion level.
During the procedure, a fan-shaped sweep of the prostate using the US probe generated a volumetric US image [21]. Suspicious targets that were selected pre-procedurally were displayed on the T2W imaging and fused with the real-time TRUS image. The rigid registration was adjusted with translation or rotation by the urologist or the interventional radiologist. All pathological specimens obtained were evaluated by a single experienced genitourinary pathologist (M.J.M.).
Statistical Analysis
Statistical analysis was performed using JMP 9.0 (SAS Institute, Cary, NC, USA). The detection rates for cancer overall and for clinically significant cancers, as well as the Gleason score, and the proportional cancer involvement for positive cores was compared between axial and sagittal cores using an unpaired analysis. Equivalence testing was performed using the two one-sided tests (TOST) approach with a maximum tolerable difference of 5%.
Paired analysis was also performed on a per-target basis. Overall agreement and κ statistics were computed for the detection of all cancers, for cancers ≥3 + 4, and for cancers ≥4 + 3. The McNemar’s test was used to test for concordance on a per-lesion basis. The percentage of cases where biopsy in one imaging approach upgraded the Gleason score or detected a cancer that was missed on the other was calculated as well on a per-biopsy target basis, as well as on a per patient basis with the inclusion of systematic TRUS-biopsy cores. Among sessions with ≥3 MRI-US-fusion targets, cancer detection rate, percentage of cases where biopsies from one imaging approach upgraded the overall Gleason score, and percentage of cases where biopsies from the highest suspicion target(s) upgraded the overall Gleason score were calculated. Detection rates and rates of discordance were computed for the cohort as stratified by prior biopsy status and MRI suspicion level. Detection rates and rates of discordance were also computed for sessions with only one MRI-US fusion target. Detection rates and rates of discordance were computed for targets from patients with a prostate volume of <40 mL and targets from patients with a prostate volume of ≥40 mL.
After stratification by MRI suspicion level, the rates of discordance were compared between the first half and second half of our institutional experience using the two-tailed Fisher’s exact test. Age, PSA level, prostate volume, race, prior biopsy history, location of the lesion (anterior vs not anterior), and MRI suspicion level were assessed as possible predictors for discordance of cancer detection between axial and sagittal cores using univariate logistic regression. Significant predictors were then included in the multivariable logistic regression analysis. A significance level of 0.05 was used for all statistical testing.
Results
In all, 893 encounters from 791 patients who underwent MRI-US fusion-guided biopsy between August 2007 and June 2013 were reviewed (Table 1). In all, 2690 targets were biopsied under MRI-US-fusion guidance; however, 211 lesions lacked the sagittal biopsy and 135 lesions lacked the axial biopsy, and these biopsies were excluded from the analysis. This yielded 4688 biopsy cores that were obtained from 2344 targets under MRI-US fusion guidance for analysis. The mean age and PSA level was 61.8 years and 9.7 ng/mL (median 6.45 ng/mL). In all, 89.5% of patients had only one MRI-US fusion-biopsy session.
Table 1.
Characteristics of the study population.
| Characteristic | Value |
|---|---|
| Total number of encounters | 893 |
| Mean (sd): | |
| Age, years | 61.8 (7.7) |
| PSA level, ng/dL | 9.7(14.8 |
| N (%): | |
| Ethnicity: | |
| White | 706 (79.1) |
| Black | 138 (15.5) |
| Other | 49 (5.5) |
| Prior biopsy status: | |
| no prior biopsy | 168 (18.8) |
| positive prior biopsy | 352 (39.4) |
| negative prior biopsy | 373 (41.8) |
| Number of biopsy targets: | |
| 1 | 163 (18.3) |
| 2 | 218 (24.4) |
| 3 | 241 (27.0) |
| 4 | 150 (16.8) |
| 5 | 68 (7.6) |
| ≥6 | 53 (5.9) |
| Number of biopsy sessions: | |
| 1 | 708 (89.5) |
| 2 | 66 (8.3) |
| 3 | 15 (1.9) |
| 4 | 2 (0.3) |
Cancer detection rates overall and for clinically significant cancers from axial and sagittal cores was statistically equivalent (Table 2). Comparable Gleason scores and percentage of core involvement were obtained when the biopsy core was positive. However, the combination of both cores which detected cancers in 31.7% of targets significantly outperformed either the axial core (25.9%, P < 0.001) or the sagittal core alone (26.1%, P < 0.001). Gleason ≥3 + 4 = 7 were detected in 17.2% of axial cores and 17.6% of sagittal cores. Gleason ≥4 + 3 = 7 were detected in 8.1% of axial cores and 8.6% of sagittal cores. However, the combination of cores detected Gleason ≥3 + 4 = 7 and Gleason ≥4 + 3 = 7 cancers in 10.2% and 9.2% of targets, respectively. For overall cancer detection, the agreement between the axial and sagittal cores was 88.6% (κ 0.703, P < 0.001, Table 3a). Considering only ≥3 + 4 = 7 cancers, agreement between axial and sagittal cores was 93.0% (κ 0.769, P < 0.001, Table 3b), respectively. When considering only ≥4 + 3 = 7 cancers, agreement between axial and sagittal cores was 96.5% (κ 0.769, P < 0.001, Table 3c).
Table 2.
Unpaired comparison of detection rates for all cancers, ≥3 + 4 = 7 cancers, and ≥4 + 3 = 7 cancers, as well as Gleason scores obtained and cancer involvement between axial and sagittal cores. Axial and sagittal cores are statistically equivalent for cancer detection (P < 0.001).
| Outcome | Both, % (n/N) | Axial, % (n/N) | Sagittal, % (n/N) |
|---|---|---|---|
| Overall detection rate per-core | 31.7 (743/2344) | 25.9 (606/2344) | 26.1 (612/2344) |
| Detection rate for Gleason ≥3 + 4 = 7 | 20.9 (490/2344) | 17.2 (403/2344) | 17.6 (413/2344) |
| Detection rate for Gleason ≥4 + 3 = 7 | 10.2 (238/2344) | 8.1 (191/2344) | 8.6 (202/2344) |
| Detection rate for Gleason ≥4 + 4 = 8 | 9.2 (216/2344) | 7.4 (174/2344) | 8.0 (187/2344) |
| Gleason scores obtained among positive cores: | |||
| 3 + 3 = 6 | 34.1 (253/743) | 33.5 (203/606) | 32.5 (199/612) |
| 3 + 4 = 7 | 33.9 (252/743) | 35.0 (212/606) | 34.5 (211/612) |
| 4 + 3 = 7 | 3.0 (22/743) | 2.8 (17/606) | 2.5 (15/612) |
| ≥4 + 4 = 8 | 29.1 (216/743) | 28.7 (174/606) | 30.6 (187/612) |
| Cancer involvement ≥50% when positive | 46.7 (347/743) | 44.6 (270/606) | 42.6 (261/612) |
Table 3.
Paired comparison showing the level of concordance between axial and sagittal cores obtained from the same lesion in detecting cancer overall (a), Gleason ≥3 + 4 = 7 cancers (b), and Gleason ≥4 + 3 = 7 cancers (c).
| (a) | Sagittal positive, % (n/N) | Sagittal negative, %(n/N) |
| Axial positive | 20.3 (475/2344) | 5.6 (131/2344) |
| Axial negative | 5.8 (137/2344) | 68.3 (1601/2344) |
| (b) | Sagittal Gleason ≥3 + 4 = 7, % (n/N) | Sagittal negative or Gleason <3 + 4 = 7, % (n/N) |
| Axial ≥3 + 4 = 7 | 13.9 (326/2344) | 3.7 (87/2344) |
| Axial negative or <3 + 4 = 7 | 3.3 (77/2344) | 79.1 (1854/2344) |
| (c) | Sagittal Gleason ≥4 + 3 = 7,% (n/N) | Sagittal negative or Gleason <4 + 3 = 7, % (n/N) |
| Axial Gleason ≥4 + 3 = 7 | 6.6 (155/2344) | 1.5 (36/2344) |
| Axial negative or Gleason <4 + 3 = 7 | 2.0 (47/2344) | 89.8 (2106/2344) |
The addition of the axial core detected a cancer missed by the sagittal core or upgraded the Gleason score in 8.3% (195/2344) of targets. The addition of the sagittal core detected a cancer missed by the axial core or upgraded the Gleason score in 8.6% (202/2344) of targets.
On a per-patient basis without systematic biopsies, agreement between axial and sagittal targeted cores for the detection of cancer was 88.6% (791/893). Axial cores detected or upgraded the Gleason score of cancer that would have been missed by the combination of sagittal cores and systematic biopsy in 4.7% of patients (42/893). Sagittal cores did so for the combination of axial cores and systematic biopsy in 5.2% (46/893) of patients. Among the 512 encounters where ≥3 targets were biopsied, as suspicion level of the target with the highest suspicion increased, cancer detection rate, as well as the proportion of cases where fusion biopsies from either approach upgraded the overall Gleason score increased (Table 4). In sessions where the index target(s) were of moderate or high suspicion, the highest Gleason score was obtained only from MRI-US-fusion biopsies of the index target(s) in 20.8% and 26.1% of cases respectively, and upgraded the overall Gleason score.
Table 4.
Per-patient analysis of encounters with ≥3 MRI-US fusion targets, stratified by MRI suspicion level. The proportion of cases where axial or sagittal cores upgraded the overall Gleason score otherwise is shown, as well as the proportion of cases where the highest Gleason score was obtained only from the highest suspicion target(s).
| MRI suspicion level | Number of encounters | Cancer detection rate, % | Axial cores upgraded Gleason score, % | Sagittal cores upgraded Gleason score, % | Highest suspicion targets upgraded Gleason score, % |
|---|---|---|---|---|---|
| Low | 62 | 51.6 | 3.2 | 3.2 | 8.1 |
| Moderate | 356 | 65.2 | 5.3 | 7.3 | 20.8 |
| High | 92 | 85.9 | 10.9 | 6.5 | 26.1 |
In all, 24.4% (572/2344) of targets were from anterior lesions. The mean prostate volume was 60.1 mL (median 51 mL). In all, 29.2% (639/2344) targets were graded as low suspicion, 60.6% (1325/2344) targets as moderate suspicion, and 10.2% (224/2344) targets as high suspicion. Stratified analyses of per-target discordance were performed based on prior biopsy status and MRI suspicion level (Table 5). Among each cohort based on prior biopsy status, cancer detection rate increased with higher MRI suspicion level (P < 0.001). High-suspicion targets in patients with no prior biopsy had a very high cancer detection rate of 90.4%, and discordance was lower than in low- or moderate-suspicion targets. However, in patients with a history of prior negative biopsies, discordance was lower in low- and moderate-suspicion targets vs high-suspicion targets. When considering only encounters with a single MRI-US target, the rate of discordance was 9.5% among moderate-suspicion targets (Table 6). Discordance was seen in neither low-suspicion targets where axial and sagittal cores were mostly both negative, nor high-suspicion targets where in more than half of cases axial and sagittal cores were both positive.
Table 5.
Subgroup analyses of per-target discordance between axial and sagittal cores. The population was stratified based on prior biopsy status and MRI suspicion level.
| Prior biopsy status | MRI suspicion level | Number of biopsy targets | Overall cancer detection rate, % | Discordance, % |
|---|---|---|---|---|
| No prior biopsy | Low | 131 | 12.2 | 3.1 |
| Moderate | 261 | 44.1 | 17.6 | |
| High | 73 | 90.4 | 8.2 | |
| Prior negative biopsy | Low | 237 | 6.8 | 3.0 |
| Moderate | 517 | 23.4 | 7.7 | |
| High | 84 | 65.5 | 14.3 | |
| Prior positive biopsy | Low | 271 | 21.8 | 14.8 |
| Moderate | 547 | 36.7 | 14.8 | |
| High | 67 | 71.6 | 14.9 |
Table 6.
Analysis of overall cancer detection rate and MRI-US fusion biopsy discordance between axial and sagittal cores in encounters with a single MRI-US fusion target, stratified by MRI suspicion level.
| MRI suspicion level | Number of encounters | Cancer detection rate, % | Both fusion cores negative, % | Both fusion cores positive, % | Discordance, % |
|---|---|---|---|---|---|
| Low | 37 | 27.0 | 97.3 | 2.7 | 0 |
| Moderate | 116 | 27.6 | 81.9 | 8.6 | 9.5 |
| High | 7 | 71.4 | 42.9 | 57.1 | 0 |
The cancer detection rate and rate of discordance was 44.3% and 13.0% (637 targets) in prostates of <40 mL, and was 27.1% and 10.9% in prostates of ≥40 mL. Stratified by MRI suspicion level, rates of discordance did not differ significantly between the first half and second half of our institutional experience (Table 7).
Table 7.
Subgroup analyses of per-target discordance between axial and sagittal cores comparing results from the first half of the cohort to the second half of the cohort, stratified by MRI suspicion level. P values are shown for significance testing of the difference in discordance (two-tailed Fisher’s exact test).
| MRI suspicion level | Institutional experience | Number of biopsy targets | Overall cancer detection rate, % | Discordance, % | P |
|---|---|---|---|---|---|
| Low | First half | 467 | 13.9 | 7.3 | 0.32 |
| Second half | 172 | 15.1 | 9.9 | ||
| Moderate | First half | 481 | 29.7 | 12.1 | 0.67 |
| Second half | 844 | 34.8 | 12.9 | ||
| High | First half | 107 | 71.0 | 11.2 | 0.69 |
| Second half | 117 | 79.5 | 13.7 |
On univariate logistic regression, prostate volume, prior biopsy status, and MRI suspicion level were significant predictors for the presence/absence of discordant biopsy findings between axial and sagittal cores (Table 8a). These three variables remained significant on multivariable analysis (Table 8b).
Table 8.
Univariate (a) and multivariable (b) logistic regression in assessing various predictors for discordance of cancer detection between axial and sagittal cores. Only significant variables on univariate analysis were included in the multivariable analysis. Odds ratios (OR) and P values are provided.
| (a) | Univariate logistic regression | ||
| OR | P | ||
| Age/year | 1.012 | 0.185 | |
| PSA/ng/mL | 0.987 | 0.058 | |
| Prostate volume/cc | 0.989 | <0.001* | |
| Race | White | 1 (reference) | _ |
| Black | 1.193 | 0.319 | |
| Other | 0.795 | 0.468 | |
| Prior biopsy result | None | 1 (reference) | - |
| Negative | 0.563 | 0.003* | |
| Positive | 1.393 | 0.073 | |
| Anterior lesion | No | 1 (reference) | - |
| Yes | 0.991 | 0.952 | |
| MRI Suspicion | Low | 1 (reference) | - |
| Moderate | 1.663 | 0.002* | |
| High | 1.647 | 0.050 | |
| (b) | Multivariable logistic regression | ||
| OR | P | ||
| Prostate volume/cc | 0.992 | 0.004* | |
| Prior biopsy result | None | 1 (reference) | - |
| Negative | 0.592 | 0.009* | |
| Positive | 1.310 | 0.115 | |
| MRI Suspicion | Low | 1 (reference) | - |
| Moderate | 1.670 | 0.036* | |
| High | 1.719 | 0.002* | |
Asterisks indicate P values less than 0.05.
Discussion
Although fusion-guided biopsy is a rapidly growing field, biopsy is currently performed with no standardisation of either the number of cores or the imaging approach used. The premise of MRI-US fusion guidance is to combine the high resolution of MRI with the real-time capabilities of US. This requires that a volumetric US image be obtained for registration of the real-time TRUS with the previously obtained diagnostic MRI study. Because US inherently acquires a fan-shaped image (which can be axially or sagittally oriented), when registering this to an MR image, which is obtained as a series of parallel axial slices, a certain level of distortion occurs. This is especially apparent near the lowest apical portions and most lateral margins of the prostate.
The manual adjustment by the biopsy team allows for added accuracy in the registration of the MRI to the real-time TRUS by slightly adjusting the automatic registration particularly attending to the apical and lateral margins, which most often require adjustments, especially in larger prostate glands. However, improving the volumetric registration in one two-dimensional imaging plane at a time may worsen the registration in another, making perfect registration difficult to achieve. In addition, imaging in the sagittal view is typically in a lateral plane because the image at this plane is composed almost entirely of peripheral zone. Movement of the prostate can also adversely affect the quality of the registration in the axis of movement, which may be difficult to predict. This implies that for a given registration and set of biopsies, one imaging approach may be more accurate than the other. The results of the present study show that over a large number of patients, there is no systematic difference that would favour one approach over the other, suggesting this is difficult to predict a priori. MRI is currently limited to identifying lesions that are at ≥3 mm in diameter. Because the registration error is estimated to be about 2.4 ± 1.2 mm [13], registration error can affect cancer detection, and small foci of cancer may be still missed by the MRI-US fusion-guided biopsy approach[24].
Although biopsy outcomes from axial and sagittal approaches are equivalent with about 90–95% agreement, obtaining multiple cores can improve characterisation. The use of more cores, either in the same plane or in different planes, may be one way to increase the likelihood of overcoming registration error, which may be most important for smaller lesions. These results suggest that discordance among the axial and sagittal core is highest when there is most uncertainty about the pre-test expected biopsy result. High-suspicion targets in high-risk patients are not likely to be discordant because it is expected that both biopsy cores will yield cancer. However, low-suspicion targets in low-risk patients are also not likely to be discordant because it is expected that both biopsy cores will be negative. It is intermediate-risk scenarios where uncertainty and discordance is highest, where the use of multiple biopsy cores can be particularly helpful. However, as per-core detection rate is usually <50%, clinical scenarios where the pre-test probability of a positive result is higher, such as moderate or high suspicion on MRI, or a prior positive biopsy result, will usually increase the odds of discordance where one core is positive and the other is negative. Conversely, scenarios where the pre-test probability of a positive result is lower, such as low-suspicion MRI, larger prostate volumes that inherently decrease cancer detection rate, or a prior negative biopsy result, will usually decrease the odds of discordance, as it is more likely both cores are negative [25–27]. Additional cores are most helpful when there is most uncertainty of the expected biopsy result, e.g. the scenario where clinical parameters and imaging findings are in disagreement or suggest intermediate risk. This was the case for most patients in the present series. This finding is further supported by the fact that in sessions with only one MRI-US fusion target (eliminating the discordance that may occur from multiple targets of differing suspicion levels in the same gland), discordance was seen primarily in moderate-suspicion targets.
Agreement is higher when considering only higher grade cancers, but this is primarily due to more stringent criteria for a ‘positive’ biopsy, and in this situation, a discordant result implies potentially missing clinically important cancer. In addition, obtaining only one biopsy core underestimates the presence of cancer or its grade in about 8–9% of targets. Even with the inclusion of systematic biopsies, this results in under-characterisation or missed cancer in ≈5% of patients, which is especially important in the setting of patient selection for active surveillance [28,29]. Among patients with ≥3 MRI-US-fusion targets, if the index lesion(s) are of moderate or high suspicion, the Gleason score obtained from fusion biopsy of these target(s) upgraded the overall Gleason score in 20–26% of cases despite accounting for only a minority of the biopsy cores. Thus, it is vital that the highest suspicion targets be biopsied as accurately as possible.
The present study has several limitations, including its absence of whole mount prostatectomy specimens as a ‘gold standard’ comparison. As biopsy locations were designated as point targets, lesion size could not be accounted for. A minority of men had multiple biopsy sessions; however, due to the comparative nature of the present analysis, the results of other cores in each session served as an internal control, and their inclusion increased our sample size and the strength of these results. Results of the systematic TRUS biopsies were only included for the per-patient analysis. However, each location targeted for MRI-US biopsy represents different MRI-visible targets that are suspicious for prostate cancer, and these locations may have little correspondence to the anatomical sextants where systematic TRUS biopsy is taken. Regardless, given that MRI-US-fusion guidance has been reported to improve detection rates of clinically significant cancer, systematic biopsy should not be relied on to compensate for cancers that are not detected on MRI-US-fusion technology. It is also possible that the effect we see is simply due to the addition of a second biopsy, and that two axial or two sagittal biopsies rather than an axial and a sagittal biopsy would have similar results. It is not possible to evaluate this scenario with the present data. The present study is also affected by inherent limitations of MRI-US-fusion guidance including endorectal coil deformation and registration error, which would affect clinical practice as well. Some of the men described in this work have been reported in previous analyses from our group. The heterogeneity of our patient population is a limitation as well, which is addressed by various stratified and multivariable analyses and our large sample size. Of note, the proportion of high-suspicion targets in patients with a prior positive biopsy is lower than the other two subgroups. This is explained by the fact that if a prior positive biopsy has been documented, high-grade cancer would probably have been referred for definite treatment instead, leaving behind a cohort that is more consistent with patients on active surveillance.
As significant procedural time is spent on patient preparation and image registration, an increase in the number of cores does not substantially increase the length of the procedure. In addition, changing the imaging approach requires little beyond rotation of the US probe, as the same anatomical location is being biopsied, and has the potential to improve biopsy coverage. In comparison to a single axial or sagittal core, the second core detects a missed cancer or upgrades the Gleason score in about 8–9% of lesions. This translated to a clinically meaningful benefit in tissue characterisation in 5% of patients in our present cohort. Although the absence of whole mount pathology prevents definitive evaluation of the accuracy of these MRI-US fusion-guided biopsies, it has been shown that obtaining at least two cores helps to improve the accuracy of pathological characterisation. As MRI-US-fusion technology matures and becomes increasingly available efforts will be directed towards establishing guidelines for its role in cancer detection and clinical decision making. Further long-term studies will better define the optimal usage of this technology.
Acknowledgments
Conflict of Interest
NIH and Philips Healthcare have a cooperative research and development agreement. NIH and Philips Healthcare share intellectual property in the field. This work was supported by the National Cancer Institute, the Center for Interventional Oncology, and the Intramural Research Program of the NIH. This research was made possible through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc., The Leona M and Harry B. Helmsley Charitable Trust, and the Howard Hughes Medical Institute, as well as other private donors. For a complete list, please visit the Foundation website at (http://www.fnih.org/work/programs-development/medical-research-scholars-program).
Abbreviations:
- ADC
apparent diffusion coefficient
- DW
diffusion-weighted
- mpMRI
multi-parametric MRI
- NIH
National Institutes of Health
- T2W
T2-weighted
- US
ultrasound/ultrasonography
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63: 11–30 [DOI] [PubMed] [Google Scholar]
- 2.Raja J, Ramachandran N, Munneke G, Patel U. Current status of transrectal ultrasound-guided prostate biopsy in the diagnosis of prostate cancer. Clin Radiol 2006; 61: 142–53 [DOI] [PubMed] [Google Scholar]
- 3.Patel AR, Jones JS. Optimal biopsy strategies for the diagnosis and staging of prostate cancer. Curr Opin Urol 2009; 19: 232–7 [DOI] [PubMed] [Google Scholar]
- 4.Naughton CK, Miller DC, Yan Y. Impact of transrectal ultrasound guided prostate biopsy on quality of life: a prospective randomized trial comparing 6 versus 12 cores. J Urol 2001; 165: 100–3 [DOI] [PubMed] [Google Scholar]
- 5.Presti JC Jr, O’Dowd GJ, Miller MC, Mattu R, Veltri RW. Extended peripheral zone biopsy schemes increase cancer detection rates and minimize variance in prostate specific antigen and age related cancer rates: results of a community multi-practice study. J Urol 2003; 169: 125–9 [DOI] [PubMed] [Google Scholar]
- 6.Yacoub JH, Verma S, Moulton JS, Eggener S, Aytekin O. Imaging-guided prostate biopsy: conventional and emerging techniques. Radiographics 2012; 32: 819–37 [DOI] [PubMed] [Google Scholar]
- 7.Turkbey B, Pinto PA, Choyke PL. Imaging techniques for prostate cancer: implications for focal therapy. Nat Rev Urol 2009; 6: 191–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turkbey B, Shah VP, Pang Y et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology 2011; 258: 488–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rastinehad AR, Baccala AA Jr, Chung PH et al. D’Amico risk stratification correlates with degree of suspicion of prostate cancer on multiparametric magnetic resonance imaging. J Urol 2011; 185: 815–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rais-Bahrami S, Siddiqui MM, Turkbey B et al. Utility of multiparametric magnetic resonance imaging suspicion levels for detecting prostate cancer. J Urol 2013; 190: 1721–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beyersdorff D, Winkel A, Hamm B, Lenk S, Loening SA, Taupitz MM. R imaging-guided prostate biopsy with a closed MR unit at 1.5 T: initial results. Radiology 2005; 234: 576–81 [DOI] [PubMed] [Google Scholar]
- 12.Pondman KM, Fütterer JJ, ten Haken B et al. MR-guided biopsy of the prostate: an overview of techniques and a systematic review. Eur Urol 2008; 54: 517–27 [DOI] [PubMed] [Google Scholar]
- 13.Xu S, Kruecker J, Turkbey B et al. Real-time MRI-TRUS fusion for guidance of targeted prostate biopsies. Comput Aided Surg 2008; 13: 255–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinto PA, Chung PH, Rastinehad AR et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol 2011; 186: 1281–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sonn GA, Natarajan S, Margolis DJ et al. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol 2013; 189: 86–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walton Diaz A, Hoang AN, Turkbey B et al. Can magnetic resonance-ultrasound fusion biopsy improve cancer detection in enlarged prostates? J Urol 2013; 190: 2020–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vourganti S, Rastinehad A, Yerram NK et al. Multiparametric magnetic resonance imaging and ultrasound fusion biopsy detect prostate cancer in patients with prior negative transrectal ultrasound biopsies. J Urol 2012; 188: 2152–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siddiqui MM, Rais-Bahrami S, Truong H et al. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol 2013; 64: 713–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Logan JK, Rais-Bahrami S, Turkbey B et al. Current status of magnetic resonance imaging (MRI) and ultrasonography fusion software platforms for guidance of prostate biopsies. BJU Int 2014; 114: 641–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore CM, Robertson NL, Arsanious N et al. Image-guided prostate biopsy using magnetic resonance imaging-derived targets: a systematic review. Eur Urol 2013; 63: 125–40 [DOI] [PubMed] [Google Scholar]
- 21.Hong CW, Amalou H, Xu S et al. Prostate biopsy for the interventional radiologist. J Vasc Interv Radiol 2014; 25: 675–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turkbey B, Mani H, Aras O et al. Correlation of magnetic resonance imaging tumor volume with histopathology. J Urol 2012; 188: 1157–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turkbey B, Pinto PA, Mani H et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection – histopathologic correlation. Radiology 2010; 255: 89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rais-Bahrami S, Türkbey B, Rastinehad AR et al. Natural history of small index lesions suspicious for prostate cancer on multiparametric MRI: recommendations for interval imaging follow-up. Diagn Interv Radiol 2014; 20: 293–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uzzo RG, Wei JT, Waldbaum RS, Perlmutter AP, Byrne JC, Vaughan ED Jr. The influence of prostate size on cancer detection. Urology 1995; 46: 831–6 [DOI] [PubMed] [Google Scholar]
- 26.Ung JO, San Francisco IF, Regan MM, DeWolf WC, Olumi AF. The relationship of prostate gland volume to extended needle biopsy on prostate cancer detection. J Urol 2003; 169: 130–5 [DOI] [PubMed] [Google Scholar]
- 27.Karakiewicz PI, Bazinet M, Aprikian AG et al. Outcome of sextant biopsy according to gland volume. Urology 1997; 49: 55–9 [DOI] [PubMed] [Google Scholar]
- 28.Hong CW, Walton-Diaz A, Rais-Bahrami S et al. Imaging and pathology findings after an initial negative MRI-US fusion-guided and 12-core extended sextant prostate biopsy session. Diagn Interv Radiol 2014; 20: 234–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stamatakis L, Siddiqui MM, Nix JW et al. Accuracy of multiparametric magnetic resonance imaging in confirming eligibility for active surveillance for men with prostate cancer. Cancer 2013; 119: 3359–66 [DOI] [PMC free article] [PubMed] [Google Scholar]


