Abstract
Epidermal growth factor (EGF) receptors are commonly expressed on the cell membrane of cancer cells and activity of these receptors results in accelerated cell growth and carcinogenesis. A variety of targeted molecules have been developed to block ligand binding and/or inhibit the function of these receptor tyrosine kinases, and several have proven therapeutic benefits. Along with the advent of new therapeutic agents comes a need for non-invasive tools to diagnose, characterize, and monitor tumor responsiveness to therapy. Imaging EGF receptors with radionuclides has been performed for decades. However, recently this area has advanced considerably with the development of EGF receptor-targeted optical imaging probes. Herein, we review recent advances in molecular imaging of the EGF receptor family, focusing specifically on optical imaging. Such agents provide the opportunity for earlier diagnosis, improved tumor characterization, and the ability to measure and monitor tumor responsiveness to anti-EGF receptor treatment strategies.
Keywords: Optical imaging, cancer, epidermal growth factor receptor, HER2, positron emission tomography, fluorescence, multiple color imaging, monoclonal antibody, affibody, activatable imaging probe, near infrared, molecular imaging, radionuclide imaging
INTRODUCTION
Epithelial growth factor receptors (EGFR) represent an important family of proteins overexpressed on the cell surface of some cancers [1]. They have become the focus of drug development aimed at blocking or inhibiting receptor function [2, 3]. The ability to identify tumors that express one or more types of EGFR could be important in selecting patients for EGFR-based therapies, as well as for developing new therapies based on EGFR or monitoring the effects of current anti-EGFR therapies. Development of imaging methods that could identify EGFR-related cell surface expression in vivo, might lead to earlier diagnosis, better prognosis and individual cancer patient management. Currently, a variety of molecular imaging modalities exist and each offers complementary information about the molecular status of a tumor [4]. It is known, for instance, that the expression of EGFR in a primary lesion does not necessarily mean that it will appear in secondary metastases. Moreover, even if it does appear in one metastasis, it may not appear in all of them. Therefore, imaging offers the ability to measure EGFR expression throughout the entire body, not just in the primary lesion. Much of the work in tumor receptor imaging has been performed with radionuclide probes [5]. Such probes offer high sensitivity but limited temporal and spatial resolution. However, more recently, optical imaging has made considerable advances in this area. In this review, we focus on two receptors from the human epidermal growth factor receptor (HER) family, namely EGFR (HER1) and HER2. Following a brief overview of the HER family of receptors and their related physiological functions, we will discuss recent progress in optical imaging of EGFR and HER2.
THE HER BIOLOGY AND ITS ROLE IN CARCINOGENESIS
The epithelial growth factor receptor family of tyrosine kinase proteins (also known as HER or ErbB) is one of the most extensively studied cell surface marker due to its long recognized role in the formation of common human cancers. The family comprises four homologous transmembrane receptors: EGFR/HER1/ErbB1, HER2/ErbB2; HER3/ErbB3; HER4/ErbB4 that share a similar structure composed of an extracelluar ligand-binding domain, a transmembrane domain, and an intracellular tyrosine kinase domain with a C-terminal non-catalytic signaling tail. A defining feature of the EGFR network is that two members of the family are non-autonomous. For instance, the HER2 receptor does not interact with a known extracellular ligand, and the HER3 receptor exhibits defective tyrosine kinase activity and may therefore function as a decoy [6].
Dimerization after ligand binding is essential for initiating receptor function and signaling transduction. Therefore, ligand binding to the extracellular domain initiates a conformational rearrangement in the ectodomain that facilitates homo- or heterodimerization between members of the family, thus, triggering tyrosine kinase phosphorylation. This, in turn, leads to signal transduction and activation of downstream signaling pathways that control various cell activities, such as cell proliferation, migration, adhesion, differentiation, apoptosis, and angiogenesis [7]. The major downstream signaling routes of the HER family include the Ras/Raf/mitogen-activated protein kinase (MAPK) cascade, which is usually associated with cell proliferation, survival, transformation and motility, and the phosphatidylinositol 3-kinase (PI3K)-AKT route that mediates processes such as cell survival, gene expression, and cell-cycle progression [2].
EGFR/HER1
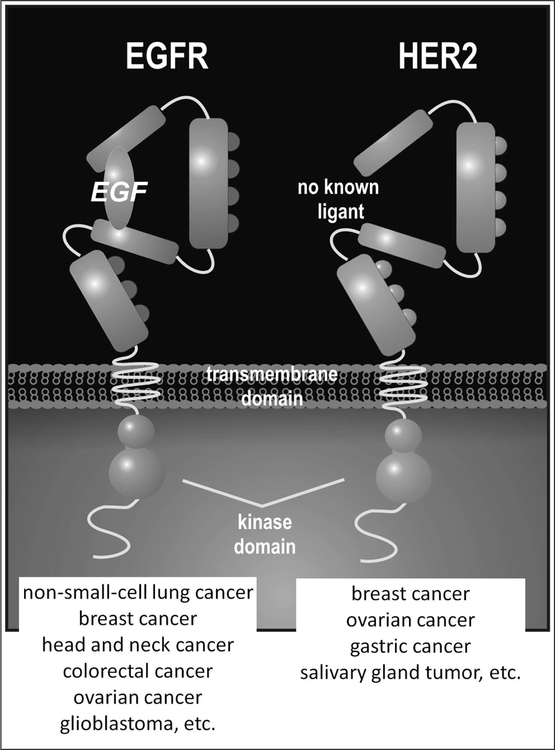
The epidermal growth factor receptor is a 170 kDa transmembrane glycoprotein that binds to multiple ligands (EGF, TGF-a, amphiregulin, betacellulin, heparin-binding EGF like growth factor, epiregulin) and forms homo- and heterodimers Fig. (1). The EGFR is constitutively expressed in many normal epithelial tissues, including skin, hair follicles, and the gastrointestinal tract [8] where the receptor expression ranges from 40,000 to 100,000 receptors per cell [9]. Deregulation of EGFR signaling plays a pivotal role in initiating the pathways that direct the behavior of various types of tumors including: breast cancer, head and neck, non-small-cell lung cancer (NSCLC), ovarian, colon cancer and glioblastoma multiforme (GBM) [9, 10]. EGFR expression on tumors is approximately 100-times that of normal cells [11], and this overexpression is associated with a more aggressive phenotype and generally worse patient survival. Therefore, it is not surprising that EGFR was one of the first cell surface markers identified as an important target for drug development. For this same reason it is also very attractive as a target for molecular imaging.
Fig. (1).
A schema for structures of HER1 and HER2 receptors with references of each receptor over-expressing cancers.
To date, two drug design strategies have emerged to interfere with the signaling transduction of EGFR: blockade of the ligand-receptor binding and inhibition of the receptor tyrosine kinase. Examples of the former include the monoclonal antibody, cetuximab (C225, Erbitux, ImClone LLC) and panitumumab (ABX-EGF), which sterically hinders ligand binding, preventing receptor activation [12]. Tyrosine kinase inhibitors are represented by small molecules such as gefitinib (Iressa, ZD1839, AstraZeneca) and erlotinib (Traceva, Genentech), which target the intracellular ATP-binding pocket of the receptor tyrosine kinase. Recent data, both in vitro and in vivo, indicate that the responsiveness of EGFR-positive tumors to EGFR targeted therapies does not correlate well with EGFR status. Also, clinical trials based on EGFR targeted therapies indicate that the expression of EGFR in tumors is, by itself, insufficient to account for their sensitivity to treatment. But even though, the EGFR level in tumors is not sufficient to predict response to therapy, in many cases its overexpression is a prerequisite for the initiation of appropriate treatment. Thus, the ability to non-invasively detect the receptor expression of tumors could facilitate the selection of patients, who are more likely to benefit from anti-EGFR-targeted therapy.
HER2
The HER2 receptor is a 185 kDa transmembrane glycoprotein that does not have a binding site of the EGF ligand and forms homo- and heterodimers with other HER family receptors Fig. (1). The HER2 receptor is amplified or overexpressed in a number of cancers including 15–20% of human breast cancers, as well as in a subset of patients with ovarian cancers, gastric carcinoma and salivary gland tumors [13]. It has been demonstrated that HER2 is a preferred heterodimerization partner of the other members of EGFR family, and that HER2-based heterodimers are characterized by a higher affinity and broader specificity for various ligands than the other heterodimeric receptor complexes [6]. After heterodimerization, HER2 overexpression promotes cell survival, proliferation, angiogenesis, invasion and metastasis, and has been implicated in the development of drug resistance and radiation resistance. Numerous efforts have been made to develop HER2-targeted therapies [14], however, the only one approved by the FDA is trastuzumab (Herceptin; Genentech Inc). Trastuzumab is in widespread use for the treatment of women with HER2 overexpressing breast cancer. To date, the efficacy of trastuzumab as a single agent has been disappointing [15, 16]. However, trastuzumab in conjunction with chemotherapy has been shown to dramatically improve survival when given concomitantly or sequentially with chemotherapy [17]. Thus, the clinical efficacy of trastuzumab as a molecularly targeted therapy depends on the accurate assessment of HER2 status.
In daily practice HER2 evaluation is measured ex vivo in tissue samples obtained from primary tumors by immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH). Both are valuable tools, but require the procurement of tumor tissues through biopsies, which is not only invasive but is also limited by sampling error that results from tumor heterogeneity and other confounding factors such as inflammation. Furthermore, as noted previously there can be inconsistencies between HER2 expression in primary and metastatic lesions. Lower et al. reported that in a group of 382 patients with primary breast cancer, 140 tumors were HER2-positive, whereas metastases from these patients were HER2-positive in only 50 based on IHC scoring. Similarly, among 242 patients with HER2-negative primary tumors, 37 had HER2-positive metastases [18]. This discrepancy is particularly important especially considering treatment decisions about trastuzumab are often made on the basis of the HER2 status of the primary tumor alone; metastases are often not rebiopsied. Therefore, method to effectively and accurately measure HER2 expression in primary and secondary sites using non-invasive molecular imaging would be beneficial.
IMAGING OF THE EGFR FAMILY OF RECEPTORS TARGETING
Molecular imaging modalities using agents that specifically target EGFR and HER2 receptors might provide a complementary, noninvasive methods to assess receptor status not only in the tumor but also in sites of metastatic disease that are not easily biopsied. Moreover, it could also help to prevent false positive or false negative results due to heterogeneity of receptor expression that occurs in individual biopsy specimens. Finally, such technologies would facilitate the selection of patients for targeted therapies as well as provide a tool to gauge the effectiveness of therapeutic interventions so that adjustments could be made to treatment protocols based individual patients responses.
There are a few requirements for targeting agents to be successfully used in molecular imaging. Each targeting moiety must be labeled appropriately depending on the imaging modality that is employed. For instance, Positron Emission Tomography (PET) and Single Photon Emission Tomography (SPECT) agents are labeled with radionuclides, whereas Magnetic Resonance Imaging (MRI) requires labeling with paramagnetic contrast agents, and optical imaging requires labeling with fluorescent dyes. To obtain optimal resolution and quantitative accuracy, the labeled agent should have high affinity and specificity for the target (even after labeling), with low uptake in normal tissue. In the case of radionuclide imaging, the physical half-life of radioisotope should match the biological half-life of the labeled molecule to achieve the optimal tumor-to-background ratio (contrast). Moreover, agent production should be straightforward and not overly costly.
Antibodies are the traditional targeting moiety choice. Multiple humanized antibodies exist for the EGFR family and several are in clinical use. However, the problem with full-sized antibodies is their large size (150 kDa) results in slow clearance from the bloodstream and leads to significant background signal from the unbound agent when employing conventional labeling/signaling systems. Therefore, to improve the imaging performance, alternative targeting agents have been developed and extensively studied. Among these advances, antibody fragments and engineered variants such as F(ab’)2, F(ab’), single-chain Fv, diabodies, and minibodies have been proven very useful. Single-chain Fv (27 kDa) are rapidly cleared via renal excretion (the kidney cut-off is about 60 kDa), and demonstrate better tumor penetration than the corresponding full-sized antibody. Other non-IgG proteins have recently been developed that are based on protein scaffolds. Examples include avimers [19], aptamers [20], and affibody molecules [21]. Affibody molecules are small (6–7 kDa) high affinity ligands based on 58 amino acids that are derived from the B domain of IgG-binding domain of staphylococcal protein A. This cysteine-free stable three-helix bundle is mutated at a single site in helix 2 to achieve increased chemical stability. Affibodies lose Fab binding activity, but retain the capability to bind Ig Fc-regions [22]. Affibody libraries are constructed by combinatorial randomization of the 13 amino acid positions in helix 1 and 2 that results in deleting and repelling the parental Fc-binding surface [23]. Selection against a number of targets has generated affibody molecules recognizing HER2 as well as EGFR among other targets [24].
SPECIFIC DETECTION OF EGFR OR HER2 EXPRESSING CANCER CELLS USING PET IMAGING
The volume of preclinical studies during the last few years dedicated to the development of radionuclide imaging agents, particularly labeled with positrons emitters, as a tool for in vivo molecular imaging of EGFR and HER2 receptors, speaks to the high level of interest in this area. Positrons are conventional monochrome and “always on” signaling moieties. Therefore, strategies for success are relatively simple and use either small molecular ligands or long half-life positrons. Herein we describe recent examples of successful PET imaging of EGF receptors.
EGFR TARGETING
The greatest experience has been achieved by targeting EGFR with labeled antibodies. 64Cu-DOTA-cetuximab was first reported by Cai et al. in 2007 as a potential PET tracer [25]. It is important to note the choice of 64Cu as the labeling agent rather than the more common 18F. The longer biologic half life of the antibody requires a longer lived radioisotope (18F T1/2=1.8h vs. 64Cu T1/2=12.7h). MicoPET images showed that 64Cu-DOTA-cetuximab had increased tumor activity in EGFR-positive tumors compared to EGFR-negative tumors. Moreover, there was a correlation between tumor uptake and the EGFR expression measured ex vivo by Western blot. Both cetuximab and panitumimab (anti-HER1) have been radiolabled with a variety of PET emitters with similar results.
There have been also several studies in which EGF (6 kDa), the natural ligand for EGFR, was radiolabeled labeled with 76Br, 68Ga, or 111In [26–28]. The use of small radiopeptides based on natural ligands or their analogs, provides rapid targeting kinetics but their rather short biological half-life and the risk of agonist effects significantly limits their applicability. EGFR-specific affibody molecules have also been developed. 111In-(ZEGFR:955) Affibody demonstrated better tumor-to-blood ratios (9.1) compared to the antibody (1.5) and the natural ligand EGF (3.0), at 24 h post injection [29]. Those results are in line with recent findings published by Miao et al. who showed that 64Cu-DOTA-ZEGFR:1907 Affibody had superior pharmacokinetics compared with 64Cu-Cetuximab [30]. The rapid clearance of the affibody is an advantage since accuracy depends on the tumor-to-background ratio (TBR) of radioactivity. The rapid excretion of smaller molecules leads to higher TBRs.
Attempts at imaging the internal domain of EGFR have also been made. For instance, radiolabeled gefitinib, an EGFR tyrosine kinase inhibitor has been tested, but the multiplicity of tyrosine kinase expression sites and associated nonspecific binding have made the probe development challenging. The results presented by Su et at. clearly indicated that 18F-gefitinib does not accumulate specifically in human tumor xenografts [31].
HER2 IMAGING
Similar to EGFR labeling, HER2 imaging has been performed with both large and small molecules. Several groups have described the performance of radiolabeled trastuzumab as a targeting molecule for HER2 imaging. Dijrkes et al. labeled trastuzumab with 89Zr (T1/2= 78.4h) [32] and later used this tracer in a clinical trial carried out to optimize dosage and time of administration of the 89Zr-trastuzumab for PET imaging of HER2-positive lesions [33].
To improve the imaging performance, a variety of different ligands have been studied over the last few years. F(ab’)2 fragments of trastuzumab were labeled with 68Ga for sequential PET imaging of changes in HER2 expression after 17-AAG (geldanamycin analog), an Hsp90 inhibitor [34]. 124I labeled C6.5, a noncovalent anti-HER2 single-chain Fv dimer was recently evaluated for predicting the response to trastuzumab therapy in mice bearing SKOV-3 tumors [35]. Several studies, demonstrated that radiolabeled HER2-specific affibodies were successfully used for imaging metastatic HER2 overexpressing tumors in the lung Fig. (2). The robust structure of affibody molecules enables radiolabeling with different radioisotopes, including 99mTc, 111In, 90Y, 125I, without degrading the binding capacity. Moreover, the first clinical studies using synthetic 111In- or 68Ga-labeled ABY-002 affibody molecules for molecular imaging of HER2-expressing malignant tumors in breast cancer patients have already been carried out by Baum et al. [36].
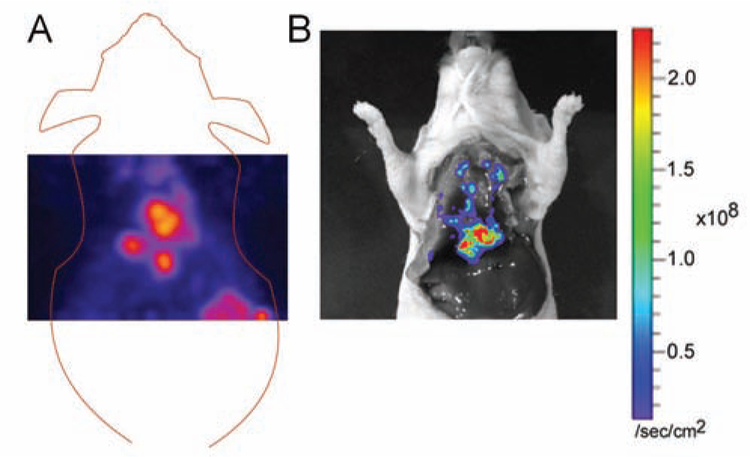
Fig. (2).
A) Representative coronal section of 18F-AffibodyHER2 uptake. The HER2-positive lung metastases are visualized 1 h post tracer injection. B) Ex vivo bioluminescence image of the same mouse shows photon signals consistent with PET signals shown in A.
ADVANTAGES AND LIMITATIONS OF RADIONUCLIDE IMAGING COMPARED WITH OPTICAL IMAGING
(Table 1) emphasizes the strengths and weaknesses of PET and optical imaging. The strength of PET is specificity and exquisite sensitivity (~10−11−10−12 moles/l), as well as the flexible chemistry that together with the decay scheme lead to favorable imaging properties. Moreover, PET produces a three dimensional image or map of functional processes in the whole body and allows for quantitative analysis of the tracer distribution. The drawbacks of PET are largely related to the limited spatial and temporal resolution (4–8 mm3 in clinical and, 1–2 mm3 in small animal imaging systems; and 5–20 min for a single imaging session), high cost and limited accessibility.
Table 1.
Strengths and Weaknesses in Terms of Spatial Resolution, Sensitivity
| Imaging Modality | Sensitivity [moles/l] | Spatial Resolution | Relative Cost | Strengths | Weaknesses |
|---|---|---|---|---|---|
| PET | ~10−11–10−12 | 1–2 mm (microPET); 4–5 mm (clinical PET) |
$ $ $ $ | Highly Sensitive Unlimited Depth Penetration Clinical Translation |
Low spatial and temporal resolution Cyclotron required for most of isotopes used |
| SPECT | ~10−11–10−11 | ~1 mm (microSPECT); 12–15 mm (clinical PET) |
$ $ $ | Simultaneous imaging of more than one tracer Clinical Translation |
Low spatial and temporal resolution |
| CT | ~10−4 | ~100 μm | $ $ | High spatial resolution (bone/lung) Fast |
Radiation No target-specific imaging Poor soft-tissue contrast |
| MRI | ~10−3–10−5 | ~20 μm | $ $ $ | Clinical translation High spatial and temporal resolution High tissue contrast |
Relatively insensitive Imaging time |
| US | A Single Bubble | ~50 μm | $ $ | Clinical translation High spatial and temporal resolution |
A few probes available (bubbles of micron size) |
| Optical Imaging | ~10−15 | ~100 μm (Endoscope) 2–3mm (in vivo imaging) |
$ | High sensitivity Harmless High-throughput capability |
Limited clinical translation Depth detection limits |
Considering applications, PET cannot be used in real-time, i.e., to guide surgical resection or biopsy, whereas optical imaging can be employed intra-operatively for simultaneous viewing of the tumor and the anatomical surface. Additionally, optical imaging is highly sensitive (10−15 moles/l), quick and unlike PET, optical imaging is an easy to perform technique with relatively low instrumentation costs. These advantages make optical imaging a modality particularly well-suited for drug development and validation processes. However, the limited tissue penetration of the optical signal limits the usefulness of optical imaging in quantitative biodistribution analysis (Table 1).
OPTICAL IMAGING OF THE EGFR FAMILY
Optical imaging can be performed at various wavelengths of light. However, due to particular absorbencies of water and hemoglobin in tissue, the near infrared (NIR) section of the optical spectrum (650–900nm) has proven to be the most useful. NIR light also is less apt to result from autofluorescence arising from endogenous fluorophores. Therefore, among other technologies being used to image the EGFR family, NIR fluorescence optical imaging is particularly promising and can be efficiently used to visualize and investigate the EGFR family surface receptors.
Unlike many radiological techniques, optical imaging can be used to guide surgical resection or biopsy because it is obtained in real-time [37]. Importantly, detection and pathologic confirmation of metastatic disease during a surgical procedure may change patient outcomes by promoting minimally invasive procedures. Moreover, this imaging modality can aid in vivo molecular profiling of the various EGFR subtypes [38], and promote the development of strategies that combine therapeutics with optical imaging into a single platform [39]. Optical imaging is attractive for translation into intervention-based applications because the signaling beacon does not emit ionizing energy, sparing both the patient and the physician/health care worker radiation exposure.
Several types of EGFR-expressing tumors are well suited for optical imaging as they are in superficial anatomical locations amenable to endoscopy. Specific applications that could be envisioned include bronchoscopy for non-small cell lung cancer and/or lung metastases from breast cancers, endoscopy for esophageal cancer [40], laparoscopy for ovarian cancer, and external surface optical imaging for breast cancer.
Similar to radionuclide molecular imaging, a variety of attempts have been made to conjugate fluorescent dyes with targeting moieties including: antibodies and their fragments (F(ab’)2, F(ab’), single-chain Fv,) diabodies, minibodies, as well as protein scaffolds such as affibodies [41–43] Fig. (3).
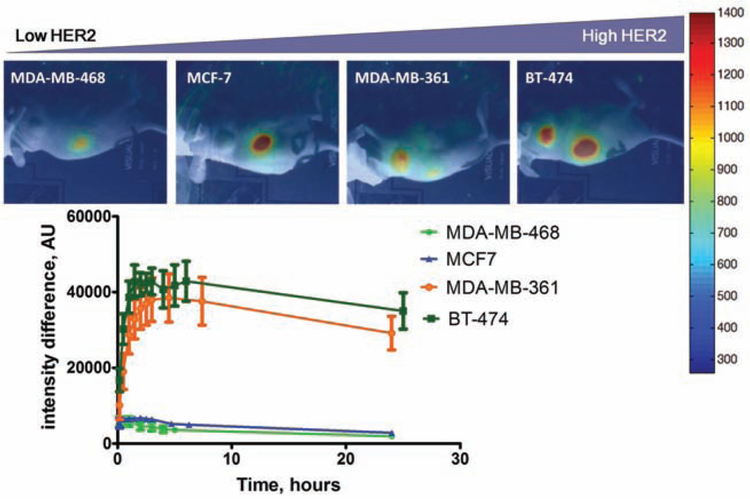
Fig. (3).
Accumulation of DyLight750-AffibodyHER2 in xenografts was well correlated with HER2 expression levels. (Figures courtesy of Dr. Rafal Zielinski.)
Folli and Ballou were the first to demonstrate the use of fluorescence imaging in experimental animal model with cyanine dyes conjugated to antibodies [44, 45]. EGFR-specific affibody molecules have been labeled with IRDye800CW and Cy5.5 and provided receptor–specific molecular imaging of EGFR-positive tumors [46, 47]. The agents showed good tumor-to-background ratios with rapid tumor targeting and quick blood clearance.
Chernomordik et al. [48] investigated temporal signal changes detected in tumor xenografts following injection of a HER2-specific affibody-based fluorescence contrast agent in order to monitor receptor status. A compartmental model was used to calculate HER2 expression from imaging data and showed that the initial slope, characterizing the temporal dependence of the fluorescence intensity, linearly depends on HER2 expression, as measured ex vivo by an ELISA assay for the same tumor. This suggests that kinetic optical imaging can be mathematically modeled to noninvasively monitor HER2 expression in vivo.
Recently, multifunctional probes have been developed. Agents that are dual labeled with nuclear and optical reporters provide unique opportunities for noninvasive pre-operative imaging with PET or SPECT and intraoperative imaging with NIR fluorophores. They both possess complementary advantages that offset the other’s limitations. NIR light has limited tissue penetration whereas gamma rays from radioisotopic decay can easily penetrate the entire body. On the other hand the physical half-life of radionuclide labeled agents limits the shelf life and imaging window, while NIR labeled agents have a long shelf life (if kept in darkened containers) and depend only on the biologic half-life rather than the physical half-life. During in vivo nuclear and optical imaging of SKBR3-luc xenografts injected with 111In-(DTPA)n-trastuzumab-(IRDye800)m significantly more uptake in the tumor region was demonstrated than in the contralateral normal muscle region [49], indicating potential as a diagnostic biomarker capable of tracking HER2-positive tumors. Ogawa et al. labeled panitumumab (anti-HER1) and trastuzumab (anti-HER2) for SPECT and optical imaging using both 111In and indocyanine green (ICG), which is activated upon internalization [50]. The multimodality imaging studies showed that while the biodistribution profile of the injected antibody was provided by nuclear imaging, only the optical imaging provided target-specific tumor uptake based on the different wavelengths of the emitted light. Therefore, simultaneous visualization, characterization, and measurement of biological processes could be obtained in a single imaging session.
As with radionuclide imaging, strategies exist for optical imaging to reduce the background signal associated with long circulating antibodies. Avidin has been used as a “chase” to clear the unbound, circulating biotinylated antibody. The biotinylated antibody is first injected and sufficient time is allowed to bind to the target, whereupon the Avidin chase is administered to clear the vasculature of unbound antibody, by shuttling the avidin bound antibody to the liver. Additionally, a combined approach using quenching (a process whereby the fluorescence signal is hindered) and an “avidin chase” has been used to further improve TBRs [50].
NOVEL OPTICAL SIGNAL ACTIVATION
While one usually thinks of optical imaging as a simple “image”, optical signal can be activated under certain conditions. Within optical molecular imaging, many signaling options exist and this has become a very active area of research. For instance, spectral separation techniques can be used to individually identify specific fluorescent probes based on their wavelength. In general, fluorophores emitting light in the NIR spectrum are optimal as this range has the greatest depth of penetration through tissue and, there is little naturally occurring fluorescence within this range.
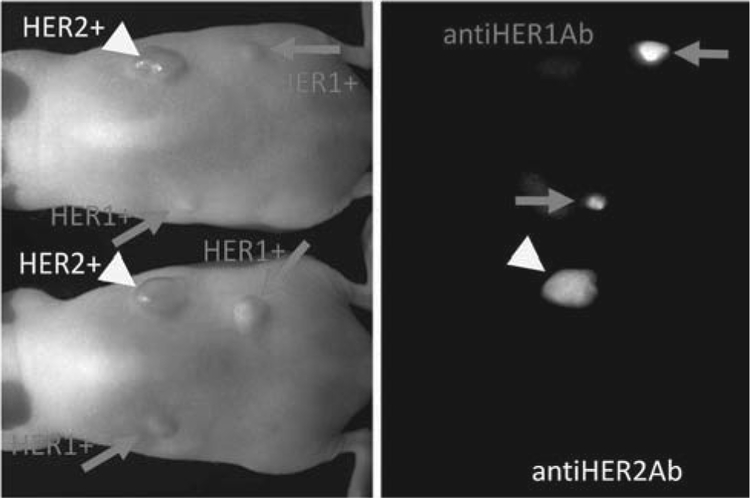
New signaling strategies include multicolor optical imaging, which has generated a lot of interest for imaging multiple receptor sub-types within the EGFR family. By simultaneously deploying multiple probes, each targeting a different receptor, and each probe characterized by a unique color, it has been demonstrated that a cocktail of optically labeled monoclonal antibodies can selectively differentiate tumors based on cell surface expression profiles, EGFR versus HER2 [42, 51] Fig. (4). Additionally, the use of multiple filter sets can increase the signal to noise ratio to permit effective differentiation of dyes within the narrow limit of the NIR spectrum [42].
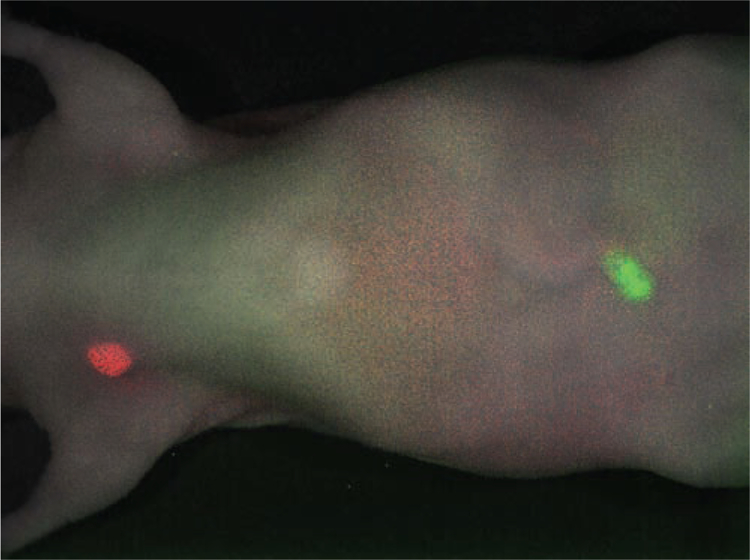
Fig. (4).
Two-color HER1 and HER2 image is shown. A cocktail injection of Cy5.5-labeled panitumumab (anti-HER1; shown in red) and Cy7-labeled trastuzumab (anti-HER2; shown in green) clearly depicts MDA-MB-468 breast cancer (HER1-positive) in the right breast and SKOV3 ovarian cancer (HER2-positive) in the navel in the tumor-bearing mice in distinct colors.
An elegant and emerging area of optical imaging is the creation of probes that are selectively activated upon binding to target cells [52] Fig. (5). Activation strategies rely on harnessing aspects of cellular biology as well as properties of optical physics and chemistry, such as quenching of the fluorescence signal by fluorophore-fluorophore and/or fluorophore-conjugated protein interactions [53]. Activatable probes are attractive because they yield higher tumor-to-background ratios as there is little to no background signal emitted from the unbound agent. Hama et al. developed an activatable agent targeting the EGFR, using a two-step activation process in which EGFR was first targeted with a highly specific non-fluorescent biotinylated monoclonal antibody, cetixumab then “chased” by a second probe containing a fluorophore [54]. Upon binding the cetuximab, the fluorophore increased in signal, yielding a 20-fold amplification of optical fluorescence signal compared to the unbound agent [54].
Fig. (5).
Activatable ICG-labeling of panitumumab (anti-HER1) and trastuzumab (anti-HER2) clearly depicted respective antigen-positive tumors in tumors-bearing mice.
Other probes have been designed so that they are activated by target cells after internalization within the cell [43]. For instance, highly specific in vivo cancer imaging of HER2 positive lung cancer cells was achieved using a pH-sensitive probe [55]. Herein, the probe was delivered to the tumor by targeting with a monoclonal antibody, trastuzumab, which, after binding to HER2, is internalized via the endosomsal-lysosomal degradation pathway. Acidification within the endolysosome leads to activation of the fluorescent signal [55]. Such probes are highly specific for HER2-positive tumors and have the advantage of producing minimal background signal, therefore yielding very high tumor-to-background ratios. The process of acidification relies on intact cellular processes and therefore the strategy selectively images viable cells [55]. Additionally, strategies harnessing quenching combined with target cell activation have been developed, yielding a dual-controlled activatable strategy with an even greater increase in tumor-to-background ratio [56]. Of note, research evaluating specific activable optical probe constructs targeting EGFR receptors has demonstrated that different fluorophores conjugated to the same antibody differ with regard to activation potential. Specifically, it has been shown that multiple Alexa680 fluorophores conjugated to a single antibody result in better activation than similar constructs with different fluorophores [57].
As discussed above, EGFR family members are important therapeutic targets. Optical imaging can be used for semi-quantitative assessment of targeted drug delivery [58]. Uniform antibody microdistribution throughout tumor nodules is crucial for antibody-targeted therapy, because non-uniform microdistribution leads to suboptimal therapeutic effects, a commonly observed limitation of therapeutic antibodies [58]. Using a mouse model of disseminated ovarian cancer, microdistribution of different doses of intraper-itoneally injected fluorescence-labeled full-antibody trastuzumab and its Fab fragments where compared using a semiquantitative approach to determine the optimal delivery agent and dosing to achieve uniform tumor distribution [58]. Such innovations may assist in optimization of dosing and delivery strategies.
Additionally, imaging modalities can be combined to harness the strengths of the individual platforms for multimodality imaging. For example, multimodality imaging was performed by a combination of a quantitative radiolabeled probe and an activatable optical probe using the monoclonal antibody panitumumab trastuzumab, labeled with 111In and ICG. These agents were tested in both EGFR and HER2 tumor bearing mice. The tumor was visualized by optical imaging, while nuclear imaging provided the biodistribution profile of the injected antibody, again important when evaluating drug delivery [59]. Another exciting area related to therapeutics is nanophototheranostics, a strategy that combines targeted phototherapy and molecular diagnostics. It has been shown that activation of certain fluorophores can result in death of targeted cells by mechanisms that remain to be elucidated. Such probes provide both imaging and therapeutic abilities, without the use of ionizing radiation [39].
PRECLINICAL EXPERIENCE
Preclinical research in optical imaging of the EGFR family of receptors has demonstrated promise for improving diagnosis and treatment of numerous types of cancer. Recent advances in animal models suggest that optical imaging may improve identification and removal of primary tumors and metastatic disease. For example, NIR fluorescence optical imaging with trastuzumab conjugated either with cyanine 5.5 or AF750 was able to evaluate the therapeutic dose effects of trastuzumab in an orthotopic human breast cancer tumor [60]. A Cy5.5-labeled anti-EGFR antibody or a rhodamine green-labeled anti-HER2 antibody bound specifically to small islands of respective receptor-positive tumors in the lung parenchyma and fluoresced in a distinct miliary pattern in a pulmonary metastasis model, whereas the use of an isotype control IgG or a receptor-negative metastatic tumor model showed diffuse background fluorescence in the lung [61].
Optical imaging of metastatic ovarian tumors in animal models has improved disease identification and tumor removal [37, 62]. Additionally, resection of limited lung metastasis is now an important clinical intervention for certain cancer types and it has been shown that HER2-targeted optical imaging probes accurately identify metastasis in mouse models of disease [61]. As mentioned previously, molecular characterization of cell-surface receptor expression in metastatic lesions could have great clinical utility. To achieve this, small foci of disease must be both detected and accurately characterized. Trastuzumab (targeting HER2) conjugated to both a fluorophore and a quenching moiety has been shown to be an effective activatable probe for identifying small tumor foci in mouse models of HER2 positive malignancies [63].
Additionally, there is evidence that strategies derived from optical imaging research in the EGFR family may be successfully applied to similar receptor groups. For example, parallels exist between prostate-membrane specific antigen (PSMA) and EGFR, which include overexpression in malignant disease, enzymatic activation, and activation upon dimerazation [64]. Using strategies initially applied to EGFR-expressing tumors, probes for prostate cancer imaging have been developed [65], suggesting that experience derived from EGFR research may expedite the development of optical imaging applications in these related areas.
SUMMARY AND PROSPECTS TOWARD CLINICAL APPLICATION
Optical imaging offers much promise in EGFR-expressing cancers. Promising avenues of translation include earlier diagnosis, optimized treatment regimens based on EGFR expression, optimization of EGFR-targeting therapeutics, and detection and characterization of metastatic disease. Additionally, we anticipate that there will be a dramatic expansion of technologies that combine optical imaging and therapeutics for so-called nanotheranostics.
Even though the theoretical rationales for imaging the EGFR family are strong, the translation of this approach to the clinic is complex and pace is slow. Nonetheless, this area of investigation promises to expand the ability of physicians to identify and treat malignancy with a high degree of specificity in humans in the near future.
ACKNOWLEDGEMENTS
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and Center for Cancer Research.
Footnotes
CONFLICT OF INTEREST
The authors do not have any conflict of interests for writing this article.
REFERENCES
- [1].Ciardiello F; Tortora G, EGFR antagonists in cancer treatment. N Engl J Med, 2008, 358, 1160–1174. [DOI] [PubMed] [Google Scholar]
- [2].Jones HE; Gee JM; Taylor KM; Barrow D; Williams HD; Rubini M; Nicholson RI, Development of strategies for the use of anti-growth factor treatments. Endocr Relat Cancer, 2005, 12 Suppl 1, S173–182. [DOI] [PubMed] [Google Scholar]
- [3].Lurje G; Lenz HJ, EGFR signaling and drug discovery. Oncology, 2009, 77, 400–410. [DOI] [PubMed] [Google Scholar]
- [4].Fass L, Imaging and cancer: a review. Mol Oncol, 2008, 2, 115–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cai W; Niu G; Chen X, Multimodality imaging of the HER-kinase axis in cancer. Eur J Nucl Med Mol Imaging, 2008, 35, 186–208. [DOI] [PubMed] [Google Scholar]
- [6].Citri A; Yarden Y, EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol, 2006, 7, 505–516. [DOI] [PubMed] [Google Scholar]
- [7].Yarden Y; Sliwkowski MX, Untangling the ErbB signalling network. Nat Rev Mol Cell Biol, 2001, 2, 127–137. [DOI] [PubMed] [Google Scholar]
- [8].Wujcik D, EGFR as a target: rationale for therapy. Semin Oncol Nurs, 2006, 22, 5–9. [DOI] [PubMed] [Google Scholar]
- [9].Herbst RS, Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys, 2004, 59, 21–26. [DOI] [PubMed] [Google Scholar]
- [10].Hatanpaa KJ; Burma S; Zhao DW; Habib AA, Epidermal Growth Factor Receptor in Glioma: Signal Transduction, Neuropathology, Imaging, and Radioresistance. Neoplasia, 2010, 12, 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Levitzki A, EGF receptor as a therapeutic target. Lung Cancer, 2003, 41 Suppl 1, S9–14. [DOI] [PubMed] [Google Scholar]
- [12].Chan SK; Hill ME; Gullick WJ, The role of the epidermal growth factor receptor in breast cancer. J Mammary Gland Biol Neoplasia, 2006, 11, 3–11. [DOI] [PubMed] [Google Scholar]
- [13].Capala J; Bouchelouche K, Molecular imaging of HER2-positive breast cancer: a step toward an individualized ‘image and treat’ strategy. Curr Opin Oncol, 2010, 22, 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bange J; Zwick E; Ullrich A, Molecular targets for breast cancer therapy and prevention. Nat Med, 2001, 7, 548–552. [DOI] [PubMed] [Google Scholar]
- [15].Nagata Y; Lan KH; Zhou X; Tan M; Esteva FJ; Sahin AA; Klos KS; Li P; Monia BP; Nguyen NT; Hortobagyi GN; Hung MC; Yu D, PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell, 2004, 6, 117–127. [DOI] [PubMed] [Google Scholar]
- [16].Vogel CL; Cobleigh MA; Tripathy D; Gutheil JC; Harris LN; Fehrenbacher L; Slamon DJ; Murphy M; Novotny WF; Burchmore M; Shak S; Stewart SJ; Press M, Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol, 2002, 20, 719–726. [DOI] [PubMed] [Google Scholar]
- [17].Romond EH; Perez EA; Bryant J; Suman VJ; Geyer CE Jr.; Davidson NE; Tan-Chiu E; Martino S; Paik S; Kaufman PA; Swain SM; Pisansky TM; Fehrenbacher L; Kutteh LA; Vogel VG; Visscher DW; Yothers G; Jenkins RB; Brown AM; Dakhil SR; Mamounas EP; Lingle WL; Klein PM; Ingle JN; Wolmark N, Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med, 2005, 353, 1673–1684. [DOI] [PubMed] [Google Scholar]
- [18].Lower EE; Glass E; Blau R; Harman S, HER-2/neu expression in primary and metastatic breast cancer. Breast Cancer Res Treat, 2009, 113, 301–306. [DOI] [PubMed] [Google Scholar]
- [19].Silverman J; Liu Q; Bakker A; To W; Duguay A; Alba BM; Smith R; Rivas A; Li P; Le H; Whitehorn E; Moore KW; Swimmer C; Perlroth V; Vogt M; Kolkman J; Stemmer WP, Multivalent avimer proteins evolved by exon shuffling of a family of human receptor domains. Nat Biotechnol, 2005, 23, 1556–1561. [DOI] [PubMed] [Google Scholar]
- [20].Hicke BJ; Stephens AW; Gould T; Chang YF; Lynott CK; Heil J; Borkowski S; Hilger CS; Cook G; Warren S; Schmidt PG, Tumor targeting by an aptamer. J Nucl Med, 2006, 47, 668–678. [PubMed] [Google Scholar]
- [21].Nord K; Gunneriusson E; Ringdahl J; Stahl S; Uhlen M; Nygren PA, Binding proteins selected from combinatorial libraries of an alpha-helical bacterial receptor domain. Nat Biotechnol, 1997, 15, 772–777. [DOI] [PubMed] [Google Scholar]
- [22].Nilsson FY; Tolmachev V, Affibody molecules: new protein domains for molecular imaging and targeted tumor therapy. Curr Opin Drug Discov Devel, 2007, 10, 167–175. [PubMed] [Google Scholar]
- [23].Tolmachev V; Orlova A; Nilsson FY; Feldwisch J; Wennborg A; Abrahmsen L, Affibody molecules: potential for in vivo imaging of molecular targets for cancer therapy. Expert Opin Biol Ther, 2007, 7, 555–568. [DOI] [PubMed] [Google Scholar]
- [24].Ahlgren S; Tolmachev V, Radionuclide molecular imaging using Affibody molecules. Curr Pharm Biotechnol, 2010, 11, 581–589. [DOI] [PubMed] [Google Scholar]
- [25].Cai W; Chen K; He L; Cao Q; Koong A; Chen X, Quantitative PET of EGFR expression in xenograft-bearing mice using 64Cu-labeled cetuximab, a chimeric anti-EGFR monoclonal antibody. Eur J Nucl Med Mol Imaging, 2007, 34, 850–858. [DOI] [PubMed] [Google Scholar]
- [26].Carlsson J; Gedda L; Gronvik C; Hartman T; Lindstrom A; Lindstrom P; Lundqvist H; Lovqvist A; Malmqvist J; Olsson P; et al. , Strategy for boron neutron capture therapy against tumor cells with over-expression of the epidermal growth factor-receptor. Int J Radiat Oncol Biol Phys, 1994, 30, 105–115. [DOI] [PubMed] [Google Scholar]
- [27].Reilly RM; Kiarash R; Sandhu J; Lee YW; Cameron RG; Hendler A; Vallis K; Gariepy J, A comparison of EGF and MAb 528 labeled with 111In for imaging human breast cancer. J Nucl Med, 2000, 41, 903–911. [PubMed] [Google Scholar]
- [28].Velikyan I; Sundberg AL; Lindhe O; Hoglund AU; Eriksson O; Werner E; Carlsson J; Bergstrom M; Langstrom B; Tolmachev V, Preparation and evaluation of (68)Ga-DOTA-hEGF for visualization of EGFR expression in malignant tumors. J Nucl Med, 2005, 46, 1881–1888. [PubMed] [Google Scholar]
- [29].Nordberg E; Orlova A; Friedman M; Tolmachev V; Stahl S; Nilsson FY; Glimelius B; Carlsson J, In vivo and in vitro uptake of 111In, delivered with the affibody molecule (ZEGFR:955)2, in EGFR expressing tumour cells. Oncol Rep, 2008, 19, 853–857. [DOI] [PubMed] [Google Scholar]
- [30].Miao Z; Ren G; Liu H; Jiang L; Cheng Z, Small-animal PET imaging of human epidermal growth factor receptor positive tumor with a 64Cu labeled affibody protein. Bioconjug Chem, 2010, 21, 947–954. [DOI] [PubMed] [Google Scholar]
- [31].Su H; Seimbille Y; Ferl GZ; Bodenstein C; Fueger B; Kim KJ; Hsu YT; Dubinett SM; Phelps ME; Czernin J; Weber WA, Evaluation of [(18)F]gefitinib as a molecular imaging probe for the assessment of the epidermal growth factor receptor status in malignant tumors. Eur J Nucl Med Mol Imaging, 2008, 35, 1089–1099. [DOI] [PubMed] [Google Scholar]
- [32].Dijkers EC; Kosterink JG; Rademaker AP; Perk LR; van Dongen GA; Bart J; de Jong JR; de Vries EG; Lub-de Hooge MN, Development and characterization of clinical-grade 89Zr-trastuzumab for HER2/neu immunoPET imaging. J Nucl Med, 2009, 50, 974–981. [DOI] [PubMed] [Google Scholar]
- [33].Dijkers EC; Oude Munnink TH; Kosterink JG; Brouwers AH; Jager PL; de Jong JR; van Dongen GA; Schroder CP; Lub-de Hooge MN; de Vries EG, Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther, 2010, 87, 586–592. [DOI] [PubMed] [Google Scholar]
- [34].Smith-Jones PM; Solit DB; Akhurst T; Afroze F; Rosen N; Larson SM, Imaging the pharmacodynamics of HER2 degradation in response to Hsp90 inhibitors. Nat Biotechnol, 2004, 22, 701–706. [DOI] [PubMed] [Google Scholar]
- [35].Reddy S; Shaller CC; Doss M; Shchaveleva I; Marks JD; Yu JQ; Robinson MK, Evaluation of the anti-HER2 C6.5 diabody as a PET radiotracer to monitor HER2 status and predict response to trastuzumab treatment. Clin Cancer Res, 2011, 17, 1509–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Baum RP; Prasad V; Muller D; Schuchardt C; Orlova A; Wennborg A; Tolmachev V; Feldwisch J, Molecular imaging of HER2-expressing malignant tumors in breast cancer patients using synthetic 111In- or 68Ga-labeled affibody molecules. J Nucl Med, 2010, 51, 892–897. [DOI] [PubMed] [Google Scholar]
- [37].Longmire M, K. N, Ogawa M, Choyke PL, Kobayashi H, Establishment of multiple color in vivo molecular fluorescence imaging guided real-time surgery for the removal HER2 specific micrometastasis. Cancer Science, 2009, 100(6), 1099–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kosaka N; Ogawa M; Longmire MR; Choyke PL; Kobayashi H, Multi-targeted multi-color in vivo optical imaging in a model of disseminated peritoneal ovarian cancer. J Biomed Opt, 2009, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mitsunaga M, O. M, Kosaka N, Rosenburm LT, Choyke PL, Kobayashi H, Cancer Cell-Selective In Vivo Near Infrared Photoimmunotherapy Targeting Specific Membrane Molecules. Nature Medicine; in press, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yu WW; Guo YM; Zhu M; Cai XW; Zhu ZF; Zhao WX; Fu XL, Clinicopathological and prognostic significance of EGFR over-expression in esophageal squamous cell carcinoma: a meta-analysis. Hepatogastroenterology, 2011, 58, 426–431. [PubMed] [Google Scholar]
- [41].Koyama Y; Hama Y; Urano Y; Nguyen DM; Choyke PL; Kobayashi H, Spectral fluorescence molecular imaging of lung metastases targeting HER2/neu. Clin Cancer Res, 2007, 13, 2936–2945. [DOI] [PubMed] [Google Scholar]
- [42].Koyama Y; Barrett T; Hama Y; Ravizzini G; Choyke PL; Kobayashi H, In vivo molecular imaging to diagnose and subtype tumors through receptor-targeted optically labeled monoclonal antibodies. Neoplasia, 2007, 9, 1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ogawa M; Kosaka N; Choyke PL; Kobayashi H, In vivo molecular imaging of cancer with a quenching near-infrared fluorescent probe using conjugates of monoclonal antibodies and indocyanine green. Cancer Res, 2009, 69, 1268–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Folli S; Westermann P; Braichotte D; Pelegrin A; Wagnieres G; van den Bergh H; Mach JP, Antibody-indocyanin conjugates for immunophotodetection of human squamous cell carcinoma in nude mice. Cancer Res, 1994, 54, 2643–2649. [PubMed] [Google Scholar]
- [45].Ballou B; Fisher GW; Waggoner AS; Farkas DL; Reiland JM; Jaffe R; Mujumdar RB; Mujumdar SR; Hakala TR, Tumor Labeling in-Vivo Using Cyanine-Conjugated Monoclonal-Antibodies. Cancer Immunol Immun, 1995, 41, 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Miao Z; Ren G; Liu H; Jiang L; Cheng Z, Cy5.5-labeled Affibody molecule for near-infrared fluorescent optical imaging of epidermal growth factor receptor positive tumors. J Biomed Opt, 2010, 15, 036007. [DOI] [PubMed] [Google Scholar]
- [47].Gong H; Kovar J; Little G; Chen H; Olive DM, In vivo imaging of xenograft tumors using an epidermal growth factor receptor-specific affibody molecule labeled with a near-infrared fluorophore. Neoplasia, 2010, 12, 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chernomordik V; Hassan M; Lee SB; Zielinski R; Gandjbakhche A; Capala J, Quantitative analysis of Her2 receptor expression in vivo by near-infrared optical imaging. Mol Imaging, 2010, 9, 192–200. [PMC free article] [PubMed] [Google Scholar]
- [49].Sampath L; Kwon S; Ke S; Wang W; Schiff R; Mawad ME; Sevick-Muraca EM, Dual-labeled trastuzumab-based imaging agent for the detection of human epidermal growth factor receptor 2 overexpression in breast cancer. J Nucl Med, 2007, 48, 1501–1510. [DOI] [PubMed] [Google Scholar]
- [50].Ogawa M; Kosaka N; Choyke PL; Kobayashi H, Tumor-specific detection of an optically targeted antibody combined with a quencherconjugated neutravidin “quencher-chaser”: a dual “quench and chase” strategy to improve target to nontarget ratios for molecular imaging of cancer. Bioconjug Chem, 2009, 20, 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Barrett T; Koyama Y; Hama Y; Ravizzini G; Shin IS; Jang BS; Paik CH; Urano Y; Choyke PL; Kobayashi H, In vivo diagnosis of epidermal growth factor receptor expression using molecular imaging with a cocktail of optically labeled monoclonal antibodies. Clin Cancer Res, 2007, 13, 6639–6648. [DOI] [PubMed] [Google Scholar]
- [52].Kobayashi H; Choyke PL, Target-cancer-cell-specific activatable fluorescence imaging probes: rational design and in vivo applications. Acc Chem Res, 2011, 44, 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ogawa M; Kosaka N; Choyke PL; Kobayashi H, H-type dimer formation of fluorophores: a mechanism for activatable, in vivo optical molecular imaging. ACS Chem Biol, 2009, 4, 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hama Y; Urano Y; Koyama Y; Choyke PL; Kobayashi H, Activatable fluorescent molecular imaging of peritoneal metastases following pretargeting with a biotinylated monoclonal antibody. Cancer Res, 2007, 67, 3809–3817. [DOI] [PubMed] [Google Scholar]
- [55].Urano Y; Asanuma D; Hama Y; Koyama Y; Barrett T; Kamiya M; Nagano T; Watanabe T; Hasegawa A; Choyke PL; Kobayashi H, Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nat Med, 2009, 15, 104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ogawa M; Kosaka N; Regino CA; Mitsunaga M; Choyke PL; Kobayashi H, High sensitivity detection of cancer in vivo using a dual-controlled activation fluorescent imaging probe based on H-dimer formation and pH activation. Mol Biosyst, 2010, 6, 888–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ogawa M; Regino CA; Choyke PL; Kobayashi H, In vivo target-specific activatable near-infrared optical labeling of humanized monoclonal antibodies. Mol Cancer Ther, 2009, 8, 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kosaka N; Ogawa M; Paik DS; Paik CH; Choyke PL; Kobayashi H, Semiquantitative assessment of the microdistribution of fluorescence- labeled monoclonal antibody in small peritoneal disseminations of ovarian cancer. Cancer Sci, 2010, 101, 820–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ogawa M; Regino CA; Seidel J; Green MV; Xi W; Williams M; Kosaka N; Choyke PL; Kobayashi H, Dual-modality molecular imaging using antibodies labeled with activatable fluorescence and a radionuclide for specific and quantitative targeted cancer detection. Bioconjug Chem, 2009, 20, 2177–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gee MS; Upadhyay R; Bergquist H; Alencar H; Reynolds F; Maricevich M; Weissleder R; Josephson L; Mahmood U, Human breast cancer tumor models: molecular imaging of drug susceptibility and dosing during HER2/neu-targeted therapy. Radiology, 2008, 248, 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Gleysteen JP; Newman JR; Chhieng D; Frost A; Zinn KR; Rosenthal EL, Fluorescent labeled anti-EGFR antibody for identification of regional and distant metastasis in a preclinical xenograft model. Head Neck, 2008, 30, 782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kosaka N; Ogawa M; Longmire MR; Choyke PL; Kobayashi H, Multi-targeted multi-color in vivo optical imaging in a model of disseminated peritoneal ovarian cancer. J Biomed Opt, 2009, 14, 014023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ogawa M; Kosaka N; Longmire MR; Urano Y; Choyke PL; Kobayashi H, Fluorophore-quencher based activatable targeted optical probes for detecting in vivo cancer metastases. Mol Pharm, 2009, 6, 386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Schulke N; Varlamova OA; Donovan GP; Ma D; Gardner JP; Morrissey DM; Arrigale RR; Zhan C; Chodera AJ; Surowitz KG; Maddon PJ; Heston WD; Olson WC, The homodimer of prostate-specific membrane antigen is a functional target for cancer therapy. Proc Natl Acad Sci U S A, 2003, 100, 12590–12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Nakajima T; Mitsunaga M; Bander NH; Heston WD; Choyke PL; Kobayashi H, Targeted, Activatable, In Vivo Fluorescence Imaging of Prostate-Specific Membrane Antigen (PSMA) Positive Tumors Using the Quenched Humanized J591 Antibody-Indocyanine Green (ICG) Conjugate. Bioconjug Chem, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]