Abstract
Background
Guidelines recommend coronary artery bypass graft surgery (CABG) over percutaneous coronary intervention (PCI) for multivessel disease and severe left ventricular (LV) systolic dysfunction. However, CABG has not been compared with PCI in such patients in randomized trials.
Methods and Results
Patients with multivessel disease and severe LV systolic dysfunction (ejection fraction ≤35%) who underwent either PCI with everolimus-eluting stent (EES) or CABG were selected from the New York State registries. The primary outcome was long-term all-cause death. Secondary outcomes were individual outcomes of MI, stroke and repeat revascularization.
Among the 4,616 patients who fulfilled our inclusion criteria (1,351 EES and 3,265 CABG), propensity score matching identified 2,126 patients with similar propensity scores. At short-term, PCI was associated with a lower risk of stroke [HR=0.05; 95% CI 0.01–0.39; P=0.004] when compared with CABG. At long-term follow-up (median-2.9 years), PCI was associated with a similar risk of death (HR=1.01; 95% CI 0.81–1.28; P=0.91), a higher risk of MI (HR=2.16; 95% CI 1.42–3.28; P=0.0003), a lower risk of stroke (HR=0.57; 95% CI 0.33–0.97; P=0.04) and a higher risk of repeat revascularization (HR=2.54; 95% CI 1.88–3.44; P<0.0001). The test for interaction was significant (P=0.002) for completeness of revascularization, such that in patients where complete revascularization was achieved with PCI, there was no difference in MI between PCI and CABG.
Conclusions
Among patients with multivessel disease and severe LV systolic dysfunction, PCI with EES had comparable long-term survival when compared with CABG. PCI was associated with higher risk of MI (in those with incomplete revascularization) and repeat revascularization, and CABG was associated with higher risk of stroke.
Keywords: coronary artery bypass graft surgery, everolimus, percutaneous coronary intervention, systolic dysfunction
Introduction
The 2014 European Society of Cardiology (ESC) and the European Association of Cardio-Thoracic Surgery (EACTS) guidelines on myocardial revascularization gives a class I recommendation for coronary artery bypass graft surgery (CABG) but a class IIb recommendation for percutaneous coronary intervention (PCI) for revascularization in patients with chronic heart failure and systolic left ventricular (LV) dysfunction (ejection fraction ≤35%).1 However, the American College of Cardiology Foundation (ACCF)/American Heart Association (AHA) stable ischemic heart disease guidelines gives a class IIb recommendation for CABG for improving survival in patients with severe left ventricular (LV) systolic dysfunction (EF <35%) with no recommendations for PCI.2 The ACCF/AHA guidelines state “the choice of revascularization in patients with CAD and LV systolic dysfunction is best based on clinical variables (e.g., coronary anatomy, presence of diabetes mellitus, presence of CKD), magnitude of LV systolic dysfunction, patient preferences, clinical judgment, and consultation between the interventional cardiologist and the cardiac surgeon.”2 In addition, the 2009 ACCF/AHA appropriate use criteria for coronary revascularization considered patients with 3-vessel CAD and depressed LVEF as appropriate indication for CABG but uncertain for PCI in patients with CCS angina >Class III, and/or evidence of intermediate- to-high-risk findings on noninvasive testing.3 However, this was removed by the 2012 focused update and LVEF is not used as a variable to select between CABG or PCI.4
The reason to recommend CABG and not PCI for patients with LV systolic dysfunction and multivessel disease is based on the fact that data from older studies reported a survival benefit of CABG over medical therapy.5 However, there is lack of clinical trials testing PCI versus medical therapy in patients with LV systolic dysfunction. Moreover, trials comparing CABG versus PCI have routinely excluded patients with severe LV systolic dysfunction. In the absence of randomized trials comparing CABG versus PCI, we sought to assess the comparative effectiveness of CABG when compared with PCI in patients with severe LV systolic dysfunction, using data from the New York State registries. Our primary objective was to assess if CABG affords a survival advantage over PCI with a second generation drug eluting stent, everolimus-eluting stent.
Methods
Patient Selection
The patients were selected from the New York State Percutaneous Coronary Intervention Reporting System (PCIRS) and the Cardiac Surgery Reporting System (CSRS) registries, which are mandatory reporting systems for all PCI and CABG procedures performed in non-federal hospitals in New York State. For this analysis, patients with multi-vessel disease and severe left ventricular systolic dysfunction (defined as ejection fraction 35% or less) who underwent either PCI with EES or isolated CABG surgery between January 1, 2008 and December 31, 2011 in New York State were considered for inclusion Trained coordinators at participating hospitals enter the data for the registries. LVEF was measured by echocardiography, LV angiography, radionuclide studies, transesophageal echocardiogram or via other methods. Sites are instructed to enter the ejection fraction taken closest to the intervention. Samples of medical records are audited regularly to ensure data quality. Data validation for LVEF measurement was performed by periodic query of any cases with a missing or unusual ejection fraction entered in the database. The institutional review board at New York University School of Medicine approved the study.
Patient Inclusion and Exclusion Criteria
Patients were included if: 1) they had multivessel disease defined as severe stenosis (≥70%) in at least 2 major epicardial coronary arteries; 2) they had LV systolic dysfunction defined as an ejection fraction of 35% or less; and 3) they underwent PCI with implantation of EES or those who underwent isolated CABG.
Patients were excluded if: 1) they had severe (degree of stenosis ≥50%) left main coronary artery disease as these patients preferentially undergo CABG; 2) they had prior cardiac surgery (CABG or valve surgery) as such patients are unlikely to undergo repeat surgery; 3) they had myocardial infarction within 24 hours preceding the index procedure as these patients preferentially undergo PCI; 4) they had PCI with a stent other than EES or using a mixture of stents; 5) they had revascularization within 1 year prior to the index procedure; and 6) they had unstable hemodynamics or were in cardiogenic shock.
Patient Follow-up
Both in-hospital and long term follow up was obtained on the patients. The PCIRS and CSRS registries collect data on in-hospital events. The PCIRS and CSRS registries were linked across time and with each other to capture subsequent revascularization procedures (long-term). In addition, we linked the registries with the New York State Vital Statistics registry to obtain information on mortality. Finally, the registries were also linked with the Statewide Planning and Research Cooperative System (SPARCS) registry to obtain outcomes of MI and stroke during follow-up. The SPARCS registry collects comprehensive information on discharges from all nonfederal hospitals in New York State and contains information on patient diagnoses, procedures, admission and discharge dates, and discharge disposition for hospital discharges, ambulatory surgery, and emergency department admissions. Data are edited monthly to identify errors, audit reports are generated following monthly updates, and related data are verified with 2 data sources for consistency.6
Outcomes
Both short (within 30-days) and long-term outcomes were evaluated. The primary outcome of the study was long-term all-cause death. Secondary outcomes were individual outcomes of MI, stroke and repeat revascularization. Myocardial infarction included both procedural MI (defined as new Q waves after a procedure in both the PCIRS and the CSRS) and spontaneous MI (defined as an emergency admission with a principal diagnosis of MI or principal diagnosis of cardiogenic shock with a secondary diagnosis of MI). Similarly stroke was identified either as a complication at the time of index procedure or at readmission (principal diagnosis of stroke). Repeat revascularization was identified as any unstaged revascularization after the index procedure. Staged revascularization was defined as a non-emergent, non-target vessel revascularization within 90 days of the index procedure that was coded as intended to be staged in the index procedure and at the time of the staged procedure.
Statistical Analysis
Categorical variables were compared using the chi-square test and continuous variables were compared using the Student t-test.
Propensity Score Matching
The EES and CABG groups differed in baseline characteristics (Table 1). Propensity score matching was therefore used to assemble a paired cohort of patients who underwent PCI with EES or CABG with similar baseline characteristics. The propensity score is a conditional probability of having a particular exposure (EES vs. CABG) given a set of baseline measured covariates.7, 8 Propensity scores were calculated using a non-parsimonious multivariable logistic regression model9 using EES use as the dependent variable and the baseline characteristics outlined in Table 1 as covariates. Propensity score matching was performed using a 1:1 matching protocol without replacement (Greedy matching algorithm) using a caliper width equal to 0.2 of the standard deviation of the logit of the propensity score. Absolute standardized differences (ASD) were estimated for all the baseline covariates before and after matching to assess pre-match and post-match imbalance.10 ASD<10% for a given covariate indicates a relatively small imbalance.10 The risks of outcomes were analyzed in the matched cohort using a Cox proportional regression model after stratifying on the matched pairs.
Table 1.
Baseline characteristics of patients with severe LV systolic dysfunction and multivessel disease before and after propensity score matching
| Pre-Matching | Post-Matching | |||||
|---|---|---|---|---|---|---|
| Variables | EES (n=1,351) | CABG (n=3,265) | ASD | EES (n=1,063) | CABG (n=1,063) | ASD |
| Age (%) | ||||||
| <59 | 28.35% | 31.49% | 6.9% | 29.07% | 30.29% | 2.7% |
| 60–69 | 28.50% | 31.70% | 7.0% | 30.20% | 30.95% | 1.6% |
| 70–79 | 27.61% | 27.04% | 1.3% | 27.19% | 25.87% | 3.0% |
| >=80 | 15.54% | 9.77% | 17.4% | 13.55% | 12.89% | 1.9% |
| Mean age | 66.6 (12.1) | 65.1 (11.0) | 13.7% | 66.1 (11.9) | 65.6 (11.3) | 4.6% |
| Sex (%) | ||||||
| Men | 73.95% | 78.41% | 10.5% | 75.63% | 75.16% | 1.1% |
| Body Surface Area | 2.0 (0.3) | 2.0 (0.3) | 6.1% | 2.0 (0.3) | 2.0 (0.3) | 2.3% |
| Hispanic ethnic background | 13.92% | 9.83% | 12.7% | 12.51% | 14.68% | 6.3% |
| Race (%) | ||||||
| White | 75.65% | 83.64% | 20.0% | 78.27% | 78.83% | 1.4% |
| Black | 15.99% | 9.16% | 20.7% | 13.83% | 13.08% | 2.2% |
| Other | 8.36% | 7.20% | 4.4% | 7.90% | 8.09% | 0.7% |
| Diabetes Mellitus (%) | 42.26% | 45.57% | 6.7% | 42.33% | 44.12% | 3.6% |
| Ejection Fraction (%) | ||||||
| <20% | 6.96% | 8.48% | 5.7% | 6.77% | 7.06% | 1.1% |
| 20–29% | 36.71% | 35.62% | 2.3% | 35.84% | 35.37% | 1.0% |
| 30–35% | 56.33% | 55.90% | 0.9% | 57.38% | 57.57% | 0.4% |
| Previous MI | ||||||
| within 1–7 days before treatment | 23.61% | 25.67% | 4.8% | 25.59% | 23.99% | 3.7% |
| within 8–14 days before treatment | 3.70% | 9.49% | 23.5% | 4.61% | 4.99% | 1.8% |
| within 15–20 days before treatment | 0.89% | 2.36% | 11.7% | 1.03% | 1.41% | 3.4% |
| >20 days before treatment | 32.57% | 33.11% | 1.2% | 32.55% | 33.87% | 2.8% |
| No previous MI | 39.23% | 29.37% | 20.9% | 36.22% | 35.75% | 1.0% |
| Cerebrovascular Disease | 3.33% | 7.14% | 17.2% | 4.05% | 3.95% | 0.5% |
| Peripheral arterial disease | 13.10% | 14.89% | 5.1% | 14.30% | 14.77% | 1.3% |
| COPD | 9.99% | 15.38% | 16.2% | 11.48% | 11.01% | 1.5% |
| Malignant ventricular arrhythmia | 1.92% | 2.14% | 1.6% | 2.07% | 1.88% | 1.4% |
| Renal Failure | ||||||
| Requiring dialysis | 4.15% | 4.32% | 0.9% | 4.05% | 4.23% | 0.9% |
| Creatinine <1.3 | 64.47% | 62.88% | 3.3% | 63.97% | 63.88% | 0.2% |
| Creatinine 1.3–1.5 | 17.10% | 16.51% | 1.6% | 17.87% | 16.84% | 2.7% |
| Creatinine 1.6–2.0 | 9.70% | 11.15% | 4.8% | 9.31% | 10.25% | 3.2% |
| Creatinine >2.0 | 4.59% | 5.15% | 2.6% | 4.80% | 4.80% | 0.0% |
| No. of diseased vessel | ||||||
| 2, with proximal LAD artery | 21.69% | 16.81% | 12.4% | 25.68% | 25.49% | 0.4% |
| 2, without proximal LAD artery | 44.86% | 12.50% | 76.6% | 32.64% | 32.74% | 0.2% |
| 3, with proximal LAD artery | 12.81% | 39.36% | 63.4% | 16.27% | 16.18% | 0.3% |
| 3, without proximal LAD artery | 20.65% | 31.33% | 24.5% | 25.40% | 25.59% | 0.4% |
| Prior PCI | 35.16% | 17.64% | 40.5% | 27.94% | 28.60% | 1.5% |
CABG = coronary artery bypass graft surgery; COPD = chronic obstructive pulmonary disease; EES = everolimus eluting stent; LAD= left anterior descending coronary artery; PCI = percutaneous coronary intervention.
Subgroup Analyses
Subgroup analyses were performed based on anatomy (3-vessel disease vs. 2-vessel disease) and completeness of revascularization (complete vs. incomplete revascularization in the PCI cohort). For the subgroup analysis only the corresponding matched pairs in a subgroup were chosen in order to maintain the baseline balance between EES and CABG groups. A sensitivity analysis was performed using the entire matched population with terms for each subgroup and for the treatment-subgroup interaction term for each subgroup.
All reported P values are two-sided and are not adjusted for multiple testing. All analyses were performed with SAS version 9.3 (SAS Institute, Cary, NC). The event rates presented are Kaplan-Meier estimates.
Results
Among the patients in the registries, 4,616 patients with multivessel disease and severe LV systolic dysfunction satisfied our entry criteria. Among them, 1,351 (29%) patients underwent PCI with EES whereas 3,265 (71%) patients underwent isolated CABG. The baseline characteristics of the two groups before and after propensity score matching are outlined in Table 1. Prior to propensity score matching, there were differences in baseline characteristics between the two groups. Propensity score matching matched 1,063 patients who underwent PCI with EES with 1063 patients who underwent CABG. Post matching the ASD was <10% for all measured baseline variables indicating an adequate match (Table 1). Among patients who underwent PCI, completeness of revascularization was achieved in 277 (20%) of patients in the cohort prior to propensity score matching and 211 (20%) of patients in the matched cohort. The median follow-up was 2.9 years.
Short-term (within 30 days) Outcomes
Among the 2126 patients in the matched cohort, at short-term follow-up, PCI was associated with a 95% lower risk of stroke [0.1% vs. 1.8%; HR=0.05; 95% CI 0.01–0.39; P=0.004] with no significant difference in death (HR=0.62; 95% CI 0.31–1.24; P=0.17), myocardial infarction (HR=1.60; 95% CI 0.52–4.89; P=0.41) or repeat revascularization (HR=1.20; 95% CI 0.37–3.93; P=0.76) when compared with CABG.
Long-term Outcomes
Primary Outcome (Death)
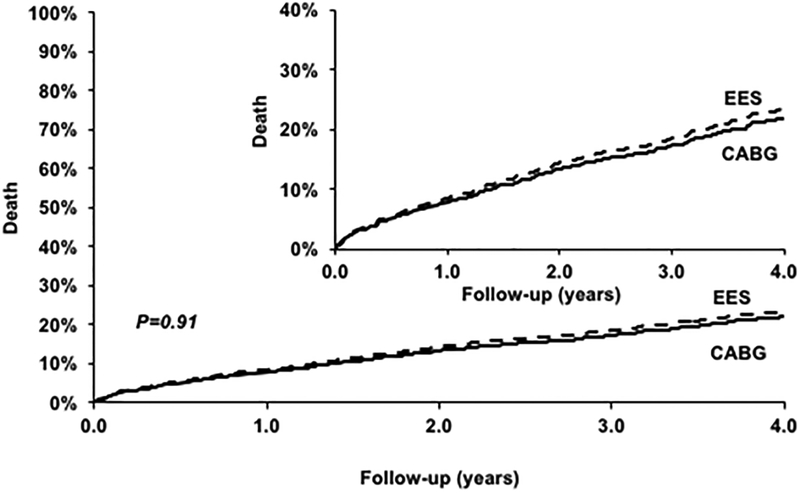
PCI was associated with a similar risk of death (HR=1.01; 95% CI 0.81–1.28; P=0.91) when compared with CABG (Figure 1) (Table 2). The test for interaction was significant (P=0.03) for number of vessels diseased such that there was a trend towards higher mortality with PCI in those with three vessel disease (HR=1.60; 95% CI 0.84–3.05; P=0.15) but lower mortality in those with two vessel disease (HR=0.67; 95% CI 0.84–3.05; P=0.15) but with no difference based on completeness of revascularization (Table 3). However, this test for interaction in the subgroup with two versus three vessel disease was no longer significant (P=0.26) in a sensitivity analysis using the entire set of matched data (eTable 1).
Figure 1.
Everolimus eluting stent (EES) versus Coronary Artery Bypass Graft surgery (CABG) for the risk of death.
Table 2.
Long-term outcomes with EES vs. CABG in the propensity score matched cohort
| Variables | No. of Patients | No. of Events | Event Rate* (4-year) | Hazard Ratio (95% CI) | P-value |
|---|---|---|---|---|---|
| Outcome: Death | |||||
| EES | 1063 | 185 | 25.22% | 1.01 [0.81, 1.28] | 0.91 |
| CABG | 1063 | 196 | 21.03% | Ref | |
| Outcome: MI | |||||
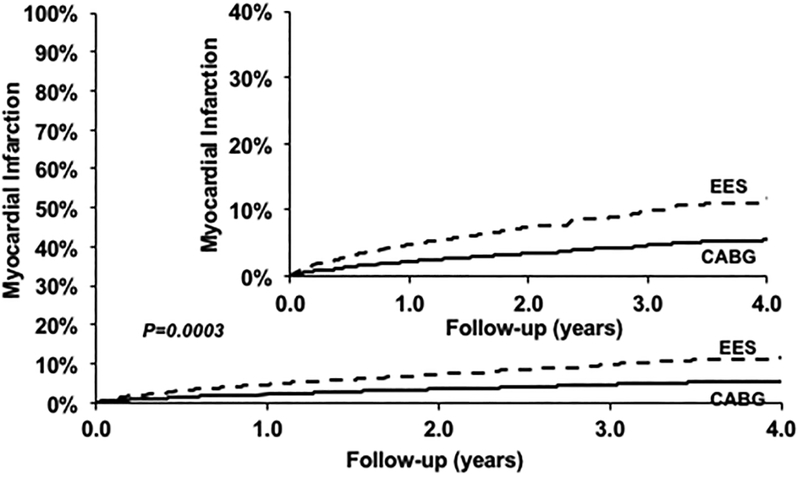
| EES | 1063 | 87 | 11.28% | 2.16 [1.42, 3.28] | 0.0003 |
| CABG | 1063 | 46 | 5.58% | Ref | |
| Outcome: Stroke | |||||
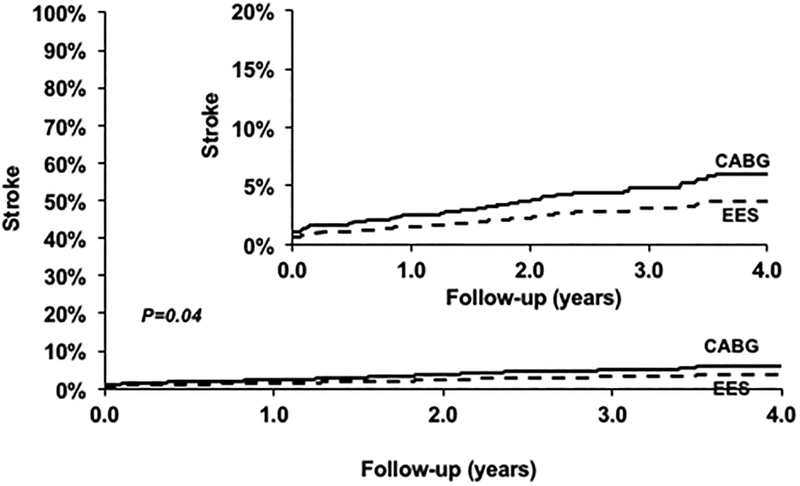
| EES | 1063 | 28 | 3.90% | 0.57 [0.33, 0.97] | 0.04 |
| CABG | 1063 | 51 | 5.92% | Ref | |
| Outcome: Revascularization | |||||
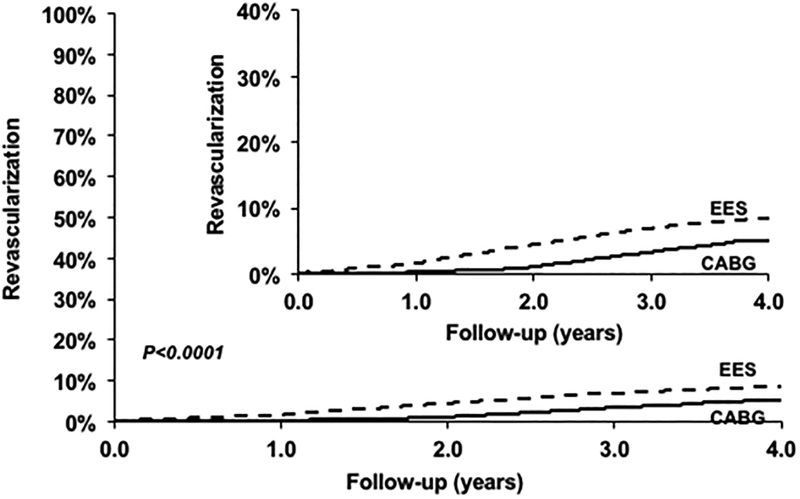
| EES | 1063 | 180 | 22.27% | 2.54 [1.88, 3.44] | <0.0001 |
| CABG | 1063 | 91 | 11.53% | Ref | |
Event rates are based on 4 year Kaplan Meier estimates. CABG = coronary artery bypass graft surgery; EES = everolimus eluting stent
Table 3.
Long-term outcomes in subgroups
| Variables | No. of Patients | No. of Events | Event Rate* (4-year) | Hazard Ratio (95% CI) | P-value | P-value for interaction |
|---|---|---|---|---|---|---|
| Outcome: Death | ||||||
| 3 Diseased Vessels | 0.03 | |||||
| EES | 166 | 29 | 26.30 | 1.60 [0.84, 3.05] | 0.15 | |
| CABG | 166 | 23 | 16.11 | Reference | ||
| 2 Diseased Vessels | ||||||
| EES | 300 | 46 | 23.11 | 0.67 [0.44, 1.03] | 0.07 | |
| CABG | 300 | 65 | 24.82 | Reference | ||
| Complete Revascularization | 0.52 | |||||
| EES | 211 | 33 | 19.01 | 0.88 [0.53, 1.45] | 0.61 | |
| CABG | 211 | 44 | 20.68 | Reference | ||
| Incomplete Revascularization | ||||||
| EES | 852 | 152 | 26.36 | 1.05 [0.81,1.36] | 0.69 | |
| CABG | 852 | 152 | 21.19 | Reference | ||
| Outcome: Myocardial Infarction | ||||||
| 3 Diseased Vessels | 0.07 | |||||
| EES | 166 | 14 | 11.89 | 6.00 [1.34, 26.81] | 0.02 | |
| CABG | 166 | 5 | 3.21 | Reference | ||
| 2 Diseased Vessels | ||||||
| EES | 300 | 17 | 10.14 | 1.22 [0.51, 2.95] | 0.66 | |
| CABG | 300 | 13 | 6.43 | Reference | ||
| Complete Revascularization | 0.002 | |||||
| EES | 211 | 9 | 5.77 | 0.58 [0.23, 1.48] | 0.26 | |
| CABG | 211 | 15 | 8.42 | Reference | ||
| Incomplete Revascularization | ||||||
| EES | 852 | 78 | 12.59 | 3.10 [1.87,5.13] | <0.0001 | |
| CABG | 852 | 31 | 4.81 | Reference | ||
| Outcome: Stroke | ||||||
| 3 Diseased Vessels | 0.48 | |||||
| EES | 166 | 3 | 2.58 | 0.40 [0.08, 2.06] | 0.27 | |
| CABG | 166 | 6 | 4.44 | Reference | ||
| 2 Diseased Vessels | ||||||
| EES | 300 | 9 | 6.01 | 0.83 [0.25, 2.73] | 0.76 | |
| CABG | 300 | 9 | 4.26 | Reference | ||
| Complete Revascularization | 0.50 | |||||
| EES | 211 | 5 | 3.28 | 0.38 [0.10,1.41] | 0.15 | |
| CABG | 211 | 12 | 6.68 | Reference | ||
| Incomplete Revascularization | ||||||
| EES | 852 | 23 | 4.01 | 0.62 [0.34,1.12] | 0.11 | |
| CABG | 852 | 39 | 5.71 | Reference | ||
| Outcome: Repeat Revascularization | ||||||
| 3 Diseased Vessels | 0.89 | |||||
| EES | 166 | 33 | 24.49 | 2.64 [1.32, 5.28] | 0.01 | |
| CABG | 166 | 16 | 12.45 | Reference | ||
| 2 Diseased Vessels | ||||||
| EES | 300 | 41 | 17.73 | 2.46 [1.29, 4.69] | 0.01 | |
| CABG | 300 | 24 | 11.64 | Reference | ||
| Complete Revascularization | 0.82 | |||||
| EES | 211 | 27 | 17.63 | 2.33 [1.07, 5.09] | 0.03 | |
| CABG | 211 | 15 | 10.18 | Reference | ||
| Incomplete Revascularization | ||||||
| EES | 852 | 153 | 23.35 | 2.58 [1.86,3.58] | <0.0001 | |
| CABG | 852 | 76 | 11.82 | Reference | ||
Event rates are based on 4 year Kaplan Meier estimates. CABG = coronary artery bypass graft surgery; EES = everolimus eluting stent; LAD= left anterior descending coronary artery.
Myocardial Infarction
PCI was associated with a higher risk of MI (HR=2.16; 95% CI 1.42–3.28; P=0.0003) when compared with CABG (Figure 2) (Table 2). This relationship was consistently seen in subgroups based on number of vessels diseased (Table 3, eTable 1). However, the test for interaction was significant (P=0.002) for completeness of revascularization, such that in patients where complete revascularization was achieved with PCI, there was no difference in MI between PCI and CABG (Table 3).
Figure 2.
Everolimus eluting stent (EES) versus Coronary Artery Bypass Graft surgery (CABG) for the risk of myocardial infarction.
Stroke
PCI was associated with a lower risk of stroke (HR=0.57; 95% CI 0.33–0.97; P=0.04) when compared with CABG (Figure 3) (Table 2). This relationship was consistently seen in subgroups based on number of vessels diseased and completeness of revascularization (Table 3, eTable 1).
Figure 3.
Everolimus eluting stent (EES) versus Coronary Artery Bypass Graft surgery (CABG) for the risk of stroke.
Repeat Revascularization
PCI was associated with a higher risk of repeat revascularization (HR=2.54; 95% CI 1.88–3.44; P<0.0001) when compared with CABG (Figure 4) (Table 2). This relationship was consistently seen in subgroups based on number of vessels diseased and completeness of revascularization (Table 3, eTable 1).
Figure 4.
Everolimus eluting stent (EES) versus Coronary Artery Bypass Graft surgery (CABG) for the risk of repeat revascularization.
Discussion
In patients with multivessel disease and severe left ventricular systolic dysfunction who underwent revascularization, we found no difference in long-term survival between PCI using the latest generation stents (EES) versus CABG. PCI was associated with increased risk of repeat revascularization and MI (in those with incomplete revascularization) whereas CABG was associated with higher upfront risk of stroke. These results were consistently seen in anatomic subgroups.
Revascularization in Patients with Severe Left Ventricular Systolic Dysfunction
Guideline recommendations favoring CABG and not PCI for patients with multivessel disease and LV systolic dysfunction are based on older studies showing a survival benefit of CABG over medical therapy in patients with LV systolic dysfunction (predominantly mild to moderate). In a subgroup analysis of the CASS registry, CABG prolonged survival when compared with medical treatment in a subgroup of 651 patients with severe LV systolic dysfunction.5 In a meta-analysis of seven randomized trials of CABG versus medical management, the survival advantage of CABG was apparent in subgroups at high-risk including those with LV systolic dysfunction.11 Similar survival advantage of CABG over medical therapy in patients with LV systolic dysfunction was also shown in observational studies.12, 13 However in a more contemporary trial- the STICH trial of subjects with LVEF <35%, there was no survival benefit of CABG when compared with optimal medical therapy after 5 years of follow-up, although there was a reduction in secondary endpoint of cardiovascular mortality and also a reduction in all-cause mortality in the as-treated group with CABG.14
The data to support PCI over medical therapy in patients with LV systolic dysfunction is scant with no randomized trials performed. Evidence from observational studies seems to suggest a higher survival with PCI compared with no revascularization.13 The guideline recommendations favoring CABG in patients with LV systolic dysfunction are largely based on the fact there is some evidence from randomized trials about the benefit of CABG over medical therapy whereas no such evidence exists for PCI. However, the common interpretation of these guidelines has been that CABG is superior to PCI and should be preferred for patients with LV systolic dysfunction. However, clinical trials of CABG versus PCI largely excluded patients with severe LV systolic dysfunction or included a very small proportion of such patients. As such comparison of CABG vs. PCI in such cohorts is based on observational studies. In the APPROACH registry, the survival with PCI was intermediate between CABG and no revascularization.13 Similar higher survival with CABG over PCI was reported in other observational studies.15, 16 However, other studies have shown similar survival between CABG and PCI in patients with LV systolic dysfunction.17 The limitations of these observational studies are the small sample size of the patients with LV systolic dysfunction, comparison of CABG versus balloon angioplasty only or PCI using bare metal stents or older generation drug eluting stents. Newer generation drug eluting stents have been shown to be superior to older generation drug eluting stents and bare metal stents in not only reducing the risk of restenosis but also death, MI or stent thrombosis.18–20 As such the mortality gap between CABG and PCI can potentially be minimized using newer generation stents. In fact, prior studies and analyses indicate that PCI with newer generation stents confer similar survival as that of CABG for the overall cohort.21–23
The current study extends these observations to patients with severe LV systolic dysfunction. Our study showed similar survival between CABG and PCI using second generation drug eluting stents. CABG was associated with a higher risk of stroke whereas PCI using EES was associated with a higher risk of revascularization. Of note, PCI was also associated with a higher risk of MI in those where complete revascularization was not achieved with PCI. The result of the present study, although hypothesis generation, suggests that PCI with a second generation DES may be an acceptable alternative to CABG in patients where complete revascularization can be achieved by PCI. Given the lower upfront risk of stroke (and perhaps death) and faster recovery period, it should perhaps be the preferred choice for the majority of patients with multivessel disease and severe LV systolic dysfunction. Randomized trials are needed to compare the outcomes between CABG and PCI in patients with LV systolic dysfunction.
Study Limitations
Although we used robust statistical techniques such as propensity score matching to account for baseline imbalance between the PCI and CABG groups, this does not account for unmeasured confounders such as frailty. Moreover we did not have data on anatomic (SYNTAX) risk score or clinical risk scores (STS or EuroSCORE) which are used to decide between PCI or CABG. The registry does not make a distinction between the cobalt chromium everolimus eluting stent versus the platinum chromium everolimus eluting stent nor captured the endeavor resolute stent which is another second generation DES. Moreover, the study results are mainly applicable to the type of stent used (everolimus eluting stent) and the CABG performed (largely single arterial bypass graft). Moreover, we do not have information on viability testing in these patients. In addition, we did not compare revascularization with medical therapy.
Conclusions
In patients with multivessel disease and severe LV systolic dysfunction, PCI with an everolimus-eluting stent resulted in similar survival as that of CABG. CABG was associated with higher upfront risk of stroke whereas PCI was associated with higher long-term risk of repeat revascularization and perhaps MI (in those with incomplete revascularization). PCI with newer generation drug eluting stents, in selected patients with left ventricular dysfunction, may therefore be an acceptable alternative to CABG in patients where complete revascularization is possible. The results are hypothesis generating and should be tested in future clinical trials.
Acknowledgments
Funding Source:
The study was funded by Abbott-Vascular.
Role of the funding source:
Study design, data analysis and interpretation, as well as preparation, review, and approval of the manuscript, were done independently by academic authors who were not governed by the funding sponsors. The funding source had no role in the design, conduct of this analysis, interpretation of the data; and preparation, review, or approval of this manuscript.
Footnotes
Disclosures:
Dr. Sripal Bangalore: Ad hoc consultant/speaker: Abbott Vascular. Research Grant: Abbott Vascular, NHLBI.
Yu Guo: None
Dr. Zaza Samadashvili: None
Dr. Saul Blecker: Supported by Agency for Healthcare Research and Quality (AHRQ) grant K08 HS23683
Dr. Edward L. Hannan: None
Reference
- 1.Authors/Task Force m, Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 esc/eacts guidelines on myocardial revascularization: The task force on myocardial revascularization of the european society of cardiology (esc) and the european association for cardio-thoracic surgery (eacts) developed with the special contribution of the european association of percutaneous cardiovascular interventions (eapci). Eur Heart J. 2014;35:2541–2619 [DOI] [PubMed] [Google Scholar]
- 2.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB 3rd, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR Jr., Smith SC Jr., Spertus JA, Williams SV, American College of Cardiology F, American Heart Association Task Force on Practice G, American College of P, American Association for Thoracic S, Preventive Cardiovascular Nurses A, Society for Cardiovascular A, Interventions, Society of Thoracic S. 2012 accf/aha/acp/aats/pcna/scai/sts guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the american college of cardiology foundation/american heart association task force on practice guidelines, and the american college of physicians, american association for thoracic surgery, preventive cardiovascular nurses association, society for cardiovascular angiography and interventions, and society of thoracic surgeons. J Am Coll Cardiol. 2012;60:e44–e164 [DOI] [PubMed] [Google Scholar]
- 3.Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. Accf/scai/sts/aats/aha/asnc 2009 appropriateness criteria for coronary revascularization: A report by the american college of cardiology foundation appropriateness criteria task force, society for cardiovascular angiography and interventions, society of thoracic surgeons, american association for thoracic surgery, american heart association, and the american society of nuclear cardiology endorsed by the american society of echocardiography, the heart failure society of america, and the society of cardiovascular computed tomography. J Am Coll Cardiol. 2009;53:530–553 [DOI] [PubMed] [Google Scholar]
- 4.Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. Accf/scai/sts/aats/aha/asnc/hfsa/scct 2012 appropriate use criteria for coronary revascularization focused update: A report of the american college of cardiology foundation appropriate use criteria task force, society for cardiovascular angiography and interventions, society of thoracic surgeons, american association for thoracic surgery, american heart association, american society of nuclear cardiology, and the society of cardiovascular computed tomography. J Am Coll Cardiol. 2012;59:857–881 [DOI] [PubMed] [Google Scholar]
- 5.Alderman EL, Fisher LD, Litwin P, Kaiser GC, Myers WO, Maynard C, Levine F, Schloss M. Results of coronary artery surgery in patients with poor left ventricular function (cass). Circulation. 1983;68:785–795 [DOI] [PubMed] [Google Scholar]
- 6.NYS Department of Health. Sparcs operations guide. https://www.Health.Ny.Gov/statistics/sparcs/training/docs/sparcs_operations_guide.Pdf. 2014; Access date May 10, 2015
- 7.Rosenbaum P, Rubin D. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55 [Google Scholar]
- 8.Rubin D Using propensity score to help design observational studies: Application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2:169–188 [Google Scholar]
- 9.Ahmed A, Husain A, Love TE, Gambassi G, Dell’Italia LJ, Francis GS, Gheorghiade M, Allman RM, Meleth S, Bourge RC. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: An observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: A matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398 [DOI] [PubMed] [Google Scholar]
- 11.Yusuf S, Zucker D, Peduzzi P, Fisher LD, Takaro T, Kennedy JW, Davis K, Killip T, Passamani E, Norris R, et al. Effect of coronary artery bypass graft surgery on survival: Overview of 10-year results from randomised trials by the coronary artery bypass graft surgery trialists collaboration. Lancet. 1994;344:563–570 [DOI] [PubMed] [Google Scholar]
- 12.O’Connor CM, Velazquez EJ, Gardner LH, Smith PK, Newman MF, Landolfo KP, Lee KL, Califf RM, Jones RH. Comparison of coronary artery bypass grafting versus medical therapy on long-term outcome in patients with ischemic cardiomyopathy (a 25-year experience from the duke cardiovascular disease databank). Am J Cardiol. 2002;90:101–107 [DOI] [PubMed] [Google Scholar]
- 13.Tsuyuki RT, Shrive FM, Galbraith PD, Knudtson ML, Graham MM, Investigators A. Revascularization in patients with heart failure. CMAJ. 2006;175:361–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, Ali IS, Pohost G, Gradinac S, Abraham WT, Yii M, Prabhakaran D, Szwed H, Ferrazzi P, Petrie MC, O’Connor CM, Panchavinnin P, She L, Bonow RO, Rankin GR, Jones RH, Rouleau JL. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brener SJ, Lytle BW, Casserly IP, Schneider JP, Topol EJ, Lauer MS. Propensity analysis of long-term survival after surgical or percutaneous revascularization in patients with multivessel coronary artery disease and high-risk features. Circulation. 2004;109:2290–2295 [DOI] [PubMed] [Google Scholar]
- 16.O’Keefe JH Jr., Allan JJ, McCallister BD, McConahay DR, Vacek JL, Piehler JM, Ligon R, Hartzler GO. Angioplasty versus bypass surgery for multivessel coronary artery disease with left ventricular ejection fraction < or = 40%. Am J Cardiol. 1993;71:897–901 [DOI] [PubMed] [Google Scholar]
- 17.Gioia G, Matthai W, Gillin K, Dralle J, Benassi A, Gioia MF, White J. Revascularization in severe left ventricular dysfunction: Outcome comparison of drug-eluting stent implantation versus coronary artery by-pass grafting. Catheter Cardiovasc Interv. 2007;70:26–33 [DOI] [PubMed] [Google Scholar]
- 18.Bangalore S, Toklu B, Amoroso N, Fusaro M, Kumar S, Hannan EL, Faxon DP, Feit F. Bare metal stents, durable polymer drug eluting stents, and biodegradable polymer drug eluting stents for coronary artery disease: Mixed treatment comparison meta-analysis. BMJ. 2013;347:f6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bangalore S, Kumar S, Fusaro M, Amoroso N, Attubato MJ, Feit F, Bhatt DL, Slater J. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: A mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation. 2012;125:2873–2891 [DOI] [PubMed] [Google Scholar]
- 20.Kaul U, Bangalore S, Seth A, Arambam P, Abhaychand RK, Patel TM, Banker D, Abhyankar A, Mullasari AS, Shah S, Jain R, Kumar PR, Bahuleyan CG, Investigators TU-I. Paclitaxel-eluting versus everolimus-eluting coronary stents in diabetes. N Engl J Med. 2015;373:1709–1719 [DOI] [PubMed] [Google Scholar]
- 21.Bangalore S, Guo Y, Samadashvili Z, Blecker S, Xu J, Hannan EL. Everolimus eluting stents versus coronary artery bypass graft surgery for patients with diabetes mellitus and multivessel disease. Circ Cardiovasc Interv. 2015;8:e002626. doi: 10.1161/CIRCINTERVENTIONS.115.002626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bangalore S, Guo Y, Samadashvili Z, Blecker S, Xu J, Hannan EL. Everolimus-eluting stents or bypass surgery for multivessel coronary disease. N Engl J Med. 2015;372:1213–1222 [DOI] [PubMed] [Google Scholar]
- 23.Park SJ, Ahn JM, Kim YH, Park DW, Yun SC, Lee JY, Kang SJ, Lee SW, Lee CW, Park SW, Choo SJ, Chung CH, Lee JW, Cohen DJ, Yeung AC, Hur SH, Seung KB, Ahn TH, Kwon HM, Lim DS, Rha SW, Jeong MH, Lee BK, Tresukosol D, Fu GS, Ong TK, Investigators BT. Trial of everolimus-eluting stents or bypass surgery for coronary disease. N Engl J Med. 2015;372:1204–1212 [DOI] [PubMed] [Google Scholar]