Abstract
Background
In patients with diabetes and multivessel disease, coronary artery bypass graft surgery (CABG) and percutaneous coronary intervention (PCI) are treatment options. However, there is paucity of data comparing CABG against newer generation stents.
Methods and Results
Patients included in the New York State registries who had diabetes and underwent isolated CABG or PCI with everolimus eluting stent (EES) for multivessel disease were included. Propensity score matching was used to assemble a cohort with similar baseline characteristics. The primary outcome was all-cause mortality. Secondary outcomes were myocardial infarction (MI), stroke and repeat revascularization. Short-term (within 30 days) and long-term outcomes were evaluated.
Among 16,089 patients with diabetes and multivessel disease, 8,096 patients with similar propensity scores were included. At short-term, EES was associated with a lower risk of death (HR=0.58; 95% CI 0.34–0.98; P=0.04) and stroke (HR=0.14; 95% CI 0.06–0.30; P<0.0001) but higher risk of myocardial infarction (HR=2.44; 95% CI 1.13–5.31; P=0.02). At long-term, EES was associated with a similar risk of death [425(10.50%) vs. 414(10.23%) events; HR=1.12; 95% CI 0.96–1.30; P=0.16], a lower risk of stroke [118(2.92%) vs. 157(3.88%) events; HR=0.76; 95% CI 0.58–0.99; P=0.04] but a higher risk of myocardial infarction [260(6.42%) vs. 166(4.10%) events; HR=1.64; 95% CI 1.32–2.04; P<0.0001] and repeat revascularization [889(21.96%) vs. 421(10.40%) events; HR=2.42; 95% CI 2.12–2.76; P<0.0001]. The higher risk of myocardial infarction was not seen in the subgroup of EES patients who underwent complete revascularization (HR=1.37; 95% CI 0.76–2.47; P=0.30).
Conclusion
In patients with diabetes and multivessel disease, EES was associated with lower upfront risk of death and stroke when compared with CABG. However at long-term, EES was associated with similar risk of death, a higher risk of MI (in those with incomplete revascularization) and repeat revascularization but a lower risk of stroke.
Keywords: coronary artery bypass graft surgery, multivessel disease, percutaneous coronary intervention
Subject codes: [7] Chronic ischemic heart disease, [24] Catheter-based coronary interventions: stents, [36] CV surgery: coronary artery disease, [190] Type 2 diabetes
Introduction
In patients with diabetes and coronary artery disease (CAD), coronary artery bypass graft surgery (CABG) and percutaneous coronary intervention (PCI) are revascularization options. The 2014 American College of Cardiology/American Heart Association (ACC/AHA) guidelines updated its previous recommendation in favor of CABG over PCI for patients with diabetes and multivessel disease from a class IIa to a class I indication,1, 2 driven largely by the results of the Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial. Similarly, the 2014 European Society of Cardiology/European Association for Cardio-Thoracic Surgery guidelines on Myocardial Revascularization recommends CABG over PCI in patients with diabetes and stable multivessel disease (Class I, Level of evidence: A).3
In the only well-powered, well conducted trial in patients with diabetes and multivessel disease-FREEDOM trial, with 1900 patients, CABG significantly reduced primary composite outcome of death, myocardial infarction (MI) or stroke at 5 years compared with 1st generation drug eluting stents (DES) (sirolimus eluting stent 51%, paclitaxel eluting stent 43%) (18.7% vs 26.6%; P=0.005), driven by reduction in MI (6.0% vs 13.9%, P<0.0001) and all-cause mortality (10.9% vs 16.3%, P=0.049).4 However, it is not known if the mortality benefit seen in FREEDOM extends to PCI with current generation stents such as the everolimus eluting stent (EES). We used data from the New York State registries to assess the comparative effectiveness of CABG when compared with PCI using EES on short and long-term cardiovascular outcomes.
Methods
Study Population
Patients with diabetes who underwent either PCI with EES or isolated CABG surgery for multivessel disease between January 1, 2008 and December 31, 2011 in New York State were included. The inclusion criteria were the following: 1) Patients with diabetes; 2) Patients with multivessel disease defined as severe stenosis (≥70%) in at least 2 major epicardial coronary arteries; and 3) Patients undergoing PCI with implantation of EES or those undergoing CABG. The exclusion criteria were the following: 1) Revascularization within 1 year prior to the index procedure; 2) Prior cardiac surgery (CABG or valve surgery) as such patients are unlikely to undergo repeat surgery; 3) Severe left main coronary artery disease (degree of stenosis ≥50%) as these patients preferentially undergo CABG; 4) PCI with a stent other than EES or using a mixture of stents; 5) Myocardial infarction (MI) within 24 hours preceding the index procedure as these patients preferentially undergo PCI; and 6) Unstable hemodynamics or in cardiogenic shock. The institutional review board at New York University School of Medicine approved the study.
Registries
The patients were identified using the New York State Department of Health’s (DOH) Percutaneous Coronary Intervention Reporting System (PCIRS) and the Cardiac Surgery Reporting System (CSRS) registries. These are mandatory reporting systems for all PCI and CABG procedures performed in non-federal hospitals in New York State. Data is entered by trained data coordinators at participating hospitals. Data quality is ensured by regular audits of a sample of medical records by DOH’s utilization review agent with regular feedback to sites.
Follow-up information on the patients undergoing PCI or CABG was obtained by linking the above registries with a number of other registries. The PCIRS and CSRS provide data on in-hospital events and on subsequent revascularization procedures. In addition, the registries were linked with the New York State Vital Statistics Death registry and to the Statewide Planning and Research Cooperative System (SPARCS) registry to obtain follow-up information. For the SPARCS registry, data are edited monthly to identify errors, audit reports are generated and related data are verified with 2 data sources for consistency.
Outcomes
The primary outcome of the study was all-cause death. Secondary outcomes were MI, stroke and repeat revascularization tabulated separately. Short-term (within 30 days) and long-term (including first 30 days) outcomes were evaluated. The definitions of outcomes are below.
MI was defined as either complication during the index admission after the procedure (procedural MI-defined as new Q waves in both the PCIRS and the CSRS) or MI at readmission (defined as an emergency admission with a principal diagnosis of MI or principal diagnosis of cardiogenic shock with a secondary diagnosis of MI). Similarly stroke was identified either as a complication at the time of index procedure or at readmission (principal diagnosis of stroke). Repeat revascularization was identified as any unstaged revascularization after the index procedure. Staged revascularization was defined as a non-target vessel revascularization within 90 days of the index procedure.
Statistical Analysis
Propensity Score Matching
Given baseline differences in characteristics between participants in the 2 groups (Table 1, Table S1), propensity score matching was used to identify a cohort of patients with similar baseline characteristics. The propensity score is a conditional probability of having a particular exposure (EES vs. CABG) given a set of baseline measured covariates.5, 6 A non-parsimonious multivariable logistic regression model7 using EES use as the dependent variable and all the baseline characteristics outlined in Table 1 and Table S1 as covariates was used to estimate the propensity scores. Matching was performed using a 1:1 matching protocol without replacement (Greedy matching algorithm) using a caliper width equal to 0.2 of the standard deviation of the logit of the propensity score. Absolute standardized differences (ASD) were estimated for all the baseline covariates before and after matching to assess pre-match and post-match imbalance.8 ASD<10% for a given covariate indicate a relatively small imbalance.8
Table 1.
Baseline characteristics before and after propensity score matching
| Pre-Matching | Post-Matching | |||||
|---|---|---|---|---|---|---|
| EES | CABG | ASD | EES | CABG | ASD | |
| Variables | (N=7,326) | (N=8,763) | (N=4,048) | (N=4,048) | ||
| Mean Age (yr) | 64.8±10.5 | 64.6±10.2 | 1.7 | 64.9±10.5 | 64.7±10.3 | 2.3 |
| Sex (%) | ||||||
| Male | 66 | 70 | 10.1 | 68 | 68 | 0.4 |
| Female | 34 | 30 | 10.1 | 32 | 32 | 0.4 |
| Race (%) | ||||||
| White | 70 | 81 | 26.1 | 76 | 75 | 1.4 |
| Black | 15 | 10 | 15.4 | 12 | 12 | 0.5 |
| Other | 15 | 9 | 18.7 | 12 | 13 | 1.4 |
| Ejection Fraction (%) | ||||||
| <20% | 1 | 2 | 11.8 | 1 | 1 | 0.0 |
| 20–29% | 3 | 7 | 17.9 | 5 | 5 | 0.9 |
| 30–39% | 5 | 13 | 25.6 | 8 | 8 | 0.4 |
| 40–49% | 12 | 19 | 18.0 | 15 | 16 | 2.1 |
| >=50% | 73 | 59 | 30.5 | 70 | 70 | 1.5 |
| missing | 5 | 0 | 30.0 | 1 | 1 | 0.3 |
| Previous myocardial infarction (%) | ||||||
| Within 1–7days | 14 | 17 | 10.2 | 16 | 16 | 0.1 |
| Within 8–14 days | 1 | 6 | 23.6 | 2 | 2 | 0.5 |
| Within 15–20 days | 0 | 1 | 10.0 | 0 | 1 | 1.3 |
| >20 days | 18 | 24 | 14.1 | 20 | 20 | 0.3 |
| No previous MI | 66 | 52 | 30.1 | 61 | 60 | 0.2 |
| Peripheral arterial disease (%) | 10 | 13 | 8.9 | 11 | 11 | 0.9 |
| Congestive heart failure (%) | ||||||
| None | 92 | 81 | 30.5 | 88 | 89 | 0.4 |
| At current admission | 5 | 15 | 33.0 | 8 | 8 | 0.3 |
| Before current admission | 3 | 4 | 3.3 | 4 | 4 | 1.0 |
| Prior PCI (%) | 35 | 20 | 34.0 | 27 | 27 | 0.2 |
| Renal Failure (%) | ||||||
| dialysis | 4 | 4 | 0.6 | 4 | 5 | 1.8 |
| <1.3 | 73 | 68 | 9.3 | 71 | 70 | 1.6 |
| 1.3–1.5 | 13 | 14 | 3.1 | 14 | 14 | 0.6 |
| 1.6–2.0 | 7 | 8 | 5.5 | 7 | 7 | 0.8 |
| >2.0 | 3 | 5 | 8.1 | 4 | 4 | 0.3 |
| No. of diseased vessels (%) | ||||||
| 2, with proximal LAD artery | 17 | 16 | 2.7 | 22 | 22 | 0.5 |
| 2, without proximal LAD artery | 54 | 15 | 89.5 | 31 | 31 | 0.2 |
| 3, with proximal LAD artery | 9 | 36 | 66.9 | 16 | 17 | 1.7 |
| 3, without proximal LAD artery | 19 | 33 | 31.1 | 31 | 30 | 0.8 |
Plus-minus values are means±SD. ASD = absolute standardized differences; CABG = coronary artery bypass graft surgery; COPD = chronic obstructive pulmonary disease; EES = everolimus eluting stent; LAD = left anterior descending artery. ASD <10% for a given covariate indicate a relatively small imbalance.
The risks of primary and secondary outcomes were further assessed in the matched cohort using a Cox proportional hazards regression model after stratifying on the matched pair. Unless otherwise specified, the event rates reports are raw events rates.
Subgroup Analyses
The following subgroup analyses based on anatomy were performed: 1) 3-vessel disease vs. 2-vessel disease; 2) with or without proximal left anterior descending (LAD) territory involvement; and 3) based on completeness of revascularization in the PCI cohort. For the subgroup analysis, only the corresponding match pairs in a subgroup were chosen in order to maintain the baseline balance between EES and CABG groups.
A P value <0.05 was used to denote statistical significance except for the subgroup analyses where a Bonferroni adjustment was used and a threshold of 0.006 (0.05/8) was used to denote statistical significance. All analyses were performed with SAS version 9.3 (SAS Institute, Cary, NC).
Results
We identified 16,089 patients with diabetes and multivessel disease who satisfied the inclusion criteria and none of the exclusion criteria. Of the 16,089 patients, 7,326 (45%) underwent PCI with EES and 8,763 (55%) patients underwent CABG. The baseline characteristics are outlined in table 1. Prior to propensity score matching there were differences (as indicated by ASD ≥10%) between the 2 groups. Propensity score matching matched 4,048 EES patients with 4,048 CABG patients with similar propensity scores. Post matching the ASD was <10% for all variables (Table 1 and Table S1).
Short-term (within 30 days) Outcomes
In the matched cohort, at short-term, EES was associated with a lower risk of death [23(0.57%) vs. 45(1.11%) events; HR=0.58; 95% CI 0.34–0.98; P=0.04] and stroke [10(0.25%) vs. 57(1.41%) events; HR=0.14; 95% CI 0.06–0.30; P<0.0001] but higher risk of myocardial infarction [18(0.44%) vs. 11(0.27%) events; HR=2.44; 95% CI 1.13–5.31; P=0.02] when compared with CABG (Figure 1).
Figure 1.
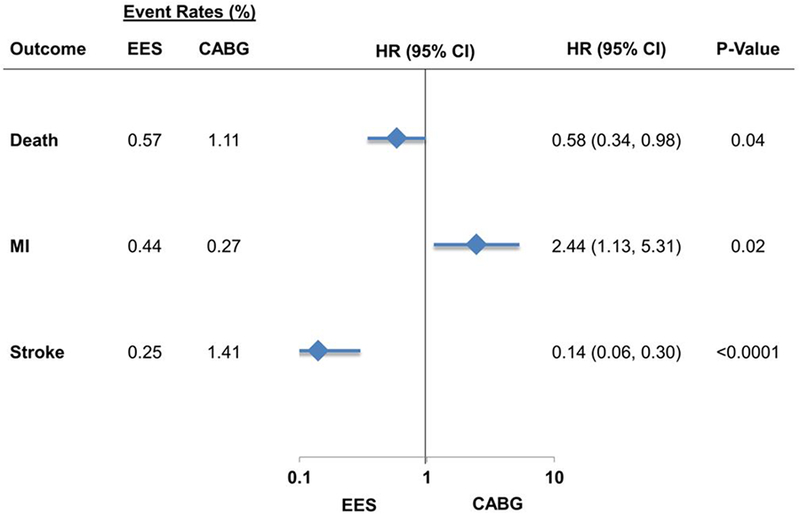
EES vs. CABG: Short-term (within 30 days) outcomes
Long-term (includes first 30 days) Outcomes
Death
In the matched cohort, at long-term follow-up, EES was associated with a similar risk of death [425(10.50%) vs. 414(10.23%) events; HR=1.12; 95% CI 0.96–1.30; P=0.16] when compared with CABG (Figure 2). This was true across anatomic subgroups based on number of vessel disease or proximal LAD involvement (Pinteraction >0.05) (Table 2).
Figure 2.
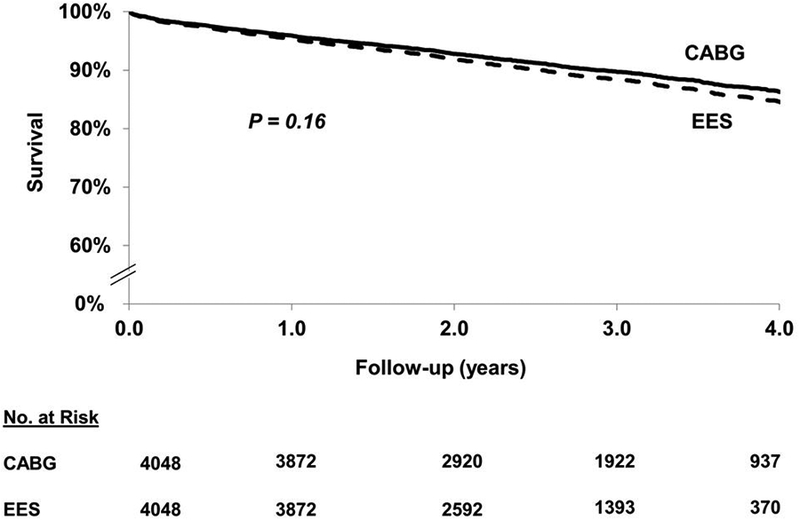
EES vs. CABG: long-term (includes first 30 days) death
Table 2.
Risk of death in anatomic subgroups
| Variables | No. of Patients | No. of Events | Event Rate (K-M estimate) | Hazard Ratio (95% CI) | P-value | P-value for interaction |
|---|---|---|---|---|---|---|
| 3 Diseased Vessels | 0.14* | |||||
| With or without proximal LAD artery | ||||||
| EES | 773 | 80 | 14.8 | 1.24(0.85,1.80) | 0.26 | |
| CABG | 773 | 69 | 11.9 | Reference | ||
| With proximal LAD artery | 0.70† | |||||
| EES | 278 | 26 | 11.3 | 1.14(0.64,2.02) | 0.66 | |
| CABG | 278 | 28 | 12.3 | Reference | ||
| Without proximal LAD artery | ||||||
| EES | 495 | 54 | 16.7 | 1.32(0.81,2.16) | 0.27 | |
| CABG | 495 | 41 | 11.8 | Reference | ||
| 2 Diseased Vessels | ||||||
| With or without proximal LAD artery | ||||||
| EES | 1008 | 72 | 10.9 | 0.85(0.60,1.19) | 0.34 | |
| CABG | 1008 | 89 | 11.3 | Reference | ||
| With proximal LAD artery | 0.55† | |||||
| EES | 250 | 20 | 12.8 | 1,00(0.52,1.92) | 0.99 | |
| CABG | 250 | 21 | 10.0 | Reference | ||
| Without proximal LAD artery | ||||||
| EES | 758 | 52 | 10.1 | 0.79(0.53,1.19) | 0.26 | |
| CABG | 758 | 68 | 11.8 | Reference | ||
| Complete Revascularization | 0.05‡ | |||||
| EES | 748 | 64 | 11.7 | 0.80(0.56,1.15) | 0.23 | |
| CABG | 748 | 81 | 13.3 | Reference | ||
| Incomplete Revascularizationϯ | ||||||
| EES | 3300 | 361 | 16.8 | 1.20(1.01,1.42) | 0.03 | |
| CABG | 3300 | 333 | 13.5 | Reference | ||
Test for interaction for the number of diseased vessels (3 diseased vessels vs. 2 diseased vessels);
Test for interaction based on the proximal LAD disease status (with vs. without proximal LAD).
Test for interaction based on completeness of revascularization (complete vs. incomplete) in the PCI cohort.
Based on incomplete revascularization in the PCI group. CABG = coronary artery bypass graft surgery; EES = everolimus eluting stent; LAD = left anterior descending artery.
Myocardial Infarction
In the matched cohort, EES was associated with a higher risk of myocardial infarction [260(6.42%) vs. 166(4.10%) events; HR=1.64; 95% CI 1.32–2.04; P<0.0001] when compared with CABG (Figure 3). The test for interaction was significant (Pinteraction =0.02) for the number of vessel disease such that the increased risk of MI with EES was seen in those with 3-vessel disease but not in those with 2-vessel disease (HR=1.34; 95% CI 0.85–2.12; P=0.21) (Table 2). The higher risk of myocardial infarction was not seen in the subgroup of EES patients who underwent complete revascularization (HR=1.37; 95% CI 0.76–2.47; P=0.30) although the test for interaction was not significant (Table 3).
Figure 3.
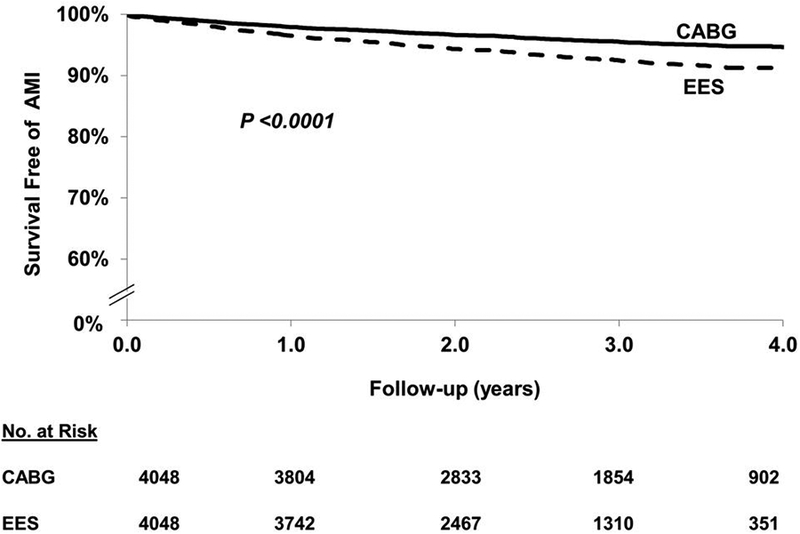
EES vs. CABG: long-term (includes first 30 days) myocardial infarction
Table 3.
Risk of myocardial infarction in anatomic subgroups
| Variables | No. of Patients | No. of Events | Event Rate (K-M estimate) | Hazard Ratio (95% CI) | P-value | P-value for interaction |
|---|---|---|---|---|---|---|
| 3 Diseased Vessels | 0.02* | |||||
| With or without proximal LAD artery | ||||||
| EES | 773 | 57 | 10.2 | 3.33(1.87,5.94) | <0.0001 | |
| CABG | 773 | 23 | 4.7 | Reference | ||
| With proximal LAD artery | 0.12† | |||||
| EES | 278 | 42 | 6.7 | 14.0(1.84,106.4) | 0.01 | |
| CABG | 278 | 18 | 3.8 | Reference | ||
| Without proximal LAD artery | ||||||
| EES | 495 | 15 | 12.1 | 2.57(1.39,4.77) | 0.003 | |
| CABG | 495 | 5 | 5.2 | Reference | ||
| 2 Diseased Vessels | ||||||
| With or without proximal LAD artery | ||||||
| EES | 1008 | 52 | 6.0 | 1.34(0.85,2.12) | 0.21 | |
| CABG | 1008 | 42 | 5.2 | Reference | ||
| With proximal LAD artery | 0.99† | |||||
| EES | 250 | 10 | 4.3 | 1.33(0.46,3.84) | 0.59 | |
| CABG | 250 | 9 | 4.4 | Reference | ||
| Without proximal LAD artery | ||||||
| EES | 758 | 42 | 6.5 | 1.35(0.81,2.24) | 0.25 | |
| CABG | 758 | 33 | 5.4 | Reference | ||
| Complete Revascularization | 0.52‡ | |||||
| EES | 748 | 33 | 5.6 | 1.37(0.76,2.47) | 0.30 | |
| CABG | 748 | 25 | 3.9 | Reference | ||
| Incomplete Revascularizationϯ | ||||||
| EES | 3300 | 227 | 9.5 | 1.69(1.33,2.14) | <0.0001 | |
| CABG | 3300 | 141 | 5.7 | Reference | ||
Test for interaction for the number of diseased vessels (3 diseased vessels vs. 2 diseased vessels);
Test for interaction based on the proximal LAD disease status (with vs. without proximal LAD).
Test for interaction based on completeness of revascularization (complete vs. incomplete) in the PCI cohort.
Based on incomplete revascularization in the PCI group. CABG = coronary artery bypass graft surgery; EES = everolimus eluting stent; LAD = left anterior descending artery.
Stroke
In the matched cohort, EES was associated with a lower risk of stroke [118(2.92%) vs. 157(3.88%) events; HR=0.76; 95% CI 0.58–0.99; P=0.04] when compared with CABG (Figure 4).
Figure 4.
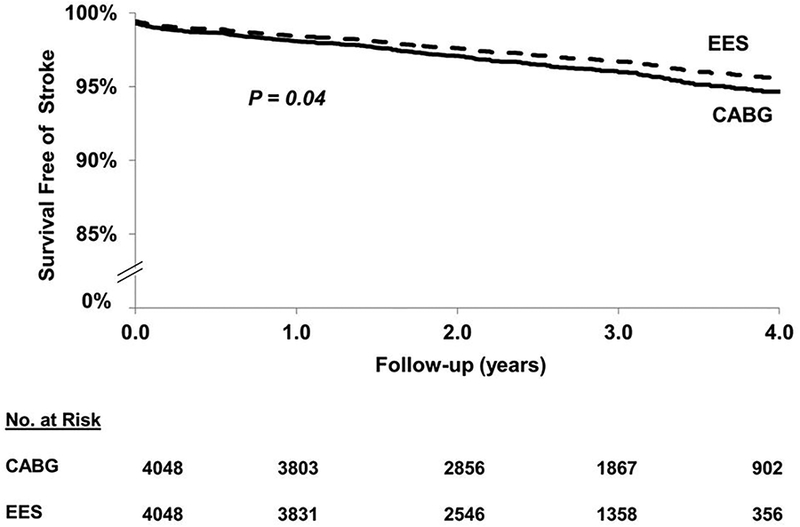
EES vs. CABG: long-term (includes first 30 days) stroke
Repeat Revascularization
In the matched cohort, EES was associated with a higher risk of repeat revascularization [889(21.96%) vs. 421(10.40%) events; HR=2.42; 95% CI 2.12–2.76; P<0.0001] when compared with CABG (Figure 5). The test for interaction was significant both for the number of vessel disease and completeness of revascularization for the magnitude of effect size rather than the direction such that the risk of repeat revascularization with EES (vs. CABG) was significantly higher in those with 3-vessel disease (vs. 2-vessel disease) and in those with incomplete revascularization (vs. complete revascularization) (Table 4).
Figure 5.
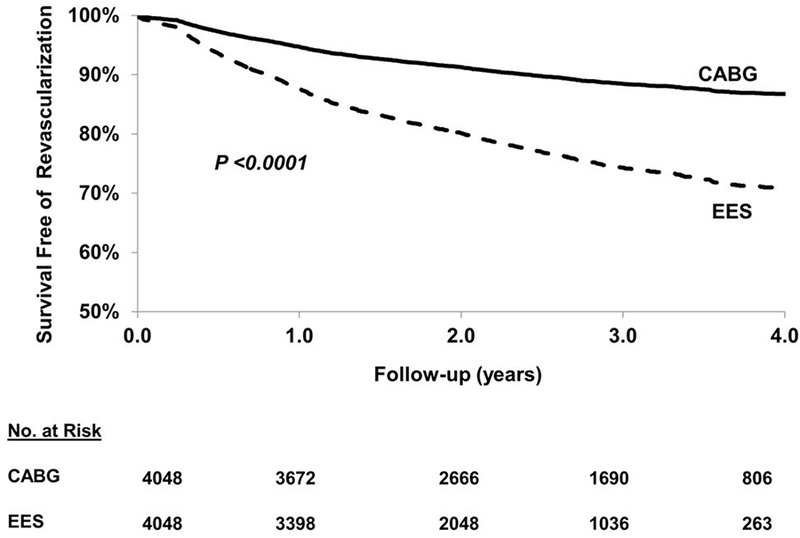
EES vs. CABG: long-term (includes first 30 days) repeat revascularization
Table 4.
Risk of repeat revascularization in anatomic subgroups
| Variables | No. of Patients | No. of Events | Event Rate (K-M estimate) | Hazard Ratio (95% CI) | P-value | P-value for interaction |
|---|---|---|---|---|---|---|
| 3 Diseased Vessels | 0.01* | |||||
| With or without proximal LAD artery | ||||||
| EES | 773 | 202 | 32.8 | 3.30(2.43,4.49) | <0.0001 | |
| CABG | 773 | 79 | 13.6 | Reference | ||
| With proximal LAD artery | 0.31† | |||||
| EES | 278 | 65 | 30.2 | 2.67(1.62,4.40) | 0.0001 | |
| CABG | 278 | 33 | 16.4 | Reference | ||
| Without proximal LAD artery | ||||||
| EES | 495 | 137 | 34.2 | 3.72(2.52,5.49) | <0.0001 | |
| CABG | 495 | 46 | 12.1 | Reference | ||
| 2 Diseased Vessels | ||||||
| With or without proximal LAD artery | ||||||
| EES | 1008 | 213 | 28.0 | 1.92(1.49,2.48) | <0.0001 | |
| CABG | 1008 | 115 | 14.8 | Reference | ||
| With proximal LAD artery | 1.44(0.86,2.40) | 0.16 | 0.21† | |||
| EES | 250 | 50 | 29.7 | |||
| CABG | 250 | 29 | 149 | Reference | ||
| Without proximal LAD artery | ||||||
| EES | 758 | 163 | 26.9 | 2.11(1.57,2.84) | <0.0001 | |
| CABG | 758 | 86 | 14.9 | Reference | ||
| Complete Revascularization | 0.005‡ | |||||
| EES | 748 | 120 | 23.3 | 1.56(1.12,2.16) | 0.01 | |
| CABG | 748 | 76 | 13.0 | Reference | ||
| Incomplete Revascularizationϯ | ||||||
| EES | 3300 | 769 | 29.8 | 2.62(2.26,3.03) | <0.0001 | |
| CABG | 3300 | 345 | 13.6 | Reference | ||
Test for interaction for the number of diseased vessels (3 diseased vessels vs. 2 diseased vessels);
Test for interaction based on the proximal LAD disease status (with vs. without proximal LAD).
Test for interaction based on completeness of revascularization (complete vs. incomplete) in the PCI cohort.
Based on incomplete revascularization in the PCI group. CABG = coronary artery bypass graft surgery; EES = everolimus eluting stent; LAD = left anterior descending artery.
Discussion
In a contemporary cohort of patients with diabetes (predominantly non-insulin dependent) and multivessel disease, with a sample size >4 times that enrolled in the FREEDOM trial, PCI with EES when compared with CABG was associated with lower short-term risk of death and stroke at the expense of a higher risk of MI. However, PCI with EES was associated with similar long-term risk of death, lower risk of stroke but higher risk of MI (in those with incomplete revascularization) and repeat revascularization when compared with CABG.
Revascularization in Patients with Diabetes
Patients with diabetes often have a high burden of atherosclerosis with extensive CAD and multivessel involvement.9 In addition, atherosclerosis tend to progress rapidly, leading to long and diffuse lesions in small caliber coronary arteries which renders revascularization challenging.10 Moreover, following revascularization, patients with diabetes are more likely to have increased risk of adverse consequences. For example, patients with diabetes undergoing PCI are more likely to develop restenosis, stent thrombosis and have higher rates of death and MI when compared with patients without diabetes.10, 11 Similarly, patients with diabetes undergoing CABG are more likely to have increased risk of perioperative complications such as deep sternal wound infections, renal failure and fatal and non-fatal cardiovascular events when compared with patients without diabetes.12, 13
In the Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) study, roughly similar percentage of follow-up events were attributable to the culprit lesion (12.9%) and non-culprit lesion (11.6%), attesting to the importance of both.14 Most non-culprit lesions that resulted in an event were angiographically mild, consistent with similar prior observations.15 Patients with diabetes have greater plaque burden16 with higher proportion of mixed plaques which have increased amount of necrotic core16 and hence a greater propensity to rupture (vulnerable plaque). CABG therefore offers better protection against future MI by bypassing a larger extent of potentially vulnerable plaque than the ‘spot’ treatment afforded by PCI. Moreover, PCI in patients with diabetes is associated with poor outcomes when compared with patients without diabetes with increased risk of restenosis and stent thrombosis and consequently increased risk of death or MI (due to stent related events). Both the above factors widen the gap in the outcomes between PCI and CABG. However, it can be hypothesized that stents which reduce the later risk, i.e. the risk of restenosis and stent thrombosis, can potentially bridge this gap between CABG and PCI.
In the FREEDOM trial, CABG significantly reduced the primary composite outcome compared with PCI driven by reduction in MI and all-cause mortality.4 Similarly, in the subgroup analysis of 452 patients with diabetes from the Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery (SYNTAX) trial, CABG was associated with numerically lower mortality (12.9% vs 19.5%; p=0.065), and MI (5.4% vs 9.0%; p=0.20) when compared with PCI at 5 years.17, 18 Consequently in a meta-analysis of 8 trials, revascularization of patients with diabetes and multivessel disease by CABG decreased long-term mortality compared with PCI using either BMS or DES.19 The DES used in the above studies were first generation DES. The newer generation DES (such as EES) have thinner struts (81 μm vs. 132–140 μm), thinner and more biocompatible polymer (7.8 μm vs. 13.7–17.8 μm) both of which reduce inflammation, thrombogenicity and promote rapid endothelialization when compared with the 1st generation DES.20 Data from randomized controlled trials,21 observational registries22 and meta-analyses of randomized trials21, 23 indicate reduction in morbidity and even mortality with newer generation stents when compared with older generation stents in the overall cohort of patients who underwent PCI. In the largest analysis so far in patients with diabetes, with data from 42 randomized trials and 22,844 patient years of follow-up we had shown that EES was the most efficacious (defined as lowest rate of restenosis) and safest (defined as lowest rate of stent thrombosis) when compared with all FDA approved stents including the bare metal stent.24 Consequently, in an indirect comparison analysis of 68 randomized trials that enrolled 24 015 patients with diabetes with a total of 71 595 patient-years of follow-up, there was similar mortality between CABG and PCI using EES, with CABG associated with numerically excess stroke and PCI with EES with numerically increased repeat revascularization and concluded that this hypothesis needs to be tested in future trials.25 The current study offers additional insights into the comparative effectiveness of CABG and PCI using newer generation DES. The current study reiterates the excess upfront risk of CABG with significant increase in death and stroke within 30 days when compared with PCI. However, PCI with EES was associated with similar risk of long term death as that of CABG. The results are largely concordant with the data from the BEST trial (overall cohort)26 and our publication on the overall cohort27 where PCI with EES was associated with increased risk of MI and repeat revascularization without any mortality difference when compared with CABG. However, data on individual endpoints for the subgroup of patients with diabetes was not presented. Our study with a sample size which is 22 folds larger than the 363 patients with diabetes included in the BEST trial, offers important additional insights on individual endpoints.
It therefore appears that the selection between PCI and CABG for patients with multivessel disease and diabetes should be based on weighing the risks of future myocardial infarction and repeat revascularization with PCI and the upfront risk of death and stroke with CABG. However, in patients with complete revascularization, the increased risk of MI with PCI was no longer present and the magnitude of increase in repeat revascularization diminished. It is therefore prudent to conclude that in contemporary clinical practice the decision between PCI and CABG in patients with diabetes should be based on the ability to achieve complete revascularization with PCI. If complete revascularization is not achievable for any reason with PCI, patients should be considered for CABG.
Study Limitations
This is a non-randomized study and therefore is limited by selection and ascertainment bias despite propensity score matching. It is conceivable that the highest risk patients are referred for CABG (resulting in worse outcomes in the CABG cohort). However, it is also conceivable that patients who are poor candidates for CABG (due to comorbidities) are referred for PCI (resulting in worse outcomes in the PCI cohort). The New York state registries do not make a distinction between the zotarolimus eluting Endeavor stent from the zotarolimus eluting Resolute stent and hence this was not included in the analysis even though the Resolute stent is a 2nd generation DES. Moreover, the registry does not distinguish between cobalt chromium and platinum chromium EES. Furthermore stent thrombosis is not captured in the database. However, most stent thrombosis present as death or MI-both of the outcomes were tracked in the current analysis. The long-term insulin use status was captured from the SPARCS registry using ICD-9 codes and is likely underestimated. The sample size of matched patients using insulin was too small to perform subgroup analysis based on insulin use status. However, the results are largely applicable to patients with non-insulin dependent diabetes. Although, there was no statistically significant difference in mortality between PCI and CABG differences may emerge with longer term follow-up or with larger sample size (Type 2 error). The Kaplan-Meier estimator for MI and repeat revascularization likely over-estimates the event rates for these outcomes as it does not account for the competing risk of death.
Conclusions
In a contemporary cohort of patients with diabetes and multivessel disease, CABG was associated with an upfront risk of death and stroke. However, PCI with EES was associated with similar risk of long-term death, higher risk of MI (in those with incomplete revascularization) and repeat revascularization but lower risk of stroke when compared with CABG. The decision between PCI and CABG in patients with diabetes should therefore be based on ability to achieve complete revascularization by PCI. Randomized controlled trials are needed to test these associations.
Supplementary Material
Acknowledgments
Funding Source:
The study was funded by Abbott-Vascular.
Role of the funding source:
Study design, data analysis and interpretation, as well as preparation, review, and approval of the manuscript, were done independently by academic authors who were not governed by the funding sponsors. The funding source had no role in the design, conduct of this analysis, interpretation of the data; and preparation, review, or approval of this manuscript.
Footnotes
Disclosures:
Dr. Sripal Bangalore: Ad hoc consultant/speaker: Abbott Vascular. Research Grant: Abbott Vascular.
Yu Guo: None
Dr. Zaza Samadashvili: None
Dr. Saul Blecker: Agency for Healthcare Quality and Research grant K08 HS23683
Dr. Jinfeng Xu: None
Dr. Edward L. Hannan: None
Contributor Information
Sripal Bangalore, New York University School of Medicine, New York, NY
Yu Guo, New York University School of Medicine, New York, NY
Zaza Samadashvili, School of Public Health, State University of New York at Albany, Albany, New York
Saul Blecker, New York University School of Medicine, New York, NY
Jinfeng Xu, New York University School of Medicine, New York, NY
Edward L. Hannan, School of Public Health, State University of New York at Albany, Albany, New York
References
- 1.Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, Fonarow GC, Lange RA, Levine GN, Maddox TM, Naidu SS, Ohman EM, Smith PK. 2014 acc/aha/aats/pcna/scai/sts focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the american college of cardiology/american heart association task force on practice guidelines, and the american association for thoracic surgery, preventive cardiovascular nurses association, society for cardiovascular angiography and interventions, and society of thoracic surgeons. Circulation. 2014;130:1749–1767 [DOI] [PubMed] [Google Scholar]
- 2.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB 3rd, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR Jr., Smith SC Jr., Spertus JA, Williams SV, American College of Cardiology F, American Heart Association Task Force on Practice G, American College of P, American Association for Thoracic S, Preventive Cardiovascular Nurses A, Society for Cardiovascular A, Interventions, Society of Thoracic S. 2012 accf/aha/acp/aats/pcna/scai/sts guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the american college of cardiology foundation/american heart association task force on practice guidelines, and the american college of physicians, american association for thoracic surgery, preventive cardiovascular nurses association, society for cardiovascular angiography and interventions, and society of thoracic surgeons. J Am Coll Cardiol. 2012;60:e44–e164 [DOI] [PubMed] [Google Scholar]
- 3.Authors/Task Force m, Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A, Authors/Task Force m. 2014 esc/eacts guidelines on myocardial revascularization: The task force on myocardial revascularization of the european society of cardiology (esc) and the european association for cardio-thoracic surgery (eacts)developed with the special contribution of the european association of percutaneous cardiovascular interventions (eapci). European heart journal. 2014;35:2541–2619 [DOI] [PubMed] [Google Scholar]
- 4.Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ, Rosenberg Y, Solomon SD, Desai AS, Gersh BJ, Magnuson EA, Lansky A, Boineau R, Weinberger J, Ramanathan K, Sousa JE, Rankin J, Bhargava B, Buse J, Hueb W, Smith CR, Muratov V, Bansilal S, King S 3rd, Bertrand M, Fuster V, Investigators FT. Strategies for multivessel revascularization in patients with diabetes. The New England journal of medicine. 2012;367:2375–2384 [DOI] [PubMed] [Google Scholar]
- 5.Rosenbaum P, Rubin D. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55 [Google Scholar]
- 6.Rubin D Using propensity score to help design observational studies: Application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2:169–188 [Google Scholar]
- 7.Ahmed A, Husain A, Love TE, Gambassi G, Dell’Italia LJ, Francis GS, Gheorghiade M, Allman RM, Meleth S, Bourge RC. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: An observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: A matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398 [DOI] [PubMed] [Google Scholar]
- 9.Nicholls SJ, Tuzcu EM, Crowe T, Sipahi I, Schoenhagen P, Kapadia S, Hazen SL, Wun CC, Norton M, Ntanios F, Nissen SE. Relationship between cardiovascular risk factors and atherosclerotic disease burden measured by intravascular ultrasound. J Am Coll Cardiol. 2006;47:1967–1975 [DOI] [PubMed] [Google Scholar]
- 10.Kip KE, Faxon DP, Detre KM, Yeh W, Kelsey SF, Currier JW. Coronary angioplasty in diabetic patients. The national heart, lung, and blood institute percutaneous transluminal coronary angioplasty registry. Circulation. 1996;94:1818–1825 [DOI] [PubMed] [Google Scholar]
- 11.Wenaweser P, Daemen J, Zwahlen M, van Domburg R, Juni P, Vaina S, Hellige G, Tsuchida K, Morger C, Boersma E, Kukreja N, Meier B, Serruys PW, Windecker S. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. 4-year results from a large 2-institutional cohort study. Journal of the American College of Cardiology. 2008;52:1134–1140 [DOI] [PubMed] [Google Scholar]
- 12.Thourani VH, Weintraub WS, Stein B, Gebhart SS, Craver JM, Jones EL, Guyton RA. Influence of diabetes mellitus on early and late outcome after coronary artery bypass grafting. The Annals of thoracic surgery. 1999;67:1045–1052 [DOI] [PubMed] [Google Scholar]
- 13.Whang W, Bigger JT Jr. Diabetes and outcomes of coronary artery bypass graft surgery in patients with severe left ventricular dysfunction: Results from the cabg patch trial database. The cabg patch trial investigators and coordinators. Journal of the American College of Cardiology. 2000;36:1166–1172 [DOI] [PubMed] [Google Scholar]
- 14.Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW, Investigators P. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235 [DOI] [PubMed] [Google Scholar]
- 15.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–671 [DOI] [PubMed] [Google Scholar]
- 16.Pundziute G, Schuijf JD, Jukema JW, van Werkhoven JM, Nucifora G, Decramer I, Sarno G, Vanhoenacker PK, Reiber JH, Wijns W, Bax JJ. Type 2 diabetes is associated with more advanced coronary atherosclerosis on multislice computed tomography and virtual histology intravascular ultrasound. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2009;16:376–383 [DOI] [PubMed] [Google Scholar]
- 17.Banning AP, Westaby S, Morice MC, Kappetein AP, Mohr FW, Berti S, Glauber M, Kellett MA, Kramer RS, Leadley K, Dawkins KD, Serruys PW. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: Comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol. 2010;55:1067–1075 [DOI] [PubMed] [Google Scholar]
- 18.Kappetein AP, Head SJ, Morice MC, Banning AP, Serruys PW, Mohr FW, Dawkins KD, Mack MJ, Investigators S. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the syntax trial. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2013;43:1006–1013 [DOI] [PubMed] [Google Scholar]
- 19.Verma S, Farkouh ME, Yanagawa B, Fitchett DH, Ahsan MR, Ruel M, Sud S, Gupta M, Singh S, Gupta N, Cheema AN, Leiter LA, Fedak PW, Teoh H, Latter DA, Fuster V, Friedrich JO. Comparison of coronary artery bypass surgery and percutaneous coronary intervention in patients with diabetes: A meta-analysis of randomised controlled trials. The lancet. Diabetes & endocrinology. 2013;1:317–328 [DOI] [PubMed] [Google Scholar]
- 20.Kolandaivelu K, Swaminathan R, Gibson WJ, Kolachalama VB, Nguyen-Ehrenreich KL, Giddings VL, Coleman L, Wong GK, Edelman ER. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation. 2011;123:1400–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dangas GD, Serruys PW, Kereiakes DJ, Hermiller J, Rizvi A, Newman W, Sudhir K, Smith RS Jr., Cao S, Theodoropoulos K, Cutlip DE, Lansky AJ, Stone GW. Meta-analysis of everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease: Final 3-year results of the spirit clinical trials program (clinical evaluation of the xience v everolimus eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions). JACC Cardiovasc Interv. 2013;6:914–922 [DOI] [PubMed] [Google Scholar]
- 22.Räber L, Magro M, Stefanini G, Kalesan B, van Domburg R, Onuma Y, Wenaweser P, Daemen J, Meier B, Jüni P, Serruys P, Windecker S. Very late coronary stent thrombosis of a newer-generation everolimus-eluting stent compared with early-generation drug-eluting stents: A prospective cohort study. Circulation. 2012;125:1110–1121 [DOI] [PubMed] [Google Scholar]
- 23.Bangalore S, Toklu B, Amoroso N, Fusaro M, Kumar S, Hannan EL, Faxon DP, Feit F. Bare metal stents, durable polymer drug eluting stents, and biodegradable polymer drug eluting stents for coronary artery disease: Mixed treatment comparison meta-analysis. BMJ. 2013;347:f6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bangalore S, Kumar S, Fusaro M, Amoroso N, Kirtane AJ, Byrne RA, Williams DO, Slater J, Cutlip DE, Feit F. Outcomes with various drug eluting or bare metal stents in patients with diabetes mellitus: Mixed treatment comparison analysis of 22 844 patient years of follow-up from randomised trials. BMJ. 2012;345:e5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bangalore S, Toklu B, Feit F. Outcomes with coronary artery bypass graft surgery versus percutaneous coronary intervention for patients with diabetes mellitus: Can newer generation drug-eluting stents bridge the gap? Circ Cardiovasc Interv. 2014;7:518–525 [DOI] [PubMed] [Google Scholar]
- 26.Park SJ, Ahn JM, Kim YH, Park DW, Yun SC, Lee JY, Kang SJ, Lee SW, Lee CW, Park SW, Choo SJ, Chung CH, Lee JW, Cohen DJ, Yeung AC, Hur SH, Seung KB, Ahn TH, Kwon HM, Lim DS, Rha SW, Jeong MH, Lee BK, Tresukosol D, Fu GS, Ong TK, Investigators BT. Trial of everolimus-eluting stents or bypass surgery for coronary disease. N Engl J Med. 2015;372:1204–1212 [DOI] [PubMed] [Google Scholar]
- 27.Bangalore S, Guo Y, Samadashvili Z, Blecker S, Xu J, Hannan EL. Everolimus-eluting stents or bypass surgery for multivessel coronary disease. N Engl J Med. 2015;372:1213–1222 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


