Abstract
Background
Blood transfusions are administered to children and adults with sickle cell anemia (SCA) for secondary stroke prevention, or as treatment for recurrent pain crises or acute anemia, but transfusion effects on cerebral hemodynamics and metabolism are not well-characterized.
Purpose
To compare blood transfusion-induced changes in hemo-metabolic parameters, including oxygen extraction fraction (OEF) and cerebral blood flow (CBF), within and between adults and children with SCA.
Study type
Prospective, longitudinal study.
Subjects
Adults with SCA (n=16) receiving simple (n=7) or exchange (n=9) transfusions and children with SCA (n=11) receiving exchange transfusions were scanned once when hematocrit was near nadir and again within seven days of transfusion. Adult controls without SCA or sickle trait (n=7) were scanned twice on separate days.
Field strength/sequence
3.0 Tesla T1-weighted, T2-weighted, and T2-relaxation-under-spin-tagging (TRUST) imaging, and phase contrast angiography.
Assessment
Global OEF was computed as the relative difference between venous oxygenation (from TRUST) and arterial oxygenation (from pulse oximetry). Global CBF was computed as total blood flow to the brain normalized by intracranial tissue volume.
Statistical tests
Hemo-metabolic variables were compared using two-sided Wilcoxon signed-rank tests; associations were analyzed using two-sided Spearman’s correlation testing.
Results
In adults with SCA, post-transfusion OEF=0.38±0.05 was lower (p=0.001) than pre-transfusion OEF=0.45±0.09. Change in OEF was correlated with increases in hematocrit (p=0.02; rho=−0.62) and with pre-transfusion hematocrit (p=0.02; rho=0.65). OEF changes after transfusion were greater (p=0.002) in adults receiving simple versus exchange transfusions. Post-transfusion CBF=77.7±26.4 ml/100g/min was not different (p=0.27) from pre-transfusion CBF=82.3±30.2 ml/100g/min. In children with SCA, both post-transfusion OEF=0.28±0.04 and CBF=76.4±26.4 were lower than pre-transfusion OEF=0.36±0.06 (p=0.004) and CBF=96.4±16.5 (p=0.004).
Data conclusion
Cerebral OEF reduces following transfusions in adults and children with SCA. CBF reduces following transfusions more often in children compared to adults, indicating that vascular reserve capacity may remain near exhaustion post-transfusion in many adults.
Keywords: oxygen extraction fraction, cerebral blood flow, blood transfusion, sickle cell anemia, phase contrast angiography
INTRODUCTION
Sickle cell anemia (SCA) is a genetically-inherited blood disorder that results in the production of erythrocytes homozygous for the pathological variant hemoglobin-S (HbS), phenotype HbSS, in the place of normal hemoglobin phenotype AA (HbAA) (1). The presence of HbS causes erythrocytes to sickle, resulting in pathological effects including chronic anemia (2) and an elevated risk for cerebral vasculopathy, silent cerebral infarction, and stroke (3–5). In children with SCA, transcranial Doppler ultrasound is often used to screen for those at increased risk for stroke (6), and regular blood transfusion therapy is initiated for primary stroke prevention in those with time-averaged maximum velocities greater than 200 cm/s in the middle cerebral artery (7). In adults with SCA, no validated method of stroke risk assessment exists; however, after initial stroke, regular blood transfusions are often administered for secondary stroke prevention. Regular transfusion therapy is also used for severe complications of SCA such as management of frequent pain episodes unresponsive to hydroxyurea therapy and for treatment of acute or persistent severe anemia, but the effects of transfusions on cerebral hemodynamics are not fully understood. Investigating hemodynamic and metabolic responses to transfusions could help identify avenues for stratifying adults with SCA at high risk for stroke to transfusion therapy.
Cerebral oxygen extraction fraction (OEF) has been identified as a potential marker of hemo-metabolic impairment in SCA (8). OEF is defined as the ratio of oxygen consumed (CMRO2; mL O2/100g tissue/minute) to oxygen delivered (product of cerebral blood flow and blood oxygen content) and therefore reflects the balance of metabolic demand and hemodynamic oxygen delivery. Recently, OEF and CBF responses to blood transfusions have been elegantly characterized with MRI in children with SCA (9); however, given that transcranial Doppler ultrasound has not been shown to be an effective method for assessing stroke risk in adults as it has in children, it is unclear whether these MRI findings translate to adults with SCA.
Adults with SCA receiving transfusions exhibit higher OEF and CBF at baseline than those treated with oral hydroxyurea (8). Such elevations in CBF (10) are expected to compensate for reduced hematocrit (Figure 1). However, similar to progression of impairment in arterial steno-occlusive disease (11,12), CBF increases may be insufficient to compensate for anemia, resulting in increases in OEF (Figure 1). This issue is fundamental, as even while undergoing routine blood transfusions, many patients remain at elevated risk for new or recurrent stroke (13). In these patients, it is hypothesized that the hemo-metabolic response to transfusion may be suboptimal, and if so, such patients may be candidates for more aggressive therapies such as stem cell transplant (14). To understand this possibility, characterizing how OEF and CBF respond to blood transfusions is a necessary prerequisite. Furthermore, the hemodynamic consequences of simple transfusions (i.e., infusion of erythrocytes with hemoglobin A; HbA), which serve to primarily increase hematocrit but also have the effect of diluting hemoglobin S (HbS), versus exchange transfusions (i.e., removal of HbS blood and replacement with HbA blood), which serve to primarily decrease HbS and blood viscosity but also maintain baseline hematocrit, are not well-characterized.
Figure 1. Physiological model.
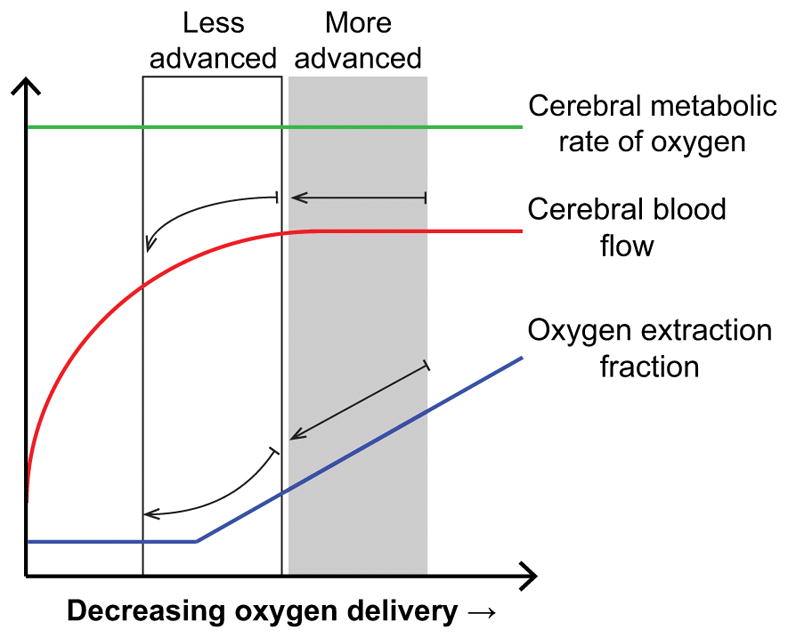
This schematic displays hypothesized behavior of cerebral metabolic rate of oxygen (CMRO2), cerebral blood flow (CBF), and oxygen extraction fraction (OEF) as hematocrit decreases in individuals with sickle cell anemia (SCA) based on models adapted from the arterial steno-occlusive disease literature (11,12). When autoregulatory increases in CBF are sufficient to compensate for anemia and reduced oxygen carrying capacity of blood, OEF is not expected to increase. In patients with SCA who cannot increase CBF sufficiently due to exhaustion of autoregulatory reserve capacity or arterial steno-occlusion, and who are in more advanced stages of disease, OEF will increase for unchanging CMRO2. Depending on the extent of impairment, small improvements in hematocrit following transfusion may produce variable changes in cerebral hemodynamics. In those who have less advanced disease (black box), both CBF and OEF are expected to reduce, while in those who have more advanced disease (gray box), transfusions will reduce OEF, but have little change on CBF.
The primary objective of this study was to quantify hemo-metabolic response to blood transfusions in patients with SCA and to evaluate whether this response is different between adults and children with SCA. We hypothesized that OEF would reduce following transfusion in adults with SCA, while CBF would remain unchanged on average. The premise for this hypothesis is that autoregulatory capabilities are exhausted in adults relative to children with SCA due to disease chronicity; therefore, even moderate improvements in hematocrit due to transfusion do not fully meet cerebral metabolic requirements, causing CBF to remain elevated in adults with SCA (Figure 1).
MATERIALS AND METHODS
Participant Demographics
All participants provided informed, written consent for this prospective Institutional Review Board-approved study. Adults (age range=16–40 years) with SCA and age- and race-matched controls and children (age range=6–15 years) with SCA were recruited. In SCA participants, hemoglobin phenotypes SS or S/Beta0 thalassemia served as inclusion criteria; other hemoglobin phenotypes, inability to complete two scans, independent major neurological or psychiatric condition (e.g., including but not limited to Parkinson’s, schizophrenia, and multiple sclerosis), and contraindications to MRI, including pregnancy or claustrophobia, served as exclusion criteria. In adult control participants, hemoglobin phenotype AA served as inclusion criteria; other phenotypes, including sickle trait (i.e., hemoglobin phenotype AS), prior stroke or silent cerebral infarction, or other major neurological conditions as described above were exclusions.
Data Acquisition
MRI scans were acquired at 3.0T (Philips Achieva; Best, The Netherlands) in adult and pediatric SCA participants receiving regular blood transfusion therapy and in adult control participants. In SCA participants, pre-transfusion MRI data were acquired late in the transfusion cycle when the participant’s hematocrit was near nadir, and post-transfusion data were acquired within seven days after transfusion. In control participants, MRI data were acquired on two separate days less than one year apart without any interval intervention. Hematocrit and hemoglobin values, including percentage hemoglobin S (HbS%), were obtained from venipuncture for both time points in SCA participants and during the first time point only, the day of the first MRI, in controls. Control and SCA participants were requested to refrain from consuming caffeine or alcohol within 24 hours of their study MRI.
Anatomical Imaging
T1-weighted imaging (magnetization-prepared-rapid-gradient-echo; TR/TE=8.2/3.7 ms; spatial resolution=1.0×1.0×1.0 mm3), T2-weighted fluid-attenuated inversion recovery (FLAIR) imaging (TR/TI/TE=11,000/2800/120 ms; spatial resolution=1.0×1.1×4.0 mm3), and time-of-flight (TOF) MR angiography (MRA) of the head (TR/TE=23/3.5 ms; spatial resolution=0.5×0.8×0.7 mm3) and neck (TR/TE=18.6/3.2 ms; spatial resolution=0.9×0.9×3.0 mm3) were performed for volumetric analysis, prior infarct assessment, to assess intracranial arterial stenosis, and to assess cervical arterial stenosis and guide placement of quantitative flow measurements, respectively.
OEF Measurement
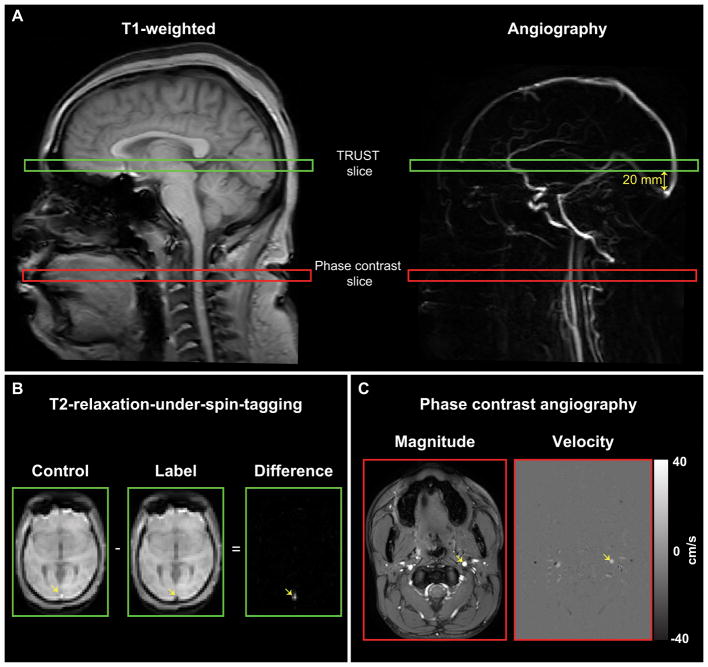
T2-relaxation-under-spin-tagging (TRUST) (15,16) data were acquired twice per session (spatial resolution=3.4×3.4×5 mm3, τCPMG=10 ms, eTE=0, 40, 80, and 160 ms, TR/TE=1978/3.6 ms, averages=3) (8,16). Control and venous-labeled (transfer insensitive labeling technique; TILT) TRUST images were acquired from a slice containing the superior sagittal sinus, approximately 20 mm superior to the confluence of the sinuses; the image slices were positioned parallel to the anterior commissure/posterior commissure line (Figure 2A).
Figure 2. Imaging methods.
The locations of the T2-relaxation-under-spin-tagging (TRUST) and phase contrast (PC) imaging slices are shown overlaid on sagittal T1-weighted and angiographic images (A). The imaging slices for the TRUST sequence were oriented parallel to the anterior commissure/posterior commissure line and were located approximately 20 mm superior to the confluence of the sinuses. The slices for the PC imaging sequences were placed approximately at the location of the C2 vertebra and were orthogonal to the vessel of interest (as shown for the left internal carotid artery; ICA). Sample control and label images for the TRUST sequence are shown for one effective echo-time (eTE) (B). The superior sagittal sinus (yellow arrow) appears bright in the control image and dark in the label image. The subtracted signal between control and label pairs at four eTEs was used to calculate the venous oxygenation inside the sagittal sinus for oxygen extraction fraction (OEF) measurement. Sample magnitude and velocity images for the PC imaging sequence are shown for the left ICA (yellow arrows); positive velocity indicated inferior-to-superior flow direction and negative velocity indicated superior-to-inferior flow direction) (C). Each vessel was segmented, and the mean velocity and cross-sectional area of each vessel were used to calculate global cerebral blood flow (CBF).
CBF Measurement
Phase contrast (PC) angiography data were acquired from the left and right internal carotid arteries (ICAs) and left and right vertebral arteries (TR/TE=20/7 ms; spatial resolution=0.5×0.5 mm2; velocity encoding gradient (venc)=40 cm/s). Imaging slices were planned using the TOF neck MRA, and the slices were placed orthogonal to the orientation of the vessels at the approximate location of the C2 vertebra (Figure 2A) (17). Magnitude images and phase images, which were subsequently converted to velocity images, were recorded (18).
Data Processing
Anatomical Imaging
To evaluate vasculopathy presence and extent, T2-weighted FLAIR MRI and TOF head MRA data were independently examined by two board-certified neuroradiologists to identify presence of infarcts and stenosis of a major intracranial artery (intracranial internal carotid artery segments and/or first segment of the middle cerebral artery, posterior cerebral artery, or anterior cerebral artery), respectively.
OEF Measurement
TRUST data were pair-wise subtracted (Figure 2B), and venous blood water T2 was quantified in the superior sagittal sinus (8); venous blood water T2 values were then converted to venous oxygen saturation (Yv) using previously-characterized calibration curves generated with bovine blood, which exhibits similar magnetic properties as blood with HbA (15). Yv was then utilized along with arterial oxygenation saturation (Ya), which was acquired from two-wavelength pulse oximetry, to calculate OEF=(Ya−Yv)/Ya. Finally, the OEF values from both TRUST measurements were averaged to obtain the OEF value for each participant. The observable from this analysis was a global measure of the OEF. As a secondary analysis, we compared OEF values pre- and post-transfusion in adults and children with SCA from the bovine model with a recently-reported TRUST calibration model specific for HbS (21).
CBF Measurement
Flow velocities inside the left and right ICAs along with the left and right vertebral arteries were computed from the phase contrast magnitude images as previously described (Figure 2C) (17). T1-weighted images were brain-extracted (19) and segmented (20) to identify regions of gray matter parenchyma, white matter parenchyma, and cerebrospinal fluid. Global CBF values were quantified from phase contrast data as the total blood flow to the brain (summation of the product of the mean velocity in each vessel and the vessel cross-sectional area) normalized to the total tissue volume (total gray and white matter). The observable from this analysis was a global measure of CBF (ml/100g/min).
Data Analysis and Statistical Considerations
Statistical analyses were performed using non-parametric methods and R Statistical Software (R Foundation for Statistical Computing, Vienna, Austria). Continuous variables are reported as mean ± standard deviation. All statistical tests required a two-tailed p<0.05 for significance.
Repeatability and Temporal Stability of OEF Measurements
Global OEF measurements from TRUST data acquired in healthy controls were assessed for repeatability (within the same scan session) and temporal stability (with longitudinal measurements) using data from control participants. Repeatability was assessed using Bland-Altman analysis (22) comparing OEF measurements computed from the two separate TRUST acquisitions. Temporal stability was assessed via a Wilcoxon-signed-rank rest between OEF measurements made from two different scan sessions. Intra-class correlation coefficients (ICC) were computed for both analyses. Agreement between values were defined as: poor (ICC<0.40), fair (ICC=0.40–0.59), good (ICC=0.60–0.74), or excellent (ICC>0.74) (23).
Hemo-metabolic Responses to Transfusion
Post-transfusion OEF and CBF values in adults and children with SCA were compared separately to pre-transfusion values using a Wilcoxon signed-rank test. In adults with SCA, post-transfusion OEF and CBF values were compared to adult control values using a Wilcoxon rank-sum test.
Effects of Age on Response to Transfusion
The effects of age on hemo-metabolic responses to transfusion were assessed using Spearman’s partial correlation analysis, independently for OEF and CBF. Age was used as the independent variable, and the transfusion-related change in OEF or CBF was used as the dependent variable, while controlling for the effects of change in hematocrit and severity of intracranial vasculopathy.
Associations with Hematologic Variables
Transfusion-related changes in OEF and CBF in adults with SCA were examined using Spearman’s correlation tests as a function of (i) hematocrit change from pre- to post-transfusion, (ii) pre-transfusion hematocrit, (iii) HbS change from pre- to post-transfusion, and (iv) pre-transfusion HbS. OEF and CBF changes were assessed identically in children with SCA. Corrections for multiple comparisons were made using the Benjamini-Hochberg procedure for controlling false discovery rate (24).
Transfusion Type
An exploratory analysis was conducted between subgroups of adults with SCA receiving simple versus exchange transfusions. Changes in hematocrit, HbS, and OEF from pre- to post-transfusion were assessed for each subgroup using a Wilcoxon signed-rank test. Finally, the change in OEF from pre- to post-transfusion was compared between the two subgroups using a Wilcoxon rank-sum test.
RESULTS
Demographic and Hematologic Variables
Data analysis was conducted in a total cohort of 34 participants: adult SCA participants on blood transfusion therapy (n=16; age=24.6±7.0 years; sex=56% female), healthy age- and race-matched adult controls scanned at two time points (n=7; age=26.1±6.2 years; sex=57% female), and pediatric SCA participants on blood transfusion therapy (n=11; age=9.6±2.7 years; sex=55% female). In SCA participants, pre-transfusion MRI data were acquired 4.9±19.8 days before a scheduled blood transfusion, when hematocrit was near nadir; one subject missed their subsequent transfusion cycle, resulting in a large variance for this measurement. Post-transfusion MRI data were acquired 2.1±2.7 days after a blood transfusion. In control participants, MRI data were acquired at two time points 202.4±67.7 days apart. Hematocrit values and hemoglobin concentrations were obtained from each participant within 0.7±1.4 days of pre-transfusion MRI and within 1.4±2.3 days of post-transfusion MRI. Three of 15 adult SCA participants exhibited cervical carotid artery stenosis >50%. All three had significant intracranial stenosis (bilateral moyamoya). A summary of demographic, hematologic, and neurologic variables of this cohort is provided in Table 1. In summary, 44% of adults with SCA received a simple transfusion while 56% received an exchange transfusion; 100% of children with SCA received an exchange transfusion.
Table 1.
Summary of control and sickle cell anemia participants.
| Adult Control participants (n=7) | Adult SCA participants (n=16) | Pediatric SCA participants (n=11) | |
|---|---|---|---|
| Demographic variables | |||
| Age (years) | 26.1 ± 6.2 | 24.6 ± 7.0 | 9.6 ± 2.7 |
| Sex (% Female) | 57 | 56 | 55 |
| Race (% Black) | 100 | 100 | 100 |
| Hematologic variables | |||
| Transfusion type (%) | |||
| Simple | - | 44 | 0 |
| Exchange | - | 56 | 100 |
| Indication for transfusion (%) | |||
| Secondary stroke prevention | - | 81 | 100 |
| Recurrent pain crisis | - | 25 | 9 |
| Anemia | - | 50 | 0 |
| Other | - | 6 | 0 |
| Imaging Timing (Days) | |||
| Time point 1 to Transfusion | - | 4.9 ± 19.8* | |
| Transfusion to Time point 2 | - | 2.1 ± 2.7 | |
| Hematocrit (%) | |||
| Time point 1 | 42.2 ± 6.8 | 24.8 ± 4.4 | 27.5 ± 3.2 |
| Time point 2 | - | 28.6 ± 4.2 | 33.4 ± 2.9 |
| Hemoglobin-S (%) | |||
| Time point 1 | 0 | 43.0 ± 10.3 | 39.1 ± 13.2 |
| Time point 2 | - | 26.2 ± 14.0 | 26.2 ± 13.2 |
| Neurologic variables | |||
| Overt Stroke (%) | 0 | 69 | 9 |
| Silent cerebral infarction (%) | 0 | 75 | 45 |
| Intracranial stenosis>50% (%) | 0 | 50 | 0 |
| Extracranial stenosis >50% (%)** | 0 | 20 | 0 |
one subject missed their subsequent transfusion cycle, resulting in a large variance for this measurement
Neck MRA was performed in 15 of 16 adults and 9 of 11 children
Repeatability and Temporal Stability of OEF Measurements
Intra-session venous oxygenation measurements demonstrated excellent agreement (Figure 3A; ICC=0.98; 95% confidence interval (CI): 0.97–0.99; p<0.001). OEF measurements in controls were not different between scans at two time points (Figure 3B); the inter-session OEF measurements also demonstrated excellent agreement (ICC=0.87; 95% CI: 0.51–0.98; p=0.001). CBF measurements in controls showed that CBF was not different between scans at two time points (Figure 3C); the inter-session CBF measurements also demonstrated excellent agreement (ICC=0.90; 95% CI: 0.42–0.99; p=0.004).
Figure 3. Repeatability and temporal stability of OEF and CBF measurements.
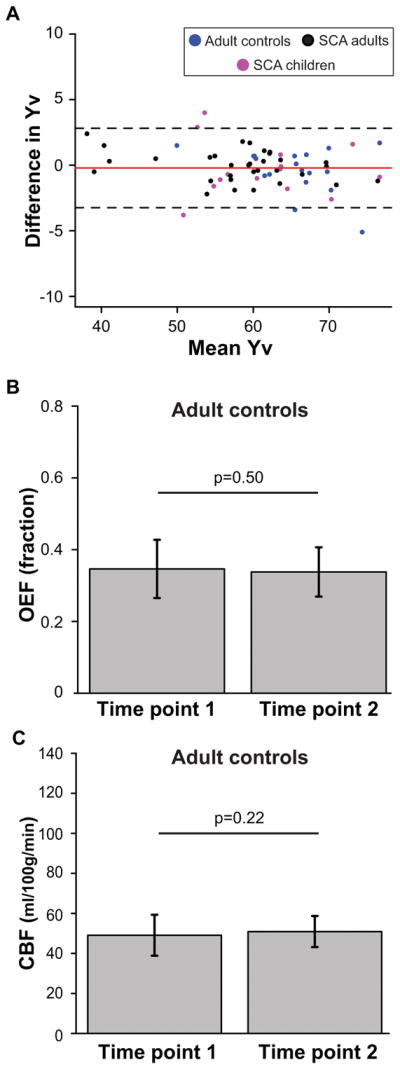
A Bland-Altman plot for repeatability analysis (repeats=2) for venous oxygen saturation (Yv), the key measurement to assess OEF, is shown here (A). Yv measurements are shown for adult controls (blue), adults with SCA (black), and children with SCA (purple) at two time points along with the mean (red) and 95% confidence intervals (black) of the difference. Bar graphs of mean global OEF (B) and CBF (C) measurements are shown at two time points. These analyses support that OEF measurements with TRUST are repeatable within a scan session; furthermore, OEF and CBF measurements are largely stable over time in healthy adults who did not receive interventions affecting cerebral hemodynamics.
Hemo-metabolic Responses to Transfusion
Oxygen Extraction Fraction Response to Transfusion
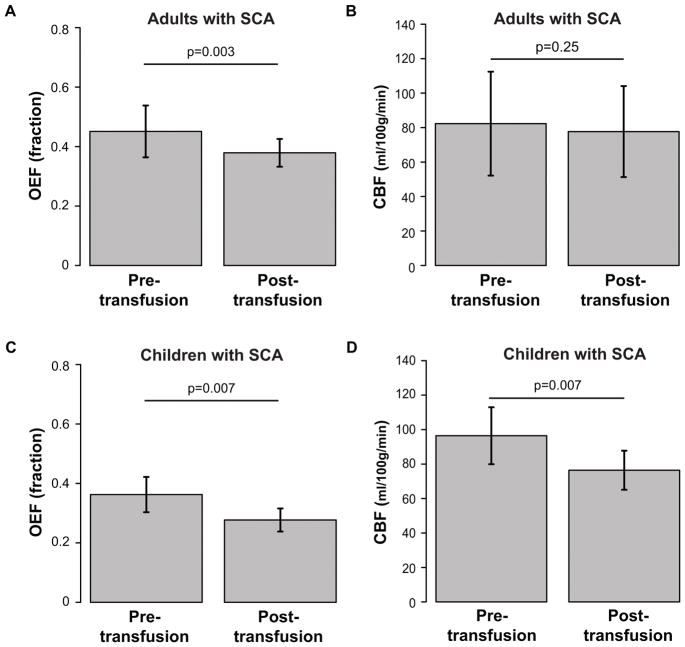
In adults with SCA (n=16), mean post-transfusion OEF=0.38±0.05 was significantly (p=0.001) lower than pre-transfusion OEF=0.45±0.09 (Figure 4A). OEF decreased after transfusion (ΔOEF=−0.10±0.05) in 12 of 16 participants (75%) and was unchanged or increased very slightly (ΔOEF=0.01±0.01) in 4 of 16 participants (25%).
Figure 4. Physiological changes.
Bar graphs of mean oxygen extraction fraction (OEF; left) and cerebral blood flow (CBF; right) are shown in adults with SCA (top) and children with SCA (bottom) from pre- and post-transfusion scans. Error bars represent one standard deviation. OEF significantly (n=16; p<0.001) reduces post-transfusion compared to pre-transfusion in adults with SCA (n=16; p=0.003) (A) and in children with SCA (n=10; p=0.007) (C); CBF remains unchanged on average in adults with SCA (n=14; p=0.25) (B) but significantly reduces post-transfusion in children with SCA (n=10; p=0.007) (D).
In children with SCA (n=10), mean post-transfusion OEF=0.28±0.04 was significantly (p=0.004) lower than pre-transfusion OEF=0.36±0.06 (Figure 4C); OEF measurements were not available in one pediatric participant. OEF decreased after transfusion (ΔOEF=−0.10±0.05) in 9 of 10 participants (90%) and was slightly higher (ΔOEF=0.03) in 1 of 10 participants (10%).
Post-transfusion OEF in adults with SCA (OEF=0.38±0.05) exhibited a strong trend (p=0.07) for not reducing to levels observed in controls (OEF=0.34±0.07), while OEF in children with SCA (OEF=0.28±0.04) reduced following transfusion to a level found in healthy age-matched controls (OEF=0.28) (9).
For completeness, we compared OEF values from the conventional TRUST calibration (presented above) to a recent HbS calibration model (21). When using the HbS calibration model, pre-transfusion OEF values in adults with SCA (OEF=0.26±0.05) were lower at baseline than with the more commonly-used bovine model (8,16) and with PET (25) and were not different from post-transfusion OEF values (OEF=0.25±0.07). In addition, pre-transfusion (OEF=0.22±0.06) and post-transfusion (OEF=0.22±0.04) OEF values in children with SCA when using the HbS model were lower than what was recently reported in a similar pediatric SCA cohort (9).
Cerebral Blood Flow Response to Transfusion
In adults with SCA (n=14), mean post-transfusion CBF=77.7±26.4 ml/100g/min was not significantly different from pre-transfusion CBF=82.3±30.2 ml/100g/min (p=0.27) (Figure 4B) but was significantly elevated compared to control CBF=50.2±7.7 ml/100g/min. CBF decreased after transfusion (ΔCBF=−13.6±6.4 ml/100g/min) in 9 of 14 participants (64%) and was unchanged or increased (ΔCBF=11.5±11.5 ml/100g/min) in 5 of 14 participants (36%).
By contrast, in children with SCA (n=10), mean post-transfusion CBF=76.4±11.3 ml/100g/min was significantly (p=0.004) lower than pre-transfusion CBF=96.4±16.5 ml/100g/min (Figure 4D); CBF data were not available in one pediatric participant. Post-transfusion CBF was lower than pre-transfusion CBF (ΔCBF=−22.3±9.9 ml/100g/min) in 9 of 10 participants (90%) and was unchanged (ΔCBF=0.1 ml/100g/min) in 1 of 10 participants (10%).
Post-transfusion CBF in adults with SCA (CBF=77.7±26.4 ml/100g/min) exhibited a strong trend (p=0.06) for not reducing to levels observed in controls (CBF=50.2±7.7 ml/100g/min), while post-transfusion CBF in children with SCA (CBF=76.4±11.3 ml/100g/min) was similar or slightly elevated compared to recently published results in age-matched controls (median CBF=71.3 ml/100g/min) (9).
Effects of Age on Response to Transfusion
In participants with SCA (n=26), there was no significant relationship between age and transfusion-related changes in OEF (p=0.13; rho=−0.23). In participants with SCA (n=24), a significant positive relationship was observed between age and transfusion-related changes in CBF (p=0.03; rho=0.45).
Associations with Hematologic Variables
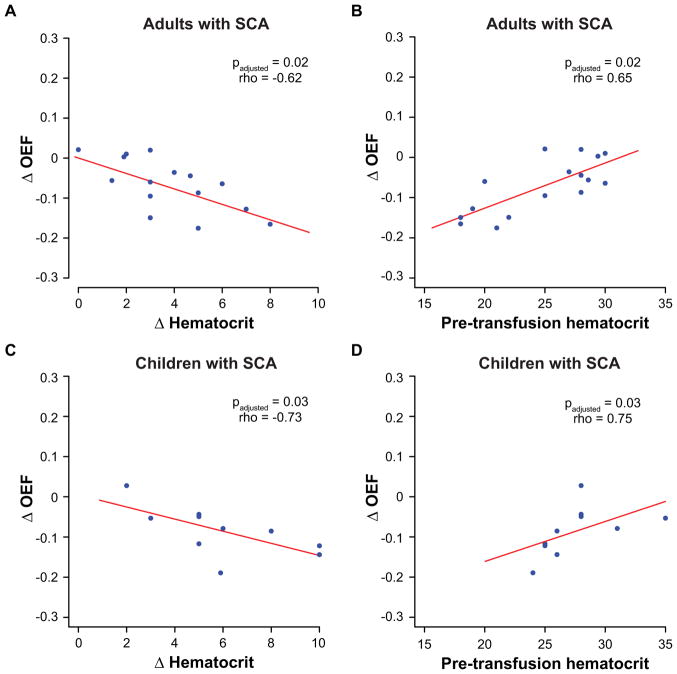
In adults with SCA, the change in OEF was significantly correlated with the change in hematocrit (padjusted=0.02; rho=−0.62) and with pre-transfusion hematocrit (padjusted=0.02; rho=0.65) (Figure 5A–B) but was not associated with either the change in HbS% (padjusted=0.56) or with pre-transfusion HbS% (padjusted=0.33). Pre-transfusion hematocrit trended to be marginally lower (p=0.09) in the subgroup with an OEF decrease (hematocrit=23.7±4.5%) compared to the subgroup in whom OEF did not change (hematocrit=27.7±2.5%); furthermore, change in hematocrit was significantly greater (p=0.02) in the subgroup with an OEF decrease (Δhematocrit=4.4±1.9%) compared to the subgroup in whom OEF did not change (Δhematocrit=1.7±1.3%). No analogous differences in hematologic variables were observed between adult subgroups with varying CBF changes.
Figure 5. Associations with hematologic variables.
Scatter plots are shown comparing the change in oxygen extraction fraction (OEF) (post-pre) versus change in hematocrit (A) and pre-transfusion hematocrit (B) in adults with SCA (top); similar plots comparing change in OEF in versus change in hematocrit (C) and pre-transfusion hematocrit (D) are also shown for children with SCA (bottom). Spearman’s correlation testing was performed, and the appropriate p- and rho-values are reported for each comparison after correction for false discovery rate. Lines-of-best-fit (red) are shown for each comparison simply as an aid to visualize the direction (direct or inverse) of the trends between variables. In adults with SCA (n=16), the change in OEF is significantly associated with the change in hematocrit and with pre-transfusion hematocrit; these correlations are also seen in children with SCA (n=10).
In children with SCA, the change in OEF was inversely correlated with the change in hematocrit (padjusted=0.03; rho=−0.73) and directly with pre-transfusion hematocrit (padjusted=0.03; rho=0.75) (Figure 5C–D) and was also not associated with either the change in HbS% (padjusted=0.46) or with pre-transfusion HbS% (padjusted=0.28). No analogous associations were present between these parameters and change in CBF in either adults or children with SCA. Pre-transfusion hematocrit was similar in the subgroup with an OEF decrease (hematocrit=28.0±3.5%) and the participant in whom OEF did not change (hematocrit=28.0%); however, change in hematocrit trended to be higher (p=0.11) in the subgroup with an OEF decrease (Δhematocrit=6.4±2.4%) compared to the subgroup in whom OEF did not change (Δhematocrit=2.0%). No analogous differences in hematologic variables were observed between pediatric subgroups with varying CBF changes.
Transfusion type
In adults receiving a simple transfusion, post-transfusion hematocrit=25.0±2.3% was higher than pre-transfusion hematocrit=20.4±2.5% (p=0.02); post-transfusion HbS% was unavailable in most receiving simple transfusions. In adults with SCA receiving exchange transfusions, post-transfusion hematocrit=31.3±2.9% was higher than pre-transfusion hematocrit=28.2±1.6% (p=0.006), and post-transfusion HbS%=23.4±13.4% was lower than pre-transfusion HbS%=42.1±10.0% (p=0.02). Adults with SCA from the two transfusion groups exhibited similar levels of impairment about silent cerebral infarction (simple=71%, exchange=75%), intracranial vasculopathy (simple=43%, exchange=56%), and history of overt stroke (simple=71%, exchange=78%) (Supplementary Tables 1–2) but were more impaired than children with SCA: 45% with SCI, 9% with overt stroke, and no intracranial vasculopathy (Table 1).
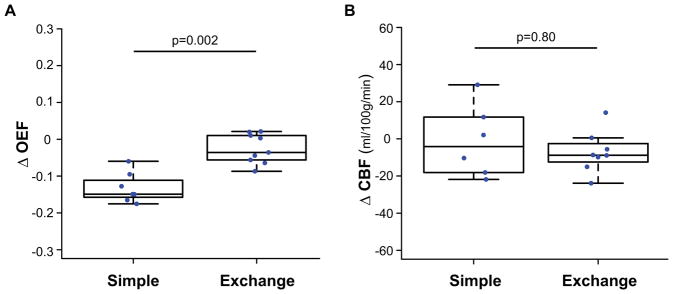
In adults with SCA receiving simple transfusions, post-transfusion OEF=0.40±0.04 was significantly lower than pre-transfusion OEF=0.54±0.06 (p=0.02), while in adults with SCA receiving exchange transfusions, post-transfusion OEF=0.36±0.04 trended lower than pre-transfusion OEF=0.39±0.03 (p=0.11). OEF changes following transfusion were significantly (p=0.002) greater in those receiving simple transfusions versus those receiving exchange transfusions (Figure 6A), but no analogous differences in CBF changes were observed in those receiving simple versus exchange transfusions (Figure 6B).
Figure 6. Transfusion type.
Box plots of the change in OEF (A) and CBF (B) are shown for adults with SCA receiving simple transfusions and exchange transfusions. Changes in OEF following transfusion were significantly (p=0.002) greater in those receiving simple transfusions (n=7) versus those receiving exchange transfusions (n=9) (A). No such differences were observed in CBF between adults with SCA receiving simple transfusions (n=6) and exchange transfusions (n=8) (B).
DISCUSSION
Study findings support that, in adults with SCA, cerebral OEF generally reduces following transfusions, while CBF is unchanged on average. This pattern in adults is different than what has been observed in pediatric SCA patients, both in this current study and in a recent study (9), in which both OEF and CBF reduced post-transfusion in pediatric SCA patients. This finding is consistent with the hypothesis that many adult patients may be operating in a more advanced stage of hemo-metabolic impairment, presumably related to a higher burden of cerebral injury and vasculopathy. Furthermore, the magnitude of OEF change was associated with the magnitude of change in hematocrit, but not change in HbS, and with pre-transfusion hematocrit. Preliminary results comparing transfusion types indicate that the magnitude of the OEF change may be larger in those receiving simple transfusions than in those receiving exchange transfusions. This difference may be related to the larger, though not statistically significant given the sample size of this study, change in hematocrit with simple transfusion and also the significantly lower pre-transfusion hematocrit in simple transfusion participants.
In individuals with SCA, blood oxygen carrying capacity is reduced, triggering autoregulatory increases in CBF in compliant parenchyma to compensate for the resulting anemia (26,27). In more chronically anemic patients or those with advanced sickle cell-related cerebrovascular disease, it is possible that these autoregulatory capabilities are exhausted (28), and thus increases in OEF may occur in the face of maintained CMRO2 and reduced oxygen delivery (11,12). Since blood transfusions serve to increase hemoglobin levels and blood oxygen content, OEF should decrease following transfusion. We confirmed this hypothesis and further demonstrated that changes in OEF due to transfusion may be dependent on the change in hematocrit. This finding can be explained by the inverse relationship between hematocrit and OEF, which indicates that increases in hematocrit should lead to decreases in OEF when CBF and CMRO2 are unchanged.
CBF, as measured with phase contrast MRA, was found to be unchanged from pre- to post-transfusion measurements in adults with SCA receiving regular blood transfusions. This is consistent with the hypothesis that CBF changes due to variations in hematocrit in severely anemic adults with SCA are small, likely due to the microvasculature operating in a maximally vasodilated state even following small transfusion-induced increases in hematocrit. A recent study in children with SCA (mean age=13.1 years) by Guilliams et al. observed a decrease in both OEF (from 34% to 31%) and CBF (from 88 ml/100g/min to 82 ml/100g/min) after transfusion. In addition, another recent study by Kosinski et al. has also shown decreased CBF and also increased cerebrovascular reactivity in children with SCA (mean age=12.8 years) following transfusion (29). We have demonstrated similar findings of reduced OEF and CBF in a comparable population of children (mean age=10.2 years) but not adults. In addition, we have shown preliminary results of partial correlation analysis indicating that CBF changes following transfusion may be more pronounced in younger versus older subjects, while OEF changes may exhibit the opposite trend. These differences in physiological response to transfusion, coupled with the differences in baseline hematocrit between the adult and pediatric populations in our studies lend further support for the proposed model.
We also observed trends for differences in hemo-metabolic response with respect to the type of transfusion given, albeit in a small sample of each group. Simple transfusions serve to primarily increase hematocrit but also have the effect of diluting HbS, while exchange transfusions serve to primarily decrease HbS but have a secondary effect of maintaining a preset baseline level of hematocrit. Although most adults with severe complications of SCA receive exchange transfusions, simple transfusions are also routinely used for different clinical indications, such as severe chronic or episodic anemia. In this study, SCA adults who received a simple transfusion (n=7) versus an exchange transfusion (n=9) were included. Results suggest that those receiving simple transfusions demonstrate a greater decrease in OEF post-transfusion than those receiving exchange transfusions, presumably due to the lower baseline hematocrit and larger transfusion-related increase in hematocrit observed in these subjects. However, future studies with larger sample sizes will be required to better characterize this possibility.
Prior work with positron emission tomography (PET) has demonstrated that blood transfusions reduce OEF in anemic patients with subarachnoid hemorrhage along a similar physiological mechanism as proposed in this study (i.e., increase in oxygen content of blood without a decrease in blood flow) (30,31). Previous work in SCA using 133Xenon inhalation found reduced CBF in a small cohort (n=2) of patients after transfusion therapy compared with non-transfused patients, but the post-transfusion CBF remained higher than in age-matched control volunteers (26). However, these patients were on average less anemic (mean hematocrit=30.5) at baseline compared with participants in this study (mean hematocrit=24.5). According to the model proposed, increased hyperemia through autoregulatory vasodilation may be sufficient compensation in these less anemic patients without complementary elevations in OEF.
While we observed on average OEF decreases and CBF stability pre- vs. post-transfusion in adults with SCA, there was some disparity in individual participants. A subset of adults with SCA did not have the expected decrease in OEF following transfusion. These four subjects were less anemic, exhibited a larger than average drop in post-transfusion CBF (CBF change=14.2 ml/100g/min), and exhibited a lower pre-transfusion OEF=0.37 compared to the group average of OEF=0.45.
TRUST MRI was utilized in this study for OEF computation with calibration models previously derived from bovine blood over a hematocrit range of 35–55% (16) and extrapolated for lower hematocrit. Bovine blood is reported to have many magnetic resonance relaxation properties similar to human blood with HbAA; the OEF values reported in this study assume applicability of previously reported calibration models to the SCA patient population. While this assumption is not unreasonable, small variations between susceptibility effects in HbS and HbA blood may exist. Preliminary results from Jordan et al. (8) report nonsignificant differences in blood water TRUST-T2 within measurement error (τCPMG=10 ms; HbA T2=0.0810s vs. HbS T2=0.0825s) between blood containing HbS and blood HbA, in which both samples were collected from human subjects at identical times with similar hematocrit (HbAA hematocrit=37.0% vs. HbSS hematocrit=36.5%) and oxygenation (HbAA oxygenation=67% vs. HbSS oxygenation=68%) and differed only in HbSS fraction (HbAA HbS%=0 vs. HbSS HbS%=78%). These findings are consistent with independent work from Eldeniz et al. (32), which reported insignificant differences in magnetic susceptibility between blood containing HbS versus HbA; these calibration models have been applied in other recent SCA studies of OEF pre- and post-transfusion (9). However, two recent studies have developed different calibration values for HbS blood. Preliminary ex vivo results from Li et al. (33) indicated that utilizing previous calibration models could result in underestimations of venous oxygenation (and OEF overestimation) by up to 20% in SCA. Other work from Bush et al. (21) reported approximately a 20% difference between venous oxygenation values calculated with existing calibration models and their HbS model; this work resulted in a lower mean OEF=0.24 in SCA patients using a very similar experimental protocol as used here (21). It should be noted that previous studies in healthy adults from multiple modalities, including MRI and gold-standard PET, have reported OEF ranges from 0.35 to 0.44 (11,34) and CMRO2 ranges from 2.8 to 5.0 ml O2/100g/min (34,35) in healthy brain tissue (36). The lower OEF values reported by Bush et al. were attributable to potential lower CMRO2 found (21) and potential capillary shunting phenomena; however, separate work found no relationship between shunting artifacts on arterial spin labeling MR signal and metabolism (37), albeit using calibration models from bovine blood. Prior work with gold-standard PET has shown a normal-to-high OEF in SCA patients (range = 0.37 to 0.52) (25). The OEF values from this study are in good agreement with these prior studies using PET. However, given the discrepant findings among multiple groups, more work is required in this area.
Phase contrast MRA was used in this study for CBF computation; phase contrast has been investigated for quantification of global CBF (38,39) and has been previously utilized to measure global CBF in SCA (27). CBF measurements with phase contrast could be affected by two factors: the measurement of bulk blood flow through the cervical vessels and the normalization of this bulk flow to viable tissue. We have addressed these points through the use of a low flow-encoding velocity (40 cm/s) and a segmentation algorithm that separated tissue and fluid, allowing for the bulk flow to be normalized to approximately the amount of viable brain tissue.
The findings should be considered in the context of the following limitations. First, while the volunteers in this study were very well characterized and followed longitudinally, the sample size of control (n=7) and adult SCA participants (n=16) utilized in this study is small. A larger longitudinal study with multivariate analyses is required to fully understand the clinical relevance of these physiological parameters as biomarkers of therapy response. Second, phase contrast MRA data for flow velocity calculation and subsequent determination of CBF was only available in 14 of 16 SCA participants. Third, conventional pulse oximetry was utilized in this study for measuring arterial oxygen saturation; corrections for dyshemoglobin levels were not performed. While Guilliams et al. have demonstrated a significant difference in dyshemoglobin levels pre- versus post-transfusion in a pediatric SCA cohort, they reported a median difference in dyshemoglobin levels of 0.4% (9); this small change is unlikely to explain the OEF differences pre- versus post-transfusion we observed. Finally, global measures of OEF and CBF are utilized in this study. While this may present a problem in patients with lateralizing cerebrovascular disease, regional vasculopathy severity that could confound global measurements were well-matched between the simple and exchange transfusion cohorts.
In conclusion, we observed that mean cerebral OEF reduces following blood transfusions in adults with SCA. These changes in OEF parallel the change in hematocrit and are associated with pre-transfusion hematocrit. Future work that focuses on how these hemo-metabolic changes following transfusion adjust in those with vs. without recurrent stroke could be useful for developing biomarkers that could have relevance for triaging patients for personalized therapies.
Supplementary Material
Acknowledgments
Grant Support: American Heart Association (14CSA20380466); NIH/NINDS (5R01NS078828); NIH/NINDS (R01NS096127)
We are grateful to Charles Nockowski, Clair Jones, Kristen George-Durrett, Christopher Thompson, and Leslie McIntosh for experimental support.
References
- 1.Kassim AA, DeBaun MR. Sickle cell disease, vasculopathy, and therapeutics. Annu Rev Med. 2013;64:451–466. doi: 10.1146/annurev-med-120611-143127. [DOI] [PubMed] [Google Scholar]
- 2.Seakins M, Gibbs WN, Milner PF, Bertles JF. Erythrocyte Hb-S concentration. An important factor in the low oxygen affinity of blood in sickle cell anemia. J Clin Invest. 1973;52(2):422–432. doi: 10.1172/JCI107199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arkuszewski M, Krejza J, Chen R, Melhem ER. Sickle cell anemia: reference values of cerebral blood flow determined by continuous arterial spin labeling MRI. Neuroradiol J. 2013;26(2):191–200. doi: 10.1177/197140091302600209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Debaun MR, Derdeyn CP, McKinstry RC., 3rd Etiology of strokes in children with sickle cell anemia. Ment Retard Dev Disabil Res Rev. 2006;12(3):192–199. doi: 10.1002/mrdd.20118. [DOI] [PubMed] [Google Scholar]
- 5.Strouse JJ, Lanzkron S, Urrutia V. The epidemiology, evaluation and treatment of stroke in adults with sickle cell disease. Expert Rev Hematol. 2011;4(6):597–606. doi: 10.1586/ehm.11.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams R, McKie V, Nichols F, et al. The use of transcranial ultrasonography to predict stroke in sickle cell disease. N Engl J Med. 1992;326(9):605–610. doi: 10.1056/NEJM199202273260905. [DOI] [PubMed] [Google Scholar]
- 7.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339(1):5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 8.Jordan LC, Gindville MC, Scott AO, et al. Non-invasive imaging of oxygen extraction fraction in adults with sickle cell anaemia. Brain. 2016;139(Pt 3):738–750. doi: 10.1093/brain/awv397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guilliams KP, Fields ME, Ragan DK, et al. Red cell exchange transfusions lower cerebral blood flow and oxygen extraction fraction in pediatric sickle cell anemia. Blood. 2018;131(9):1012–1021. doi: 10.1182/blood-2017-06-789842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prohovnik I, Hurlet-Jensen A, Adams R, De Vivo D, Pavlakis SG. Hemodynamic etiology of elevated flow velocity and stroke in sickle-cell disease. J Cereb Blood Flow Metab. 2009;29(4):803–810. doi: 10.1038/jcbfm.2009.6. [DOI] [PubMed] [Google Scholar]
- 11.Derdeyn CP, Videen TO, Yundt KD, et al. Variability of cerebral blood volume and oxygen extraction: stages of cerebral haemodynamic impairment revisited. Brain. 2002;125(Pt 3):595–607. doi: 10.1093/brain/awf047. [DOI] [PubMed] [Google Scholar]
- 12.Powers WJ. Cerebral hemodynamics in ischemic cerebrovascular disease. Ann Neurol. 1991;29(3):231–240. doi: 10.1002/ana.410290302. [DOI] [PubMed] [Google Scholar]
- 13.Hulbert ML, McKinstry RC, Lacey JL, et al. Silent cerebral infarcts occur despite regular blood transfusion therapy after first strokes in children with sickle cell disease. Blood. 2011;117(3):772–779. doi: 10.1182/blood-2010-01-261123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordan LC, DeBaun MR. Cerebral hemodynamic assessment and neuroimaging across the lifespan in sickle cell disease. J Cereb Blood Flow Metab. 2017 doi: 10.1177/0271678X17701763. 271678X17701763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu H, Ge Y. Quantitative evaluation of oxygenation in venous vessels using T2-Relaxation-Under-Spin-Tagging MRI. Magn Reson Med. 2008;60(2):357–363. doi: 10.1002/mrm.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu H, Xu F, Grgac K, Liu P, Qin Q, van Zijl P. Calibration and validation of TRUST MRI for the estimation of cerebral blood oxygenation. Magn Reson Med. 2012;67(1):42–49. doi: 10.1002/mrm.22970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juttukonda MR, Jordan LC, Gindville MC, et al. Cerebral hemodynamics and pseudo-continuous arterial spin labeling considerations in adults with sickle cell anemia. NMR Biomed. 2017;30(2) doi: 10.1002/nbm.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aslan S, Xu F, Wang PL, et al. Estimation of labeling efficiency in pseudocontinuous arterial spin labeling. Magn Reson Med. 2010;63(3):765–771. doi: 10.1002/mrm.22245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 21.Bush AM, Coates TD, Wood JC. Diminished cerebral oxygen extraction and metabolic rate in sickle cell disease using T2 relaxation under spin tagging MRI. Magn Reson Med. 2017 doi: 10.1002/mrm.27015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 23.Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assessment. 1994;6(4):284–290. [Google Scholar]
- 24.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met. 1995;57(1):289–300. [Google Scholar]
- 25.Herold S, Brozovic M, Gibbs J, et al. Measurement of regional cerebral blood flow, blood volume and oxygen metabolism in patients with sickle cell disease using positron emission tomography. Stroke. 1986;17(4):692–698. doi: 10.1161/01.str.17.4.692. [DOI] [PubMed] [Google Scholar]
- 26.Prohovnik I, Pavlakis SG, Piomelli S, et al. Cerebral hyperemia, stroke, and transfusion in sickle cell disease. Neurology. 1989;39(3):344–348. doi: 10.1212/wnl.39.3.344. [DOI] [PubMed] [Google Scholar]
- 27.Bush AM, Borzage MT, Choi S, et al. Determinants of resting cerebral blood flow in sickle cell disease. Am J Hematol. 2016;91(9):912–917. doi: 10.1002/ajh.24441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim YS, Nur E, van Beers EJ, et al. Dynamic cerebral autoregulation in homozygous Sickle cell disease. Stroke. 2009;40(3):808–814. doi: 10.1161/STROKEAHA.108.531996. [DOI] [PubMed] [Google Scholar]
- 29.Kosinski PD, Croal PL, Leung J, et al. The severity of anaemia depletes cerebrovascular dilatory reserve in children with sickle cell disease: a quantitative magnetic resonance imaging study. Br J Haematol. 2017;176(2):280–287. doi: 10.1111/bjh.14424. [DOI] [PubMed] [Google Scholar]
- 30.Dhar R, Zazulia AR, Derdeyn CP, Diringer MN. RBC Transfusion Improves Cerebral Oxygen Delivery in Subarachnoid Hemorrhage. Crit Care Med. 2017;45(4):653–659. doi: 10.1097/CCM.0000000000002266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhar R, Zazulia AR, Videen TO, Zipfel GJ, Derdeyn CP, Diringer MN. Red blood cell transfusion increases cerebral oxygen delivery in anemic patients with subarachnoid hemorrhage. Stroke. 2009;40(9):3039–3044. doi: 10.1161/STROKEAHA.109.556159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eldeniz C, Binkley M, Ragan D, et al. Sickle hemoglobin vs. normal hemoglobin: any changes in susceptiblity? International Society for Magnetic Resonance in Medicine; Honolulu: 2017. [Google Scholar]
- 33.Li W, Xu X, Liu P, et al. Determination of Oxygenation Extraction Fraction for People with Sickle Cell Anemia Using Calibration Model Specific to SCA Blood. International Society for Magnetic Resonance in Medicine; Honolulu: 2012. [Google Scholar]
- 34.Leenders KL, Perani D, Lammertsma AA, et al. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age Brain. 1990;113(Pt 1):27–47. doi: 10.1093/brain/113.1.27. [DOI] [PubMed] [Google Scholar]
- 35.Hatazawa J, Fujita H, Kanno I, et al. Regional cerebral blood flow, blood volume, oxygen extraction fraction, and oxygen utilization rate in normal volunteers measured by the autoradiographic technique and the single breath inhalation method. Ann Nucl Med. 1995;9(1):15–21. doi: 10.1007/BF03165003. [DOI] [PubMed] [Google Scholar]
- 36.Gottstein U, Bernsmeier A, Sedlmeyer I. the Carbohydrate Metabolism of the Human Brain. I. Studies with Substrate-Specific Enzymatic Methods in Normal Brain Circulation. Klin Wochenschr. 1963;41:943–948. doi: 10.1007/BF01478536. [DOI] [PubMed] [Google Scholar]
- 37.Juttukonda MR, Donahue MJ, Davis LT, et al. Preliminary evidence for cerebral capillary shunting in adults with sickle cell anemia. Journal of Cerebral Blood Flow and Metabolism. 2017 doi: 10.1177/0271678X17746808. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu P, Lu H, Filbey FM, et al. Automatic and reproducible positioning of phase-contrast MRI for the quantification of global cerebral blood flow. PLoS One. 2014;9(5):e95721. doi: 10.1371/journal.pone.0095721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng SL, Su P, Wang FN, et al. Optimization of phase-contrast MRI for the quantification of whole-brain cerebral blood flow. J Magn Reson Imaging. 2015;42(4):1126–1133. doi: 10.1002/jmri.24866. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.