Abstract
Background
Early recognition of bowel and mesenteric injury following blunt abdominal trauma remains difficult. We hypothesized that patients with intra-abdominal adhesions from prior laparotomy would be subjected to visceral sheering deceleration forces and increased risk for bowel and mesenteric injury following blunt abdominal trauma.
Methods
We performed a multicenter retrospective cohort analysis of 267 consecutive adult trauma patients who underwent operative exploration following moderate-critical (abdominal injury score 2–5) blunt abdominal trauma, comparing patients with prior laparotomy (n = 31) to patients with no prior laparotomy (n = 236). Multivariable regression was performed to identify predictors of bowel or mesenteric injury.
Results
There were no significant differences between groups for injury severity scores or findings on abdominal ultrasound, diagnostic peritoneal aspirate/lavage, pelvic radiography, or preoperative CT scan. The prior laparotomy cohort had greater incidence of full thickness bowel injury (26 vs. 9%, p = 0.010) and mesenteric injury (61 vs. 31%, p = 0.001). The proportion of bowel and mesenteric injuries occurring at the ligament of Treitz or ileocecal region was greater in the no prior laparotomy group (52 vs. 25%, p = 0.003). Prior laparotomy was an independent predictor of bowel or mesenteric injury (OR 5.1, 95% CI 1.6–16.8) along with prior abdominal inflammation and free fluid without solid organ injury (model AUC: 0.81, 95% CI 0.74–0.88).
Conclusions
Patients with a prior laparotomy are at increased risk for bowel and mesenteric injury following blunt abdominal trauma. The distribution of bowel and mesenteric injuries among patients with no prior laparotomy favors embryologic transition points tethering free intraperitoneal structures to the retroperitoneum.
Introduction
The incidence of intra-abdominal injury among adult patients with blunt abdominal trauma is approximately 13% [1], with bowel and mesenteric injuries occurring in 1–5% [2, 3]. Identifying patients who will benefit from operative exploration remains difficult. With the development of several prediction models [4–11] and the introduction of multidetector computed tomography (CT) technology [12, 13], the reported rate of non-therapeutic laparotomy has decreased from 27.1% in 1995 to 3.9% in 2012 [14, 15]. Despite these advances, the incidence of complications following non-therapeutic laparotomy ranges from 15 to 41%, and the incidence of incisional ventral hernia or small bowel obstruction requiring hospital admission within 10 years of trauma laparotomy is at least 15%, underscoring the potential benefits of more precise patient selection [14–16]. In addition, delayed or missed diagnosis of a bowel or mesenteric injury remains a significant problem and is associated with increased morbidity and mortality [17].
Clinicians cannot rely on imaging findings alone in diagnosing bowel and mesenteric injury following blunt trauma. The sensitivity and specificity of multidetector CT for bowel and mesenteric injury are approximately 64 and 80%, respectively, and 13% of all patients with small bowel perforations following blunt abdominal trauma have normal preoperative CT scans [18, 19]. Diagnosis by history and physical exam is also difficult, especially in cases of altered mental status due to traumatic brain injury, distracting injuries, drug and alcohol use, and analgesic and sedative medications [10, 20]. Regardless of mental status, the presence of soft tissue bruising in the distribution of a seatbelt may be elicited by inspection of the abdomen and has been associated with bowel injury following blunt abdominal trauma [21, 22]. Similarly, abdominal inspection may reveal a scar from a prior laparotomy. Although prior laparotomy has not been previously described as a risk factor for bowel or mesenteric injury following blunt trauma, the pathogenesis of blunt traumatic aortic injury suggests that investigation of this relationship is warranted. In high-speed deceleration injuries, the aorta is most commonly injured just distal to the left subclavian artery, where it is tethered by the ligamentum arteriosum; the next most common sites of injury are the aortic root and diaphragmatic hiatus, also representing transition points from fixed to mobile tissue [23–25].
We hypothesized that patients with a prior laparotomy, who are likely have to developed adhesions at the operative site [26] and between the viscera and abdominal wall [27], would be vulnerable to shearing forces on the visceral block, placing them at increased risk for bowel and mesenteric injury following blunt abdominal trauma.
Methods
We performed a multicenter retrospective cohort analysis of 267 consecutive adult trauma patients who underwent operative exploration following blunt abdominal trauma. Derivation of the study population is illustrated in Supplementary Fig. 1. Institutional review board approval was obtained. Data registries at St. Mary’s Medical Center in West Palm Beach, Florida, and University of Florida Health in Gainesville, Florida, were searched for patients age ≥18 years who had abdominal injury score 2 (moderate), 3 (serious), 4 (severe), or 5 (critical) and Current Procedural Terminology codes for laparotomy or laparoscopy from July 2011–January 2017, excluding patients with penetrating injuries, burn injuries, or incomplete records. Patients who had a history of laparotomy prior to traumatic injury (n = 31) were compared to patients with no history of prior laparotomy (n = 236), and prior laparotomy was assessed for the ability to predict bowel and mesenteric injuries identified on operative exploration.
Data were collected from institutional data registries and by retrospective review of the electronic medical record. Baseline demographics included age, sex, injury severity score, abdominal injury score, vital signs, and history of an abdominal inflammatory process (e.g., diverticulitis, pelvic inflammatory disease, bacterial peritonitis), laparotomy, or laparoscopy. Risk factors for intra-abdominal injury identified by previous studies were assessed, including low Glasgow Coma Scale score, anemia, seatbelt sign, and computed tomography (CT) scan evidence of pelvic fracture or free fluid in the absence of solid organ injury [4–11, 21, 22, 28]. Initial findings on focused assessment with sonography for trauma (FAST), diagnostic peritoneal aspirate/lavage (DPA/DPL), and pelvic radiography were also assessed. Abdominal pain and tenderness were not considered in this analysis because the history and physical examination of a trauma patient is often hindered by altered mental status due to traumatic brain injury, distracting injuries, drug and alcohol use, and analgesic and sedative medications, such that the absence of abdominal pain and tenderness has limited utility in ruling out abdominal injury [10]. Assessment of intra-operative findings included solid organ injury, diaphragmatic hernia, partial and full thickness bowel injuries, mesenteric injuries, and the location of bowel and mesenteric injuries. Assessment of intra-operative management included the performance of bowel repair, bowel resection, and mesentery hemostasis or repair.
Statistical analysis was performed in SPSS version 24 (IBM, Armonk, NY). Continuous variables were compared by the Kruskal–Wallis test and reported as median [interquartile range]. Discrete variables were compared by Fisher’s Exact test and reported as n (%). The association between history of an abdominal inflammatory process and prior laparotomy was assessed by Spearman’s rank correlation coefficient. Univariable logistic regression was performed to identify conditions present on admission that were associated with bowel or mesenteric injury identified on operative exploration, reported as odds ratios (OR) with 95% confidence intervals (CI). To avoid false positive results due to the family-wise error rate associated with multiple comparisons, the regression analysis was limited to prior laparotomy, prior laparoscopy, prior abdominal inflammation, factors which were significantly different between prior laparotomy and no prior laparotomy cohorts, and previously identified risk factors for bowel and mesenteric injury, as above. Factors that were statistically significant on univariable analysis were entered into a multivariable regression model to identify independent predictors of bowel or mesenteric injury. A multivariable regression model should contain approximately ten outcome events for each variable in the model [29]. There were 107 patients with a bowel or mesenteric injury and four model covariates, indicating that this analysis was adequately powered. Model strength was assessed by calculating area under the receiver operating characteristic curve.
Results
Baseline patient characteristics are listed in Table 1. The prior laparotomy cohort was older (age 58 vs. 38 years, p < 0.001) and had lower admission hemoglobin levels (11.1 vs. 12.2 g/dL, p = 0.010) than the no prior laparotomy cohort. There was a greater proportion of patients with a history of an abdominal inflammatory process in the prior laparotomy cohort (32 vs. 9%, p = 0.001), which correlated directly with prior laparotomy (r = 0.226, p < 0.001). Of the 32 patients with prior abdominal inflammation, the etiology was appendicitis in ten patients, diverticulitis in seven patients, cholecystitis in four patients, pancreatitis in three patients, spontaneous bacterial peritonitis in three patients, granulomatous disease in three patients, and tubo-ovarian abscess in two patients. There were no significant differences between groups for injury severity score, abdominal injury score, or admission vital signs. There were no significant differences in the incidence of positive FAST, DPA, DPL, pelvic fractures, or open book deformities between groups.
Table 1.
Summary of baseline characteristics for the study population
| Patient characteristics | All patients (n = 267) | Prior laparotomy (n = 31) | No prior laparotomy (n = 236) | p |
|---|---|---|---|---|
| Age (years) | 40 [26–58] | 58 [52–74] | 38 [25–54] | <0.001 |
| Female/male | 81/186 | 18/13 | 63/173 | 0.001 |
| Prior abdominal inflammation | 32 (12%) | 10 (32%) | 22 (9%) | 0.001 |
| Prior laparoscopy/laparotomy | 14 (5%) | 3 (10%) | 11 (5%) | 0.213 |
| Injury severity score | 29 [18–38] | 24 [22–41] | 29 [17–38] | 0.508 |
| Abdominal injury score | 3 [3, 4] | 3 [3, 4] | 3 [3, 4] | 0.192 |
| Glasgow Coma Scale score | 15 [3–15] | 15 [5–15] | 14 [3–15] | 0.414 |
| Intubated in field | 82 (31%) | 7 (23%) | 75 (32%) | 0.408 |
| Intubated in trauma bay | 43 (16%) | 7 (23%) | 36 (15%) | 0.302 |
| Cardiac arrest in field | 16 (6%) | 2 (6%) | 14 (6%) | >0.999 |
| Cardiac arrest in trauma bay | 4 (1%) | 1 (3%) | 3 (1%) | 0.391 |
| Admission heart rate | 101 [83–118] | 100 [76–119] | 101 [83–118] | 0.770 |
| Admission SBP (mmHg) | 119 [97–138] | 127 [104–140] | 119 [95–138] | 0.179 |
| Admission Hb (g/dL) | 12.0 [10.4–13.7] | 11.1 [9.1–12.3] | 12.2 [10.4–13.7] | 0.010 |
| Seatbelt sign | 29 (11%) | 4 (13%) | 25 (11%) | 0.757 |
| FAST performed | 173 (65%) | 25 (81%) | 148 (63%) | 0.070 |
| Negative | 79 (30%) | 10 (32%) | 69 (29%) | 0.836 |
| Equivocal | 9 (3%) | 1 (3%) | 8 (3%) | >0.999 |
| Positive | 85 (32%) | 14 (45%) | 71 (30%) | 0.151 |
| DPA/DPL performed | 29 (11%) | 1 (3%) | 28 (12%) | 0.220 |
| Negative | 8 (3%) | 0 (0%) | 8 (3%) | 0.602 |
| Positive | 21 (8%) | 1 (3%) | 20 (9%) | 0.485 |
| FAST or DPA/DPL positive | 106 (40%) | 15 (48%) | 91 (39%) | 0.331 |
| Pelvic radiography performed | 219 (82%) | 25 (81%) | 194 (82%) | 0.806 |
| Normal | 160 (60%) | 19 (61%) | 141 (60%) | >0.999 |
| Pelvic fracture | 54 (20%) | 7 (23%) | 47 (20%) | 0.812 |
| Open book deformity | 17 (6%) | 2 (6%) | 15 (6%) | >0.999 |
Data are presented as median [interquartile range] or n (%)
SBP systolic blood pressure, Hb hemoglobin, FAST focused assessment with sonography for trauma, DPA diagnostic peritoneal aspirate, DPL diagnostic peritoneal lavage
Preoperative CT scan findings are listed in Table 2. One hundred and seventy-six patients (66%) had a preoperative CT scan. Four scans (2%) identified no injuries; operative exploration demonstrated that one of these patients had a full thickness bowel injury at the ligament of Treitz, one had a partial thickness cecal injury, one had a jejunal mesenteric injury, and one had a splenic laceration. Free fluid was identified in the absence of a solid organ injury on 29% of all CT scans, including 38% of all scans in the prior laparotomy cohort and 28% of all scans in the no prior laparotomy cohort. Mesenteric stranding was more frequent in the prior laparotomy cohort, though the difference was not statistically significant (33 vs. 14%, p = 0.053). CT evidence of bowel injury was similar between groups.
Table 2.
Summary of abdominal and pelvic computed tomography (CT) scan findings
| CT findings | All patients (n = 267) | Prior laparotomy (n = 31) | No prior laparotomy (n = 236) | p |
|---|---|---|---|---|
| Had a preoperative CT scana | 176 (66%) | 21 (68%) | 155 (66%) | >0.999 |
| Normal CT scan | 4 (2%) | 1 (5%) | 3 (2%) | 0.401 |
| Solid organ injury | 106 (60%) | 9 (43%) | 97 (63%) | 0.099 |
| Bleeding pseudoaneurysm | 29 (16%) | 4 (19%) | 25 (16%) | 0.755 |
| Free fluid | 131 (74%) | 14 (67%) | 117 (75%) | 0.426 |
| Without solid organ injury | 51 (29%) | 8 (38%) | 43 (28%) | 0.318 |
| Free air | 14 (8%) | 3 (14%) | 11 (7%) | 0.224 |
| Pelvic fracture | 47 (27%) | 8 (38%) | 39 (25%) | 0.291 |
| Bladder injury | 10 (6%) | 0 (0%) | 10 (6%) | 0.363 |
| Intraperitoneal | 7 (4%) | 0 (0%) | 7 (5%) | >0.999 |
| Extraperitoneal | 3 (2%) | 0 (0%) | 3 (2%) | >0.999 |
| Mesenteric stranding | 29 (16%) | 7 (33%) | 22 (14%) | 0.053 |
| Mesenteric hematoma | 28 (16%) | 4 (19%) | 24 (15%) | 0.750 |
| Bowel injury | 28 (16%) | 6 (29%) | 22 (14%) | 0.111 |
| Equivocal | 25 (14%) | 6 (29%) | 19 (12%) | 0.087 |
| Probable/diagnostic | 3 (2%) | 0 (0%) | 3 (2%) | >0.999 |
Data are presented as n (%) or median [interquartile range]
Subsequent analyses in this table consider only subjects who had a preoperative CT scan (176 total patients, 21 patients with prior laparotomy, and 155 patients with no prior laparotomy)
Operative findings and management are listed in Table 3. Laparoscopy was attempted for two patients in the prior laparotomy cohort, both of whom required conversion to laparotomy. Laparoscopy was attempted for eleven patients in the no prior laparotomy cohort, eight of whom required conversion to laparotomy. Adhesions were noted in 55% of all patients with prior laparotomy, compared with 5% of all patients with no prior laparotomy (p < 0.001). The incidence of solid organ injury was higher in the no prior laparotomy cohort, though the difference was not statistically significant (65 vs. 48%, p = 0.079). Partial and full thickness bowel injuries were identified in 14 and 11% of all patients, respectively, with greater incidence of full thickness bowel injury in the prior laparotomy cohort (26 vs. 9%, p = 0.010). Mesenteric injuries were identified in 34% of all patients, with greater incidence in the prior laparotomy cohort (61 vs. 31%, p = 0.001). Forty percent of all patients had a bowel or mesenteric injury, including 77% of all patients in the prior laparotomy cohort and 35% of all patients in the no prior laparotomy cohort (p < 0.001).
Table 3.
Summary of operative findings and management
| Operative findings and management | All patients (n = 267) | Prior laparotomy (n = 31) | No prior laparotomy (n = 236) | p |
|---|---|---|---|---|
| Laparotomy | 254 (95%) | 29 (94%) | 225 (95%) | 0.213 |
| Laparoscopy | 3 (1%) | 0 (0%) | 3 (1%) | >0.999 |
| Converted to open | 10 (4%) | 2 (6%) | 8 (3%) | 0.327 |
| Days from admission to surgery | 0 [0–1] | 0 [0–1] | 0[0–1] | 0.155 |
| Identified solid organ injury | 168 (63%) | 15 (48%) | 153 (65%) | 0.079 |
| Missed on CT scana | 6 (3%) | 1 (5%) | 5 (3%) | 0.539 |
| Identified diaphragm hernia | 21 (8%) | 3 (10%) | 18 (8%) | 0.720 |
| Missed on CT scana | 5 (3%) | 1 (5%) | 4 (3%) | 0.474 |
| Identified bowel injury | 66 (25%) | 11 (35%) | 55 (23%) | 0.182 |
| Partial thickness | 37 (14%) | 3 (10%) | 34 (14%) | 0.590 |
| Full thickness | 29 (11%) | 8 (26%) | 21 (9%) | 0.010 |
| Missed on CT scana | 36 (20%) | 7 (33%) | 29 (19%) | 0.148 |
| Required bowel repair | 32 (12%) | 5 (16%) | 27 (11%) | 0.393 |
| Required bowel resection | 24 (9%) | 5 (16%) | 19 (8%) | 0.173 |
| Identified mesenteric injury | 91 (34%) | 19 (61%) | 72 (31%) | 0.001 |
| Missed on CT scana | 24 (14%) | 4 (19%) | 20 (13%) | 0.495 |
| Required hemostasis/repair | 60 (22%) | 13 (42%) | 47 (20%) | 0.010 |
| Required bowel resectionb | 17 (6%) | 4 (13%) | 13 (6%) | 0.120 |
| Any bowel or mesenteric injury | 107 (40%) | 24 (77%) | 83 (35%) | <0.001 |
| Non-diagnostic laparotomy | 7 (3%) | 0 (0%) | 7 (3%) | >0.999 |
| Non-therapeutic laparotomy | 18 (7%) | 0 (0%) | 18 (7%) | 0.608 |
Data are presented as n (%) or median [interquartile range]
CT computed tomography
Considering only patients with a preoperative CT scan (176 total patients, 21 patients with prior laparotomy, and 155 patients with no prior laparotomy)
Devascularizing mesenteric injuries that required bowel resection in the absence of a full thickness bowel injury
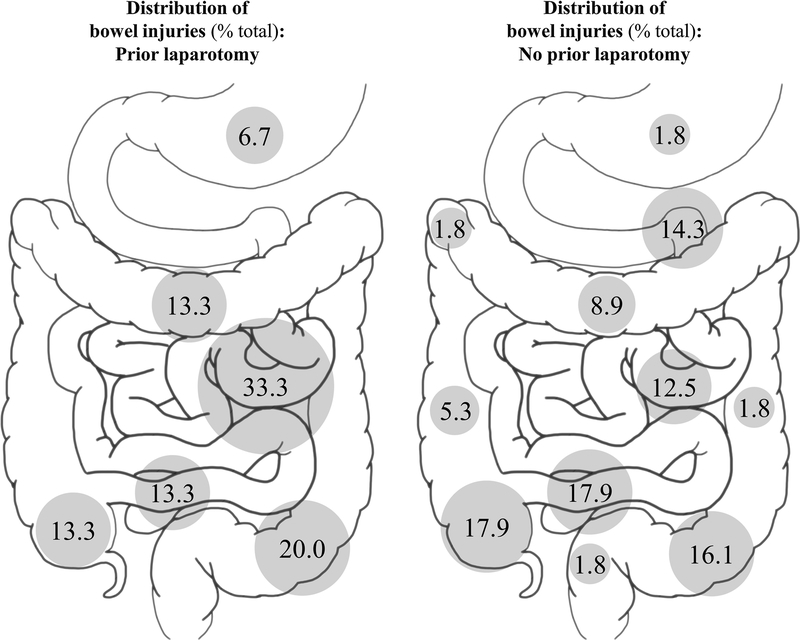
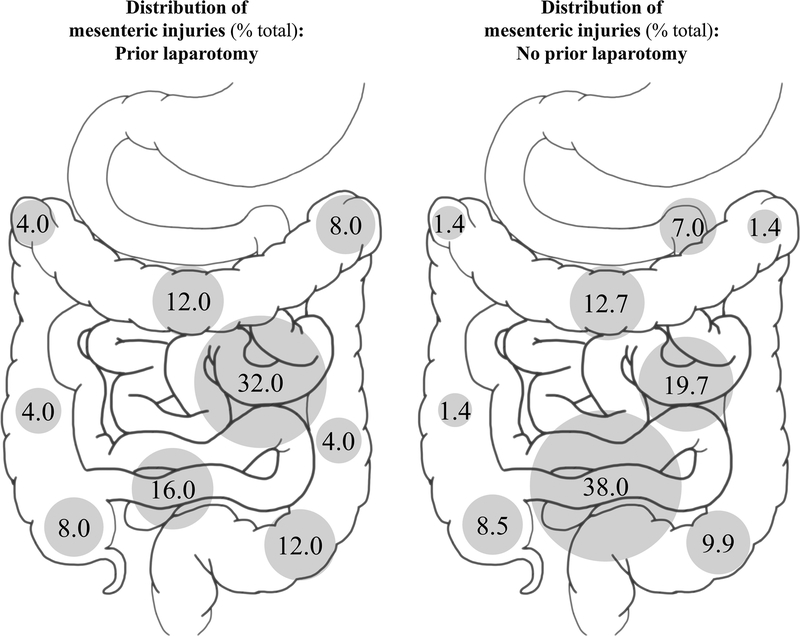
Distributions of bowel injuries are illustrated in Fig. 1. Jejunal and proximal ileal injuries accounted for one-third of all bowel injuries in the prior laparotomy cohort. The most common sites of bowel injury in patients with no prior laparotomy were the terminal ileum and cecum, with 36% of all injuries occurring at these locations. The proportion of bowel injuries occurring at the ligament of Treitz, terminal ileum, or cecum was greater among patients with no prior laparotomy, though the difference was not statistically significant (50 vs. 27%, p = 0.147). Distributions of mesenteric injuries are illustrated in Fig. 2. Jejunal and proximal ileal injuries accounted for 32% of all mesenteric injuries in the prior laparotomy cohort. The most common location for mesenteric injury in the no prior laparotomy cohort was the terminal ileum, accounting for 38% of all mesenteric injuries. The proportion of mesenteric injuries occurring at the ligament of Treitz, terminal ileum, or cecum was significantly greater among patients with no prior laparotomy (54 vs. 24%, p = 0.019). Considering all bowel and mesenteric injuries, among patients with no prior laparotomy, 52% of all injuries occurred at the ligament of Treitz, terminal ileum, or cecum; among patients with prior laparotomy, 25% of all injuries occurred at these locations (p = 0.003).
Fig. 1.
Distribution of bowel injuries identified on operative exploration for patients with and without a history of laparotomy prior to traumatic injury
Fig. 2.
Distribution of mesenteric injuries identified on operative exploration for patients with and without a history of laparotomy prior to traumatic injury
Predictors of bowel or mesenteric injury are listed in Table 4. On univariable regression, prior laparotomy was associated with increased odds of identifying a bowel or mesenteric injury on operative exploration (OR 6.3, 95% CI 2.6–15.3). A prior abdominal inflammatory process, a seatbelt sign, and CT evidence of a solid organ injury without free fluid were each associated with increased odds of bowel or mesenteric injury on univariable analysis and were included in the multivariable model. Intra-operative identification of adhesions was associated with increased odds of bowel or mesenteric injury (OR 6.6, 95% CI2.6–17.0), but was not considered in the multivariable model because this information is not available preoperatively. Seatbelt sign did not contribute significantly to the multivariable model, but the other three factors were independent predictors of bowel or mesenteric injury. CT evidence of free fluid without solid organ injury was the strongest predictor (OR 10.8, 95% CI 4.6–25.2) followed by prior laparotomy (OR 5.1, 95% CI 1.6–16.8) and prior abdominal inflammation (OR 3.8, 95% CI1.2–11.8). Together, these factors composed a strong prediction model with area under the receiver operating characteristic curve 0.81 (95% CI 0.74–0.88).
Table 4.
Predictors of bowel or mesenteric injury
| Factors | Univariable regression | Multivariable regression | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Prior laparotomy | 6.32 | 2.61–15.29 | <0.001 | 5.11 | 1.56–16.81 | 0.007 |
| Age (years) | 1.01 | 1.00–1.02 | 0.218 | − | − | − |
| Female sex | 1.30 | 0.76–2.20 | 0.337 | − | − | − |
| Prior abdominal inflammation | 3.88 | 1.76–8.58 | 0.001 | 3.75 | 1.19–11.83 | 0.024 |
| Prior laparoscopy | 1.13 | 0.38–3.35 | 0.827 | − | − | − |
| Glasgow Coma Scale score | 1.03 | 0.99–1.08 | 0.163 | − | − | − |
| Hemoglobin (g/dL) | 1.03 | 0.93–1.15 | 0.531 | − | − | − |
| Seatbelt sign | 5.66 | 2.32–13.79 | <0.001 | 2.32 | 0.70–7.71 | 0.168 |
| FAST/DPA/DPL positive | 1.03 | 0.63–1.71 | 0.894 | - | - | - |
| Any pelvic fracture | 0.68 | 0.38–1.19 | 0.173 | − | − | − |
| Open book pelvic deformity | 1.75 | 0.65–4.68 | 0.268 | − | − | − |
| CT free fluid, no solid organ injury | 11.43 | 5.15–25.38 | <0.001 | 10.76 | 4.60–25.18 | <0.001 |
Factors that were statistically significant on univariable analysis were entered into the multivariable regression model. Area under the receiver operating characteristic curve for the multivariate model was 0.81 (95% CI 0.74–0.88)
OR odds ratio, CI confidence interval, FAST focused assessment with sonography for trauma, DPA diagnostic peritoneal aspirate, DPL diagnostic peritoneal lavage, CT computed tomography of the abdomen and pelvis
Discussion
These results suggest that patients with intra-abdominal adhesions from a prior laparotomy may be at increased risk for bowel and mesenteric injury following blunt abdominal trauma. CT scan evidence of free fluid without solid organ injury was also a strong predictor of bowel or mesenteric injury. The clinical significance of this finding remains controversial, with recommendations for [30, 31] and against [32, 33] routine laparotomy. Adjunctive imaging studies like the FAST exam and DPA/DPL may not detect small amounts of enteric contents or a large mesenteric defect with devascularized bowel. Therefore, the history and physical examination is essential, but is often hindered by altered mental status due to traumatic brain injury, distracting injuries, drug and alcohol use, and analgesic and sedative medications. However, similar to the seatbelt sign, the presence of a laparotomy scar is readily apparent on inspection of the abdomen, indicating the likely presence of intra-abdominal adhesions and increased probability of bowel or mesenteric injury. These findings have impacted our clinical practice. Among patients with blunt abdominal trauma, history of prior laparotomy is now an indication for close observation and consideration of interval CT scan with oral contrast.
Patients with no history of abdominal surgery also suffer bowel and mesenteric injuries following blunt trauma. In our study, more than half of these injuries occurred at the ligament of Treitz, terminal ileum, or cecum, representing embryologic transition points tethering free intraperitoneal structures to the retroperitoneum. Among patients with a prior laparotomy, only one quarter of all bowel and mesenteric injuries occurred at these sites.
Previous studies have investigated the relationship between prior abdominal surgery and adhesive disease in non-trauma patients. Twelve years after Dr. Harrith M. Hasson [34] described his technique for open trocar placement under direct vision, Chi et al. [35] reported that previous abdominal surgery was associated with difficult abdominal entry. Rafii et al. [36] subsequently performed a prospective analysis of 477 patients undergoing laparoscopy and found that subjects with previous abdominal surgery had increased risk for complicated port placement and failure to achieve pneumoperitoneum. In our study, prior laparoscopy was not associated with increased risk for bowel or mesenteric injury following blunt abdominal trauma. Laparoscopy may induce less adhesion formation than laparotomy by decreasing the total length of incised parietal peritoneum, limiting tissue desiccation, and providing a magnified view of the operative field, which may allow for more precise tissue handling [37, 38]. In a porcine model of laparoscopic versus open nephrectomy, Moore et al. [26] found that adhesions occurred at the operative site in 75% of open nephrectomies and 13% of all laparoscopic nephrectomies. A similar phenomenon occurs at the abdominal wall. In a rabbit model of laparoscopic versus open cecal deserosalization, Jorgensen et al. [27] identified no abdominal wall adhesions in the laparoscopic group; adhesions to the abdominal wall developed in 70% of the open group. The impact of abdominal inflammatory processes is less clear, with the weight of evidence suggesting that generalized peritonitis involving visceral and parietal peritoneum may be more likely to produce adhesions.
This study was limited by a small sample size and selection bias inherent to its retrospective design. The prior laparotomy cohort had advanced age and a greater proportion of female subjects; although age and sex were not associated with bowel or mesenteric injury on the uni-variable analysis, a larger sample may detect a difference. Patients from two centers were included to increase sample size and improve generalizability, and the power analysis indicates that the sample size was adequate to build the multivariable model. All consecutive patients meeting study criteria were included to limit selection bias. In addition, the lack of long-term follow-up and surveillance hinders assessment of the long-term morbidity associated with laparotomy and bowel or mesenteric injury in this study. Future research should seek to validate these findings in a larger dataset and should continue to investigate aspects of the history and physical examination that may be useful for decision-making.
Conclusions
Patients with a prior laparotomy and intra-abdominal adhesions were at increased risk for bowel and mesenteric injury following blunt abdominal trauma. The distribution of bowel and mesenteric injuries among patients with no prior laparotomy favored embryologic transition points tethering free intraperitoneal structures to the retroperitoneum at the ligament of Treitz and ileocecal region. CT evidence of free fluid without solid organ injury was a strong predictor of bowel or mesenteric injury and may be used in combination with history and physical exam findings for optimal prognostication.
Supplementary Material
Acknowledgements
This work was supported in part by Grants R01 GM113945–01 (PAE), R01 GM105893–01A1 (AMM), and P50 GM111152–01 (FAM, SCB, PAE, AMM) awarded by the National Institute of General Medical Sciences (NIGMS). TJL was supported by a post-graduate training grant (T32 GM-008721) in burns, trauma and perioperative injury by NIGMS. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Compliance with ethical standards
Conflict of interest
The Authors declare that they have no conflict of interest.
References
- 1.Nishijima DK, Simel DL, Wisner DH et al. (2012) Does this adult patient have a blunt intra-abdominal injury? JAMA 307(14):1517–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watts DD, Fakhry SM, Group EM-IHVIR (2003) Incidence of hollow viscus injury in blunt trauma: an analysis from 275,557 trauma admissions from the East multi-institutional trial. J Trauma 54(2):289–294 [DOI] [PubMed] [Google Scholar]
- 3.Rizzo MJ, Federle MP, Griffiths BG (1989) Bowel and mesenteric injury following blunt abdominal trauma: evaluation with CT. Radiology 173(1):143–148 [DOI] [PubMed] [Google Scholar]
- 4.Beck D, Marley R, Salvator A et al. (2004) Prospective study of the clinical predictors of a positive abdominal computed tomography in blunt trauma patients. J Trauma 57(2):296–300 [DOI] [PubMed] [Google Scholar]
- 5.Udekwu PO, Gurkin B, Oller DW (1996) The use of computed tomography in blunt abdominal injuries. Am Surg 62(1):56–59 [PubMed] [Google Scholar]
- 6.Nelson JB, Bresticker MA, Nahrwold DL (1992) Computed tomography in the initial evaluation of patients with blunt trauma. J Trauma 33(5):722–727 [DOI] [PubMed] [Google Scholar]
- 7.Mackersie RC, Tiwary AD, Shackford SR et al. (1989) Intra-abdominal injury following blunt trauma. Identifying the high-risk patient using objective risk factors. Arch Surg 124(7):809–813 [DOI] [PubMed] [Google Scholar]
- 8.Grieshop NA, Jacobson LE, Gomez GA et al. (1995) Selective use of computed tomography and diagnostic peritoneal lavage in blunt abdominal trauma. J Trauma 38(5):727–731 [DOI] [PubMed] [Google Scholar]
- 9.Richards JR, Derlet RW (1998) Computed tomography for blunt abdominal trauma in the ED: a prospective study. Am J Emerg Med 16(4):338–342 [DOI] [PubMed] [Google Scholar]
- 10.Poletti PA, Mirvis SE, Shanmuganathan K et al. (2004) Blunt abdominal trauma patients: can organ injury be excluded without performing computed tomography? J Trauma 57(5):1072–1081 [DOI] [PubMed] [Google Scholar]
- 11.Holmes JF, Wisner DH, McGahan JP et al. (2009) Clinical prediction rules for identifying adults at very low risk for intra-abdominal injuries after blunt trauma. Ann Emerg Med 54(4):575–584 [DOI] [PubMed] [Google Scholar]
- 12.Atri M, Hanson JM, Grinblat L et al. (2008) Surgically important bowel and/or mesenteric injury in blunt trauma: accuracy of multidetector CT for evaluation. Radiology 249(2):524–533 [DOI] [PubMed] [Google Scholar]
- 13.Park MH, Shin BS, Namgung H (2013) Diagnostic performance of 64-MDCT for blunt small bowel perforation. Clin Imaging 37(5):884–888 [DOI] [PubMed] [Google Scholar]
- 14.Renz BM, Feliciano DV (1995) Unnecessary laparotomies for trauma: a prospective study of morbidity. J Trauma 38(3):350–356 [DOI] [PubMed] [Google Scholar]
- 15.Schnuriger B, Lam L, Inaba K et al. (2012) Negative laparotomy in trauma: are we getting better? Am Surg 78(11):1219–1223 [PubMed] [Google Scholar]
- 16.Li T, Robertson-More C, Maclean AR et al. (2015) Bowel obstructions and incisional hernias following trauma laparotomy and the nonoperative therapy of solid organ injuries: a retrospective population-based analysis. J Trauma Acute Care Surg 79(3):386–392 [DOI] [PubMed] [Google Scholar]
- 17.Fakhry SM, Brownstein M, Watts DD et al. (2000) Relatively short diagnostic delays (<8 hours) produce morbidity and mortality in blunt small bowel injury: an analysis of time to operative intervention in 198 patients from a multicenter experience. J Trauma 48(3):408–414 (discussion 414–405) [DOI] [PubMed] [Google Scholar]
- 18.Landry BA, Patlas MN, Faidi S et al. (2016) Are we missing traumatic bowel and mesenteric injuries? Can Assoc Radiol J 67(4):420–425 [DOI] [PubMed] [Google Scholar]
- 19.Fakhry SM, Watts DD, Luchette FA et al. (2003) Current diagnostic approaches lack sensitivity in the diagnosis of perforated blunt small bowel injury: analysis from 275,557 trauma admissions from the EAST multi-institutional HVI trial. J Trauma 54(2):295–306 [DOI] [PubMed] [Google Scholar]
- 20.Beal AL, Ahrendt MN, Irwin ED et al. (2016) Prediction of blunt traumatic injuries and hospital admission based on history and physical exam. World J Emerg Surg 11(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jost E, Roberts DJ, Penney T et al. (2017) Accuracy of clinical, laboratory, and computed tomography findings for identifying hollow viscus injury in blunt trauma patients with unexplained intraperitoneal free fluid without solid organ injury. Am J Surg 213(5):874–880 [DOI] [PubMed] [Google Scholar]
- 22.Chandler CF, Lane JS, Waxman KS (1997) Seatbelt sign following blunt trauma is associated with increased incidence of abdominal injury. Am Surg 63(10):885–888 [PubMed] [Google Scholar]
- 23.Parmley LF, Mattingly TW, Manion WC et al. (1958) Nonpenetrating traumatic injury of the aorta. Circulation 17(6):1086–1101 [DOI] [PubMed] [Google Scholar]
- 24.Feczko JD, Lynch L, Pless JE et al. (1992) An autopsy case review of 142 nonpenetrating (blunt) injuries of the aorta. J Trauma 33(6):846–849 [DOI] [PubMed] [Google Scholar]
- 25.Burkhart HM, Gomez GA, Jacobson LE et al. (2001) Fatal blunt aortic injuries: a review of 242 autopsy cases. J Trauma 50(1):113–115 [DOI] [PubMed] [Google Scholar]
- 26.Moore RG, Partin AW, Adams JB et al. (1995) Adhesion formation after transperitoneal nephrectomy: laparoscopic v open approach. J Endourol 9(3):277–280 [DOI] [PubMed] [Google Scholar]
- 27.Jorgensen JO, Lalak NJ, Hunt DR (1995) Is laparoscopy associated with a lower rate of postoperative adhesions than laparotomy? A comparative study in the rabbit. Aust N Z J Surg 65(5):342–344 [DOI] [PubMed] [Google Scholar]
- 28.Mahmood I, Tawfek Z, Abdelrahman Y et al. (2014) Significance of computed tomography finding of intra-abdominal free fluid without solid organ injury after blunt abdominal trauma: time for laparotomy on demand. World J Surg 38(6):1411–1415. 10.1007/s00268-013-2427-5 [DOI] [PubMed] [Google Scholar]
- 29.Peduzzi P, Concato J, Kemper E et al. (1996) A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 49(12):1373–1379 [DOI] [PubMed] [Google Scholar]
- 30.Ng AK, Simons RK, Torreggiani WC et al. (2002) Intra-abdominal free fluid without solid organ injury in blunt abdominal trauma: an indication for laparotomy. J Trauma 52(6):1134–1140 [DOI] [PubMed] [Google Scholar]
- 31.Brasel KJ, Olson CJ, Stafford RE et al. (1998) Incidence and significance of free fluid on abdominal computed tomographic scan in blunt trauma. J Trauma 44(5):889–892 [DOI] [PubMed] [Google Scholar]
- 32.Hulka F, Mullins RJ, Leonardo V et al. (1998) Significance of peritoneal fluid as an isolated finding on abdominal computed tomographic scans in pediatric trauma patients. J Trauma 44(6):1069–1072 [DOI] [PubMed] [Google Scholar]
- 33.Livingston DH, Lavery RF, Passannante MR et al. (1998) Admission or observation is not necessary after a negative abdominal computed tomographic scan in patients with suspected blunt abdominal trauma: results of a prospective, multi-institutional trial. J Trauma 44(2):273–280 (discussion 280–272) [DOI] [PubMed] [Google Scholar]
- 34.Hasson HM (1971) A modified instrument and method for laparoscopy. Am J Obstet Gynecol 110(6):886–887 [DOI] [PubMed] [Google Scholar]
- 35.Chi I, Feldblum PJ, Balogh SA (1983) Previous abdominal surgery as a risk factor in interval laparoscopic sterilization. Am J Obstet Gynecol 145(7):841–846 [DOI] [PubMed] [Google Scholar]
- 36.Rafii A, Camatte S, Lelievre L et al. (2005) Previous abdominal surgery and closed entry for gynaecological laparoscopy: a prospective study. BJOG 112(1):100–102 [DOI] [PubMed] [Google Scholar]
- 37.Drollette CM, Badawy SZ (1992) Pathophysiology of pelvic adhesions. Modern trends in preventing infertility. J Reprod Med 37(2):107–121 (discussion 121–102) [PubMed] [Google Scholar]
- 38.Gutt CN, Oniu T, Schemmer P et al. (2004) Fewer adhesions induced by laparoscopic surgery? Surg Endosc 18(6):898–906 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.