Abstract
Fixed DC was compared to ceftriaxone, ceftriaxone with 200 μA fixed DC, or no treatment in a rat model of methicillin-susceptible S. aureus foreign-body osteomyelitis. After 3 weeks, fewer bacteria were present in bones of the ceftriaxone group (5.71 log10cfu/g [P, 0.0004]) and the ceftriaxone/DC group (3.53 log10cfu/g [P, 0.0002]) than untreated controls (6.70 log10cfu/g). Fewer bacteria were present in the ceftriaxone/DC group than in the ceftriaxone alone- and DC alone groups (P, 0.0012 and 0.0008, respectively). There were also fewer bacteria on the implanted wires in the groups treated with ceftriaxone (5.47 log10cfu/cm2) or ceftriaxone/DC (2.82 log10cfu/cm2) than in the untreated controls (6.44 log10cfu/cm2 [P, 0.0003 and 0.0002, respectively]). There were fewer bacteria in the ceftriaxone/DC rats than in the ceftriaxone alone- and fixed DC alone-treated rats (P, 0.0017 and 0.0016, respectively). Fixed DC with an antibiotic may be useful for treating foreign body infections caused by S. aureus.
Keywords: Staphylococcus aureus, prosthetic joint infection, treatment
INTRODUCTION
In today’s medical practice, it is commonplace for patients to have surgically implanted devices, ranging from prosthetic joints, to catheters, to pacemakers, with many more possibilities. Arthroplasty implantation is among the most common surgical procedures performed in the United States, with over 1 million total hip and knee arthroplasties placed in 2010 (Kremers, et al., 2015; Tande & Patel, 2014), and with this number expected to rise exponentially to over 500,000 and over 3.4 million total hip and knee arthroplasties, respectively, by the year 2030 (Kurtz, et al., 2007). Along with the increased use of prosthetic joints comes an increased number of infections and thus significant economic impact (Tande & Patel, 2014). Prosthetic joint infections (PJIs) are difficult to treat, given that the bacteria related with these infections exist in the biofilm state, in which bacteria are surrounded by an extrapolymeric substance, composed of polysaccharides, proteins, lipids, and extracellular DNA, and are in their own niche wherein the availability of nutrients and growth and death rates are altered (Gbejuade, et al., 2015; Høiby, et al., 2010). In biofilms, bacteria are 100-1000 times more resistant to antimicrobial treatment compared to their planktonic counterparts (Gbejuade, et al., 2015; Høiby, et al., 2010; Tande & Patel, 2014). In the majority of PJIs, Gram positive cocci are the causal agents, with Staphylococcus aureus and coagulase-negative staphylococci being the main culprits (Benito, et al., 2016; Tande & Patel, 2014).
Because of the poor activity of most currently available antibiotics, novel treatment strategies need to be explored. In previous studies, we have shown that low-amperage fixed direct electrical current (DC) reduced S. aureus biofilms formed on implant-associated materials in vitro (Schmidt-Malan, et al., 2015), and also in vivo in a novel model of foreign body osteomyelitis caused by Cutibacterium acnes (Schmidt-Malan, et al., 2017). In the present study, we tested S. aureus in the same in vivo model, comparing fixed DC alone, ceftriaxone alone, and fixed DC combined with ceftriaxone, to each other and to no treatment.
MATERIALS AND METHODS
Microorganism.
Methicillin-susceptible S. aureus Infectious Diseases Research Laboratory (IDRL)-4284, a clinical isolate, was studied. The isolate was saved in a Microbank™ (Pro-Lab Diagnostics, Round Rock, TX) at −80°C. The MIC of oxacillin was 0.25 μg/ml (susceptible) and that of penicillin was 2 μg/ml (resistant); the cefoxitin disc zone size was 26.5 mm (susceptible). Susceptibility to oxacillin and cefoxitin infers susceptibility to ceftriaxone (Clinical and Laboratory Standards Institute, 2018a, 2018b).
Experimental rat model.
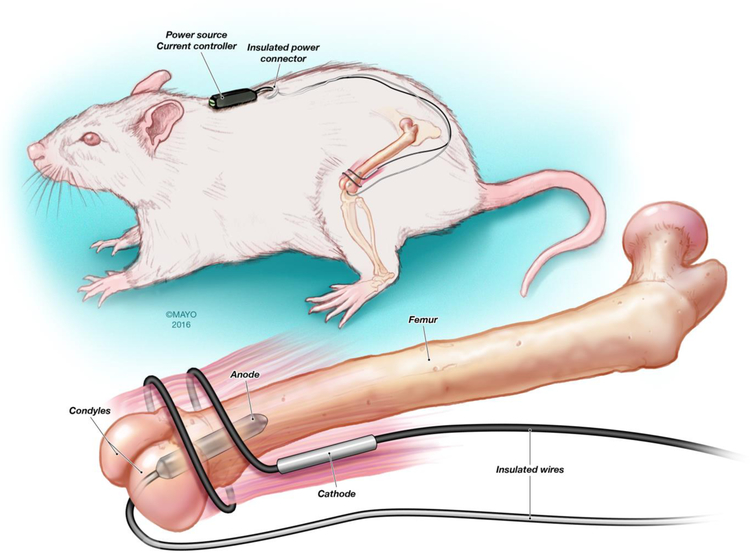
This study followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 8023, revised 1978), and was approved by Mayo Clinic Institutional Animal Care and Use Committee in Rochester, MN. Foreign body osteomyelitis was established in male Wistar rats (approximately 300 g) (Envigo, Indianapolis, IN), as previously described (Schmidt-Malan, et al., 2017). Animals underwent general anesthesia by intramuscular application of ketamine (60 mg/kg), xylazine (6 mg/kg) and acepromazine (1.5 mg/kg). For each animal, the left leg and midscapular area was clipped and washed with Hibiclens (4% chlorhexidine gluconate) (Mölnlycke Health Care, Norcross, GA). The left knee was exposed, after which a median parapatellar incision was made. A hole was created between the condyles of the femur using a 16G needle followed by insertion of a 14G needle to allow space for the wire to be implanted. Ten μl of arachidonic acid sodium salt (a sclerosing agent) (99%; Sigma-Aldrich Co., St. Louis, MO) at 50 μg/ml was injected into the femur. A platinum wire (10 mm × 3 mm) with a preformed S. aureus biofilm was implanted into the femur. The platinum wire was seeded with S. aureus biofilm (~105 cfu/cm2, as determined by quantitative culture on a separate set of wires) by incubation in tryptic soy broth (TSB) for approximately 2 hours. The remaining space in the bone was covered with dental gypsum after the wire was implanted. The cables used to supply power were tunneled under the skin to the prepared midscapular area and exposed (Figure 1); the incisions on the leg and in the midscapular area were closed with 3-0 vicryl (Ethicon, Inc., Somerville, NJ) and the exposed wires were secured to the skin with 3-0 silk (Ethicon, Inc.) (Figure 1). The leg was sprayed with AluSpray (Neogen Corporation, Lansing, MI) and Chew-Guard (Summit Hill Laboratories, Tinton Falls, NJ). Buprenorphine (slow release at 60 mg/kg) and meloxicam (slow release at 4 mg/kg) were used for analgesia.
Figure 1.
Graphic illustrating the experimental foreign body osteomyelitis model. [Reproduced with permission from (Schmidt-Malan, et al., 2017).]
One week after establishing infection, treatment was started. Thirty-six animals were randomly assigned to one of four study arms: no treatment (n=13), ceftriaxone treatment (n=7), continuous 200 μA fixed DC (n=8) or ceftriaxone with 200 μA fixed DC (n=8). The DC amperage was chosen based on our prior studies of in vitro effects on biofilms when administered in combination with antimicrobial agents (Del Pozo, et al., 2009). One animal from the ceftriaxone treatment group was removed from the study because it chewed the jacket harboring the power source and lost one of its bottom incisors. Ceftriaxone was administered at 50 mg/kg intramuscularly once daily; we have previously published pharmacokinetic data using this ceftriaxone dose (Schmidt-Malan, et al., 2017). Battery packs, set to deliver 200 μA of fixed DC, were connected to the exposed wires (Figure 2). Treatment was administered for 21 days. Twenty-four hours after the last dose of ceftriaxone, rats were sacrificed with CO2 inhalation and the left femur with the platinum implant was removed and placed into a sterile 50 ml conical tube. The femur was frozen to −80°C. Bone surrounding the implanted wire was cut (a 5 mm section), weighed and refrozen to −80°C, and then pulverized for quantitative bacterial culture. The crushed bone was placed in 2 mL of TSB, vortexed for 30 seconds, sonicated at 40 kHz for 5 minutes, vortexed for 30 seconds, serially diluted and plated on trypticase soy agar plates containing 5% sheep blood (TSA II, Becton Dickinson Franklin Lakes, NJ). The wire was removed from the bone and placed in 1 mL of TSB and cultured, as described above.
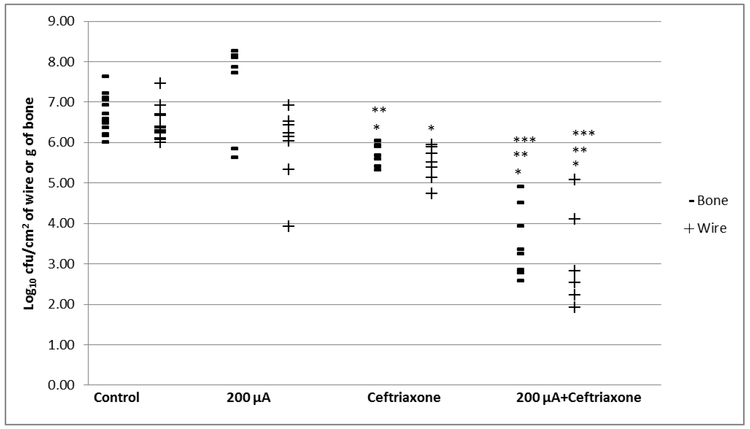
Figure 2.
Treatment of foreign-body osteomyelitis caused by S. aureus IDRL-4284. *Denotes significance compared to control, ** denotes significance compared to 200 μA alone, *** denotes significance compared to ceftriaxone alone, p<0.05.
Quantitative culture results for bone and wire were obtained after 48 hours of incubation at 37°C and expressed as log10 cfu/g or log10 cfu/cm2, respectively.
Statistical methods.
Statistical analyses were performed using SAS software (SAS Institute, Inc., Cary, NC). Using the Wilcoxon rank sum test, we compared the log10 cfu per gram of bone or cm2 of wire for the no-treatment, ceftriaxone treatment, 200 μA fixed DC treatment, and ceftriaxone with 200 μA fixed DC treatment groups. All tests were two sided; P values of <0.05 were considered statistically significant.
RESULTS
Experimental rat model.
Continuous low-amperage fixed DC alone and in combination with ceftriaxone as well as ceftriaxone alone was used to treat experimental femoral foreign body osteomyelitis.
The median quantities of bacteria were 6.59 (range 6.01 to 7.64) log10 cfu/g of bone in the untreated group, 5.70 (range 5.33 to 6.05) log10 cfu/g of bone in the ceftriaxone group, 8.00 (range 5.64 to 8.27) log10 cfu/g of bone in the 200 μA fixed DC group and 3.31 (range 2.58 to 4.92) log10 cfu/g of bone in the ceftriaxone with 200 μA fixed DC group. The bones of animals treated with ceftriaxone or ceftriaxone with 200 μA fixed DC had statistically significantly fewer bacteria than the bones of untreated animals (P, 0.0004 or 0.0002, respectively). There were fewer bacteria in the ceftriaxone with 200 μA fixed DC animals than in the ceftriaxone alone- and 200 μA fixed DC alone-treated animals (P, 0.0012 and 0.0008, respectively) (Figure 2).
The untreated group had a median quantity of 6.30 (range 6.00 to 7.46) log10 cfu/cm2 on the wires, with 5.52 (range 4.73 to 5.94) log10 cfu/cm2 of wire in the ceftriaxone group, 6.18 (range 3.92 to 6.92) log10 cfu/cm2 of wire in the 200 μA fixed DC group and 2.38 (range 1.92 to 5.07) log10 cfu/cm2 of wire in the ceftriaxone with 200 μA fixed DC group. Compared to the untreated group, the ceftriaxone alone and the ceftriaxone with 200 μA groups had statistically significant fewer bacteria on the wires (P, 0.0003 and 0.0002, respectively). There were fewer bacteria in the ceftriaxone with 200 μA fixed DC animals than in the ceftriaxone alone- and 200 μA fixed DC alone- treated animals (P, 0.0017 and 0.0016, respectively) (Figure 2).
We have presented the results using Wilcoxon rank sum test. The false discovery rate approach is more powerful than methods like the Bonferroni correction that control for multiple comparisons (Glickman, et al, 2014). However, after adjusting the p-values for multiple comparisons using approaches, such as false discovery rate and Bonferroni, the findings remain unchanged (i.e., p-values <0.05).
DISCUSSION
In this study, we have shown that when combined with ceftriaxone, fixed DC is able to significantly reduce the bacterial population in the bones and on the implants of rats infected with S. aureus in a femoral model of foreign-body osteomyelitis.
To date, our group and several others have tested low-amperage fixed DC in combination with antibiotics in vitro to reduce the bacterial load in biofilms of Pseudomonas aeruginosa, S. aureus, Staphylococcus epidermidis, and Klebsiella pneumoniae. This has been termed the ‘bioelectric effect,’ in which it is proposed that electrical current assists the penetration of antibiotics into biofilms by a form of electrophoresis (Costerton, et al., 1994; Del Pozo, et al., 2009; Stoodley & Lappin-Scott, 1997; Wellman, et al., 1996). Costerton et al. showed that amperages as low as 100 μA may be adequate in enhancing the effects of tobramycin against P. aeruginosa in a biofilm state, with a 6-log reduction in viable bacteria (Costerton, et al., 1994). In a study, similar to the work of Costerton et al., the activity of tobramycin was heightened when in the presence of 1 mA current against P. aeruginosa and K. pneumoniae biofilms, in which a 6- to 8-log reduction was recognized (Wellman, et al., 1996). Another study, by our group, found that the activity of vancomycin against methicillin-resistant S. aureus when combined with fixed DC was enhanced compared to vancomycin alone; in addition, we reported enhanced activity of daptomycin and erythromycin when combined with fixed DC against S. epidermidis (Del Pozo, et al., 2009).
The evidence provided in prior in vitro studies along with that of our current in vivo study, give promise to the possibility of combining electrical strategies with conventional antimicrobial agents for treatment of foreign body-associated osteomyelitis. Studies are currently underway addressing toxicity/adverse effects of the applied strategy.
A limitation of this study is that only one strain of S. aureus was tested. Testing a variety of S. aureus strains, as well as other PJI-associated species, would allow a better understanding of the scope of use of fixed DC in combination with antimicrobials for the treatment of PJI. A second limitation is that this model does not fully encompass the clinical conditions that are present in human patients, as a platinum electrode served as the implanted material (whereas ceramic constituents, metal alloys, and stainless steel are the most commonly used in humans). A third limitation is that only one antimicrobial agent was tested; the combined effect of antimicrobial agent and fixed DC may be further enhanced if fixed DC were to combined with an antimicrobial agent with better anti-biofilm activity than ceftriaxone. Other metals and amperages should be tested, along with different combinations of antimicrobials, in future studies. When treating with DC alone, there was an increase in the amount of bacteria in bone compared to the controls; however this was not statistically significant. The standard deviation in the fixed DC alone bone group was higher (SD 1.08) compared to the control bone group (SD 0.47), resulting in a higher median bacterial density in the fixed DC alone group.
In conclusion, adding fixed DC to conventional antimicrobial agent treatment may aid in the management of foreign body-associated methicillin-susceptible S. aureus infections.
HIGHLIGHTS.
Rat model methicillin-susceptible Staphylococcus aureus foreign body osteomyelitis
Ceftriaxone alone versus ceftriaxone with fixed direct current versus controls
Fixed direct current plus antibiotic active in S. aureus foreign body osteomyelitis
ACKNOWLEDGEMENTS
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases under Award Number R01 AI091594. RP is also supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01 AR056647. CB was supported under the National Institute of Arthritis and Musculoskeletal and Skin Diseases for the Musculoskeletal Research Training Program under award number T32 AR56950. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Benito N, Franco M, Ribera A, Soriano A, Rodriguez-Pardo D, Sorlí L, Fresco G, Fernández-Sampedro M, Del Toro MD, & Guío L. Time trends in the aetiology of prosthetic joint infections: a multicentre cohort study. Clin Microbiol Infect 2016; 22: 732.e731–732.e738. [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 11th Edition CLSI document M07, 11th ed 2018a; Clinical and Laboratory Standards Institute; Wayne, PA. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 28th ed. CLSI Supplement M100. 2018b: Clinical and Laboratory Standards Institute; Wayne, PA. [Google Scholar]
- Costerton JW, Ellis B, Lam K, Johnson F, & Khoury AE. Mechanism of electrical enhancement of efficacy of antibiotics in killing biofilm bacteria. Antimicrob Agents Chemother 1994; 38: 2803–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Pozo JL, Rouse MS, Mandrekar JN, Sampedro MF, Steckelberg JM, & Patel R. Effect of electrical current on the activities of antimicrobial agents against Pseudomonas aeruginosa, Staphylococcus aureus, and Staphylococcus epidermidis biofilms. Antimicrob Agents Chemother 2009; 53: 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gbejuade HO, Lovering AM, & Webb JC. The role of microbial biofilms in prosthetic joint infections: a review. Acta Orthop 2015; 86: 147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman ME, Rao SR, & Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol. 2014; 67:850–857. [DOI] [PubMed] [Google Scholar]
- Høiby N, Bjarnsholt T, Givskov M, Molin S, & Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 2010; 35: 322–332. [DOI] [PubMed] [Google Scholar]
- Kremers HM, Larson DR, Crowson CS, Kremers WK, Washington RE, Steiner CA, Jiranek WA, & Berry DJ. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surgery 2015; 97: 1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz S, Ong K, Lau E, Mowat F, & Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surgery 2007; 89: 780–785. [DOI] [PubMed] [Google Scholar]
- Schmidt-Malan SM, Brinkman CL, Greenwood-Quaintance KE, Karau MJ, Mandrekar JN, & Patel R. Activity of electrical current in experimental Propionibacterium acnes foreign-body osteomyelitis. Antimicrob Agents Chemother 2017; 61: e01863–01816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Malan SM, Karau MJ, Cede J, Greenwood-Quaintance KE, Brinkman CL, Mandrekar JN, & Patel R. Antibiofilm activity of low-amperage continuous and intermittent direct electrical current. Antimicrob Agents Chemother 2015; 59: 4610–4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley P, & Lappin-Scott H. Influence of electric fields and pH on biofilm structure as related to the bioelectric effect. Antimicrob Agents Chemother 1997; 41: 1876–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tande AJ, & Patel R. Prosthetic joint infection. Clin Microbiol Rev 2014; 27: 302–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman N, Fortun SM, & McLeod BR. Bacterial biofilms and the bioelectric effect. Antimicrob Agents Chemother 1996; 40: 2012–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]