Abstract
Objectives:
Women likely require higher adherence than men to pre-exposure prophylaxis (PrEP) with tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC) for similar efficacy. Pharmacologic metrics of adherence predict efficacy better than self-report, but expected drug levels (adherence benchmarks) must be established using directly-observed therapy. We sought to evaluate whether tenofovir hair concentrations differ between women and men receiving directly-observed TDF/FTC.
Methods:
We assessed tenofovir hair concentrations in HIV-uninfected volunteers randomized to receive 100%, 67%, or 33% of daily dosing of TDF/FTC for 12 weeks (DOT-DBS, NCT02022657). Hair samples were collected at dosing weeks 4, 8, and 12 and weeks 3 and 6 during a 12-week washout. Tenofovir concentrations in the proximal 1.5 centimeters of hair (representing ~6 weeks of exposure) were analyzed using liquid chromatography/tandem mass spectrometry. Linear regression was used to model tenofovir hair concentrations in terms of sex, doses over the prior 6 weeks, and number of days since last dose.
Results:
A total of 264 hair samples were analyzed from 23 female and 24 male participants. Female participants had similar tenofovir hair concentrations to males (estimated fold-difference 0.92, 95% CI 0.75-1.13, p=0.43). The estimated fold-difference in tenofovir levels for female versus male participants did not appreciably change when age (0.93, CI 0.76-1.15), weight (0.89, CI 0.71-1.11), or race/ethnicity (0.95, CI 0.77-1.17) were added to the model.
Conclusions:
Women and men have similar adherence benchmarks for tenofovir in hair samples. As pharmacokinetic metrics are increasingly used for PrEP monitoring, these findings provide guidance for assessing adherence via hair concentrations.
Keywords: HIV prevention, adherence, cumulative exposure, pharmacokinetics, PrEP in women
INTRODUCTION
Oral pre-exposure prophylaxis (PrEP) with tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC) is now recommended in global guidelines for individuals at risk of HIV acquisition. [1, 2] Adherence to PrEP is an important correlate of its success. Whereas PrEP was effective across a wide range of adherence levels in trials of men who have sex with men (MSM),[3-5] women seemed to require higher levels of adherence to PrEP for protection from HIV acquisition.[6-8] Two placebo-controlled trials of PrEP among younger sexually active women in Africa demonstrated no reduction in HIV acquisition.[9, 10] Low levels of adherence to study product (as measured via PrEP drug concentrations in plasma), despite high levels of self-reported adherence, seemed to explain the null findings in these trials. Pharmacokinetic modeling studies indicate that higher levels of adherence may be required to protect against vaginal exposure (6-7 PrEP doses per week) compared to rectal exposure (approximately 4 doses per week) because of lower concentrations of tenofovir and tenofovir-diphosphate in cervicovaginal than in colorectal tissues.[11-13]
Pharmacologic measures (e.g. PrEP drug concentrations in plasma, dried blood spots [DBS], or hair) can be used to estimate levels of adherence and exposure to PrEP among HIV-uninfected individuals (in whom HIV RNA cannot be used as a surrogate for adherence) and were essential to interpreting the results of the PrEP trials. “Adherence benchmarks” (antiretroviral drug concentrations measured in a biomatrix after a known number of doses taken) have been established for PrEP drugs in plasma,[14] DBS,[15] and hair,[16] paving the way for the use of these adherence metrics during widespread PrEP roll-out. However, to ensure that a metric is interpreted correctly for both men and women at risk, it is important to examine potential sex differences in adherence benchmarks for the particular matrix.[14, 15] In this study, we sought to evaluate whether tenofovir concentrations in hair differ between men and women receiving TDF/FTC under directly-observed dosing.
METHODS
Study design and population
The DOT-DBS study[15] (NCT02022657) enrolled healthy, HIV-uninfected volunteers at low risk of HIV acquisition with an estimated glomerular filtration rate (eGFR) >60 ml/min in two cities in the United States: Denver and San Francisco. Participants received open-label oral TDF/FTC 300/200 mg under directly-observed dosing conditions (with confirmation of pill ingestion in-person or via live video). Study procedures were approved by the institutional review boards of the University of Colorado and the University of California, San Francisco (UCSF) and all participants provided written informed consent.
Procedures
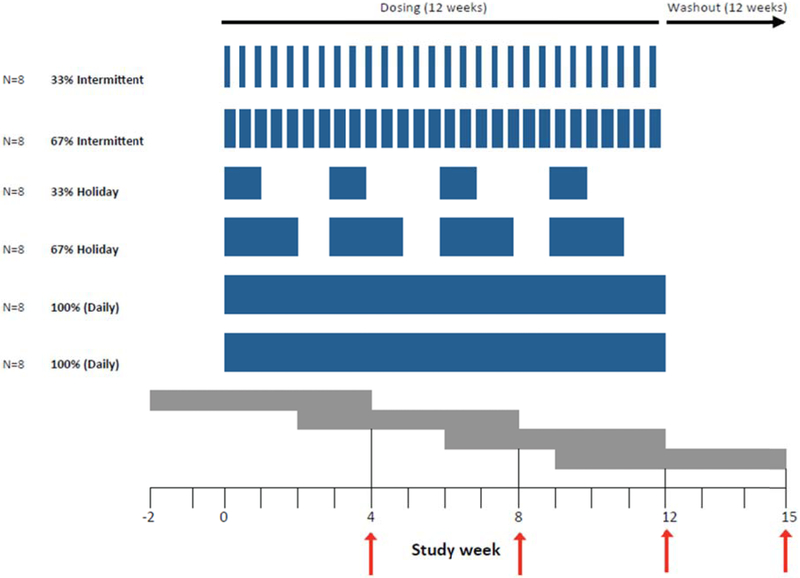
Participants were randomized to receive two of the following 12-week dosing regimens: 100%, 67%, or 33% of daily dosing, separated by a 12-week washout period. The 33% and 67% arms were split into “intermittent” dosing (which alternated by days, e.g. 1 day on/2 days off for 33% intermittent dosing) or “holiday” dosing (in which dosing weeks were separated by week-long breaks, e.g. 1 week on/2 weeks off for 33% holiday dosing). (Figure 1)
Figure 1. Schema for directly-observed administration of TDF/FTC and collection of hair samples from study participants.
Blue bars indicate doses given during each randomized study arm. Intermittent doses were separated by days (e.g. 1 day on/2 days off for 33% intermittent dosing) and holiday doses were separated by weeks (e.g. 1 week on/2 weeks off for 33% holiday dosing). Gray bars represent ~6 weeks of hair growth (reflecting drug exposure over that time) prior to hair collection (indicated by red arrows). Hair was collected at weeks 4, 8, and 12 during dosing and every 3 weeks during a 12-week washout period. Following a 12-week washout period, participants received 12 additional weeks of a different dosing regimen and hair collection.
Small hair samples were collected at weeks 4, 8, and 12 of each dosing regimen and every 3 weeks during the 12-week washout period. Tenofovir concentrations in the proximal 1.5 centimeters of hair (representing approximately 6 weeks of exposure) were analyzed using validated liquid chromatography/tandem mass spectrometry (LC-MS/MS)-based methods at the UCSF Hair Analytical Laboratory (HAL).[16] The assay has been validated from 0.002-0.400 ng tenofovir/mg hair and has been peer-reviewed and approved by the National Institute of Allergy and Infectious Diseases Division of AIDS-supported Clinical Pharmacology and Quality Assurance (CPQA) program.[17]
Statistical analyses
Linear regression models with random-person effects were used to model logarithmically transformed tenofovir hair concentrations in terms of doses taken over the prior 42 days (6 weeks), number of days since the last dose, sex at birth, weight, and race/ethnicity. Data were analyzed from hair samples collected when participants had taken at least one dose of TDF/FTC in the prior 42 days; hair samples from study weeks 18, 21, and 24 were thus excluded from analysis. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Characteristics of study participants
A total of 23 female and 24 male participants were enrolled in the study and included in this analysis (Supplemental Table 1, http://links.lww.com/QAD/B318). Among females, the median age was 29 years (range 21-49); race/ethnicity was 48% White, 17% African American, 35% Hispanic; median weight was 63 kg (range 47-114). Among males, median age was 29 years (range 22-50); race/ethnicity was 63% White, 13% African American, 25% Hispanic; median weight was 83 kg (range 60-155).
Hair collection
A total of 371 hair samples were collected during the study and participants provided a median of 10 (range 1-10) hair samples. After excluding samples collected when participants had taken 0 doses of TDF/FTC in the preceding 6 weeks, we analyzed data on tenofovir concentrations from 264 hair samples.
Fold-difference in tenofovir hair concentrations by sex
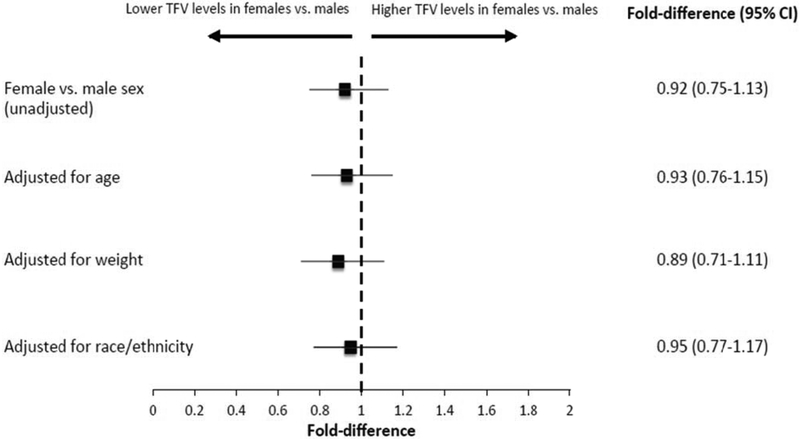
In models controlling for the number of doses taken in the previous 6 weeks and days since last dose, female participants had similar tenofovir hair concentrations to males (fold-difference 0.92, 95% CI 0.75-1.13, p=0.43), (Figure 2). The estimated fold-difference in tenofovir levels for female versus male participants did not appreciably change when age (0.93, 95% CI 0.76-1.15, p=0.50), weight (0.89, 95% CI 0.71-1.11, p=0.30), or race/ethnicity (0.95, 95% CI 0.77-1.17, p=0.65) were added to the model.
Figure 2. Estimated fold-difference in hair concentrations of tenofovir by female versus male sex.
DISCUSSION
Under directly-observed dosing conditions, we found that concentrations of tenofovir in hair samples from women were similar to those in men. This observation remained consistent when controlled for race/ethnicity, age, and weight. Our findings suggest that previously established adherence benchmarks for tenofovir concentrations in hair[16] can be similarly applied to both sexes. As PrEP implementation expands globally, these findings have implications for adherence monitoring in future PrEP studies and programs.
Adherence to PrEP appears to be particularly critical to its effectiveness in women.[18] As a result of differences in tissue concentrations of tenofovir, higher levels of adherence to PrEP may be required to protect against vaginal exposure than rectal exposure.[12] Because self-reported adherence has been unreliable across multiple PrEP trials[9, 10, 19] and open-label studies,[20, 21] and because “white coat” dosing (in which adherence increases just prior to study or clinic visits) is a possible confounder,[22] pharmacologic adherence metrics (objective measures that reflect drug ingestion) are of critical importance for interpreting results. Adherence benchmarks for pharmacologic adherence metrics that do not differ by sex and can be applied across populations may facilitate the interpretation of adherence and outcomes in future PrEP studies. The current study indicates that tenofovir concentrations in hair under directly-observed dosing conditions are similar by sex and that the same adherence benchmarks can be applied to both women and men. In addition, although our study analyzed data collected in the context of 33%, 67%, or 100% of daily dosing, our results suggest that prior estimates of tenofovir hair concentrations under different dosing patterns (<2, 2-3, 4-6, or 7 doses per week) [21, 23] can be applied to both sexes.
Young women in sub-Saharan Africa are at disproportionate risk of HIV acquisition[24] and could greatly benefit from PrEP. Adherence monitoring may be particularly valuable for this population; however, the optimal adherence metric for women on PrEP has not yet been established. Biomarkers of adherence that estimate cumulative drug adherence and exposure over weeks to months (such as hair and DBS) may be particularly valuable in programmatic settings and studies in which participants are seen infrequently, such as on a quarterly basis. Hair and DBS are less susceptible to “white coat” adherence patterns than drug levels in plasma, which reflects recent drug ingestion (yes or no) within days prior to collection. Hair samples have potential feasibility advantages in resource-limited settings in that hair collection is non-biohazardous and does not require cold storage/shipping conditions. Efforts are underway to build capacity for testing of tenofovir hair concentrations in laboratories based in Africa and to develop lower-cost methods to analyze antiretroviral concentrations in hair in resource-limited settings.[25]
Future studies are needed to determine drug concentration thresholds in any matrix above which women are protected from HIV acquisition. Although such thresholds have been established for MSM,[26] analogous levels have not been determined in women. These thresholds would have particular value in future trials of investigational agents for PrEP, in which oral TDF/FTC will serve as the comparator arm, since drug concentration thresholds can serve as surrogate endpoints. Trials powered to detect seroconversion even in high incidence settings would require large sample sizes and considerable expense, making a surrogate endpoint desirable. In addition, the use of well-established drug concentration thresholds would allow for an estimate of effects in populations disproportionately impacted by HIV in low-incidence settings, such as African American women in the United States,[27] and could lead to actionable interventions to optimize adherence and maximize the protective effect of PrEP.
In summary, our results suggest that adherence benchmarks for tenofovir in hair under directly-observed dosing conditions are similar by sex and, therefore, that adherence monitoring to PrEP via hair levels can be similarly applied to both women and men. As PrEP programs expand worldwide and pharmacokinetic metrics are increasingly used to monitor PrEP use, these findings provide guidance for assessing adherence via hair concentrations among women and men on PrEP.
Supplementary Material
Acknowledgements:
We thank the study participants for contributing their time to the study and the dedicated study staff at the University of Colorado, the San Francisco Department of Public Health, and the UCSF Hair Analytical Laboratory (HAL).
Source of funding: Support for this work was provided by grants from the National Institutes of Health (U01 AI106499 [P.L.A.], 2R01 AI098472 [M.G.], K23 AI104315 [J.C.M], T32 AI7447-23 [to support S.S.], UL TR000004 [UCSF CTSI], UL1 TR001082 [University of Colorado CTSA], and K12 HD052163 [to support C.A.K.]). Gilead Sciences provided study drug.
Footnotes
Potential conflicts of interest: Gilead Sciences donated tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) as study drug, but had no other role in the design or conduct of the study or the analysis or interpretation of data. C.A.K. has received grant support paid to her institution from the Gilead Sciences Research Scholars Program in HIV. P.L.A. has received grant and contracts from Gilead Sciences, paid to his institution. A.Y.L. has led studies in which Gilead Sciences has donated study drug.
REFERENCES
- 1.World Health Organization. WHO Technical Brief: Preventing HIV during pregnancy and breastfeeding in the context of pre-exposure prophylaxis (PrEP). Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- 2.World Health Organization; Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection, second edition. Geneva, Switzerland; 2016. [PubMed] [Google Scholar]
- 3.McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016,387:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molina JM, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 2015,373:2237–2246. [DOI] [PubMed] [Google Scholar]
- 5.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010,363:2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012,367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fonner VA, Dalglish SL, Kennedy CE, Baggaley R, O'Reilly KR, Koechlin FM, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS 2016,30:1973–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Straten A, Van Damme L, Haberer JE, Bangsberg DR. Unraveling the divergent results of pre-exposure prophylaxis trials for HIV prevention. AIDS 2012,26:F13–19. [DOI] [PubMed] [Google Scholar]
- 9.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 2015,372:509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012,367:411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louissaint NA, Cao YJ, Skipper PL, Liberman RG, Tannenbaum SR, Nimmagadda S, et al. Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue. AIDS Res Hum Retroviruses 2013,29:1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cottrell ML, Yang KH, Prince HM, Sykes C, White N, Malone S, et al. A translational pharmacology approach to predicting outcomes of preexposure prophylaxis against HIV in men and women using tenofovir disoproxil fumarate with or without emtricitabine. J Infect Dis 2016,214:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patterson KB, Prince HA, Kraft E, Jenkins AJ, Shaheen NJ, Rooney JF, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med 2011,3:112re114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hendrix CW, Andrade A, Bumpus NN, Kashuba AD, Marzinke MA, Moore A, et al. Dose frequency ranging pharmacokinetic study of tenofovir-emtricitabine after directly observed dosing in healthy volunteers to establish adherence benchmarks (HPTN 066). AIDS Res Hum Retroviruses 2016,32:32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson PL, Liu AY, Castillo-Mancilla JR, Gardner EM, Seifert SM, McHugh C, et al. Intracellular tenofovir-diphosphate and emtricitabine-triphosphate in dried blood spots following directly observed therapy. Antimicrob Agents Chemother 2018,62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu AY, Yang Q, Huang Y, Bacchetti P, Anderson PL, Jin C, et al. Strong relationship between oral dose and tenofovir hair levels in a randomized trial: Hair as a potential adherence measure for pre-exposure prophylaxis (PrEP). PLoS One 2014,9:e83736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiFrancesco R, Taylor CR, Rosenkranz SL, Tooley KM, Pande PG, Siminski SM, et al. Adding value to antiretroviral proficiency testing. Bioanalysis 2014,6:2721–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomson KA, Baeten JM, Mugo NR, Bekker LG, Celum CL, Heffron R. Tenofovir-based oral preexposure prophylaxis prevents HIV infection among women. Curr Opin HIV AIDS 2016,11:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amico KR, Marcus JL, McMahan V, Liu A, Koester KA, Goicochea P, et al. Study product adherence measurement in the iPrEx placebo-controlled trial: concordance with drug detection. J Acquir Immune Defic Syndr 2014,66:530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amico KR, Mehrotra M, Avelino-Silva VI, McMahan V, Veloso VG, Anderson P, et al. Self-reported recent PrEP dosing and drug detection in an open label PrEP study. AIDS Behav 2016,20:1535–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koss CA, Hosek SG, Bacchetti P, Anderson PL, Liu AY, Horng H, et al. Comparison of measures of adherence to HIV preexposure prophylaxis among adolescent and young men who have sex with men in the United States. Clin Infect Dis 2018, 66:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koss CA, Bacchetti P, Hillier SL, Livant E, Horng H, Mgodi N, et al. Differences in cumulative exposure and adherence to tenofovir in the VOICE, iPrEx OLE, and PrEP Demo studies as determined via hair concentrations. AIDS Res Hum Retroviruses 2017,33:778–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gandhi M, Glidden DV, Mayer K, Schechter M, Buchbinder S, Grinsztejn B, et al. Association of age, baseline kidney function, and medication exposure with declines in creatinine clearance on pre-exposure prophylaxis: an observational cohort study. Lancet HIV 2016,3:e521–e528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdool Karim Q, Kharsany AB, Leask K, Ntombela F, Humphries H, Frohlich JA, et al. Prevalence of HIV, HSV-2 and pregnancy among high school students in rural KwaZulu-Natal, South Africa: a bio-behavioural cross-sectional survey. Sex Transm Infect 2014,90:620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gandhi M, Yang Q, Bacchetti P, Huang Y. Short communication: A low-cost method for analyzing nevirapine levels in hair as a marker of adherence in resource-limited settings. AIDS Res Hum Retroviruses 2014,30:25–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012,4:151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adimora AA, Cole SR, Eron JJ. US Black Women and Human Immunodeficiency Virus Prevention: Time for New Approaches to Clinical Trials. Clin Infect Dis 2017,65:324–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.