Abstract
Purpose
While there is growing scientific evidence for and significant advances in the use of genomic technologies in medicine, there is a significant lag in the clinical adoption and sustainability of genomic medicine. Here we describe the findings from the National Human Genome Research Institute’s (NHGRI) Implementing GeNomics In pracTicE (IGNITE) Network in identifying key constructs, opportunities, and challenges associated with driving sustainability of genomic medicine in clinical practice.
Methods
Network members and affiliates were surveyed to identify key drivers associated with implementing and sustaining a genomic medicine program. Tallied results were used to develop and weigh key constructs/drivers required to support sustainability of genomic medicine programs.
Results
The top three driver–stakeholder dyads were (1) genomic training for providers, (2) genomic clinical decision support (CDS) tools embedded in the electronic health record (EHR), and (3) third party reimbursement for genomic testing.
Conclusion
Priorities may differ depending on healthcare systems when comparing the current state of key drivers versus projected needs for supporting genomic medicine sustainability. Thus we provide gap-filling guidance based on IGNITE members’ experiences. Although results are limited to findings from the IGNITE network, their implementation, scientific, and clinical experience may be used as a road map by others considering implementing genomic medicine programs.
Keywords: Sustainability, Genomics, Implementation science, IGNITE Network, Genomic education
Introduction
The Implementing GeNomics In pracTicE (IGNITE) Network (https://ignite-genomics.org/), funded by the National Human Genome Research Institute (NHGRI), strives to develop and implement strategies for using genomics in routine clinical care. Beginning in 2013, six research institutions and 14 community partners were funded to demonstrate the feasibility of genomic medicine in diverse settings. In addition, 16 affiliate institutions voluntarily collaborate with IGNITE to learn genomic medicine implementation techniques, share their experiences, and participate in network activities. IGNITE strives to identify best practices in genomic medicine implementation, and challenges to the successful adoption and sustainability of genomics programs. Utilizing the collective experiences of IGNITE and its investigators, we identified key functions and specific actions needed for sustainability. We describe the process used to prioritize the drivers as they applied to four stakeholders: providers, payers, patients, and government agencies.
Materials and methods
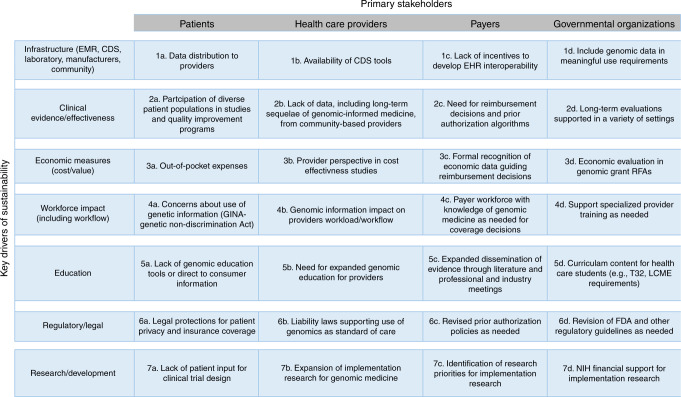
Data collection and analysis were carried out in two steps. The first step involved an open-ended survey sent to the six IGNITE site principal investigators and the six IGNITE Working Group chairs (https://ignite-genomics.org/about-ignite/ and https://ignite-genomics.org/network/working-groups/) to identify the key drivers of genomic medicine sustainability. Based on the 12 responses received, seven key drivers emerged as most important (Fig. 1). The IGNITE Sustainability Working Group, comprised of manuscript authors, then identified four primary stakeholders impacted by these key drivers (Fig. 1), thus creating a 28-dyad matrix.
Fig. 1. Seven key drivers of genomic sustainability across four primary stakeholders resulting in 28 constructs (dyads).
EMR electronic medical record, CDS clinical decision support, LCME Liaison Committee on Medical Education, FDA Food and Drug Administration, RFA Request for Application, NIH National Institutes of Health
The second survey was designed to collect a consolidated ranking of the top seven key drivers across four stakeholder groups (patients, providers, payers, and governmental organizations) from each site. Sites were provided with the 28-dyad matrix and each site was asked to select what they viewed as the most important dyad for each key driver. The sites were then asked to rank the top 7 dyads important for genomic testing sustainability with 1 being the most important and 7 being the least important dyad. The survey tool was disseminated via e-mail to 20 IGNITE member sites (6 IGNITE study site principal investigators [PIs] and 14 IGNITE affiliates); PIs and affiliate leaders were asked to collect input from their teams and submit a consolidated response. The consolidated responses from each member site was then used for analysis. Individual investigator input into the consolidated results was not received or reviewed by the authors. We received 18 responses from 20 sites (90% response rate). In addition, we received 2 survey responses from IGNITE funded study site partners, for a total of 20 completed surveys. Study site respondents included 11 PIs, including 8 physicians, all with patient care and genomic medicine experience, 1 PharmD with extensive genomic research and patient care background, and 2 PhD-trained PIs with extensive experience in genomic research. The 14-affiliate IGNITE members are comprised of physicians, PharmDs, and PhDs with extensive genomic medicine and research experience. The Sustainability Working Group tabulated the results from the 20 respondent sites and constructed two alternate measures of importance based on these responses. The first measure was the proportion of institutions that reported dyad importance (i.e., ranked any one dyad in the top 7 of the 28 possibilities), and the second measure was the proportion of institutions that reported the dyads that were ranked as a “top three” priority.
Results
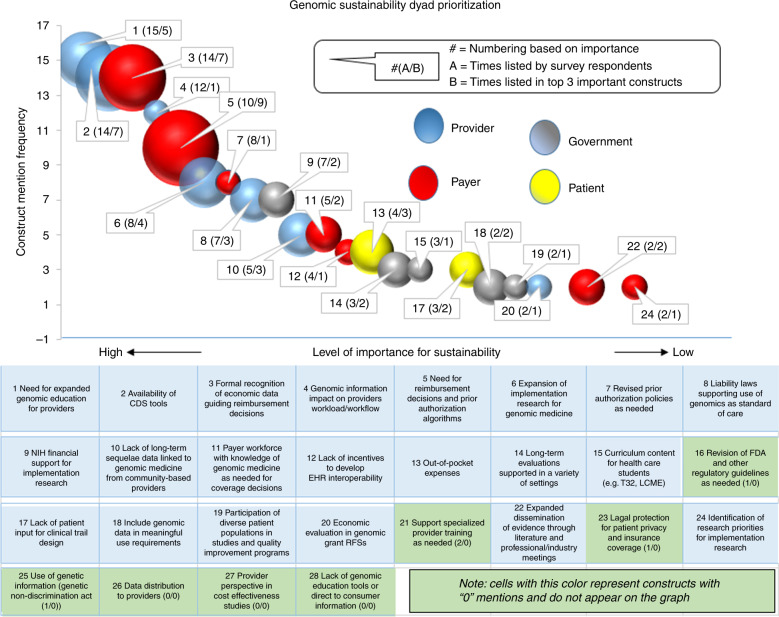
An analysis of the priority-setting survey responses revealed diverse opinions on which drivers and stakeholders were most important for genomic medicine sustainability. Of the 28 driver–stakeholder constructs, 21 (75%) were identified by 22 respondents in their top 7 ranking. A further analysis of these 21 constructs revealed that provider and payer related constructs were selected 7 times each, or 33% of the time each, as being the most important stakeholders, while governmental organizations and patients were selected 5 (24%) and 2 times (10%), respectively (see Fig. 2). It is recognized that the results from both surveys represent the consolidated opinion of IGNITE member sites and that results outside of the network might differ. The three top-ranked constructs deemed most important for sustainability were provider education, a lack of genomic-focused clinical decision support (CDS) tools, and reimbursement. The relationship between the frequency with which dyads were identified as important, and the relative importance assigned to each dyad, is depicted using a bubble chart (Fig. 2).
Fig. 2.
Constructs weighted by frequency selected as being important in genomic sustainability (a), and frequency weighted in top three of importance (b).
Discussion
The analysis of the results from the IGNITE Network’s consolidated, site-level surveys, based on its members’ experience and input, identified that their most critical sustainability strategies involve provider and payer stakeholders; however, there is considerable diversity of opinion as to what specific drivers need to be targeted by such efforts. Based on the extensive experience of IGNITE members in genomic medicine, we believe that sustainability of genomics in practice requires that local implementation processes be well planned from the onset. Findings from the IGNITE Network can assist clinical sites interested in adopting genomic testing by helping to identify key drivers, stakeholders, and expectations that must be met if genomics are to be implemented in a sustainable manner. Our findings do not negate the fact that there are many factors required for sustainability of genomics in clinical practice. Rather, we identify that there will be differences in priorities from one institution to another. Despite the fact that all survey respondents were part of the IGNITE Network and the small sample size, it is not surprising there was no consensus about which drivers were most important to the sustainability of genomic medicine in clinical practice. While most respondents thought providers and payers were the most critical stakeholders, the drivers that were thought to be most important for each stakeholder differed. For providers, important barriers were lack of education and the need for genomic-focused CDS tools; whereas for payers, the barriers were related to reimbursement. Strategies to overcome these barriers, and the contributions of the IGNITE Network to these strategies, are discussed below.
Driver: education (provider education)
Lack of trust and understanding of genomic testing results, and knowledge of how to interpret and modify treatment, can be a significant challenge among providers. The National Institutes of Health (NIH) have supported the training of the next generation of clinician-researchers by expanding genomic-focused Career Development Awards (K-awards) or Institutional Training Grants (T32 grants). Broader dissemination can be achieved by incorporating training in genomics in medical school curricula and encouraging continuing medical education (CME) programs to cover this topic.1 Educational programs addressing research advances, treatment guidelines, and related liability laws are ideal topics for physicians, nurses, and pharmacists already in practice.2 Additional programs may be needed to encourage active collaboration among nursing, counseling, and pharmacy practitioners.3 The Consolidated Framework for Implementation Research (CFIR) method was used to evaluate a guideline implementation process for the IGNITE site to support the development of such guidelines4,5 (https://ignite-genomics.org/spark-toolbox/clinicians/).
Driver: infrastructure (CDS/EHR for providers)
Obtaining the appropriate infrastructure has historically been cited as a priority for sustaining clinical pharmacogenomic testing, one example of genomic implementation.6,7 Infrastructure must include not only electronic health record (EHR) systems that have accessible locations for ordering genomic tests, but also CDS tools that reduce the time and burden of finding and interpreting genomic information. In particular, CDS tools need to ensure that genetic information is not filed in the same way as other laboratory results whose validity declines with time. NHGRI networks such as the Electronic Medical Records and Genomics Network (eMERGE8) and IGNITE9 have developed and cataloged key decision support tools that can be integrated into EHR systems (e.g., https://ignite-genomics.org/spark/clinical-decision-support-integration-genetic-information-ehr/).
Driver: economic measures (reimbursement strategies for payers)
The adoption and sustainability of genomic medicine is challenged by the quantity and types of evidence needed for payers to justify reimbursement.12,13. Payers state that they will not reimburse for new diagnostics that do not meet their thresholds for clinical validity and utility.10 Payers need to develop and communicate clear criteria for quantitative and qualitative evidence requirements so that researchers know where best to target their efforts. In addition, high-quality cost-effectiveness studies of genomic-based interventions are needed for payers to determine if genomic medicine constitutes high-value care appropriate for coverage.11 Comparative effectiveness research is needed to generate the evidence of clinical validity and utility. Findings from previous studies in genomic medicine are often limited due to small cohort size, and lack of diversity in patients and practice settings studied. Furthermore, although the cost of genetic testing has been decreasing, the economic value of genomic medicine in clinical practice will also depend on its larger impact on the overall cost of patient care throughout the healthcare system. Thus, sustainability will require that genomic researchers considerably expand the scope of their studies to include economic outcomes and underrepresented populations. Combined with the rapid pace of technological advancement in the field, the evidentiary requirements of payers will require substantial commitments of research resources, as well as material support from impacted government organizations (e.g., Centers for Medicare & Medicaid Services). The IGNITE Toolbox has archived a variety of resources to support these future research efforts (https://ignite-genomics.org/spark-toolbox/researchers/). As the needed evidence accumulates, researchers will need to work closely with other organizations to ensure the coding systems used to report medical, surgical, and diagnostic procedures, and which underlie reimbursement arrangements, are revised appropriately. The IGNITE Working Group on Clinical Validity and Utility and Economics is developing documentation that describes the process to apply for new code sets (https://ignite-genomics.org/network/working-groups/#).
Conclusion
Sustainability of a new technology is fraught with many challenges. The results of this study from the IGNITE Network demonstrate that each key driver of genomic medicine sustainability has relevance across the domain of stakeholders and ultimately should be addressed to help improve adoption, implementation, and sustainability. In our analysis of the data, the authors focused on the three most important constructs (dyads) requiring resource allocation and focus. The survey results identified provider education/training, the availability of CDS tools/EHRs that properly embed genomic data, and adequate reimbursement by third party payers for genomic testing as the three most critical areas required for sustainability. It should be noted, however, that constructs not ranked in the top three cannot be ignored, because findings of this study should be considered site-specific. Institutions considering the adoption of genomic interventions should perform their own evaluation to determine which driver–stakeholder constructs are most relevant for their specific situation.
When considering construct focus, it is also important to understand the complexity and timelines required to address stakeholder concerns associated with each key driver. For example, the workforce impact driver is complex, because it is linked to how the CDS and EHR is designed. Provider technology fatigue is a growing problem and EHRs must incorporate user-defined controls for alerts and display only clinically validated “pop-ups” to prevent undue workflow interruption. CDS tools can facilitate effective and efficient provider workflow. The adoption and sustainability of genomic medicine is challenged by the quantity and types of clinical evidence needed for implementation.12,13 Constructs associated with the drivers for strong clinical evidence and research and development are linked, requiring significant investment in time and resources to deliver the required result. To help overcome research barriers and facilitate clinical research, the IGNITE Network has developed implementation models, tools, and best practices supporting the routine application of genomic medicine to patient care.4,9,14 Constructs associated with regulatory and legal issues were not highly ranked by the respondents, and can be difficult to address without the help of national professional organizations establishing new standards of care influencing provider liability and quality standards and third party payers’ reimbursement for genomic testing. It should also be noted one limitation of this study is that survey respondents were limited to IGNITE members, although the respondents have extensive experience in genomic medicine. To validate a nonbiased result, additional survey input should include representations from the two stakeholder groups not yet surveyed (patients and payers). Based on the study results, we suggest that sustainability of genomic medicine in practice requires continued and adequate funding for clinical research, continued education of clinicians, investments in design and development by vendors supplying electronic medical record systems to the healthcare community, and acceptance of genomic medicine clinical utility by third party payers resulting in reasonable reimbursement.
Acknowledgements
This work was supported by grants from the National Institutes of Health (U01 HG007269, U01 HG007253, U01 HG007762, U01 HG007282, U01 HG007775, U01 HG007278, and by the NIH IGNITE Network (http://ignite-genomics.org/).
Disclosure
The authors declare no conflicts of interest.
References
- 1.Korf BR. Genetics and genomics education: the next generation. Genet Med. 2011;13:201–2. doi: 10.1097/GIM.0b013e31820986cd. [DOI] [PubMed] [Google Scholar]
- 2.Haga SB. Educating patients and providers through comprehensive pharmacogenetic test reports. Pharmacogenomics. 2017;18:1047–50. doi: 10.2217/pgs-2017-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haga SB. Delivering pharmacogenetic testing to the masses: an achievable goal? Pharmacogenomics. 2014;15:1–4. doi: 10.2217/pgs.13.211. [DOI] [PubMed] [Google Scholar]
- 4.Sperber NR, et al. Challenges and strategies for implementing genomic services in diverse settings: experiences from the Implementing GeNomics In pracTicE (IGNITE) network. BMC Med Genom. 2017;10:35. doi: 10.1186/s12920-017-0273-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orlando LA, et al. Developing a common framework for evaluating the implementation of genomic medicine interventions in clinical care: the IGNITE Network’s Common Measures Working Group. Genet Med. 2017;20:655–63. doi: 10.1038/gim.2017.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khelifi M, Tarczy-Hornoch P, Devine EB, Pratt W. Design recommendations for pharmacogenomics clinical decision support systems. AMIA Jt Summits Transl Sci Proc. 2017;2017:237–46. [PMC free article] [PubMed] [Google Scholar]
- 7.Klein ME, Parvez MM, Shin JG. Clinical implementation of pharmacogenomics for personalized precision medicine: barriers and solutions. J Pharm Sci. 2017;106:2368–79. doi: 10.1016/j.xphs.2017.04.051. [DOI] [PubMed] [Google Scholar]
- 8.National Human Genome Research Institute (NHGRI). Electronic Medical Records and Genomics (eMERGE) Network. 2017. Accessed 20 June 2018. https://www.genome.gov/27540473/#al-2.
- 9.Weitzel KW, et al. The IGNITE Network: a model for genomic medicine implementation and research. BMC Med Genom. 2016;9:1. doi: 10.1186/s12920-015-0162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burke W. Genetic tests: clinical validity and clinical utility. Curr Protoc Hum Genet. 2014;81:9.15.1–9.15.8. doi: 10.1002/0471142905.hg0915s81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frueh FW. Regulation, reimbursement, and the long road of implementation of personalized medicine—a perspective from the United States. Value Health. 2013;16(6 suppl):S27–31. doi: 10.1016/j.jval.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Perry CG, Shuldiner AR. Pharmacogenomics of anti-platelet therapy: how much evidence is enough for clinical implementation? J Hum Genet. 2013;58:339–45. doi: 10.1038/jhg.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips KA, et al. Making genomic medicine evidence-based and patient-centered: a structured review and landscape analysis of comparative effectiveness research. Genet Med. 2017;19:1081–91. doi: 10.1038/gim.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavallari LH, et al. The IGNITE Pharmacogenetics Working Group: an opportunity for building evidence with pharmacogenetic implementation in a real-world setting. Clin Transl Sci. 2017;10:143–6. doi: 10.1111/cts.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]