Abstract
Agrobacterium tumefaciens attaches stably to plant host tissues and abiotic surfaces. During pathogenesis, physical attachment to the site of infection is a prerequisite to infection and horizontal gene transfer to the plant. Virulent and avirulent strains may also attach to plant tissue in more benign plant associations, and as with other soil microbes, to soil surfaces in the terrestrial environment. Although most A. tumefaciens virulence functions are encoded on the tumor-inducing plasmid, genes that direct general surface attachment are chromosomally encoded, and thus this process is not obligatorily tied to virulence, but is a more fundamental capacity. Several different cellular structures are known or suspected to contribute to the attachment process. The flagella influence surface attachment primarily via their propulsive activity, but control of their rotation during the transition to the attached state may be quite complex. A. tumefaciens produces several pili, including the Tad-type Ctp pili, and several plasmid-borne conjugal pili encoded by the Ti and At plasmids, as well as the so-called T-pilus, involved in interkingdom horizontal gene transfer. The Ctp pili promote reversible interactions with surfaces, whereas the conjugal and T-pili drive horizontal gene transfer (HGT) interactions with other cells and tissues. The T-pilus is likely to contribute to physical association with plant tissues during DNA transfer to plants. A. tumefaciens can synthesize a variety of polysaccharides including cellulose, curdlan (β−1,3 glucan), β−1,2 glucan (cyclic and linear), succinoglycan, and a localized polysaccharide(s) that is confined to a single cellular pole and is called the unipolar polysaccharide (UPP). Lipopolysaccharides are also in the outer leaflet of the outer membrane. Cellulose and curdlan production can influence attachment under certain conditions. The UPP is required for stable attachment under a range of conditions and on abiotic and biotic surfaces. Other factors that have been reported to play a role in attachment include the elusive protein called rhicadhesin. The process of surface attachment is under extensive regulatory control, and can be modulated by environmental conditions, as well as by direct responses to surface contact. Complex transcriptional and post-transcriptional control circuitry underlies much of the production and deployment of these attachment functions.
Keywords: attachment, cell surface structures, biofilms, regulation
1. Introduction
A wide diversity of bacteria interact with surfaces in their environments, often forming multicellular assemblies known as biofilms. Recent years have seen an explosion of research on biofilms and surface attachment mechanisms, reflecting an appreciation of how ubiquitous these processes are and the extent to which they can influence the bacterial physiology (Visick et al. 2016). Adherent bacteria exhibit dramatically different bioactivities than they do in the unattached state and, most notably, biofilm formation can markedly increase tolerance towards antibiotics. For pathogenic bacteria, association with host surfaces is often the first step toward infection, and antibiotic resistant biofilms formed by pathogens have become a major clinical problem in human medicine (Hoiby 2017). Biofilms on non-host surfaces can also act as disease reservoirs and are conducive to horizontal gene transfer of antibiotic resistance determinants and virulence factors (Madsen et al. 2012). Structures on the bacterial cell surface, including a variety of filamentous and globular protein adhesins, and exopolysaccharides, mediate the interactions with surfaces that lead to stable colonization. Multiple types of surface fibers including pili, fimbriae, and β-amyloid filaments contribute to attachment. These fibers include flagella that drive motility for many bacteria, but in some cases can act as adhesins as well as propulsive structures. Several different types of secretion systems, such as Type III (T3S), Type IV (T4S), and Type VI (T6S) systems, can also influence surface interactions. Certain bacterial taxa produce large surface proteins with multi-repeat domains that can function as adhesins (Hinsa et al. 2003). A variety of polysaccharides, including outer membrane lipopolysaccharides, have been implicated in stable surface attachment and biofilm formation (Branda et al. 2005). The production of specific cell-surface adhesive structures is often under elaborate regulatory control.
Similar to human and animal pathogens, many pathogens of plants must productively colonize host surfaces to cause disease. Multicellular aggregates also play roles for pathogenic bacteria in foliar, vascular, and root environments (Danhorn and Fuqua, 2007). Pathogenic Agrobacterium species must physically associate with surfaces to drive interkingdom gene transfer to plants and other aspects of Agrobacterium-induced disease. For crown gall disease, the details of the physical interactions between A. tumefaciens and plant cells which lead to T4S-mediated introduction of transferred DNA (T-DNA) into the plant cell cytoplasm remain poorly understood. It is clear that A. tumefaciens is an effective colonizer of plant surfaces during pathogenic and non-pathogenic interactions, as well as associating with abiotic surfaces in the soil environment. How this general surface attachment progresses, or switches to the physical association leading to T-DNA transfer, is still being actively studied. A large cluster of so-called Attachment (Att) genes were purported to be required for A. tumefaciens association with plant tissues, and to be necessary for virulence (Matthysse et al. 2000). Subsequently, the Att genes were shown to be encoded on the pAt plasmid, and this plasmid was demonstrated to be dispensable for virulence (Nair et al. 2003). More recent work has suggested the possibility that mutations in the Att cluster may have a dominant inhibitory effect on attachment (Matthysse et al. 2008). Thus, despite their historical identification, the Att genes are currently not thought to play a direct role in attachment to plants or other surfaces. Rather, there must be other cell surface structures that function in this capacity. Flagella, several different forms of pili, and multiple complex polysaccharides are produced by A. tumefaciens, and several of these are now known to promote general attachment to surfaces. The virulence (Vir) proteins involved in interkingdom gene transfer to plants may also contribute to attachment on host tissues. As with mammalian pathogens, deployment of these and other cell surface attributes in A. tumefaciens can be elaborately regulated by transcriptional and post-transcriptional mechanisms. In this review, we describe the current understanding of A. tumefaciens cell surface structures that contribute to surface attachment mechanisms, including those for host tissues and abiotic materials, the molecular composition and biosynthesis of these structures, and the recognized systems that control their activity.
2. Flagella
Flagella play an important role in attachment in addition to their more general function in enabling diverse bacteria to propel themselves through their environment. Flagellar propulsion can enable bacteria to move towards conditions that are favorable such as high nutrients, and avoid conditions that inhibit growth or damage cells. Much of what is known about flagella structure and assembly derives from studies of the peritrichous flagella of Escherichia coli and Salmonella enterica (Chevance and Hughes 2008; MccNab 1996). However, outside of these model systems, there is a significant variety of flagellar organization, number, and composition (Schuhmacher et al. 2015). An example of this is the type strain A. tumefaciens C58, which extrudes four to six flagella that are each ~10–12 nm diameter filaments (Chesnokova et al. 1997; Shaw et al. 1991). As with several other members of the Rhizobiaceae, the A. tumefaciens flagellar filament is composed of multiple flagellin proteins, and exhibits a complex ultrastructure (Götz et al. 1982). The primary flagellin FlaA is strictly required for motility. The other three flagellins (FlaB, FlaC, and FlaD) play more ancillary roles but are proposed to be important for flagellar filament structural integrity (Deakin et al. 1999).
2.1. Flagellum structure, function, biogenesis, and regulation
The structure and activity of the bacterial flagellum is recognized as one of the molecular marvels of the natural world (prompting some individuals to conclude that their structure is evidence for divine intervention; Pallen and Matzke 2006). The biogenesis of these remarkable rotary nanomachines is a prime example of an ordered molecular assembly process. Flagella are assembled from the inside out, with their basal bodies comprised of a series of ring structures (Chevance and Hughes 2008). In Gram-negative bacteria, the C-ring is assembled near the cytoplasmic face of the inner membrane (Fig. 1). Associated with the C-ring is the MS-Ring that forms within the cytoplasmic membrane, and it houses a T3S system which exports specific flagellar components through the center of this ring. The next components to assemble are the P ring (embedded in the peptidoglycan) and the L ring (embedded in the outer membrane), containing proteins secreted into the periplasm via the general secretion system. All of these rings are made of multiple copies of the same proteins, and at least some of the structures are thought to be dynamic in the numbers of monomers that form the ring (Branch et al. 2014; Lele et al. 2012). The motor and stator complex is embedded in the inner membrane and has a large domain that associates with the C-ring (Fig. 1). Motor proteins assemble around the C-ring complex. Other proteins, including the switch proteins that can alter flagellar rotation, can associate with the inner face of the C-ring (Chevance and Hughes, 2008). The rod structure connects these rings through the bacterial envelope with the proteins that make up the flagellar filament (hook subunits, assembly chaperones, linkers and flagellins). These proteins are exported through the lumen of the rod, via the T3S system, and are added to the growing filament at the distal end. The hook is connected to the rod, and approximately 130 copies of the hook protein assemble, directed by the hook chaperone, to form this flexible universal joint (Fig. 1). Additional linker proteins and an assembly chaperone facilitate sequential addition of the flagellin subunits to the hook and subsequent extension of the helical filament bulding from the distal end, comprised of as many as 30,000 flagellin monomers (although this number varies significantly with bacterial taxon and average flagellar length; Blair 2003). Rotation of the flagellum is driven by proton translocation, and in E. coli and Salmonella spp. it is estimated that ~550 protons are translocated per single rotation of the flagellum. In the enteric model systems the rotation of the flagellum is reversible, with counterclockwise (CCW) rotation driving straight swimming, and clockwise (CW) rotation generating cellular tumbles that reorient the cell. The ratio of swimming to tumbling is under the control of the chemotaxis system, which enables directed motility (Wadhams and Armitage, 2004).
Figure 1. General structure of the bacterial flagellum.
Diagrammatic representation of a bacterial flagellum structure based on flagella from Salmonella enterica serovar Typhimurium (Salmonella typhimurium), alongside a flagellum structure determined by cryoelectron microscopy of the flagellum from Treponema primitia. Combined figure adapted with permissions from Nat Rev Microbiol (Pallen and Matzke, 2006) and Curr. Biol. (DeRosier 2006).
The general properties of the A. tumefaciens flagellum are consistent with those from the enteric model systems, but with several important differences. Among the Rhizobiaceae the best studied model is Sinorhizobium meliloti, and this system shares several distinguishing features with A. tumefaciens. For example, each of these systems has complex flagella composed of four different flagellins (Deakin et al. 1999; Götz et al. 1982). Rotation of these complex flagella is consistently in the CW direction, and in contrast to E. coli and Salmonella, this is not reversed to generate tumbles. Rather, the current model is that the rate of CW flagellar rotation is modulated by the chemotaxis system, and that asynchronous rotation of multiple flagella causes tumbling (Sourjik and Schmitt 1996).
The arrangement of the flagellar filaments varies among even closely related bacteria (Schuhmacher et al. 2015). In A. tumefaciens the flagella are organized into a polar tuft of 4–6 filaments (Chesnokova et al. 1997), whereas S. meliloti has a peritrichous organization (Götz et al. 1982). There is significant variation for flagellar placement among the rhizobia that has been recognized for many years (Leifson and Erdman, 1958). A. tumefaciens demonstrates a swimming pattern with long, straight runs (Mohari et al. 2015). The bacterium has strong positive chemotactic responses to the sugars sucrose, glucose and fructose, with slightly weaker responses to a variety of other sugars, as well as responses to the amino acids valine and arginine (Ashby et al. 1988).
The many proteins that contribute to assembly of flagella and motility are typically encoded in large operons or gene clusters. In A. tumefaciens C58, the genes encoding the structural components of the flagella, assembly factors, and several motility regulators are in one large gene cluster (~36 kbp, Atu0541-Atu0583; Table 1) on the circular chromosome (Deakin et al. 1997a; 1997b; 1999). Additionally, the core chemotaxis genes reside in a single gene cluster (>13.5 kbp, Atu0514–0526) that also includes several likely flagellar genes and the two master motility regulators visN and visR. The Che cluster is located close to the Fla cluster, separated only by ~14 kbp. Together, there are more than 50 genes in these two clusters (Table 1) and most if not all appear to be dedicated to motility, although only a subset of these have been experimentally validated for A. tumefaciens. There are a few scattered chemotaxis gene homologues throughout the genome (predominantly methyl-dependent chemotaxis protein homologues), but the majority of motility and chemotaxis functions are in these two clusters (Liu and Ochman 2007).
Table 1.
Core Chemotactic and Flagellar Gene Clusters
| Gene Number | Gene1 name | Gene length - bp (Protein length - aa)1 | Predicted Function1 |
|---|---|---|---|
| Atu0514 | 1707 (568) | Methyl-accepting chemotaxis protein | |
| Atu0515 | cheX | 300 (99) | Chemotaxis protein |
| Atu0516 | cheY1 | 366 (121) | Chemotaxis receiver protein |
| Atu0517 | cheA | 2214 (737) | Chemotaxis histidine kinase |
| Atu0518 | cheR | 909 (302) | Chemotaxis methyltransferase |
| Atu0519 | cheB | 1056 (351) | Chemotaxis methylesterase |
| Atu0520 | cheY2 | 390 (129) | Chemotaxis receiver protein |
| Atu0521 | cheD | 546 (181) | Methyl-accepting chemotaxis protein |
| Atu0522 | 184 (127) | Undefined function | |
| Atu0523 | fliF | 1701 (566) | Flagellar M-ring protein |
| Atu0524 | visN | 681 (226) | LuxR-type transcriptional regulator |
| Atu0525 | visR | 756 (251) | LuxR-type transcriptional regulator |
| Atu0526 | mclA | 1848 (615) | Methyl-accepting chemotaxis protein |
| Atu0542 | fla | 942 (313) | Flagellin |
| Atu0543 | flaB | 963 (320) | Flagellin |
| Atu0544 | 258 (85) | Undefined function | |
| Atu0545 | flaA | 921 (306) | Flagellin |
| Atu0546 | fliP | 738 (245) | Flagellar export protein |
| Atu0547 | fliL | 501 (166) | Flagellar protein |
| Atu0548 | flgH | 720 (239) | Flagellar L-ring precursor |
| Atu0549 | 537 (178) | Undefined function | |
| Atu0550 | flgI | 1122 (373) | Flagellar P-ring precursor |
| Atu0551 | flgA | 489 (162) | Flagellar P-ring protein |
| Atu0552 | flgI | 789 (262) | Flagellar rod protein |
| Atu0553 | fliE | 339 (112) | Flagellar hook-basal body protein |
| Atu0554 | flgC | 420 (139) | Flagellar body-rod protein |
| Atu0555 | flgB | 393 (130) | Flagellar basal body-rod protein |
| Atu0556 | 405 (134) | Undefined function | |
| Atu0557 | fliI | 1422 (473) | Flagellum-specific ATPase |
| Atu0558 | flgF | 735 (244) | Flagellar basal body-rod protein |
| Atu0559 | 636 (211) | Undefined function | |
| Atu0560 | motA | 873 (290) | Flagellar motor protein |
| Atu0561 | fliM | 960 (319) | Flagellar motor switch protein |
| Atu0562 | fliN | 540 (179) | Flagellar motor switch protein |
| Atu0563 | fliG | 1044 (347) | Flagellar motor switch protein |
| Atu0564 | flhB | 1083 (360) | Flagellar export protein |
| Atu0565 | 438 (145) | Undefined function | |
| Atu0566 | 213 (70) | Undefined function | |
| Atu0567 | flaD | 1293 (430) | Flagellin |
| Atu0568 | 657 (218) | Undefined function | |
| Atu0569 | motB | 1302 (433) | Flagellar motor protein |
| Atu0570 | motC | 1281 (426) | Chemotaxis protein |
| Atu0571 | motD | 1350 (449) | Chemotaxis protein |
| Atu0572 | 444 (147) | Undefined function | |
| Atu0573 | rem | 672 (223) | OmpR-type transcriptional regulator |
| Atu0574 | flgE | 1278 (425) | Flagellar hook protein |
| Atu0575 | flgK | 1479 (492) | Flagellar hook-associated protein |
| Atu0576 | flgL | 1104 (367) | Flagellar hook-associated protein |
| Atu0577 | flaF | 345 (114) | Flagellar biosynthesis regulatory protein |
| Atu0578 | flbT | 450 (149) | Flagellar biosynthesis regulatory protein |
| Atu0579 | flgD | 474 (157) | Hook formation protein |
| Atu0580 | fliQ | 267 (88) | Flagellar export protein |
| Atu0581 | flhA | 2088 (695) | Flagellar export protein |
| Atu0582 | fliR | 723 (240) | Flagellar export protein |
| Atu0583 | 420 (139) | Undefined function | |
| Atu0584 | 528 (175) | Undefined function | |
| Atu0585 | 369 (122) | Undefined function | |
| Atu8132 | 534 (177) | Undefined function |
Gene identity, length, and predicted function annotations are based on KEGG Gene Ontology (Kyoto University Bioinformatics Center) and Osterman et al. 2015.
Expression of flagellar genes is often under complex, stepwise morphogenetic control which ensures that each component of the flagellum is produced at the appropriate step of biogenesis (Chevance and Hughes 2008). Based on the enteric model systems, these genes are often divided into three classes, initiating with the master regulators of motility (Class I), the genes encoding the T3S system through which the flagellum is assembled, the hook-basal body complex (Class II), and those proteins that make up the flagellar filament (Class III). Flagellar gene expression in several rhizobia, including A. tumefaciens, is controlled through a complex regulatory hierarchy the details of which remain to be fully defined. Class IA is composed of the LuxR-type transcription factors VisN and VisR (Vital in swimming), both of which are required for flagellar motility (Sourjik et al. 2000). VisN and VisR activate expression of the class IB gene rem (regulator of exponential growth motility) (Xu et al. 2013). Rem is an OmpR-type response regulator (Rotter et al. 2006) with no recognized cognate histidine kinase, and lacking the canonical Asp residue at which most response regulators are phosphorylated. Rem activates expression of the Class II flagellar genes which include the components of the flagellar hook and basal body as well as the motor, and it is also required for expression of Class III genes, including the flagellin genes and several chemotaxis genes (Sourjik et al. 2000; Zatakia et al. 2018). In Brucella melitensis, flagellin synthesis is inversely regulated by the flagellin activator FlbT and the repressor FlaF (Ferooz et al. 2011). Indeed, this level of control may be broadly conserved throughout the Alphaproteobacteria, as a similar pathway has been delineated in C. crescentus (Mangan et al. 1999). A. tumefaciens has homologues of FlaF and FlbT, although their roles are largely unexplored. Many flagellar assembly pathways include the activity of a specialized sigma factor dedicated to the transcription of subsets of the flagellar genes (e.g. σ28 in E. coli and σD in B. subtilis), with promoter sequences that are quite distinct from those for σ70 promoters (Aldridge and Hughes 2002). The motility sigma factor is often controlled through an anti-sigma factor (Hughes and Mathee 1998). Promoters regulated by Rem in S. meliloti, and by extension in A. tumefaciens, clearly have a non-σ70 architecture (Rotter et al. 2006), suggesting the presence of an alternate sigma factor, but there is no such annotated sigma factor encoded in the motility gene cluster. Thus far, this presumptive sigma factor has not been identified for any of the rhizobia.
Numerous factors outside of the hierarchy of flagellar regulators affect A. tumefaciens motility through various mechanisms. The periplasmic succinoglycan-regulatory protein ExoR is required for motility through its effects on flagellar gene expression (Tomlinson et al. 2010); the broader role of ExoR will be discussed later in this review (Section 6.1.1). Flagella synthesis as well as flagellin expression and motility are elevated when A. tumefaciens cells are grown in the absence of light, but the mechanism for this is unclear (Oberpichler et al. 2008). Flagellar motility is also affected by the cell cycle regulators divK, pdhS1, and pleC, mutants in which form branched cells and have altered flagellar placement (Kim et al. 2013). A pdhS2 mutant does not manifest aberrant cell shape, and these cells produce flagella, but they do not swim. Given the asymmetric cell division mechanism of A. tumefaciens (Brown et al. 2012), it is not surprising that flagellar biogenesis would be integrated with control of the cell cycle.
2.2. Role of flagellar motility in attachment
Flagella play a role in A. tumefaciens attachment to model surfaces. Aflagellate mutants deleted for the hook protein FlgE, and motA deletion mutants with unpowered flagella are both highly deficient in surface attachment and biofilm formation under static conditions (Merritt et al. 2007). Given the requirement for active flagellar rotation, it was concluded that swimming motility drives the frequency or productivity of surface contact. Interestingly, an aflagellate hook mutant formed biofilms more robustly and rapidly than wild-type cells in a flow cell, suggesting that the flow regime promoted high frequency surface contact and that perhaps the lack of motility limited emigration from the surface (Merritt et al. 2007). A straight swimming cheA mutant manifested only a modest attachment defect in static culture, but quantitative analysis of flow cell biofilms revealed a different three-dimensional structure. Motility in these straight swimming cheA mutants is compromised in motility agar dispersal assays, but spontaneous suppressor mutants can be readily isolated (Mohari et al. 2015). These mutants regain tumbling activity, and hence migration through motility agar because of structural deformations in the flagellum. These changes, however, result in a dramatic loss of attachment, revealing how proper coordination of motility is important during surface colonization. Further evidence for the connection between motility and attachment of A. tumefaciens came from a screen for regulators of attachment in which null mutations of the Class IA master regulators visN and visR resulted in increased attachment, even though they abolished motility (Xu et al. 2013). The increased attachment of these mutants results from a complex regulatory pathway as detailed below (Section 6.2).
3. Pili
Many bacteria promote attachment to surfaces with proteinacious surface appendages known as pili (or sometimes called fimbriae). Although many pili are considered static appendages, they are all actively extruded, and in many cases actively retracted. These filaments can also drive twitching motility in some bacteria (Mattick 2002). A subset of pili are involved in plasmid conjugation, and are referred to as sex pili. A. tumefaciens encodes production of several different types of pili. The chromosomes of agrobacteria often carry a cluster of genes (Fig. 2) annotated as the Ctp cluster (Cpa-type pilus; named after homologues in Caulobacter crescentus; Skerker and Shapiro 2000), required to form Type IV pili of the Tad (Tight adherence) subclass, also known as common pili (Wang et al. 2014). Two distinct types of conjugative pili are encoded by the Ti plasmid: the Trb conjugal pili, required to conjugatively transfer the entire plasmid to recipient bacteria (Cook et al. 1997), and the T-pilus, required for T-DNA transfer to plants (Fullner et al. 1996). The At plasmid also encodes its own set of conjugative pili, called the AvhB system (Chen et al. 2002).
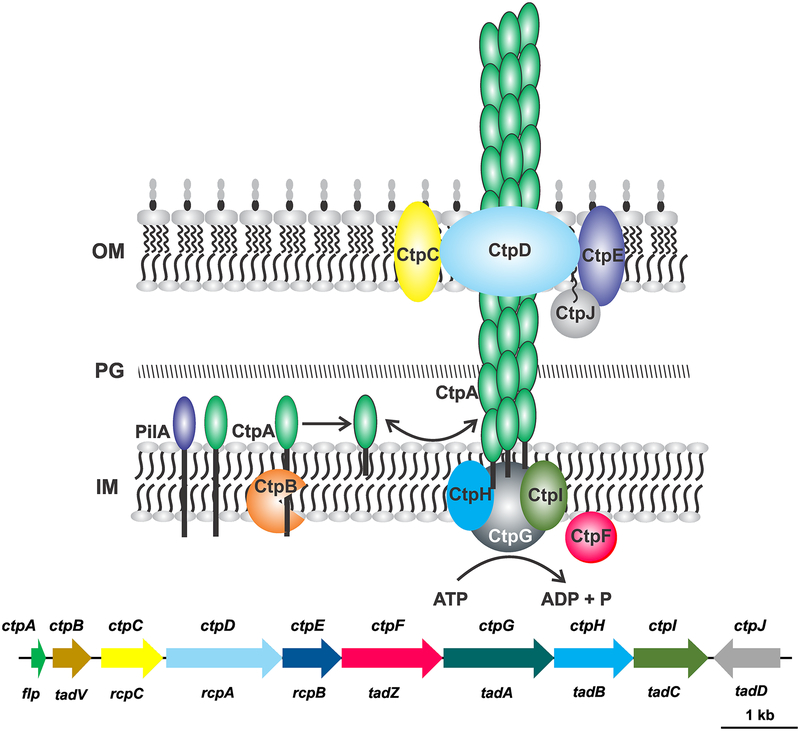
Figure 2. Genetic basis of Ctp pili and model for assembly.
Predicted localization and assembly mechanism for Ctp proteins based on the general model for Tad pilus assembly (Tomich et al. 2007). The CtpA pilin is processed by CtpB cleavage and incorporated into the emerging pilus. CtpG hydrolyzes ATP to drive pilus assembly. Protein names indicated in the figure. Gene map indicates the ctp gene names above the gene arrows, and gene names in the generalized Tad-type pilus system are provided below. Gene colors match protein colors in the diagram. OM, outer membrane; PG, peptidoglycan; IM, inner membrane.
3.1. Ctp pili
Electron microscopy of A. tumefaciens C58 reveals thin filaments (~3 nm in diameter) interspersed across the cell body (Lai et al. 2000; Wang et al. 2014). The A. tumefaciens C58 genome sequence contains a cluster of nine genes (Atu0224–0216), annotated ctpABCDEFGHI, that encode Type IV pilus assembly homologues (Fig. 2). Although initially defined as Type IVb pili, a recent study has re-classified the Ctp pili genes as Type IVc pilus assembly genes, conserved in diverse bacteria (Ellison et al. 2017). The A. tumefaciens Ctp locus is homologous and syntenous among different members of the Rhizobiaceae, as well as in more diverse Alphaproteobacteria such as C. crescentus (Skerker and Shapiro, 2000). Individual non-polar, in-frame deletions of most of the genes in the A. tumefaciens C58 Ctp cluster result in loss of piliation as evaluated by transmission electron microscopy, and these mutants are significantly inhibited for attachment and biofilm formation (Wang et al. 2014). Transcriptional fusion analysis suggests the existence of two promoters, one upstream of ctpA (ctpABCD) and a second upstream of ctpE (ctpEFGHI) (Fig. 2, Wang et al. 2014).
The ctpA gene encodes a small pilin homologue (64aa), often called Flp (fimbrial low molecular-weight protein) pilin. The protein contains the hydrophobic “Flp-motif” that includes the conserved pilin processing site (G/XXXXEY) (Kachlany et al. 2001). A second Flp pilin homologue, annotated pilA, is encoded elsewhere in the C58 genome (Atu3514), and if ectopically expressed can complement ctpA mutants for pilus assembly (Wang et al. 2014). Immediately downstream of ctpA is ctpB, a prepilin peptidase homologue, required to process pilin during assembly. The ctpC, ctpD, and ctpE genes encode proteins that are homologous to the RcpC, RcpB, and RcpA proteins, respectively, from Aggregatibacter actinomycetemcomitans which putatively encode an outer membrane complex centered around the CtpD secretin (Tomich et al. 2007). The CtpF protein is a homologue of CpaE from C. crescentus and TadZ from A. actinomycetemcomitans, proteins that have MinD/ParE homology and are predicted localization factors. Interestingly, ctpF is the only A. tumefaciens C58 gene in the Ctp cluster for which deletion does not cause loss of piliation (Wang et al. 2014). CtpG is an ATPase likely localized to the cytoplasm, and based on similarity to TadA from A. actinomycetemcomitans, is involved in powering Ctp pilus biogenesis. Finally, the CtpH and CtpI proteins are so-called platform proteins associated with the cytoplasmic membrane upon which the pilus is assembled, similar to TadB/C, and are likely to have arisen via a gene duplication event (Tomich et al. 2007). In many systems, a TadD homologue with a tetratricopeptide repeat (TPR) motif is located downstream and convergent to the pilus gene cluster. This gene is Atu0215 in A. tumefaciens C58, and here we tentatively designate it as ctpJ (Fig. 2). For the Tad system, this homologue is speculated to be a pilotin, with a lipid linkage to the outer membrane. However, the CtpJ product has no secretion signal and its role in Ctp pilus function is not known.
In the current model (Fig. 2), pools of the CtpA pilin (or alternatively PilA) associate with the cytoplasmic membrane. Pilus biogenesis requires the CtpB prepilin peptidase to cleave the CtpA monomers, and it is this processed form that interacts with the assembly machine, incorporating into the growing pilus at the base. Pilus assembly is powered by the cytoplasmic ATPase (CtpG), driving conformational changes that promote interactions of the pilin subunits on the platform proteins at the cytoplasmic face. The CtpC, CtpD, and CtpE proteins interact with the emerging pilus at the outer membrane, with the pilus spanning the membrane through the CtpD secretin. Recent work has also shown that Type IVc pili can retract, and the released pilin proteins can reassociate with the cytoplasmic membrane (Ellison et al. 2017). Many of the specific aspects of this overall model remain to be evaluated experimentally in A. tumefaciens and other similar systems.
Mutagenesis of the ctpA, ctpB, and ctpG genes leads to significant deficiencies in biofilm formation for A. tumefaciens, and this was correlated with problems in reversible attachment (Wang et al. 2014). Deletion of the entire Ctp gene cluster causes a similar loss of attachment. Individual mutations in any of the other genes in the Ctp cluster (ctpC, ctpD, ctpE, ctpF, ctpH, and ctpI), with the exception of the ctpF mutant, abolish pilus biogenesis, but surprisingly all of these mutants are stimulated rather than diminished for attachment. Disruption of ctpA in these hyperadherent Ctp mutants abolished their stimulated attachment. Likewise, mutations that prevent production of the unipolar polysaccharide (UPP) adhesin (see Section 4.2) completely prevent bacterial attachment (Wang et al. 2014). Although the reason for this hyperattachment in certain Ctp mutants remains unclear, it is possible that genetic disruption of these pilus functions, and perhaps accumulation of the CtpA pilin in the cytoplasmic membrane, causes feedback regulation in the cell to activate a pilus-independent, but UPP-dependent attachment mechanism. Similar signaling in response to pilin levels in the inner membrane have been reported for T4Pa pili in Pseudomonas aeruginosa (Kilmury and Burrows 2016)
As with other Alphaproteobacteria that produce unipolar polysaccharide adhesins which promote attachment, A. tumefaciens transitions from reversible to stable polar attachment via just-in-time production of the adhesive material (see Section 6.3). Production of the UPP is strictly surface-contact dependent (Li et al. 2012). For C. crescentus, recent studies showed that its Type IVc Cpa pilus is involved in triggering surface-contact dependent production of its polar adhesive, known as the holdfast (Ellison et al. 2017). The Cpa pili are localized to the same cellular pole from which the holdfast will be produced, and resistance to pilus depolymerization stimulates holdfast production. It is hypothesized that physical association with surfaces during reversible interactions inhibits Cpa pilus depolymerization, thereby stimulating holdfast production. In A. tumefaciens the Ctp pilus is required for reversible attachment, but electron microscopy does not thus far support a polar localization for these pili (Lai et al. 2000; Wang et al. 2014). However, it is certainly possible that a similar pilus-dependent surface-stimulation of UPP production plays a role in the transition to stable attachment for A. tumefaciens.
3.2. Conjugative pili
Conjugative plasmids have been reported to promote biofilm formation in diverse bacteria (Ghigo 2001). This is however, not attributed to the conjugative pili encoded by the plasmids, but rather, to other plasmid-encoded functions. These plasmids often drive their conjugation via cellular interaction with recipient bacteria mediated through their filamentous conjugative pili. For many years it was proposed that conjugative pili simply act in promoting the physical association of donor and recipient cells, and that DNA transfer occurred via mating pair formation and an ill-defined conduit between cells (Lessl and Lanka 1994). However, more recent work has resurrected the notion that the single-stranded DNA delivered by conjugative plasmids transits through the lumen of the conjugal pilus (Babic et al. 2008; Costa et al. 2016). Conjugative pili promote interactions between bacterial cells, and it is certainly conceivable that these surface structures could also play a role in surface attachment. Virulent A. tumefaciens produce two distinct conjugative pili encoded by the Ti plasmid (Vir and Tra/Trb) and often also a third conjugative pilus type encoded by the At plasmid (AvhB). These conjugative pili are all considered components of their respective Type IV secretion (T4S) systems. The pili that function in T4S plasmid conjugation systems should not be confused with the Ctp Type IVc pili (Section 3.1) or other Type IV pili. The Ti plasmid encodes the so-called T-pilus as a component of the machinery for T-DNA transfer to plant cells (Fullner et al. 1996). Also encoded by the Ti plasmid is the Trb conjugative pilus, required for horizontal transfer of the plasmid to other agrobacteria (Cook et al. 1997).
T-pilus production is strictly regulated along with the vir genes in response to plant wound conditions (Fullner et al. 1996). Encoded within the virB operon that also specifies the other components of the T4S system, the VirB2 protein is the pilin, and VirB3 is the prepilin peptidase (Lai et al. 2002). The VirB5 protein localizes to the terminus of the T-pilus and is proposed to mediate interactions between the T-pilus and the target plant cell (Aly and Baron 2007). Whether this interaction can be considered a component of the surface attachment process, or rather some other aspect of the intimate associations that lead to T-DNA transfer, is unclear. UPP-dependent attachment drives polar interactions with biotic and abiotic surfaces. Multiple studies from different groups, examining protein localization using VirB T4SS protein fusions with autofluorescent proteins, have suggested a unipolar localization for the T4SS secretion complex, including recognized components of the T-pilus (Judd et al. 2005). However, more recent work using immunolocalization has reported localization of VirB proteins in lateral arrays along the length of the cell, and this work has invoked a model in which T-DNA transfer occurs via these longitudinally-associated cells (Aguilar et al. 2011). It remains unclear how to accommodate these apparently conflicting observations. It is possible that the polar attachment to surfaces (both plants and abiotic surfaces) and the attachment to plant tissues that leads to T-DNA transfer are mechanistically distinct processes. Another possible model is that there is a temporal progression from polar attachment via the UPP to deployment of the VirB T4SS machinery. This transition could include a reorientation to a longitudinal association between the bacteria and the plant cells, or simply transfer of T-DNA at the site of polar associations. The polar or longitudinal association mechanisms are not mutually exclusive. Understanding the extent to which surface attachment is integrated with T-DNA transfer remains an area of active study.
The Ti plasmid conjugative pilus is encoded by the traI-trbABCDEFGHI operon and is under the strict control of conjugal opines and, through these, TraR-dependent quorum sensing (Fuqua et al. 1994; Piper et al. 1993). Thus, the Ti plasmid conjugative pilus is only produced by cells that are exposed to specific opines, and have reached a high population density. Given the strict conditionality of Trb pilus production, this structure cannot play a general role in surface interactions, but it remains uncertain whether under the appropriate conditions they might do so. In contrast, the At plasmid conjugative transfer system characterized for A. tumefaciens C58 is expressed constitutively in laboratory culture, and pAtC58 can be conjugatively transferred to recipients at a significant rate (Chen et al. 2002). However, there have been no reports indicating a role for these presumptive surface structures in surface attachment outside of interactions between bacterial cells. The At conjugative pili have never been visualized microscopically.
4. Exopolysaccharides
The process by which A. tumefaciens transitions from a planktonic, swimming cell to a sessile, surface-attached cell is determined, among other factors, by the production and subsequent extrusion of exopolysaccharides (EPS) from the cell. So far, it has been shown that A. tumefaciens C58 produces at least five different forms of EPS: cellulose, unipolar polysaccharide (UPP) adhesin, succinoglycan, cyclic-β-1,2-glucan, and curdlan (Berne et al. 2015; Li et al. 2012; Schmid et al. 2015; Xu et al. 2013), although it is possible that other yet-to-be-identified EPS species are produced. Not all the known EPS types, however, play roles in surface attachment. Succinoglycan, for instance, is necessary for symbiosis in S. meliloti (Reuber and Walker 1993) but is not required for attachment or biofilm formation in A. tumefaciens (Matthysse 2014; Tomlinson et al. 2010). Cyclic-β-1,2-glucan is a periplasmic polysaccharide believed to play a role in osmoregulation, and the inability of A. tumefaciens to synthesize this polysaccharide results in pleiotropic effects, including increased sensitivity to osmotic stress, reduced motility, and reduced attachment. The attachment deficient phenotype of a cyclic-β-1,2-glucan null mutant is likely indirect (Matthysse 2014), and no direct evidence implicating cyclic-β-1,2-glucan in attachment has been reported. This section will focus on the exopolysaccharides implicated in A. tumefaciens attachment.
4.1. Cellulose:
Cellulose is an abundant crystalline polymer that is commonly found in the plant kingdom but is also produced by a broad range of bacterial species. It is common in the genera of Proteobacteria including Komagataeibacter (formerly Gluconoacetobacter and Acetobacter), Azotobacter, Aerobacter, Escherichia, Salmonella, Rhizobium, and Agrobacterium (Arioli et al. 1998, Shoda and Sugano 2005). Cellulose, a polymer of glucose joined by β (1→4) glycosidic bonds, forms fibers where the individual cellulose chains are arranged in parallel structures held together by hydrogen bonds and Van der Waal forces. These fibers are water-insoluble and mechanically strong (Römling 2002). The physical properties of cellulose are illustrated by its presence in plant cell walls as large bundles of microfibrils and other higher-order structures, where it functions to determine plant cell shape and protect plant cells from osmotic stress and other environmental damage. In bacteria, however, cellulose is rarely contained in the cell wall or plasma membrane but rather is secreted outside of the cell as thin microfibril ribbons, the size of which is estimated at one-hundredth that of plant cellulose (Shoda and Sugano 2005; Williamson et al. 2002). Cellulose synthesis was first identified in Agrobacterium tumefaciens as thin fibrils responsible for floc formation during log-phase growth (Deinema and Zevenhuizen 1971), and then later reported to be involved in functions such as attachment and plant infection (Matthysse 1981, 1983, 1987).
4.1.1. Genetic basis and biosynthesis
Cellulose biosynthesis genes have been characterized in many different bacteria and are often encoded in conserved gene clusters. Core functionalities are found in all such systems, but there are other genes that are specific to certain subgroups. All genes involved in cellulose biosynthesis have recently been classified as the bacterial cellulose synthesis (bcs) genes (Römling and Galperin 2015). Historically, A. tumefaciens cellulose biosynthesis genes have been designated as cel genes, and for the purposes of this review we will maintain this nomenclature but also provide the corresponding bcs designation (Table 2, Fig. 3). In A. tumefaciens C58, cellulose synthesis is largely directed by the products of seven genes found in two presumptive operons convergent to one another on the linear chromosome (Fig. 3). The first operon is composed of five genes, celHABCG, and is convergent with the celDE genes (Fig. 3). The celABC genes have homologues in most bcs systems, but celH, celG, celD, and celE are found in a restricted subgroup designated Type IIIa (Römling and Galperin 2015).
Table 2.
A. tumefaciens cellulose biosynthesis genes
| Gene | Number | bp (aa) | BCS name1 | Enzymatic function1 | Predicted Localization1 |
|---|---|---|---|---|---|
| celH | Atu8187 | 900 (300) | bcsN | Periplasmic, single TM domain | Periplasmic |
| celA | Atu3309 | 2190 (789) | bcsA | Cellulose synthase, subunit A | Transmembrane (7 TMs) |
| celB | Atu3308 | 2484 (828) | bcsB | Cellulose synthase, subunit B | Periplasmic (SS+1 TM) |
| celC | Atu3307 | 1056 (352) | bcsZ | Endo-β−1,4-glucanase, (cellulase), periplasmic | Periplasmic (SS) |
| celG | Atu3306 | 2343 (781) | bcsK | Tetratricopeptide (TPR) motif – peptidoglycan interaction |
Periplasmic (SS) |
| celE | Atu3305 | 1155 (385) | bcsL | Acetyltransferase (TPR) | Cytoplasmic |
| celD | Atu3304 | 1647 (549) | bcsM | Aminohydrolase (deacetylase?) | Cytoplasmic |
Based on Römling and Galperin, 2015
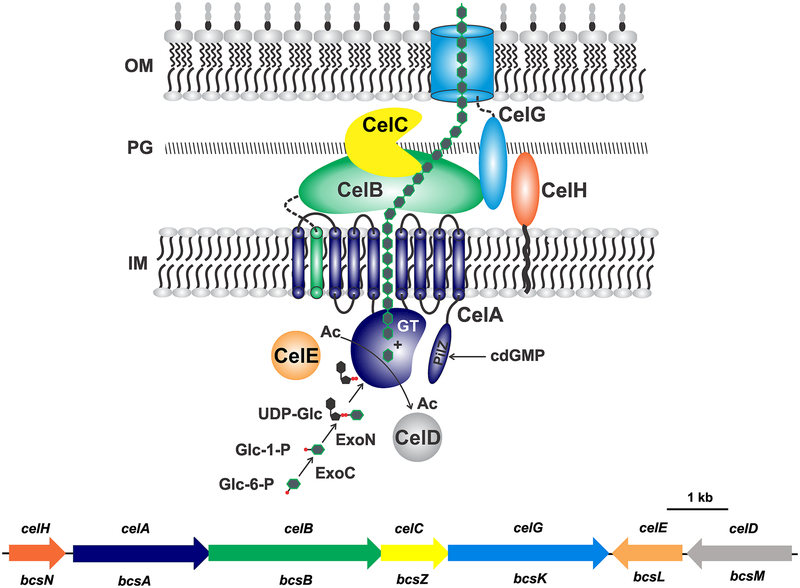
Figure 3. Cellulose: Genetic basis and biosynthesis model.
Predicted localization and mechanism of biosynthesis for cellulose in A. tumefaciens based on the generalized model (Römling and Galperin, 2015). Cellulose strand depicted as linked green hexagons. Cel protein names indicated in the figure. For CelA, GT is the predicted glycosyl transferase domain and PilZ is the cdGMP-binding domain. Black squiggle on CelH is a predicted lipid linkage. Gene colors match protein colors in the diagram; Cel names are above with corresponding Bcs nomenclature below. OM, outer membrane; PG, peptidoglycan; IM, inner membrane, Ac, acetyl groups; Glc-6-P, glucose-6-phosphate; Glc-1-P, glucose-1-phosphate; UDP-Glc, uridyl diphosphate glucose.
A tentative molecular model for cellulose biosynthesis by A. tumefaciens can be formulated based on both limited experimental data from A. tumefaciens and several extensively studied systems, most notably Komagataeibacter xylinus. The proteins involved in cellulose synthesis are believed to form multi-protein complexes, with around 50 such complexes visible in a row along the longitudinal axis of the bacterial rod as observed by cryoEM in K. xylinus (formerly Acetobacter xylinum) (Kimura et al. 2001). Precursors for cellulose synthesis derive from the glycolytic intermediate glucose-6-phosphate (G6P), which is isomerized to glucose-1-phosphate (G1P) by a phosphoglucomutase likely to be ExoC in A. tumefaciens (Fig. 3). G1P is then conjugated to the nucleotide uridyl-diphosphate (UDP) by a UTP–glucose-1-phosphate uridylyltransferase to form UDP-glucose (UDP-Glc), and it is likely that A. tumefaciens ExoN, or a paralogue, drives this reaction. CelA is predicted to be the complex cellulose synthase that utilizes UDP-Glc and adds each Glc residue to the growing cellulose molecule via a β−1,4 linkage. CelA is homologous to the cellulose synthase catalytic subunits of Rhizobium leguminosarum bv trifolii, and Sinorhizobium meliloti, and corresponds more broadly to the BcsA component of other cellulose biosynthesis systems (Römling and Galperin 2015). As such, CelA has eight transmembrane domains, a large cytoplasmic loop that comprises a glycosyl transferase (GT) domain which functions to add glucose residues to the growing glucan chain, and a C-terminal PilZ domain which regulates the enzyme (Morgan et al. 2013) (see Section 4.1.2). Although earlier models suggested a lipid-linkage for the nascent cellulose polymer (Matthysse et al. 1995a), more recent structural work with the Type IIIa BcsA protein of Rhodobacter sphaeroides suggests that there is no lipid linkage and that the cellulose chain is synthesized by addition of one glucose subunit at a time to the interior end of the molecule (Morgan et al. 2013). The growing polymer is extruded into the periplasm through a channel via a ratcheting motion within the BcsA glycosyl transferase domain. CelB (BcsB) is often considered as a second non-catalytic subunit of cellulose synthase, and it is associated with CelA via a single transmembrane domain (Fig. 3). The majority of CelB is predicted to be periplasmic and plays a role in navigation of the emerging cellulose chain through the periplasm. The cellulose strand then transits the periplasm and crosses the outer membrane via a β-barrel-type protein channel; in many Bcs systems this function is performed by the BcsC component.
In Type IIIa systems such as A. tumefaciens there are no BcsC homologues and it is now thought that CelG (BcsK) plays an analogous role, as an outer membrane secretin (Römling and Galperin 2015). As with the BcsK component in other Cel systems, CelG has a large tetratricopeptide repeat (TPR) domain, considered to drive protein-protein interactions, which extends into the periplasm presumably in contact with other periplasmic Cel proteins and perhaps peptidoglycan (Fig. 3). Although the outer membrane channel for cellulose would presumably be critical for cellulose biosynthesis, one study reported that a celG transposon mutant exhibited elevated cellulose production (Matthysse et al. 2005). The mechanistic basis for the surprising phenotype of this mutant, given the predicted central role for CelG, warrants further investigation. The CelC protein (BcsZ) belongs to a family of glycoside hydrolases that cleave glycosidic bonds between carbohydrates. Transposon insertions in celC block cellulose synthesis in A. tumefaciens, and in vitro cellulose synthesis experiments with cel mutants are consistent with a role for the celC product in the hydrolysis of glucose oligomers (Matthysse et al. 1995a,b). Immediately upstream of celA is a gene we designate as celH (bcsN), encoding a predicted periplasmic protein with a single transmembrane domain, found only in Type IIIa systems. The function of CelH remains poorly defined, but it is required for cellulose synthesis in A. tumefaciens (Kim and Fuqua, unpublished). Encoded in a second cellulose synthesis operon convergent to celHABCG are CelE and CelD, again only found in Type IIIa Bcs systems. Both CelE and CelD proteins are cytoplasmic (Matthysse et al. 1995a), and sequence similarity suggests that they may encode an acetyltransferase and a possible deacetylase, perhaps controlling the level of acetylation for cellulose precursors.
4.1.2. Regulation of cellulose synthesis
To date, the best understood form of cellulose regulation is allosteric, via the cdGMP second messenger and its interaction with the CelA C-terminal cytoplasmic domain, which contains a PilZ motif found in many cdGMP-responsive proteins. High intracellular levels of cdGMP stimulate cellulose production (Barnhart et al. 2013; Xu et al. 2013). The cdGMP signal molecule is now recognized as a nearly ubiquitous second messenger that controls various important cellular processes such as cell cycle progression, cell division, motility, attachment, and virulence in a wide range of bacteria (Jenal et al. 2017). In fact, cellulose synthase activity in A. tumefaciens was one of the first systems shown to be regulated by cdGMP (Amikam and Benziman 1989). Intracellular cdGMP levels are regulated by the activities of diguanylate cyclases (DGCs) which synthesize cdGMP from two molecules of GTP, and phosphodiesterases (PDEs) which typically break down cdGMP into pGpG molecules (Jenal et al. 2017; Römling et al. 2013) (see Section 6.2). A pair of recent studies have suggested that a particular DGC in A. tumefaciens, Atu1297 that these investigators named CelR (also called PleD), has a strong effect on A. tumefaciens cellulose biosynthesis (Barnhart et al. 2013, 2014). Several other A. tumefaciens DGCs and PDEs can also regulate cellulose biosynthesis (Feirer et al. 2015; Xu et al. 2013). Expression of the A. tumefaciens cel genes is hypothesized to be regulated by a MarR-type protein designated CelI that was identified in a genetic screen. A transposon mutant in celI overproduced cellulose, but no direct measurements were performed on cel gene expression (Matthysse et al. 2005).
4.1.3. Role in attachment
An important early step in A. tumefaciens pathogenesis is attachment to a host plant surface. Cellulose synthesis has been reported to play a central role in stable plant attachment, although it now clear that this cellulose-mediated attachment is not sufficient for virulence (Matthysse 1983, 1987). One early study showed that, although mutants unable to synthesize cellulose were easily washed off from the site of inoculation and failed to form large aggregates compared to the parent strain, they could still attach to carrot cells and were virulent (Matthysse 1983). Furthermore, washing the inoculation site drastically affected the ability of these mutants to form tumors compared to the parent strain, suggesting that cellulose may be important for properly anchoring A. tumefaciens to plant cells thereby facilitating tumorigenesis. On abiotic surfaces such as a glass or a plastic coverslip, however, cellulose deficient mutants are fully proficient for attachment and are not easily dislodged following washing (Xu et al. 2012). This result suggests a differential role for cellulose synthesis depending on the nature of the surface being colonized. Overproduction of cellulose can, however, increase biofilm formation by A. tumefaciens even on abiotic surfaces (Wang et al. 2014; Xu et al. 2013). It seems reasonable to speculate that the cellulose fibers present in plant cells may form hydrogen bonds with the cellulose fibers synthesized by A. tumefaciens upon contact with the plant surface, and this interaction may anchor A. tumefaciens tightly to its host, thereby facilitating infection (Matthysse 2014).
4.2. Unipolar Polysaccharide (UPP)
Many Gram-negative bacteria which readily can either attach to surfaces via one pole or laterally (Meadows 1971). For the well-studied pseudomonads, it is thought that the transition from reversible to irreversible attachment occurs as a switch from polar to lateral attachment (Sauer et al. 2002). However, for many bacteria, particularly those in the Alphaproteobacteria group, cells attach stably by their poles and do not readily transition to a lateral state (Li et al. 2012). Polar attachment has been observed for several Alphaproteobacteria and is often facilitated by a secreted polysaccharide-containing adhesin. The best characterized of these is the so-called holdfast localized to the end of the cellular appendage known as the stalk in the prosthecate bacterium C. crescentus (Bodenmiller et al. 2004; Fiebig et al. 2014; Toh et al. 2008), and also found in other members of the Order Caulobacterales (Berne et al. 2015; Fritts et al. 2017). Polar polysaccharides are now known to be prevalent in the order Rhizobiales but are best characterized in Rhizobium leguminosarum and A. tumefaciens. This structure is localized to a single, consistent pole (the old pole of the daughter after cell septation) and is therefore designated as the unipolar polysaccharide (UPP) in Agrobacterium tumefaciens (Gu et al. 2011; Laus et al. 2006; Tomlinson and Fuqua 2009).
4.2.1. Composition
UPP-type polysaccharides are often chemically distinct among members of the Rhizobiales, yet they share similar genetic, biosynthetic and functional characteristics as highlighted in subsequent subsections. The chemical composition of most alphaproteobacterial UPP-type polysaccharides is not clear, partly due to insolubility as well as limitations in the quantity synthesized by the bacteria (Berne et al. 2015; Tomlinson and Fuqua 2009). However, the UPP-type polysaccharide of R. leguminosarum, which functions in host legume association through binding by a host-specific lectin, is composed mostly of mannose and glucose and has thus been designated a glucomannan (Laus et al. 2006). Even with the intensely studied C. crescentus holdfast, the only information on polysaccharide chemistry is that it includes N-acetylglucosamine (GlcNAc), through its recognition by the GlcNAc-specific lectin Wheat Germ Agglutinin (WGA) (Berne et al. 2015). The A. tumefaciens UPP is not only recognized by the WGA lectin, but also by the Dolichos biflora (DBA) lectin which specifically binds N-acetylgalactosamine (Heindl et al. 2014; Tomlinson and Fuqua 2009; Xu et al. 2012). Notwithstanding, it is probable that other sugars are present in the UPP. Rhodopseudomonas palustris also produces a UPP recognized by WGA (Fritts et al. 2017). Chemical analyses, including mass spectrometry and NMR of extracted UPP-type polysaccharides, should help to determine the chemical composition of these exopolysaccharide adhesins.
4.2.2. Genetic basis and biosynthesis
In C. crescentus, holdfast production requires proteins encoded by holdfast synthesis (hfs) genes in a genomic cluster comprising hfsEFGHCBAD (Berne et al. 2015; Toh et al. 2008). Several homologues and analogues of these proteins have been identified in A. tumefaciens. Notably, uppE and uppC of A. tumefaciens have sequence similarity to hfsE and hfsD, respectively (Fritts et al. 2017). The uppE and uppC genes are contained in a cluster on the A. tumefaciens circular genome with four other genes designated uppA, uppB, uppD, and uppF spanning loci Atu1235–1240 (Fig. 4; Fritts et al. 2017; Xu et al. 2012). Deletion of the entire uppA-F gene cluster, as well as individual genes such as uppE, abolishes UPP production in A. tumefaciens. This effect can be observed as a lack of binding to surfaces, and hence a lack of biofilm production (Xu et al. 2012; Xu et al. 2013). Consistent with its role in attachment, mutations which cause UPP overproduction greatly enhance attachment and biofilm formation (Feirer et al. 2015; Feirer et al. 2017; Wang et al. 2014; Xu et al. 2013).
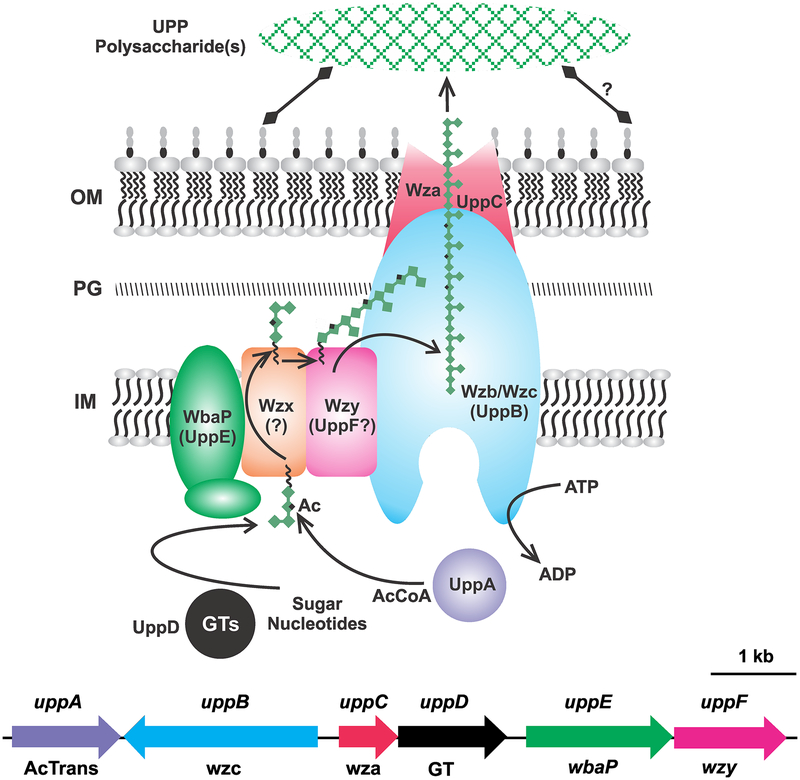
Figure 4. Wzx/Wzy polysaccharide pathway: Genetic basis and biosynthesis model for UPP.
Predicted localization and mechanism of biosynthesis for UPP in A. tumefaciens based on Wzx-Wzy generalized model (Cuthbertson et al. 2009). Branched green filament of diamonds is meant to depict the polysaccharide strand. The black square on some residues is putative acetylation. The Wzb/c protein may hydrolyze ATP to power polymerization. Upp protein names and the general Wzx-Wzy components are indicated in the figure. Black squiggle on the polysaccharide subunit is the undecaprenyl phosphate. Gene colors match protein colors in the diagram; upp gene names above and corresponding Wzx-Wzy components below (AcTrans – acetyltransferase; GT glycosyl transferase). Assembled UPP structure is represented by green cross-hatched oval outside cell, and the diamond headed lines are putative linkages to the cell pole surface. OM, outer membrane; PG, peptidoglycan; IM, inner membrane.
The occurrence of a upp-type gene cluster in members of the Rhizobiales appears to be common among plant-associated microbes, as several plant symbionts such as R. leguminosarum, B. japonicum, and S. meliloti possess highly conserved upp clusters (Fritts et al. 2017). In R. leguminosarum, these genes are designated gms because mutation of one of these genes, gmsA (homologous to uppE), abolishes synthesis of the unipolar glucomannan (Laus et al. 2006). In addition, free-living members of the Rhizobiales such as R. palustris possess a upp cluster closely similar to that of A. tumefaciens (Fig. 4). Certain alphaproteobacterial mammalian pathogens also have upp-type genes, including Ochrobacterum anthropi ATCC 49188 with a conserved and syntenous set of all six upp genes, and Brucella melitensis and B. suis that have a cluster homologous to three of the six upp genes. Conserved upp genes appear to be lacking in some obligate intracellular pathogens of the Rhizobiales, suggesting a loss of the upp cluster following adaptation to the intracellular lifestyle. Although they are required to synthesize a polar polysaccharide and are hypothesized to share a common ancestor with the hfs genes of C. crescentus, it is noteworthy that the upp-type genes are distinct and broadly distributed among the Rhizobiales (Fritts et al. 2017).
Apart from the genes that make up the core hfs cluster in C. crescentus, other genes, present elsewhere in the genome, can also play important roles in holdfast biosynthesis. The pssY and pssZ genes are paralogues of hfsE, and single deletion mutants for each gene synthesized holdfasts comparable to those of wild-type. However, a triple deletion mutant is completely deficient for attachment and holdfast synthesis (Toh et al. 2008). Furthermore, hfsC deletion results in a holdfast synthesis defect when combined with a deletion of its paralogue, hfsI, but not when each gene is singly deleted. In A. tumefaciens, the uppE gene is required for UPP production and attachment under phosphorus (Pi)-replete conditions, but under Pi-limitation uppE is functionally redundant with Atu0102, an orthologue of pssY (Xu et al. 2012). Disruption of both genes is required to prevent UPP production and attachment in Pi limitation. It is possible that additional core upp genes exist elsewhere in the A. tumefaciens genome. Preliminary evidence reveals that there are other genes outside of the A. tumefaciens uppABCDEF cluster required for UPP production (Natarajan et al., in preparation).
Bacterial polysaccharides are synthesized via one of three main pathways, largely differentiated by membrane topology, characteristic components, and the type of polysaccharides synthesized. These pathways are the Wzx/Wzy-dependent pathway, the ABC-transporter-dependent pathway, and the synthase-dependent pathway. Both the ABC-transporter- and synthase-dependent pathways synthesize mainly homopolymeric exopolysaccharides with single-sugar repeating units (with some exceptions such as certain LPS pathways), and use a mechanism that largely involves complete polysaccharide synthesis in the cytoplasm before export out of the cell (Mi et al. 2017). In contrast, the Wzx/Wzy-dependent pathway more commonly synthesizes heteropolymers with repeating units of three to six sugars. These repeating units are polymerized in the periplasm before final export out of the cell (Islam and Lam 2013; Islam and Lam 2014; Schmid et al. 2015).
The holdfast synthesis genes of C. crescentus, as well as the upp-type genes from A. tumefaciens and R. palustris, are homologous to a Wzx/Wzy-dependent polysaccharide biosynthesis pathway (Toh et al. 2008, Fritts et al. 2017, Heindl et al. 2014). The Wzx/Wzy dependent pathway is named after the integral, inner-membrane oligosaccharide-transferase protein identified in several Gram-positive and Gram-negative bacteria responsible for O-antigen assembly, encoded by the wzy gene (Islam and Lam 2013; Islam and Lam 2014; Kalynych et al. 2014). This pathway consists of cytoplasmic glycosyltransferases (GTs) which add sugar nucleotide precursors to a polyisoprenoid lipid carrier molecule, undecaprenyl phosphate (UndP), which is embedded within the cytoplasmic leaflet of the inner membrane (Fig. 4). The first sugar is added to UndP by the initiating GT (WbaP), a polyisoprenylphosphate hexose-1 phosphate (PHPT), to form a pyrophosphate linkage with the carrier (UndPP) (Cuthbertson et al. 2009). For holdfast synthesis in C. crescentus, this initiating PHPT activity is performed by the GT HfsE (encoded within the hfs gene cluster), PssY, or PssZ (Toh et al. 2008). In A. tumefaciens the PHPT is UppE, with the Atu0102 PssY homologue functioning in the same capacity under Pi limitation (Fig. 4; Xu et al. 2012). Following addition of the first sugar moiety, subsequent sugars are added by additional GTs until a complete repeating polysaccharide unit is formed. This molecule is then flipped from the cytoplasmic face of the inner membrane to its periplasmic face via a Wzx translocase, also called a flippase (Fig. 4). Flippases of this type are integral membrane proteins, usually with twelve transmembrane domains. Wzx flippases belong to the polysaccharide transport (PST) family of the larger multidrug/oligosaccharidyl-lipid/ polysaccharide (MOP) flippase superfamily (Hong and Reeves 2014). A flippase homologue is encoded within the core hfs gene cluster of C. crescentus (HfsF), but such a homologue is absent from the UPP-type gene cluster of the Rhizobiales (Fritts et al. 2017; Toh et al. 2008). Despite the vast array of O-antigen and other polysaccharides synthesized through this pathway, several studies on flippases indicate a strong specificity for their substrates, which may explain the high sequence variability among Wzx proteins in different organisms and even within a single organism (Islam and Lam 2013, 2014; Wang et al. 2012). Polymerization of the flipped repeating units occurs in the periplasm via the Wzy protein in conjunction with the chain-length determining Wzc protein (sometimes in conjunction with a Wzb-type protein), also called the polysaccharide co-polymerase or PCP (Fig. 4). In C. crescentus there are two Wzy-type proteins, HfsC and HfsI, that are functionally redundant for holdfast synthesis (Hardy et al. 2018). The PCP function is thought to be split into two proteins, HfsA (Wzc) and HfsB (Wzb). For the UPP-type systems in the Rhizobiales, there are no clear Wzy-type proteins within upp cluster, although UppF is annotated as an O-antigen ligase, which could plausibly drive a similar polymerization reaction. The UppA protein is a PCP homologue, but mutations in this gene result in only modest effects on UPP production in A. tumefaciens (Natarajan et al., in prep). There are multiple unassigned Wzx-type, Wzy-type, and Wzz-type proteins encoded in the A. tumefaciens genome that may contribute to UPP biosynthesis. The polymerizing polysaccharide strand is then exported across the outer membrane via a secretin, a β-barrel protein designated Wza or OPX for outer membrane polysaccharide export (Cuthbertson et al. 2009; Islam and Lam 2014). In C. crescentus this protein is annotated HfsD, and in the UPP-type systems this is annotated UppC. Mutations in these genes result in abolishment of polar polysaccharide synthesis. (Berne et al. 2015; Natarajan et al. in prep).
Stable association with the pole of the cell is one of the properties that distinguishes polar polysaccharides from capsular or secreted exopolysaccharides. In C. crescentus, the holdfast attachment (hfa) genes, arranged in a three gene operon, hfaABD, encode proteins that function in anchoring the holdfast to the C. crescentus stalk (Cole et al. 2003; Hardy et al. 2010). Independent mutants in several hfa genes shed their holdfasts from cells and fail to attach to abiotic surfaces. HfaA is a β-amyloid protein similar to curlin from E. coli (Blanco et al. 2012). HfaA export requires the outer membrane lipoprotein HfaB and the outer membrane protein HfaD to which it is thought to anchor. HfaA forms a structure analogous to a β-amyloid filament that tethers the holdfast to the stalk tip (Hardy et al. 2010). Whereas hfs and hfa genes are well conserved within the Caulobacterales, no hfa homologs have been identified in the A. tumefaciens genome or in the genomes of most other Rhizobiales (Fritts et al. 2017). There are several uncharacterized proteins that are predicted to adopt a β-amyloid fold, but their role, if any, is unknown. It is also plausible that the UPP is anchored to another outer membrane structure such as LPS, but it is not clear how this would be specifically retained at the pole.
4.2.3. Regulation of UPP synthesis
Thus far, no differential gene expression for the uppABCDEF cluster has been observed, even in mutants or under conditions which strongly promote or inhibit UPP production (Xu et al. 2013). Therefore, most of the regulatory effects observed are hypothesized to be post-transcriptional, and likely through allosteric control of biosynthesis.
Holdfast synthesis is intricately linked to cell cycle progression in the dimorphic life cycle of C. crescentus, where the holdfast is elaborated at the end of the stalk during cell development (Toh et al. 2008). Interactions with the surface can accelerate production of the holdfast (Li et al. 2012). In contrast to C. crescentus, UPP production in A. tumefaciens is only observed upon surface or cell-cell contact (Li et al. 2012; Xu et al. 2013). Following attachment, UPP is visible very rapidly after surface contact. Surface contact-dependent stimulation of polar polysaccharide secretion has also been observed for other Alphaproteobacteria such as Asticcacaulis biprosthecum (Li et al. 2012). The mechanism of surface contact recognition in A. tumefaciens is an area of active study.
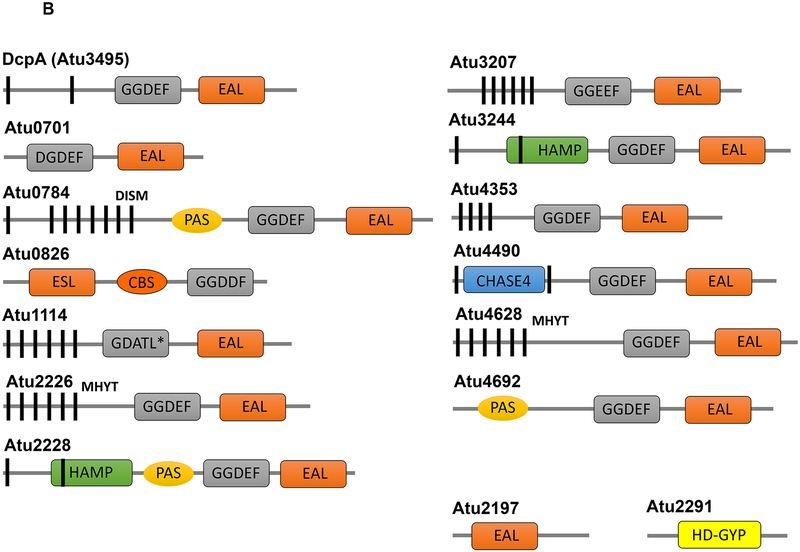
Among the widespread roles of cdGMP in many bacteria is the regulation of polysaccharide production such as cellulose (Section 4.1.2) and UPP (Jenal et al. 2017; Xu et al. 2013). Ectopic expression of the A. tumefaciens DGC PleD in A. tumefaciens resulted not only in increaseed cell-cell aggregation and biofilm formation, but also a decoupling of UPP synthesis from surface contact (Xu et al. 2013). These observations suggest that elevated intracellular cdGMP levels bypass the surface-contact dependent requirement for UPP production, and is evidence for the role of cdGMP in UPP regulation. The A. tumefaciens genome encodes over 30 DGCs that regulate intracellular cdGMP levels, and several of these have been directly implicated in control of UPP production (Heindl et al. 2014) (see Section 6.2). A number of mutants identified for dysregulated UPP production have been isolated, and thus far all of these appear to connect in some way to cdGMP pools (Feirer et al. 2015; Feirer et al. 2017; Xu et al. 2013). Several environmental conditions which modulate UPP-dependent attachment include Pi-limitation (increasing attachment) and limitation for the divalent cations Fe2+ and Mn2+ (decreased attachment) (Danhorn et al. 2004; Heindl et al. 2016; Xu et al. 2012). Interestingly, UPP production and cellulose synthesis are often, but not always, co-regulated by specific cdGMP-dependent pathways (Wang et al. 2016; Xu et al. 2013). The integration of control for these two important polysaccharides during surface colonization is a subject of active investigation.
4.2.4. Role in attachment
Biofilm formation on biotic and abiotic surfaces in A. tumefaciens follows a stepwise trend beginning with reversible attachment during which the organism must overcome repulsive electrostatic and hydrodynamic forces near the surface, followed by a more permanent attachment largely mediated by UPP (Heindl et al. 2014). In fact, surface association stimulates UPP production very rapidly, and its deployment is coincident with stable surface attachment (Li et al. 2012). Mutations of key upp genes such as uppE and uppC, as well as deletion of the uppABCDEF cluster, abolishes binding to abiotic surfaces (Xu et al. 2012) and results in loose aggregate formation and weak binding to plant tissues (Natarajan et al., manuscript in preparation), suggesting its importance in permanent, irreversible attachment. In C. crescentus and its close relatives, studies have shown that the cohesive and adhesive properites of the holdfast are determined by the degree and pattern of acetylation of the polysaccharide, regulated by the putative deacetylase HfsH (Wan et al. 2013). A putative acetyltransferase encoding gene, uppA, may play a similar role in A. tumefaciens, but this is yet to be rigorously tested. Mutations in genes that block UPP production retain virulence (Natarajan et al. in preparation; Gelvin et al., unpublished), as evaluated by assays that directly inoculate wounded plant tissues, suggesting that UPP-mediated attachment per se is not required for T-DNA transfer to plants. This is consistent with the chromosomal location of the upp genes (Xu et al. 2012), their activity in avirulent, Ti-plasmidless agrobacteria, and their conservation among diverse Rhizobiales (Fritts et al. 2017).
4.3. Curdlan
Many species and strains of Agrobacterium synthesize the β−1,3 glucan called curdlan, and several species are the industrial source for this polysaccharide, which is used as a cement additive. There is indirect evidence that curdlan may have the capacity to function in attachment.
4.3.1. Composition
Curdlan is a neutral, water-insoluble exopolysaccharide that is one of three structural classes of (1 → 3)-β-glycosidic linkages of glucose. These classes are the linear (1 → 3)-β-D-glucans, the side-chain-branched (1 → 3, 1 → 2)-β-glucans, and the cyclic (1 → 3, 1 → 6)-β-glucans. Curdlan is mostly linear, but may have a few intra- and inter-chain (1 → 6)-linkages (McIntosh et al. 2005; Zhan et al. 2012). A single curdlan molecule may have as many as 12,000 glucose units (McIntosh et al. 2005), displaying an average molecular weight of 5.3 × 104 to 2.0 × 106 Da when dissolved in alkaline solutions such as 0.3 N NaOH. Curdlan was first identified in Alcaligenes faecalis var. myxogenes (now Agrobacterium sp. ATCC31749), and has since been identified in other Agrobacterium and some Rhizobium species (Nakanishi et al. 1976).
4.3.2. Genetic basis and biosynthesis
The molecular genetics of curdlan biosynthesis has been studied predominantly in Agrobacterium sp. ATCC31749 (Zhan et al. 2012). Isolation of curdlan-deficient transposon mutants of Agrobacterium sp. ATCC31749 resulted in the identification of curdlan synthesis genes. These genes include crdA, crdS, crdC, and crdR (Karnezis et al. 2003; Ruffing et al. 2011; Stasinopoulos et al. 1999), which are necessary for curdlan synthesis, as well as pssAG (Karnezis et al. 2002), which enhances curdlan production. The crdASC genes form a cluster and are likely to be co-transcribed, whereas crdR and pssAG occur elsewhere in the genome (McIntosh et al. 2005). The crdS gene encodes a 540 amino acid integral membrane protein of the HasA family of β-glycosyl transferases, with seven transmembrane helices and a large intracellular hydrophilic region (Karnezis et al. 2003; Stasinopoulos et al. 1999). CrdS has high sequence similarity to BcsA, the bacterial cellulose synthase, and thus is almost certainly the curdlan synthase (Stasinopoulos et al. 1999). However, CrdS lacks the C-terminal PilZ cdGMP binding domain present in CelA, nor does it have any other known c-di-GMP binding domains (Barnhart et al. 2013). In contrast to crdS, crdA and crdC have no known counterparts in the sequence databases. The crdA gene is predicted to encode a membrane-anchored protein that may play a role in transfer of the polymer across the cytoplasmic membrane, whereas crdC is predicted to encode a periplasmic protein which may function in passage of the polymer through the periplasm. However, crdC mutants still make curdlan, whereas crdA and crdS mutants do not (McIntosh et al. 2005). CrdA, CrdS, and CrdC may form a multi-protein complex spanning the inner membrane and the periplasm (Karnezis et al. 2003). The pssAG gene encodes a membrane protein that plays a role in phosphatidylserine production and functions to enhance curdlan production (Karnezis et al. 2002). Phosphatidylserine is a membrane phospholipid as well as a precursor for phosphatidylethanolamine, suggesting a role for phospholipid composition in increased curdlan biosynthesis. No outer membrane secretin has been identified to function in extracellular export of curdlan. It is noteworthy that although A. tumefaciens C58 has the complete set of genes for its synthesis, curdlan is not detectably produced under standard growth conditions (Matthysse 2014).
The model for the biosynthesis of curdlan is thought to resemble that of cellulose, with UDP-Glc, derived from G6P, as the precursor for the polysaccharide synthesis (Fig 3; Stasinopoulos et al. 1999). Polymerization presumably occurs within the GT catalytic domain on the large cytoplasmic loop of CrdS followed by extrusion of the polysaccharide from the cell. The details of the curdlan export mechanism remain to be defined.
4.3.3. Regulation of curdlan synthesis.
Curdlan biosynthesis is transcriptionally activated by the helix-turn-helix protein CrdR (Yu et al. 2015). Transcriptome profiling of Agrobacterium ATCC31749 to investigate regulation of curdlan synthesis showed a 100-fold increase of crdASC expression under nitrogen limiting conditions (Ruffing and Chen 2012). This regulation occurs through the sensor kinase NtrB and its response regulator NtrC, global regulators of nitrogen metabolism (McIntosh et al. 2005). Deletion of the sigma factor rpoN, often associated with nitrogen limitation responses, led to elevated levels of curdlan synthesis (Ruffing et al. 2012). Transcriptional profiling under nitrogen-limited conditions in Agrobacterium sp. ATCC31749 also revealed increased expression of a pair of DGC proteins, predicted to impact cdGMP pools (Ruffing and Chen 2012). Mutation of one of these DGCs (AGRO_3967) resulted in markedly decreased curdlan production (Ruffing and Chen 2012). The CrdS protein lacks a PilZ domain that would impart cdGMP-responsiveness, but there are other possible inputs of cdGMP into curdlan biosynthesis (Barnhart et al. 2013).
4.3.4. Role in attachment
Presently, curdlan has not directly been shown to play a role in the attachment of cells to biotic or abiotic surfaces, although it seems a likely candidate. There is no published evidence that mutations which impact curdlan production in A. tumefaciens C58 affect virulence or root colonization. The curdlan over-producing strain ATCC31749 forms a fragile, easily detachable blanket-like structure when incubated with tomato roots or on agar plates containing Aniline Blue (Matthysse 2014).
5. Protein Adhesins
Many bacteria externalize specialized adhesin proteins, distinct from pili, that promote surface interactions. These include the large adhesin proteins (Laps) from Pseudomonas (Hinsa et al. 2003), the filamentous hemaglutinnin (FHA) proteins originally identified in Bordatella species (Locht et al. 1993), and β-amyloid curli fibers from uropathogenic E. coli (Barnhart and Chapman 2006; Kai-Larsen et al. 2010). Proteinacious adhesins have also been implicated in attachment of rhizobia, including A. tumefaciens, to root tissues. Early studies on the attachment of rhizobia to pea root hairs under conditions with high calcium ion concentrations identified a Ca2+-binding protein defined as rhicadhesin, and addition of a crude preparation of this protein to live rhizobia diminished their attachment (Smit et al. 1989). Similar activities were characterized in extracts of A. tumefaciens (Dardanelli et al. 2003). Cell surface proteins defined as the Rap (Rhizobium adhering proteins) adhesins were identified from a subset of rhizobia (Ausmees et al. 2001; Russo et al. 2006) and may be related to the earlier defined rhicadhesin activity. The predicted genes for these proteins are not conserved within the A. tumefaciens genome. It remains unclear what encodes the rhicadhesin activity initially detected in A. tumefaciens and how this putative activity relates to attachment. A. tumefaciens C58 does have a small cluster of genes that encode proteins that are predicted to adopt β-amyloid structures due to their similarity with the CsgA protein of E. coli (Barnhart and Chapman 2006), although their role in attachment, if any, remains unexplored.
6. Regulation of Attachment
The transition from a motile state to a sessile growth mode is a crucially important process for many bacteria, and for A. tumefaciens this is the case under a variety of contexts. As such, the attachment process is under complex regulatory control at multiple levels. The individual surface features are themselves often under complex transcriptional control networks. Some of the relevant expression control pathways represent global transcription regulatory networks, such as the response to decreasing pH through the RGI pathway (Heckel et al. 2014), or phosphorus (Pi)-limitation via the PhoR-PhoB system (Danhorn et al. 2004). Other control circuits are more specific to a given process, such as the VisR-VisN-Rem regulation of flagellar and chemotaxis gene expression, target functions that can also impact attachment processes (Xu et al. 2013). Beyond simply identifying transcriptional control systems that influence attachment, the understanding of how these regulatory circuits are integrated leading up to and during intimate interactions with surfaces remains rudimentary. In addition to the variety of different transcriptional pathways, post-transcriptional mechanisms, particularly allosteric control of polysaccharide biosynthesis, exert a major influence on attachment.
6.1. Transcriptional regulators that impact attachment.
Genetic analysis in A. tumefaciens C58 has identified several different transcriptional regulators, mutations in which cause significant changes in attachment proficiency or biofilm formation.
6.1.1. ExoR-ChvG-ChvI (RGI) Pathway
Transposon mutations in the exoR regulatory gene result in profound loss of attachment. As described above (Section 2.1), ExoR is not itself a transcription factor, but rather a periplasmic regulator of the ChvG sensor kinase, which in turn controls the downstream response regulator ChvI, altogether comprising the RGI pathway (Heckel et al. 2014). Under neutral conditions ExoR maintains ChvG in the inactive form. At low pH ExoR is proteolytically cleaved, resulting in active ChvG, and leading to phosphorylation of ChvI (Wu et al. 2012). Mutations in exoR lead to high level phosphorylation of ChvI, and differential expression of hundreds of A. tumefaciens genes (Heckel et al., 2014). Null mutants for exoR are non-motile, hypermucoid, and unable to attach to surfaces. Strikingly, the attachment deficiency is not due to the loss of motility or hypermucoidy (Tomlinson et al. 2010). Among the genes under exoR control there are a number that may potentially impact attachment, including genes directing motility and chemotaxis (decreased), succinoglycan biosynthesis (increased), a Type VI secretion system (T6SS) (increased), plasmid conjugation (decreased), cdGMP synthesis that would affect dcGMP (decreased), and Tad-type pili (decreased). However, no single class of genes is responsible for the severe attachment deficiency of the exoR mutant, and this phenotype may be due to the combinatorial effects on these genes. It is also unclear which target genes ChvI regulates directly, with the exception of the T6SS genes, a promoter to which ChvI has been shown to bind in vitro (Wu et al. 2012).
6.1.2. Additional environmentally responsive pathways
Ectopic expression of the response regulator PhoB simulates the Pi-limitation response and increases biofilm formation in A. tumefaciens C58, consistent with the observation that limiting Pi has the same effect (Danhorn et al. 2004). PhoR and PhoB comprise a Pi-responsive two-component system that has been intensely studied in several different systems, including the rhizobia (McDermott 2000). The PhoB-regulated target genes that lead to increased attachment of A. tumefaciens in limiting Pi are not yet known, but it is clear that Pi limitation increases the number of cells which attach at their pole via the UPP adhesin (Xu et al. 2012). It is possible that this stimulatory effect is mediated through cdGMP pools.
A genetic screen for attachment deficiencies in A. tumefaciens C58 led to the identification of a regulatory gene that was designated sinR (surface interaction Regulator), mutations in which resulted in less dense and thinner biofilms than wild-type on abiotic surfaces (Ramey et al. 2004). A. tumefaciens SinR is a transcription factor of the CRP-FNR superfamily and is one of four FNR-type regulators encoded by the A. tumefaciens C58 genome. The canonical FNR regulator characterized in E. coli has an N-terminal domain associated with an iron-sulfur (4Fe-4S) cluster via a conserved set of cysteine residues, that is responsive to oxygen levels, and a C-terminal DNA-binding domain (Körner et al. 2003). FNR is activated under limiting oxygen and its regulon is composed of many different genes that are differentially expressed under these conditions. As with several other sub-groups of regulators in the FNR family, SinR does not have the conserved Cys residues and as such is not thought to contain an iron-sulfur cluster. Transcription of the sinR gene is strongly activated under oxygen limitation and upregulated in mature biofilms, and this is dependent on FnrN, another A. tumefaciens FNR-type protein. In contrast to SinR, FnrN has the conserved Cys residues (Ramey et al. 2004). Ectopic expression of sinR increases the density of A. tumefaciens biofilms relative to those of wild-type. It is hypothesized that as surface-adherent biomass increases, oxygen limitation is sensed through FnrN, which stimulates sinR expression, thereby controlling late stages of biofilm formation. The SinR-regulated functions that impart these effects on biofilm formation have not been identified.
Analysis of the genome sequence of plant pathogen Xylella fastidiosa identified a transcriptional regulator eventually designated BigR (Biofilm growth-associated Repressor) within a cluster of genes predicted to be involved in sulfur metabolism, including hydrogen sulfide detoxification (Barbosa and Benedetti, 2007; Guimaraes et al., 2011). The regulator and most of the presumptive operon are conserved in A. tumefaciens C58 (Atu3465–68). Based on its phenotypes in X. fastidiosa, the bigR homologue (Atu3466) of A. tumefaciens was disrupted, and the mutant had elevated expression of this operon and increased attachment on abiotic and biotic surfaces (Barbosa and Benedetti, 2007). BigR is a member of the ArsR/SmtB family of repressors and has a pair of redox-reactive Cys residues. Oxidation of these Cys residues and their resulting disulfide linkage cause changes in the protein that lead to derepression of the promoter upstream of Atu3465. This promoter is also active in A. tumefaciens biofilms, and it is speculated that BigR is oxidized by unknown intermediates of sulfur metabolism under the oxygen-limitation in the biofilm. Expression of the bigR operon leads to the detoxification of hydrogen sulfide and other growth inhibitory sulfur metabolites (Guimaraes et al. 2011). It is not known whether BigR regulates any additional genes outside of its own operon.
6.2. Cyclic diguanylate monophosphate-dependent regulation of surface attachment functions
Multiple lines of evidence have indicated that cdGMP levels play a profound role during the transition of A. tumefaciens from the free-living to the surface-attached state. Early studies by Benzimann and colleagues suggested that A. tumefaciens cellulose synthase is cdGMP-regulated (Amikam and Benzimann 1989), and more recent work has shown that both cellulose and UPP production are controlled in parallel to regulate the motile-to-sessile switch (Xu et al. 2013). Over the last two decades, and particularly emerging in the last ten years, the pervasive role for cdGMP in modulating bacterial physiology and behavior has gained recognition (Wolfe and Visick 2010). Diguanylate cyclase enzymes (DGCs) synthesize cdGMP from two GTP molecules via a catalytic site that contains the GGDEF motif (sometimes GGEEF). Specific cdGMP phosphodiesterases (PDEs) typically hydrolyze the signal into pGpG, which then rapidly degrades to GMP. PDEs predominantly have an EAL catalytic motif, but a small fraction of cdGMP PDEs have an HD-GYP catalytic motif (Jenal et al. 2017). There are additional residues outside the catalytic site that define the DGC and PDE domains. It is common to identify single proteins with both DGC (GGDEF) and PDE (EAL/HD-GYP) domains. For a significant subset of these dual domain proteins, one of the two domains is degenerate and non-catalytically active. For a small fraction of these proteins, both domains are active and can catalyze synthesis of cdGMP, as well as its turnover. The bias in the ratio between DGC and PDE activity can be under regulatory control, comprising an environmentally-responsive cdGMP module. In fact, many DGC, PDE, and DGC-PDE proteins have additional domains that impart environmentally-responsive control of their catalytic activities, and it is common that these proteins contain transmembrane domains. Certain bacteria can have as few as a single DGC protein (e.g. Yersinia pestis) and others may have as many as 60 different proteins (e.g. Vibrio species) with DGC domains, with or without PDE domains. The integration of cdGMP signaling by multiple synthesis and degradation proteins in a single bacterial cell is a topic garnering significant attention, and current models suggest a complex combination of (i) conditional expression of specific DGC, PDE, and DGC-PDE genes; (ii) differential allosteric control of the proteins under varying environmental conditions; (iii) rapid fluxes of the signal across the cell by localized synthesis and degradation; and (iv) in some cases, compartmentalization. As might be expected with such a pervasive regulator, there are also multiple mechanisms by which cdGMP can control output functions in the cell. At least seven structurally distinct cdGMP binding motifs have been identified. The first identified were so-called PilZ domains, but there are now five other documented protein binding motifs as well as a cdGMP-responsive riboswitch (Jenal et al. 2017). Several of these may function simultaneously in the same bacterium.
A. tumefaciens was the second bacterial species for which cdGMP control was reported for cellulose biosynthesis (Amikam and Benziman, 1989), after its initial discovery in the bacterium now called K. xylinus (Ross et al. 1987). It is now clear that cdGMP regulates the cellulose synthase enzyme CelA in A. tumefaciens, very likely through allosteric interactions with its conserved PilZ domain. In addition, cdGMP exerts profound control over UPP biosynthesis. Mutations that increase cdGMP levels stimulate UPP production, and those that decrease cdGMP can diminish UPP-mediated attachment (Xu et al. 2013). None of the proteins known to be required for UPP biosynthesis have a recognizable PilZ domain or cdGMP binding motif (Xu et al. 2012). Production of cdGMP by A. tumefaciens depends on a suite of proteins with DGC domains. The A. tumefaciens C58 genome encodes 29 proteins with a conserved GGDEF (or GGEEF) catalytic motif (Fig. 5A), and thirteen of these also contain an EAL motif (Fig. 5B). Two proteins are presumptive solo PDEs, one with an EAL motif, and a second with an HD-GYP motif, in both cases not associated with a DGC domain. There is experimental evidence in A. tumefaciens implicating four of the DGCs (Atu1257, Atu1297, Atu1691, and Atu2179) and one DGC-PDE protein (Atu3495) in the control of polysaccharide production and attachment. Additionally, a gene from Agrobacterium sp. ATCC 31749 (AGRO_3967) homologous to the DGC homologue Atu1119 (Fig. 5A) is co-regulated with β−1,3 glucan (curdlan) production, and its mutation diminishes production of this polysaccharide (Ruffing and Chen, 2012). A recent characterization of cdGMP-related proteins in the related bacterium S. meliloti provides a useful comparison for A. tumefaciens. S. meliloti has six presumptive DGC proteins, 12 DGC-PDEs, and two PDEs (Schäper et al. 2016). A subset of 13 of these proteins have likely orthologues in A. tumefaciens, whereas seven are not found in A. tumefaciens. Conversely, 16 of the A. tumefaciens cdGMP-related proteins are not found in S. meliloti. Several of the S. meliloti proteins have roles in attachment and biofilm formation, but none are as striking in their phenotypes as several examples from A. tumefaciens.
Figure 5. Diguanylate cyclases and phosphodieterases of A. tumefaciens.
(A) DGC proteins, (B) DGC-PDE proteins and PDE/HD-GYP only proteins. Atu gene numbers and if assigned, genetic names are provided for each protein. Predicted transmembrane domains are indicated by vertical lines and any recognizable functional domains are indicated. Protein domains. REC - two-component-type receiver domain; GAF - cGMP-specific phosphodiesterases, Adenylyl cyclases and FhlA; HAMP - Histidine kinases, Adenyl cyclases, Methyl-accepting proteins and Phosphatases; PAS - Per, ARNT, Sim; CBS - Cystathionine-Beta-Synthase; CHASE4, predicted ligand binding module; MHYT, 6-transmembrane domain; DISM, 7-transmembrane domain, Diverse Intracellular Signaling Modules.
The Atu1297 gene encoding a DGC homologue (Fig. 5A) has been designated pleD on the basis of its predicted amino acid sequence and its position immediately downstream of the cell cycle regulator divK, similar to the well-studied pleD DGC from C. crescentus (Paul et al. 2004; Sommer and Newton 1989). As with its homologue, the N-terminus of the A. tumefaciens PleD includes a dual two-component response regulator domain upstream of the DGC domain (Fig. 5A). Expression of this protein in E. coli and A. tumefaciens results in strong overproduction of cdGMP, dependent upon the GGDEF catalytic motif (Xu et al. 2013). In C. crescentus, the phosphorylation state of PleD is regulated by the sensor kinases DivJ and PleC of the complex CtrA phosphorelay, and it plays a prominent role in cell cycle control. The genetic linkage of pleD to divK in A. tumefaciens suggested that it might also function in cell cycle control, as divK mutants manifest cell division defects (Kim et al. 2013). However, null mutations in pleD have minimal impacts on cell division, and only marginally impact motility and attachment (Kim et al. 2013). In contrast, ectopic expression of PleD in A. tumefaciens strongly stimulates cellulose and UPP production concomitant with elevated cdGMP levels (Xu et al. 2013). Direct measurements in a pleD mutant suggests that it is diminished for cellulose production, but this does not manifest itself as strong effects on attachment, even exhibiting mild stimulation of attachment to plants (Barnhart et al. 2013). Ectopic expression of another DGC homologue, Atu1060, which has similar domain structure to PleD (Fig. 5A), also increased polysaccharide synthesis, but mutation of Atu1060 had no significant impact (Barnhart et al. 2013). It seems likely that elevated PleD levels (and similarly Atu1060) impact attachment processes by increasing cytoplasmic pools of cdGMP, but its role under native conditions is not clear.
In genetic studies on UPP production, mutations in the motility master regulators visN and visR resulted in increased UPP and cellulose production, enhancing attachment to abiotic surfaces and bypassing surface contact dependence (Xu et al. 2013). Genetic suppressor analysis revealed that mutation of a specific DGC homologue, designated DgcA (Atu1257; Fig 5A), prevented elevated polysaccharide production in a visR mutant. Furthermore, transcriptome analysis identified two additional DGC genes, dgcB (Atu1691) and dgcC (Atu2179), that were elevated in expression in a visR mutant. DgcA and DgcB (Fig. 5A) were capable of increasing cdGMP levels when expressed in E. coli and in A. tumefaciens (dependent on their GGDEF catalytic motifs), whereas DgcC was not. Directed mutation of dgcA blocked the elevated attachment of the visR mutant, whereas mutation of dgcB had a more modest effect. In the wild-type background, mutation of dgcB significantly diminishes attachment and a dgcA mutation has a less pronounced effect. A dgcAdgcB double mutant abolished all detectable attachment. In contrast, mutations in dgcC had no effect on attachment, irrespective of the genetic background tested. Intriguingly, DgcB is orthologous to DgcB of C. crescentus, which was recently reported to be responsible for stimulation of polar holdfast production via interactions with specific biosynthetic proteins in response to the drag on flagellar rotation experienced during surface interactions (Hug et al. 2017; see Section 6.3).
In the same genetic screen that identified the role for VisNR in regulating attachment, a mutant was isolated in a dual DGC-PDE, Atu3495 (Fig. 5B), that strongly upregulated UPP and cellulose production, again bypassing surface-contact dependence (Xu et al. 2013). This protein is now designated DcpA (diguanylate cyclase/phosphodiesterase A), as it exhibits both cdGMP synthesis and degradation activities (Feirer et al. 2015). Mutations in dcpA cause elevated levels of cdGMP synthesis in laboratory-grown A. tumefaciens, indicating that the PDE activity is dominant under these conditions. However, a complex regulatory pathway has been discovered that dictates the bias between DGC and PDE activity for DcpA, and hence its control of attachment processes. Surprisingly, this pathway involves low molecular weight metabolites called pterins, compounds virtually ubiquitous in living systems and related to folic acid and its derivatives (Feirer and Fuqua, 2017). Production of a specific type of pterin derivative, known as a monapterin, is required to maintain DcpA in its PDE dominant state, and this control requires a protein encoded in the same operon as dcpA, named PruR (Pterin-responsive Upp Regulator), which contains a presumptive pterin-binding motif (Feirer et al. 2015). It is clear that this pathway plays a prominent role in regulation of attachment through modulation of the relevant cdGMP pools. Several observations have implicated the DcpA system as an important hub for environmentally–responsive attachment control (Feirer et al. 2017; Heindl et al. 2016; Wang et al. 2016). The mechanism by which the monapterin cofactors are involved, and the reason for this complex regulation, are the subject of current studies.
6.3. Surface contact-responsive control of attachment functions.
In wild-type A. tumefaciens, production of the UPP is strictly surface contact-dependent (Li et al. 2012). Elevation of cellular cdGMP levels, either through DGC expression or loss of PDE activity, leads to the uncoupling of this requisite surface contact stimulation (Feirer et al. 2015; Xu et al. 2013). This finding suggests that the response to surface contact may function through fluxes in cdGMP levels, perhaps establishing transient gradients within the cell. Recent work in C. crescentus has revealed a role for cdGMP in surface contact stimulation of holdfast production, both in response to inhibition of Cpa pilus retraction and the inhibition of flagellar rotation (Hug et al. 2017; Ellison et al. 2017). It is hypothesized that the drag on flagellar rotation is transduced through an increase in the activity of the DgcB DGC, and that cdGMP allosterically regulates the glycosyl transferase HfsJ, required for holdfast biosynthesis. The C. crescentus DgcB is orthologous to the A. tumefaciens DgcB (DgcBAt), mutations in which lead to dramatic reductions in surface attachment in otherwise wild-type A. tumefaciens. It is intriguing to speculate that DgcBAt functions similarly to stimulate deployment of the UPP, thereby initiating stable attachment.
7. Conclusions
From the breadth of this review it should be clear that there are multiple extracellular structures which can contribute to the attachment of A. tumefaciens cells to surfaces. The molecular details of these structures and their surface assembly or export continues to be a fertile area of study. Furthermore, the deployment of these specific structures is highly regulated, both at the level of gene expression and through allosteric control mechanisms. It is likely that additional attachment factors remain to be identified for A. tumefaciens and incorporated into the current models. Most importantly, the coordination of these different factors to drive productive surface attachment in benign associations, as well as disease progression, remains poorly understood. It is clear that A. tumefaciens is well adapted for transitioning to a sessile state on a variety of surfaces, and that factors such as pili, flagella, the UPP, and cellulose can play important roles in this general attachment process. However, the relationship of these general attachment mechanisms to the interactions with plant tissues that eventually lead to T-DNA transfer is still uncertain. Standard tumorigenesis assays that often involve inoculation with large numbers of cells that are either strongly induced for virulence or applied to tissues that are extensively wounded prior to inoculation do not accurately reflect the natural infection process. In these assays, the general attachment functions seem to be dispensable and only mutants which directly disrupt the T-DNA transfer process or severely compromise bacterial metabolism manifest deficiencies (Heindl et al. 2016; Kim et al. 2013). Qualitative evaluation of plant tissue attachment has revealed large scale trends and deficiencies (Matthysse et al. 2005; Ramey et al. 2004; Tomlinson et al. 2010), but these are difficult to relate to virulence. Experiments which require more natural modes of infection or that measure attachment directly are likely required to evaluate effectively the role for general attachment functions in transformation.
The mechanisms that drive general surface attachment are certainly fundamental cellular attributes, as the genes required are encoded on the chromosomes, whereas the majority of primary virulence functions are carried on the curable (unstable) and horizontally transmitted Ti plasmid (Goodner et al. 2001; Wood et al. 2001). A. tumefaciens derivatives that lack the Ti plasmid are avirulent but are still fully competent for surface attachment. Although it is logical to posit that the fundamental attachment process leading to stable polar association of cells with surfaces is functionally connected with the attachment processes that culminate in T-DNA transfer, this remains to be validated convincingly. One model would be a temporal process in which the general attachment functions promote stable, proximal association to potential infection sites on the plant, among other more benign interactions. Under the appropriate environmental signals, this attachment may be followed by induction of the Vir genes and related functions that drive pathogenesis and disease progression, including direct engagement with plant surface structures (Zhu et al. 2003). An alternative model is that the general attachment processes promote benign interactions with plants and other surfaces, but that the attachment that leads to T-DNA transfer is completely distinct. Whether one of these models is correct, or perhaps an amalgamation of the two, remains an area of active study.
8. Acknowledgements
Research on surface attachment functions for A. tumefaciens in the Fuqua lab is supported by the National Institutes of Health GM120337. We thank Justin Eagan for helpful comments on the manuscript.
9 References
- Aguilar J, Cameron TA, Zupan J, et al. (2011) Membrane and core periplasmic Agrobacterium tumefaciens virulence Type IV secretion system components localize to multiple sites around the bacterial perimeter during lateral attachment to plant cells. mBio 2: e00218–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge P, & Hughes KT (2002) Regulation of flagellar assembly. Curr Opin Microbiol 5:160–165 [DOI] [PubMed] [Google Scholar]
- Aly KA, Baron C (2007) The VirB5 protein localizes to the T-pilus tips in Agrobacterium tumefaciens. Microbiology 153:3766–3775 [DOI] [PubMed] [Google Scholar]
- Amikam D, Benziman M (1989) Cyclic diguanylic acid and cellulose synthesis in Agrobacterium tumefaciens. J Bacteriol 171: 6649–6655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arioli T, Peng L, Betzner AS et al. (1998) Molecular analysis of cellulose biosynthesis in Arabidopsis. Science 279:717–720 [DOI] [PubMed] [Google Scholar]
- Ashby AM, Watson MD, Loake GJ et al. (1988. Ti plasmid-specified chemotaxis of Agrobacterium tumefaciens C58C1 toward vir-inducing phenoliv compounds and soluble factors from monocotyledonous and dicotyledonous plants. J Bacteriol 170:4181–4187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausmees N, Jacobsson K, Lindberg M (2001) A unipolarly located, cell-surface-associated agglutinin, RapA, belongs to a family of Rhizobium-adhering proteins (Rap) in Rhizobium leguminosarum bv. trifolii. Microbiology,147: 549–559 [DOI] [PubMed] [Google Scholar]
- Babić A, Lindner AB, Vulić M et al. (2008) Direct visualization of horizontal gene transfer. Science 319:1533–1536 [DOI] [PubMed] [Google Scholar]
- Barbosa RL, Benedetti CE (2007) BigR, a transcriptional repressor from plant associated bacteria, regulates an operon implicated in biofilm growth. J Bacteriol, 189:6185–6194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart MM, Chapman MR (2006) Curdlan biogenesis and function. Annu Rev Microbiol 60:131–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart DM, Su S, Baccaro BE et al. (2013) CelR, an ortholog of the diguanylate cyclase PleD of Caulobacter, regulates cellulose synthesis in Agrobacterium tumefaciens. Appl Environ Microbiol 79:7188–7202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart DM, Su S, Farrand SK (2014) A signaling pathway involving the diguanylate cyclase CelR and the response regulator DivK controls cellulose synthesis in Agrobacterium tumefaciens. J Bacteriol 196:1257–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berne C, Ducret A, Hardy GG et al. (2015) Adhesins involved in attachment to abiotic surfaces by Gram-negative bacteria. Microbiol Spectr 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair DF (2003) Flagellar movement driven by proton translocation. FEBS Lett 545:86–95 [DOI] [PubMed] [Google Scholar]
- Blanco LP, Evans ML, Smith DR et al. (2012) Diversity, biogenesis and function of microbial amyloids. Trends Microbiol 20:66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodenmiller D, Toh E, Brun YV (2004) Development of surface adhesion in Caulobacter crescentus. J Bacteriol 186:1438–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch RW, Sayegh MN, Shen C, Nathan VSJ, Berg HC (2014) Adaptive remodeling by FliN in the bacterial rotary motor. J Mol Biol 426:3314–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda SS, Vik Å, Friedman L et al. (2005) Biofilms: the matrix revisited. Trends Microbiol 13:20–26. [DOI] [PubMed] [Google Scholar]
- Brown PJ, de Pedro MA, Kysela DT et al. (2012) Polar growth in the Alphaproteobacterial order Rhizobiales. Proc Natl Acad Sci USA 109:1697–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Chen y, Wood DW et al. (2002) A new type IV secretion system promotes conjugal transfer in Agrobacterium tumefaciens. J Bacteriol 184:4838–4845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnokova O, Coutinho JB, Khan IH et al. (1997) Characterization of flagella genes of Agrobacterium tumefaciens, and the effect of a bald strain on virulence. Mol Microbiol 23:579–590 [DOI] [PubMed] [Google Scholar]
- Chevance FF, Hughes KT (2008) Coordinating assembly of a bacterial macromolecular machine. Nat Rev Microbiol 6:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JL, Hardy GG, Bodenmiller D et al. (2003) The HfaB and HfaD adhesion proteins of Caulobacter crescentus are localized in the stalk. Mol Microbiol 49:1671–1683 [DOI] [PubMed] [Google Scholar]
- Cook DM, Li PL, Ruchaud F et al. (1997) Ti plasmid conjugation is independent of vir: reconstitution of the tra functions from pTiC58 as a binary system. J Bacteriol 179:1291–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa TR, Ilangovan A, Ukleja M et al. (2016) Structure of the bacterial sex F pilus reveals an assembly of a stoichiometric protein-phospholipid complex. Cell 166:1436–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbertson L, Mainprize IL, Naismith JH et al. (2009) Pivotal roles of the outer membrane polysaccharide export and polysaccharide copolymerase protein families in export of extracellular polysaccharides in Gram-negative bacteria. Microbiol Mol Biol R 73:155–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danhorn T, Hentzer M, Givskov M et al. (2004) Phosphorus limitation enhances biofilm formation of the plant pathogen Agrobacterium tumefaciens through the PhoR-PhoB regulatory system. J Bacteriol 186:4492–4501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danhorn T, Fuqua C (2007) Biofilm formation by plant-associated bacteria. Annu Rev Microbiol 61:401–422 [DOI] [PubMed] [Google Scholar]
- Dardanelli M, Angelini J, Fabra A (2003) A calcium-dependent bacterial surface protein is involved in the attachment of rhizobia to peanut roots. Can J Microbiol 49:399–405 [DOI] [PubMed] [Google Scholar]
- Deakin WJ, Parker VE, Wright EL et al. (1999) Agrobacterium tumefaciens possesses a fourth flagellin gene located in a large gene cluster concerned with flagellar structure, assembly and motility. Microbiology 145:1397–1407 [DOI] [PubMed] [Google Scholar]
- Deakin WJ, Furniss CS, Parker VE et al. (1997a) Isolation and characterisation of a linked cluster of genes from Agrobacterium tumefaciens encoding proteins involved in flagellar basal-body structure. Gene 189: 135–137 [DOI] [PubMed] [Google Scholar]
- Deakin WJ, Sanderson JL, Goswami T et al. (1997b) The Agrobacterium tumefaciens motor gene, motA, is in a linked cluster with the flagellar switch protein genes, fliG, fliM and fliN. Gene 189:139–141 [DOI] [PubMed] [Google Scholar]
- Deinema MH, Zevenhuizen LPTM (1971) Formation of cellulose fibrils by Gram-negative bacteria and their role in bacterial flocculation. Arch Microbiol 78:42–57 [Google Scholar]
- DeRosier D (2006). Bacterial flagellum: visualizing the complete machine in situ. Curr Biol, 16, R928–R930 [DOI] [PubMed] [Google Scholar]
- Ellison CK, Kan J, Dillard RS et al. (2017) Obstruction of pilus retraction stimulates bacterial surface sensing. Science 358:535–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feirer N, Fuqua C (2017) Pterin function in bacteria. Pteridines 28:23–36 [Google Scholar]
- Feirer N, Kim D, Xu J et al. (2017) The Agrobacterium tumefaciens CheY-like protein ClaR regulates biofilm formation. Microbiology 163:1680–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feirer N, Xu J, Allen KD et al. (2015) A pterin-dependent signaling pathway regulates a dual-function diguanylate cyclase-phosphodiesterase controlling surface attachment in Agrobacterium tumefaciens. mBio 6:e00156–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferooz J, Lemaire J, Letesson JJ (2011) Role of FlbT in flagellin production in Brucella melitensis. Microbiology 157:1253–1262 [DOI] [PubMed] [Google Scholar]
- Fiebig A, Herrou J, Fumeaux C et al. (2014) A cell cycle and nutritional checkpoint controlling bacterial surface adhesion PLoS Genet 10:e1004101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritts RK, LaSarre B, Stoner AM et al. (2017) A Rhizobiales-specific unipolar polysaccharide adhesin contributes to Rhodopseudomonas palustris biofilm formation across diverse photoheterotrophic conditions Appl Environ Microbiol 83:e03035–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullner KJ, Lara JC, Nester EW (1996) Pilus assembly by Agrobacterium T-DNA transfer genes. Science 294:2323–2328 [DOI] [PubMed] [Google Scholar]
- Fuqua WC, Winans SC (1994) A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J Bacteriol 176:2796–2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghigo JM (2001) Natural conjugative plasmids induce bacterial biofilm development. Nature 412:442. [DOI] [PubMed] [Google Scholar]
- Goodner B, Hinkle G, Gattung S et al. (2001) Genome sequence of the plant pathogen and biotechnology agent Agrobacterium tumefaciens C58. Science 294:2323–2328 [DOI] [PubMed] [Google Scholar]
- Götz R, Limmer N, Ober K et al. (1982) Motility and chemotaxis in two strains of Rhizobium with complex flagella. Microbiology 128:789–798 [Google Scholar]
- Gu X, Lee SG, Bar-Peled M (2011) Biosynthesis of UDP-xylose and UDP-arabinose in Sinorhizobium meliloti 1021: first characterization of a bacterial UDP-xylose synthase, and UDP-xylose 4-epimerase. Microbiology 157:260–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães BG, Barbosa RL, Soprano AS et al. (2011) Plant pathogenic bacteria utilize biofilm growth-associated repressor (BigR), a novel winged-helix redox switch, to control hydrogen sulfide detoxification under hypoxia. J Biol Chem 286:26148–26157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy GG, Allen RC, Toh E et al. (2010) A localized multimeric anchor attaches the Caulobacter holdfast to the cell pole. Mol Microbiol 76:409–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy GG, Toh E, Berne C et al. (2018) Mutations in sugar-nucleotide synthesis genes restore holdfast polysaccharide anchoring to Caulobacter crescentus holdfast anchor mutants J Bacteriol 200:e00597–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckel BC, Tomlinson AD, Morton ER et al. (2014) Agrobacterium tumefaciens exoR controls acid response genes and impacts exopolysaccharide synthesis, horizontal gene transfer, and virulence gene expression. J Bacteriol 196:3221–3233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindl JE, Hibbing ME, Xu J et al. (2016) Discrete responses to limitation for iron and manganese in Agrobacterium tumefaciens: influence on attachment and biofilm formation. J Bacteriol 198:816–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindl JE, Wang Y, Heckel BC et al. (2014) Mechanisms and regulation of surface interactions and biofilm formation in Agrobacterium Front Plant Sci 5:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinsa SM, Espinosa‐Urgel M, Ramos JL et al. (2003) Transition from reversible to irreversible attachment during biofilm formation by Pseudomonas fluorescens WCS365 requires an ABC transporter and a large secreted protein. Mol Microbiol 49:905–918 [DOI] [PubMed] [Google Scholar]
- Høiby N (2017) A short history of microbial biofilms and biofilm infections. APMIS, 125: 272–275 [DOI] [PubMed] [Google Scholar]
- Hong Y, Reeves PR (2014) Diversity of O-antigen repeat unit structures can account for the substantial sequence variation of Wzx translocases. J Bacteriol 196:1713–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug I, Deshpande S, Sprecher KS et al. (2017) Second messenger–mediated tactile response by a bacterial rotary motor. Science 358:531–534 [DOI] [PubMed] [Google Scholar]
- Hughes KT, Mathee K (1998) The anti-sigma factors. Annu Rev Microbiol 52:231–286 [DOI] [PubMed] [Google Scholar]
- Islam ST, Lam JS (2013) Wzx flippase‐mediated membrane translocation of sugar polymer precursors in bacteria. Environ Microbiol 15:1001–1015 [DOI] [PubMed] [Google Scholar]
- Islam ST, Lam JS (2014) Synthesis of bacterial polysaccharides via the Wzx/Wzy-dependent pathway. Can J Microbiol 60:697–716 [DOI] [PubMed] [Google Scholar]
- Jenal U, Reinders A, Lori C (2017) Cyclic di-GMP: second messenger extraordinaire. Nat Rev Microbiol 15:271. [DOI] [PubMed] [Google Scholar]
- Judd PK, Kumar RB, Das A (2005) The type IV secretion apparatus protein VirB6 of Agrobacterium tumefaciens localizes to a cell pole. Mol Microbiol 55:115–124 [DOI] [PubMed] [Google Scholar]
- Kachlany SC, Planet PJ, DeSalle R et al. (2001) flp‐1, the first representative of a new pilin gene subfamily, is required for non‐specific adherence of Actinobacillus actinomycetemcomitans. Mol Microbiol 40:542–554 [DOI] [PubMed] [Google Scholar]
- Kai-Larsen Y, Lüthje P, Chromek M et al. (2010) Uropathogenic Escherichia coli modulates immune responses and its curli fimbriae interact with the antimicrobial peptide LL-37. PLoS Pathog 6:e1001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalynych S, Morona R, Cygler M (2014) Progress in understanding the assembly process of bacterial O-antigen. FEMS Microbiol Rev 38:1048–1065 [DOI] [PubMed] [Google Scholar]
- Karnezis T, Epa VC, Stone BA et al. (2003) Topological characterization of an inner membrane (1→ 3)-β-D-glucan (curdlan) synthase from Agrobacterium sp. strain ATCC31749. Glycobiology 13:693–706 [DOI] [PubMed] [Google Scholar]
- Karnezis T, Fisher HC, Neumann GM et al. (2002) Cloning and characterization of the phosphatidylserine synthase gene of Agrobacterium sp. strain ATCC 31749 and effect of its inactivation on production of high-molecular-mass (1→ 3)-β-d-glucan (curdlan) J Bacteriol 184:4114–4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmury SL, Burrows LL (2016) Type IV pilins regulate their own expression via direct intramembrane interactions with the sensor kinase PilS. PNAS 113:6017–6022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Heindl JE, Fuqua C (2013) Coordination of division and development influences complex multicellular behavior in Agrobacterium tumefaciens PLOS One 8:e56682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Chen HP, Saxena IM et al. (2001) Localization of c-di-GMP-binding protein with the linear terminal complexes of Acetobacter xylinum. J Bacteriol 183:5668–5674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner H, Sofia HJ, Zumft WG (2003) Phylogeny of the bacterial superfamily of Crp-Fnr transcription regulators: exploiting the metabolic spectrum by controlling alternative gene programs. FEMS Microbiol Rev 27:559–592 [DOI] [PubMed] [Google Scholar]
- Lai EM, Chesnokova O, Banta LM et al. (2000) Genetic and environmental factors affecting T-pilin export and T-pilus biogenesis in relation to flagellation of Agrobacterium tumefaciens. J Bacteriol 182:3705–3716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai EM, Eisenbrandt R, Kalkum M et al. (2002) Biogenesis of T pili in Agrobacterium tumefaciens requires precise VirB2 propilin cleavage and cyclization. J Bacteriol 184:327–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laus MC, Logman TJ, Lamers GE et al. (2006) A novel polar surface polysaccharide from Rhizobium leguminosarum binds host plant lectin. Mol Microbiol 59:1704–1713 [DOI] [PubMed] [Google Scholar]
- Leifson E, Erdman LW (1958) Flagellar characteristics of Rhizobium species. A van Leeuw J Microb 24:97–110 [DOI] [PubMed] [Google Scholar]
- Lele PP, Branch RW, Nathan VJS, Berg HC (2012) Mechanism for remodeling of the bacterial flagellar switch. Proc Natl Acad Sci, USA 109:20018–20022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessl M, Lanka E (1994) Common mechanisms in bacterial conjugation and Ti-mediated T-DNA transfer to plant cells. Cell 77:321–324 [DOI] [PubMed] [Google Scholar]
- Li G, Brown PJ, Tang JX et al. (2012) Surface contact stimulates the just‐in‐time deployment of bacterial adhesins. Mol Microbiol 83:41–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Ochman H (2007) Origins of flagellar gene operons and secondary flagellar systems. J Bacteriol 189:7098–7104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locht C, Berlin P, Menozzi FD et al. (1993) The filamentous haemagglutinin, a multifaceted adhesin produced by virulent Bordetella spp. Mol Microbiol 9:653–660 [DOI] [PubMed] [Google Scholar]
- Madsen JS, Burmølle M, Hansen LH et al. (2012) The interconnection between biofilm formation and horizontal gene transfer. FEMS Immunol Med Mic 65:183–195. [DOI] [PubMed] [Google Scholar]
- Mangan EK, Malakooti J, Caballero A et al. (1999) FlbT couples flagellum assembly to gene expression in Caulobacter crescentus. J Bacteriol 181:6160–6170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse AG (1983) Role of bacterial cellulose fibrils in Agrobacterium tumefaciens infection. J Bacteriol 154:906–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse AG (1987) Characterization of nonattaching mutants of Agrobacterium tumefaciens. J Bacteriol 169:313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse AG (2014) Attachment of Agrobacterium to plant surfaces. Front Plant Sci 5:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse AG, Holmes KV, Gurlitz RH (1981) Elaboration of cellulose fibrils by Agrobacterium tumefaciens during attachment to carrot cells. J Bacteriol 145:583–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse AG, Jaeckel P, Jeter C (2008) attG and attC mutations of Agrobacterium tumefaciens are dominant negative mutations that block attachment and virulence. Can J Microbiol 54:241–247 [DOI] [PubMed] [Google Scholar]
- Matthysse AG, Marry M, Krall L et al. (2005) The effect of cellulose overproduction on binding and biofilm formation on roots by Agrobacterium tumefaciens. Mol Plant Microbe Interac 18:1002–1010 [DOI] [PubMed] [Google Scholar]
- Matthysse AG, Thomas DL, White AR (1995a) Mechanism of cellulose synthesis in Agrobacterium tumefaciens. J Bacteriol 177:1076–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse AG, White S, Lightfoot R (1995b) Genes required for cellulose synthesis in Agrobacterium tumefaciens. J Bacteriol,177:1069–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse AG, Yarnall H, Boles SB et al. (2000) A region of the Agrobacterium tumefaciens chromosome containing genes required for virulence and attachment to host cells. Biophys Biochi. Acta 1490:208–212 [DOI] [PubMed] [Google Scholar]
- Mattick JS (2002) Type IV pili and twitching motility. Annu Rev Microbiol 56:289–314 [DOI] [PubMed] [Google Scholar]
- McDermott TR (2000) Phosphorus assimilation and regulation in the rhizobia Prokaryotic Nitrogen Fixation: a Model System for the Analysis of a Biological Process (ed. Triplett EW) 529–548. Horizon Scientific Press, Norfolk, United Kingdom [Google Scholar]
- McIntosh M, Stone BA, Stanisich VA (2005) Curdlan and other bacterial (1→ 3)-β-D-glucans. Appl Microbiol Biotech 68:163–173 [DOI] [PubMed] [Google Scholar]
- Macnab RM (1996) Flagella and motility Escherichia coli and Salmonella: Cellular and Molecular Biology (ed. Neidhardt FC) 123–145. ASM Press, Washington DC [Google Scholar]
- Meadows PS (1971) The attachment of bacteria to solid surfaces. Arch Microbiol 75:374–381 [DOI] [PubMed] [Google Scholar]
- Merritt PM, Danhorn T, Fuqua C (2007) Motility and chemotaxis in Agrobacterium tumefaciens surface attachment and biofilm formation. J Bacteriol 189:8005–8014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi W, Li Y, Yoon SH et al. (2017) Structural basis of MsbA-mediated lipopolysaccharide transport. Nature 549:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohari B, Licata NA, Kysela DT et al. (2015) Novel pseudotaxis mechanisms improve migration of straight-swimming bacterial mutants through a porous environment. mBio 6:e00005–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JL, Strumillo J, Zimmer J (2013) Crystallographic snapshot of cellulose synthesis and membrane translocation. Nature 493:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair GR, Liu Z, Binns AN (2003) Reexamining the role of the accessory plasmid pAtC58 in the virulence of Agrobacterium tumefaciens strain C58. Plant Physiol 133:989–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi I, Kimura K, Suzuki T et al. (1976) Demonstration of curdlan-type polysaccharide and some other β−1, 3-glucan in microorganisms with aniline blue. J Gen Appl Microbiol 22:1–11 [Google Scholar]
- Oberpichler I, Rosen R, Rasouly A et al. (2008) Light affects motility and infectivity of Agrobacterium tumefaciens. Environ Microbiol 10:2020–2029 [DOI] [PubMed] [Google Scholar]
- Osterman IA, Dikhtyar YY, Bogdanov AA et al. (2015) Regulation of flagellar gene expression in bacteria. Biochemistry (Moscow) 80:1447–1456 [DOI] [PubMed] [Google Scholar]
- Pallen MJ, Matzke NJ (2006) From The Origin of Species to the origin of bacterial flagella. Nat Rev Microbiol, 4:784. [DOI] [PubMed] [Google Scholar]
- Paul R, Weiser S, Amiot NC et al. (2004) Cell cycle-dependent dynamic localization of a bacterial response regulator with a novel di-guanylate cyclase output domain. Gene Dev 18:715–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper KR, Beck von Bodman S, Farrand SK (1993) Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature 362:448–450. [DOI] [PubMed] [Google Scholar]
- Ramey BE, Matthysse AG, Fuqua C (2004) The FNR‐type transcriptional regulator SinR controls maturation of Agrobacterium tumefaciens biofilms. Mol Microbiol 52:1495–1511 [DOI] [PubMed] [Google Scholar]
- Reuber TL, Walker GC (1993) Biosynthesis of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Cell 74:269–280 [DOI] [PubMed] [Google Scholar]
- Römling U (2002) Molecular biology of cellulose production in bacteria. Res Microbiol 153:205–212 [DOI] [PubMed] [Google Scholar]
- Römling U, Galperin MY, Gomelsky M (2013) Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev 77:1–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römling U, Galperin MY (2015) Bacterial cellulose biosynthesis: diversity of operons, subunits, products, and functions. Trends Microbiol 23:545–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross P, Weinhouse H, Aloni Y, Michaeli D, Weinbergerohana P, Mayer R, Braun S, Devroom E, Vandermarel GA, Vanboom JH, Benziman M (1987) Regulation of cellulose synthase in Acetobacter xylinum by cyclic diguanylic acid. Nature 325:279–281 [DOI] [PubMed] [Google Scholar]
- Rotter C, Mühlbacher S, Salamon D et al. (2006) Rem, a new transcriptional activator of motility and chemotaxis in Sinorhizobium meliloti. J Bacteriol 188:6932–6942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffing AM, Castro-Melchor M, Hu WS et al. (2011) Genome sequence of the curdlan-producing Agrobacterium sp. strain ATCC 31749. J Bacteriol 193:4294–4295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffing AM, Chen RR (2012) Transcriptome profiling of a curdlan-producing Agrobacterium reveals conserved regulatory mechanisms of exopolysaccharide biosynthesis. Microb Cell Fact 11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo DM, Williams A, Edwards A et al. (2006) Proteins exported via the PrsD-PrsE type I secretion system and the acidic exopolysaccharide are involved in biofilm formation by Rhizobium leguminosarum. J Bacteriol 188:4474–4486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer K, Camper AK, Ehrlich GD et al. (2002) Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J Bacteriol 184:1140–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäper S, Krol E, Skotnicka D et al. (2016) Cyclic di-GMP regulates multiple cellular functions in the symbiotic alphaproteobacterium Sinorhizobium meliloti. J Bacteriol 198:521–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid J, Sieber V, Rehm B (2015) Bacterial exopolysaccharides: biosynthesis pathways and engineering strategies. Front Microbiol 6:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmacher JS, Thormann KM, Bange G (2015) How bacteria maintain location and number of flagella? FEMS Microbiol Rev 39:812–822 [DOI] [PubMed] [Google Scholar]
- Shaw CH, Loake GJ, Brown AP et al. (1991) Isolation and characterization of behavioural mutants and genes of Agrobacterium tumefaciens. Microbiology 137:1939–1953 [Google Scholar]
- Shoda M, Sugano Y (2005) Recent advances in bacterial cellulose production. Biotechnol Bioproc E 10:1 [Google Scholar]
- Skerker JM, Shapiro L (2000) Identification and cell cycle control of a novel pilus system in Caulobacter crescentus. EMBO J 19:3223–3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit G, Logman TJ, Boerrigter ME et al. (1989) Purification and partial characterization of the Rhizobium leguminosarum biovar viciae Ca2+-dependent adhesin, which mediates the first step in attachment of cells of the family Rhizobiaceae to plant root hair tips. J Bacteriol 171:4054–4062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer JM, Newton A (1989) Turning off flagellum rotation requires the pleiotropic gene pleD: pleA, pleC, and pleD define two morphogenic pathways in Caulobacter crescentus. J Bacteriol 171:392–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourjik V, Muschler P, Scharf B, Schmitt R (2000) VisN and VisR are global regulators of chemotaxis, flagellar, and motility genes in Sinorhizobium (Rhizobium) meliloti. J Bacteriol 182:782–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourjik V, Schmitt R (1996) Different roles of CheY1 and CheY2 in the chemotaxis of Rhizobium meliloti. Mol Microbiol 22:427–436 [DOI] [PubMed] [Google Scholar]
- Stasinopoulos SJ, Fisher PR, Stone BA et al. (1999) Detection of two loci involved in (1→ 3)-β-glucan (curdlan) biosynthesis by Agrobacterium sp. ATCC31749, and comparative sequence analysis of the putative curdlan synthase gene. Glycobiol. 9:31–41 [DOI] [PubMed] [Google Scholar]
- Toh E, Kurtz HD, Brun YV (2008) Characterization of the Caulobacter crescentus holdfast polysaccharide biosynthesis pathway reveals significant redundancy in the initiating glycosyltransferase and polymerase steps. J Bacteriol 190:7219–7231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomich M, Planet PJ, Figurski DH (2007) The tad locus: postcards from the widespread colonization island. Nat Rev Microbiol 5:363–375 [DOI] [PubMed] [Google Scholar]
- Tomlinson AD, Fuqua C (2009) Mechanisms and regulation of polar surface attachment in Agrobacterium tumefaciens. Curr Opin Microbiol 12:708–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson AD, Ramey-Hartung B, Day TW et al. (2010) Agrobacterium tumefaciens ExoR represses succinoglycan biosynthesis and is required for biofilm formation and motility. Microbiology 156:2670–2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visick KL, Schembri MA, Yildiz F et al. (2016) Biofilms 2015: Multidisciplinary approaches shed light into microbial life on surfaces. J Bacteriol 198:2553–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhams GH, Armitage JP (2004) Making sense of it all: bacterial chemotaxis. Nat Rev Mol Cell Biol 5:1024–1037 [DOI] [PubMed] [Google Scholar]
- Wan Z, Brown PJ, Elliott EN, Brun YV (2013) The adhesive and cohesive properties of a bacterial polysaccharide adhesin are modulated by a deacetylase. Mol Microbiol 88:486–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Haitjema CH, Fuqua C (2014) The Ctp type IVb pilus locus of Agrobacterium tumefaciens directs formation of the common pili and contributes to reversible surface attachment. J Bacteriol 196:2979–2988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kim SH, Natarajan R et al. (2016) Spermidine inversely influences surface interactions and planktonic growth in Agrobacterium tumefaciens. J Bacteriol 198:2682–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Yang F, von Bodman SB (2012) The genetic and structural basis of two distinct terminal side branch residues in stewartan and amylovoran exopolysaccharides and their potential role in host adaptation. Mol Microbiol 83:195–207 [DOI] [PubMed] [Google Scholar]
- Williamson RE, Burn JE, Hocart CH (2002) Towards the mechanism of cellulose synthesis. Trends Plant Sci 7:461–467 [DOI] [PubMed] [Google Scholar]
- Wolfe AJ, Visick KL (Eds.) (2010) The Second Messenger Cyclic di-GMP. ASM Press, USA [Google Scholar]
- Wood DW, Setubal JC, Kaul R et al. (2001) The genome of the natural genetic engineer Agrobacterium tumefaciens C58. Science 294:2317–2323 [DOI] [PubMed] [Google Scholar]
- Wu CF, Lin JS, Shaw GC et al. (2012) Acid-induced type VI secretion system is regulated by ExoR-ChvG/ChvI signaling cascade in Agrobacterium tumefaciens. PLoS Pathog 8:e1002938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kim J, Danhorn T et al. (2012) Phosphorus limitation increases attachment in Agrobacterium tumefaciens and reveals a conditional functional redundancy in adhesin biosynthesis. Res Microbiol 163:674–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kim J, Koestler BJ et al. (2013) Genetic analysis of Agrobacterium tumefaciens unipolar polysaccharide production reveals complex integrated control of the motile‐to‐sessile switch. Mol Microbiol 89:929–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Zhang C, Yang L et al. (2015) CrdR function in a curdlan-producing Agrobacterium sp. ATCC31749 strain. BMC Microbiol 15:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatakia HM, Arapov TD, Meier VM et al. (2018) Cellular stoichiometry of methyl-accepting chemotaxis proteins in Sinorhizobium meliloti J Bacteriol 200:e00614–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen XB, Kin CC, Zhang HT (2012) Recent advances in curdlan biosynthesis, biotechnological production and applications. Appl Microbiol Biotechnol 93:525–31 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Nam J, Humara JM et al. (2003) Identification of Arabidopsis rat mutants. Plant Physiol 132:494–505 [DOI] [PMC free article] [PubMed] [Google Scholar]