Abstract
Melanocortin 4 receptor (MC4R), a canonical melanocyte-stimulating hormone receptor, is the main responsible for monogenic obesity in humans. Previous studies in fish and avian species showed that MC4R becomes an ACTH receptor after interaction with the melanocortin receptor accessory protein 2 (MRAP2). We show that human MC4R behaves in a similar way through its interaction with MRAP2. This evolutionary conservation of MRAP2-induced ligand selectivity supports a physiological role for the interaction with MC4R. Both proteins are coexpressed in the same hypothalamic neurons, providing an anatomical substrate and molecular mechanism for the central therapeutic actions of ACTH in the treatment of infantile spasms. These neurons may link the effects of stress on the energy balance independently of glucocorticoid secretion. The complex MC4R-MRAP2 throws light on the action of ACTH and, by extension, on the relay of stress-related information to additional biological systems.
In tetrapod species, melanocortin peptides, encoded by proopiomelanocortin (POMC), signal through five related class A G-coupled protein receptors, melanocortin receptors (MCRs) 1 through 5. They usually bind α-, β-, or γ-melanocyte-stimulating hormones (MSHs) with different affinities, but MC2R is unique because it is activated exclusively by ACTH. MC2R activation is closely associated with the stress response through the synthesis and secretion of adrenal glucocorticoids, along the hypothalamic-pituitary-adrenal axis [1]. Other MCRs mediate diverse physiological functions, including skin pigmentation (MC1R), energy balance (MC3R and MC4R), and exocrine secretion (MC5R) [2]. MC4R is involved in the regulation of energy intake and expenditure in vertebrates [3], including fish [4, 5]. The targeted disruption of MC4R results in obesity [6] as well as overexpression of endogenous inverse agonists [7–9].
The functional expression of MC2R requires an adrenal-specific factor named MC2R accessory protein (MRAP) [10]. This single-transmembrane domain protein assists MC2R trafficking to the plasma membrane but is also involved in both ACTH binding [11, 12] and ACTH-induced cAMP production [11]. Specific details of the structure–activity relationship between receptor and accessory protein are unknown [13, 14], but it has been suggested that MC2R may require MRAP interaction to form a high-affinity binding pocket to ACTH or may assist MC2R to bind to the Gs subunit of the heterodimeric G protein complex [15]. Vertebrate genome has a paralog of the MRAP gene, the so-called MRAP2 [10]. Most authors continue to call the first characterized protein MRAP rather than MRAP1, but here we use the numerical nomenclature [16]. MRAP2 interplays with all human (h) MCRs and decreases receptor sensitivity to (Nle4-D-Phe7)-α-MSH [17] although, using α-MSH, the opposite results have been reported when coexpressing MRAP2 and mice MC4R [18]. Nonmammalian species exhibits ortholog genes of MRAP1 and MRAP2. In zebrafish (zf), MRAP2 shows two paralog genes (i.e., zfMRAP2a and zfMRAP2b) [19]. Our previous studies have demonstrated that zfMRAP2a is able to change the pharmacological profile of zfMC4R by increasing the receptor response to ACTH. In the presence of zfMRAP2a, zfMC4R becomes an ACTH receptor [20], and similar results have been reported recently in chicken [21]. This suggests an evolutionary conservation of the MRAP2-induced ACTH sensitivity of MC4R that points to the existence of a physiological role for MRAP2-induced ligand selectivity. To test this hypothesis and to evaluate the conservation of this mechanism, which may link regulation of the energy balance (MC4R) and stress responses (ACTH), we study the pharmacological profile of hMC4R after interaction with hMRAP2 and zfMRAP variants and demonstrate that the accessory proteins change the pharmacological profile of hMC4R, thus providing ACTH sensitivity to the receptor.
1. Materials and Methods
A. Constructs and Peptides
hMRAP2 (Clone ID: OHu17223D; ORF: NM_138409.2) and hMC4R (Clone ID: OHu24975D; ORF: NM_005912.2) in pcDNA3.1 were obtained from GenScript. Zf constructs in pcDNA 3.1 were from Josep Agulleiro et al. [20]. Peptides were obtained from Bachem.
B. Pharmacological Experiments
A HEK-293 cell clone stably expressing β-galactosidase placed downstream of tandem repetitions of cAMP-responsive elements was used to evaluate receptor activation [22]. Cells were maintained in DMEM (Gibco) supplemented with 10% fetal bovine serum (Gibco) and 1% penicillin/streptomycin (Gibco) in a humidified atmosphere of 5% CO2 at 37°C. zfMC4R or hMC4R constructs were transiently transfected alone or together with zfMRAP2a, zfMRAP2b, or hMRAP2. Transient transfections were carried out using Lipofectamine LTX (Invitrogen) with 100 ng of each construct. A construct carrying luciferase gene was also transfected to standardize transfection levels. The following day, cells were divided among 96-well plates and stimulated with human α-MSH and ACTH (1-24) from 10−6 to 10−12 M or 8-Br-cAMP 10−3 M in assay medium to standardize the cell conditions 48 hours posttransfection. After 6 hours, the medium was removed, cells were lysed, and galactosidase activity was measured as previously described [22]. Measurements were normalized to the protein content determined using the BCA protein assay kit (Pierce). Luciferase activity was determined using the luciferase assay kit (Promega). Activation assays were performed in quadruplicate wells and repeated at least three times, independently.
C. Tissue Expression
Human tissue expression patterns were obtained from GTEX portal (GTEx Analysis Release V7, dbGaP Accession phs000424.v7.p2; https://www.gtexportal.org/home/), and expressed as transcript per million. Zf expression patterns of MRAP2a and 2b were previously reported [19, 20].
D. Data Analysis and Statistics
Receptor activation data were fitted to logistic curves using GraphPad Prism; the mean effective dose values are provided in Table 1. For graphic representation, one representative experiment was selected for each gene or combination. Basal activities were compared by one-way ANOVA after standardization by transfection level (luciferase levels) and cell condition (8-Br-cAMP levels). Differences were considered significant at P < 0.05. Sequence alignments were modified from previous work [19].
Table 1.
Effect of MRAP2 on Pharmacological Profile of MC4R
| zfMC4R | zfMC4R + hMRAP2 | zfMC4R + zfMRAP2a | zfMC4R + zfMRAP2b | |
|---|---|---|---|---|
| α-MSH | 8.25 × 10−9 (4.41 × 10−9–1.50 × 10−8) | 9.60 × 10−9 (4.27 × 10−9–2.24 × 10−8) | 8.1 × 10−9 (5.32 × 10−9–1.22 × 10−8) | 5.58 × 10−9 (2.67 × 10−9–1.07 × 10−8) |
| hACTH (1-24) | 1.27 × 10−7 (5.42 × 10−8–2.92 × 10−7) | 5.44 × 10−9 (2.66 × 10−9–1.02 × 10−8) | 1.27 × 10−9 (4.66 × 10−10–4.14 × 10−9) | 3.81 × 10−8 (2.27 × 10−8–6.28 × 10−8) |
| hMC4R | hMC4R + hMRAP2 | hMC4R + zfMRAP2a | hMC4R + zfMRAP2b | |
| α-MSH | 2.66 × 10−8 (1.78 × 10−8–4.07 × 10−8) | 8.63 × 10−9 (5.97 × 10−9–1.23 × 10−8) | 7.85 × 10−9 (5.29 × 10−9–1.15 × 10−8) | 7.93 × 10−9 (5.93 × 10−9–1.05 × 10−8) |
| hACTH (1-24) | 1.33 × 10−7 (6.89 × 10−8–2.48 × 10−7) | 3.52 × 10−10 (1.98 × 10−10–6.34 × 10−10) | 4.09 × 10−9 (8.79 × 10−10–1.56 × 10−8) | 5.22 × 10−8 (2.91 × 10−8–8.87 × 10−8) |
Mean effective dose values (M) of mean reporter activation under the control of cAMP-responsive elements stably expressed in HEK-293 cells transiently expressing hMC4R or zfMC4R alone or in combination with different MRAPs after incubation with melanocortin agonist (hACTH1-24 or α-MSH). The mean of the reporter activation, expressed as percentage of the basal level, for each concentration of melanocortin agonist (hACTH1-24 or α-MSH), was calculated from four replicates of one representative experiment; the resultant data were fitted to logistic curves using GraphPad Prism software. Numbers in parentheses indicate the 95% confidence intervals of nonlinear fittings.
2. Results
A. MRAP2-Induced Pharmacological Profiles of MC4R
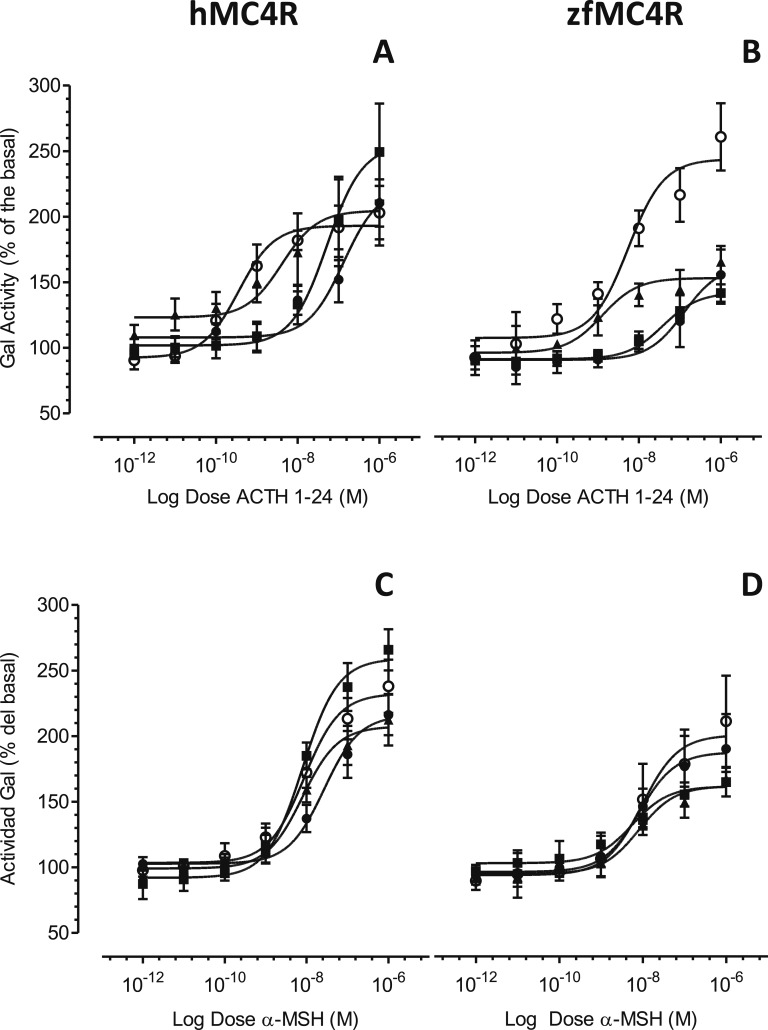
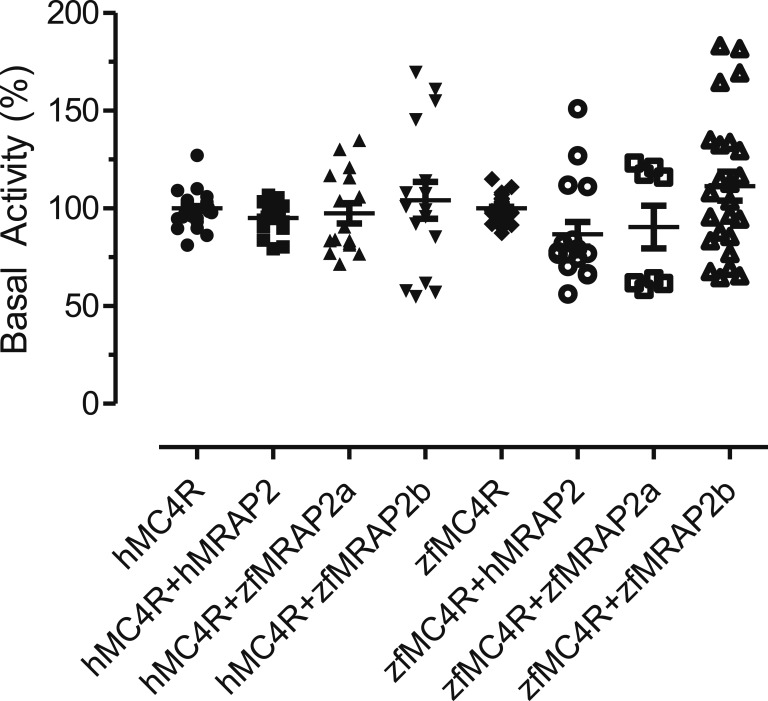
The coexpression of hMC4R with hMRAP2 increased MC4R sensitivity for ACTH more than 350-fold (Fig. 1A; Table 1). The zfMRAP2a had a more modest effect on hMC4R (32-fold change) (Fig. 1A; Table 1), whereas the zfMRAP2b produced only a 2.5-fold change in the ACTH sensitivity of MC4R (Fig. 1A; Table 1). The expression of any accessory protein with hMC4R only induced a threefold increase in the MC4R sensitivity by α-MSH (Fig. 1C; Table 1). The pharmacological profile of zfMC4R coexpressed with the zfMRAP2 paralogs as well as hMRAP2 (Fig. 1B and 1D; Table 1) were also studied. Similar to hMC4R, hMRAP2 (23-fold change) and zfMRAP2a (100-fold change) increased the zfMC4R response to ACTH (Fig. 1B; Table 1), but MRAPs had no effect at all on MC4 responsiveness to α-MSH (Fig. 1D; Table 1). MRAP2 coexpression with MC4R had no effect on the basal cAMP signaling level (Fig. 2). Data are accessible at Mendeley data repository [23].
Figure 1.
Pharmacological properties of melanocortin agonists (A, B) hACTH (1 through 24) and (C, D) α-MSH at HEK-293 transiently expressing both hMC4R (A, C) or zfMC4R (B, D) and different MRAPs (●, MC4R; ○, MC4R+hMRAP2; ▲, MC4R+zfMRAP2a; ■, MC4R+zfMRAP2b), but stably expressing a cAMP-responsive β-galactosidase reporter gene. Data were normalized to protein levels and expressed as percentage of the basal levels. A construct carrying luciferase gene under the control of a constitutive promoter was also transfected to standardize the transfection levels. Experiments were performed using quadruplicate data points and repeated at least two times independently. Data are mean ± SEM of the one representative experiment.
Figure 2.
Effects of different MRAPs on hMC4R- or zfMC4R-induced galactosidase basal activity in HEK-293 cells stably expressing galactosidase gene under the control of a constitutive promoter carrying several cAMP-responsive element sites. Data were pooled from all the pharmacological experiments performed to study the effect of melanocortin agonist on hMC4R or zfMC4R (see “Materials and Methods” for details). No significant differences were detected after one-way ANOVA (P < 0.05).
B. Tissue Expression
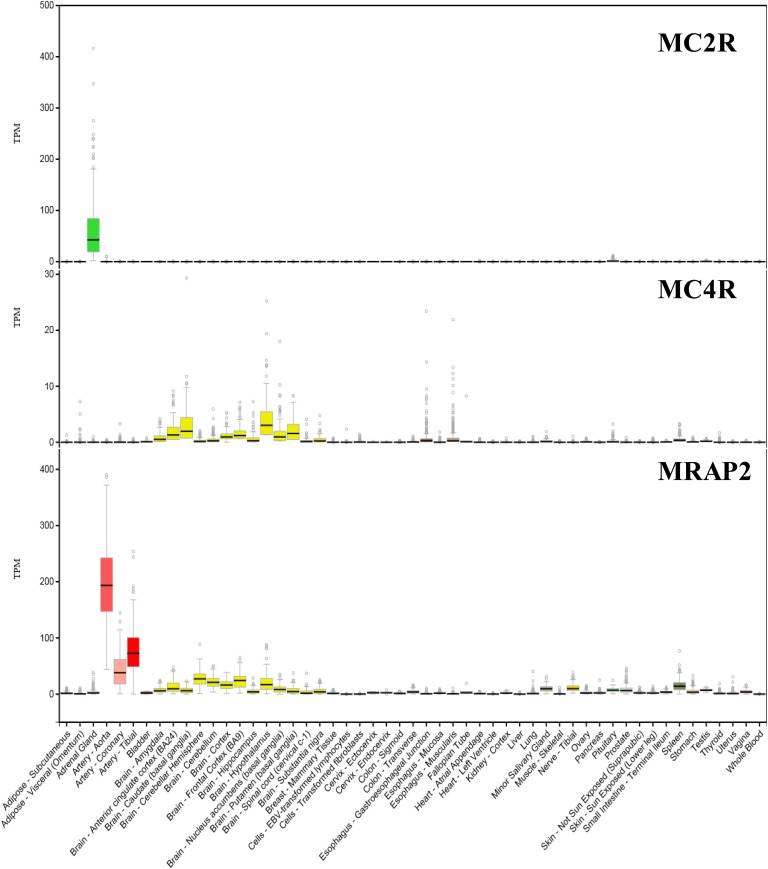
In humans, MC2R is expressed in the adrenal gland, with residual levels in other tissues (Fig. 3). By contrast, MC4R is expressed mainly in the brain, but some levels are also detected in the esophagus, spleen, minor salivary glands, and testis (Fig. 3). hMRAP2 is highly expressed in the arterial tissue, but also in the brain, minor salivary glands, spleen, transverse colon, and tibial nerve (Fig. 3). In zf, quantitative PCR experiments showed that both MRAPs are mainly expressed in the brain and interrenal tissue (the fish equivalent to the adrenal gland) together with zfMC4R. However, low levels of all three genes were observed in the gills and spleen [20].
Figure 3.
Expression profiles of human MC2R, MC4R, and MRAP2 using RNA sequencing. Data were obtained from GTExPortal (https://gtexportal.org) and expressed as TPM. TPM, transcript per million.
C. Comparison of MRAP Sequences Revealed Conserved and Nonconserved Regions Potentially Responsible for Specific Functional Properties
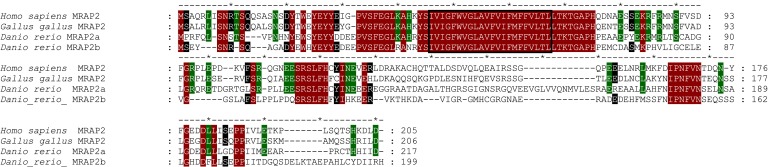
Amino acid sequence analyses revealed that tetrapod MRAP sequences exhibit 73% identity, whereas the identity of zfMRAP2s compared with tetrapod MRAP2 is <50%. zfMRAP2a was 47% identical to both chicken and hMRAP2, whereas zfMRAP2b identity was 40%, and 39% when compared with chicken MRAP2 or hMRAP2, respectively. Finally, zfMRAP2a and zfMRAP2b were 43% identical in the same range as zfMRAP2b and tetrapod MRAP2s.
Based on these comparisons, it was possible to identify those residues that are conserved between tetrapod MRAP2 and zfMRAP2a but are different in zfMRAP2b sequences. We postulate specifically that these amino acids are responsible for the MRAP2-induced ACTH sensitivity of MC4R. zfMRAP2a exhibits 24 identical residues with tetrapod MRAP2s that differs in zfMRAP2b (Fig. 4).
Figure 4.
Alignment of MRAP amino acid sequences. Amino acid numbers are indicated on the right (dashes have been introduced to improve alignment). The putative transmembrane helix is framed. Residues in red are identical in all sequences, whereas residues in green or black are identical in tetrapod MRAP2 and zfMRAP2a or MRAP2b, respectively.
3. Discussion
Our studies in zf demonstrated that zfMC4R, a canonical α-MSH receptor, binds ACTH by interplay with zfMRAP2a [20]. A recent report in chicken [21] corroborated our previous studies, suggesting the evolutionary conservation of the MRAP2-induced ACTH sensitivity of MC4R. We now demonstrate that hMRAP2 is also able to provide ACTH sensitivity to the hMC4R, extending such conservation to mammalian species and further supporting a distinctive function for the MRAP2-MC4R interaction in the vertebrate biology. If ACTH can bind MC4R under interaction with the MRAP2, the receptor could mediate stress effects independently of glucocorticoids, thus offering a molecular pathway linking stress response and the regulation of energy balance but also providing additional functions for ACTH regulated by MC4R.
Although the effects of MC4R on the energy balance are mediated at central level [3], the presence of ACTH in the brain is controversial. In the rat brain, POMC is expressed in the arcuate nucleus (ARC) and the nucleus of the solitary tract [24] and is mainly processed to α-MSH and β-endorphin [25], but some hypothalamic ACTH-IR after reversed-phase HPLC also elutes at the position of ACTH [26], suggesting its presence in the rat brain. Experiments using ACTH-specific antibodies also suggest the presence of ACTH in mouse ARC [27]. Alternatively, systemic ACTH could reach the central structures to activate the MRAP2/MC4R neurons, thus feeding back neuronal circuits regulating the stress response and energy balance and relaying peripheral stress information to the brain. Intraperitoneal administration of ACTH and vigabatrin are the only drugs by the US Food and Drug Association for the treatment of infantile spasms (IS), a neurodevelopmental epileptic syndrome of infancy [28]. The therapeutic effects of systemic or intracerebroventricular (ICV) ACTH persist when central glucocorticoid receptors are blocked but not when MCRs are antagonized, supporting the existence of a steroid-independent effect of ACTH mediated by MCRs [28–30]. Because MC2R is not expressed in the human brain, the central MC4R-MRAP2 neurons could be the targets of peripheral ACTH in the IS treatment and, by extension, potential targets for the development of therapeutic agents. In fact, central or peripheral ACTH administration has profound effects a central level. IS alters the expression of 30% of the genes in the ARC, but the peripheral administration of ACTH is able to restore the neurotransmission transcriptome to non-IS condition [31]. Peripheral ACTH reduces corticotrophin-releasing factor expression in the amygdala of adrenalectomized rats by 35% [30]. The ICV administration of ACTH4-10, which binds melanocortin receptor but does not induce glucocorticoid secretion, recapitulates peripheral ACTH effects [29]. In addition, the inhibitory effects of ICV administration on food intake in both chicken [32] and rats [33] support the central role for the ACTH. In chickens, central ACTH administration reinforces c-Fos-IR in the ARC and the lateral and ventromedial hypothalamus, which are involved in the stimulation of hunger and satiety, respectively [32]. Accordingly, MC4R-MRAP2 neurons seem to be closely associated with regulation of the energy balance because MRAP2-deficient mice are obese and disruptive mutations in the human genome are associated with early-onset obesity. Previously, it was proposed that MRAP2 enhances the MC4R-mediated synthesis of cAMP, suggesting that alterations in MC4R signaling may be one of the mechanisms underlying the association between MRAP2 disruption and obesity [18]. In our hands, MRAP2 potentiated severely ACTH-induced signaling of the hMC4R and zfMC4R (30- to 300-fold) and weakly α-MSH–induced signaling (threefold) but never the basal activity of the receptor. Therefore, the central complex MC4R-MRAP2 could mediate ACTH effects on food intake but could also segregate discrete populations of MC4R neurons (i.e., those responding only to α-MSH and those responding to α-MSH and ACTH), depending on the presence of MRAP2. Both populations could mediate different aspects of POMC neuronal function.
We further explored the coexpression and localization of MC4R and MRAP2 in different human tissues, paying special attention to non-MC2R–expressing tissues. The expression of the complex MC4R-MRAP2 in these tissues offers gates for the action of ACTH and, by extension, to the relay of stress-related information to additional biological systems. MC2R was only expressed to any important extent in the adrenal gland, although very low levels were also detected by RNA sequencing in the pituitary and testis. MC4R, together with MRAP2, are highly expressed throughout the brain, including the hypothalamus. Recent single-cell transcriptome studies have demonstrated MRAP2 colocalization with both MC3R and MC4R in the mouse hypothalamus [34], and our previous studies in zf showed that both zfMRAP2a and zfMRAP2b are coexpressed in the magnocellular preoptic nucleus, homolog to the mammalian paraventricular nucleus, and the lateral tuberal nucleus, homolog to the arcuate nucleus [20]. Therefore, hypothalamic coexpression of MC4R and MRAP2 offers a neuronal substrate to the central effects of ACTH. In addition, some levels of MR4R/MRAP2 coexpression were detected in the spleen, suggesting the involvement of the MC4R–MRPA2 complex in the regulation of hematopoietic and/or immune function. Interestingly, MRAP2 showed the highest expression levels in the vascular system, particularly in the arterial tissue, in the absence of substantial MC4R expression. The function of MRAP2 in the vascular system is unclear, but it is known that MRAP2 is able to modulate the activity of some other G-coupled receptors in mammalian species, suggesting the existence of unexplored functions for MRAP2 [35, 36]. The exhaustive phenotyping of MRAP2 (−/−) deficient mice [18] could help to unravel physiological roles for MRAP2.
Comparative sequence analysis revealed the existence of several residues conserved in all MRAP2 sequences, which conferred ACTH sensitivity to the MC4R, but not in MRAP2b. These residues are potential interplay sites between MRAP2 and MC4R, but more structural studies on MRAP2-MC4R interaction are required.
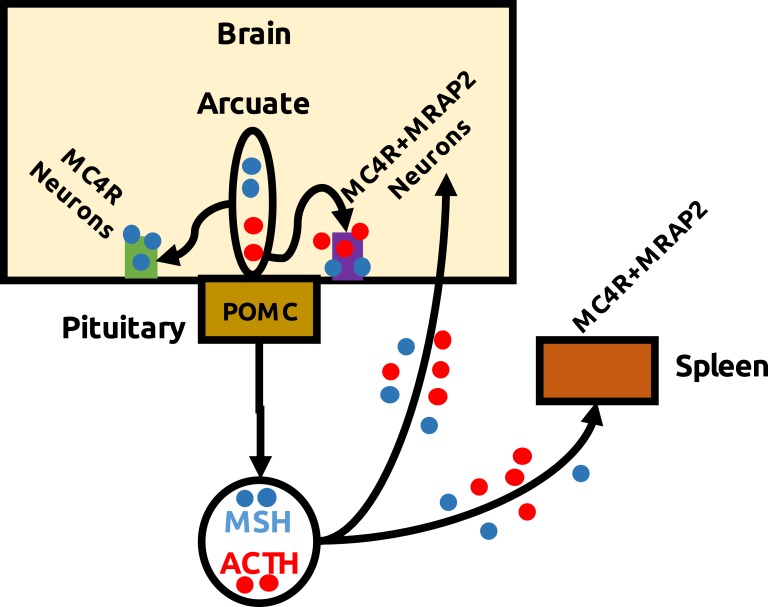
In summary, we demonstrate that ACTH is a potent agonist of hMC4R when coexpressed with MRAP2. Both proteins colocalize in the hypothalamic neurons, offering a neuronal substrate for the action of peripheral and/or central ACTH but also supporting neuronal and molecular basis for glucocorticoid-independent effects of stress (Fig. 5). Finally, we suggest that MC4R-MRAP2 hypothalamic neurons could be the target of peripheral ACTH in IS treatments, providing an alternative for the development of drugs.
Figure 5.
Scheme showing possible interactions between ACTH and MC4R/MRAP2 neurons. POMC is mainly expressed in the pituitary and arcuate nucleus of the hypothalamus. In the corticotroph cells of the pituitary, POMC is processed to ACTH, whereas in the arcuate it is cleaved to release α-MSH and β-endorphin. However, some chromatographic studies coupled to immunoreactive reactions support the presence of ACTH in the rat hypothalamus. Because MC2R is not expressed in the brain, the MC4R-MRAP2 neurons seem to mediate central ACTH effects, as demonstrated in rat and chicken by inhibitory actions on food intake of ICV administration [31, 32]. Alternatively, hypophyseal ACTH could reach central structures to activating hypothalamic MC4R–MRAP2 neurons, relaying stress information back to the brain. ACTH is one of the two treatments approved by the US Food and Drug Association for IS. Peripheral administration results in melanocortin-dependent transcriptomic changes in the arcuate nucleus, suggesting the central effects of peripheral ACTH. Therefore, we propose that MC4R-MRAP2 brain neurons are the target of peripheral ACTH in IS treatments, thus providing an alternative to develop therapeutic drugs.
Acknowledgments
Financial Support: This research was supported by grant AGL2016-74857-C3-3-R from the Spanish Research Agency (AEI) and from the European Fund of Regional Development (to J.M.C.-R.) and the DFG Cluster of Excellence “Unifying Concepts in Catalysis” (research fields D/E) (to G.K. and P.S.). L.S. was a recipient of a Formación de Personal Investigador predoctoral fellow from the Spanish AEI.
Disclosure Summary: The authors have nothing to declare.
Glossary
Abbreviations:
- ARC
arcuate nucleus
- DMEM
Dulbecco Modified Eagle Medium
- h
human
- ICV
intracerebroventricular
- IS
infantile spasm
- MCR
melanocortin receptor
- MRAP
melanocortin receptor accessory protein
- MSH
melanocyte-stimulating hormone
- POMC
proopiomelanocortin
- zf
zebrafish
References and Notes
- 1. Chan LF, Metherell LA, Clark AJ. Effects of melanocortins on adrenal gland physiology. Eur J Pharmacol. 2011;660(1):171–180. [DOI] [PubMed] [Google Scholar]
- 2. Cerdá-Reverter JM, Agulleiro MJ, R RG, Sánchez E, Ceinos R, Rotllant J. Fish melanocortin system. Eur J Pharmacol. 2011;660(1):53–60. [DOI] [PubMed] [Google Scholar]
- 3. Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27(7):736–749. [DOI] [PubMed] [Google Scholar]
- 4. Cerdá-Reverter JM, Ringholm A, Schiöth HB, Peter RE. Molecular cloning, pharmacological characterization, and brain mapping of the melanocortin 4 receptor in the goldfish: involvement in the control of food intake. Endocrinology. 2003;144(6):2336–2349. [DOI] [PubMed] [Google Scholar]
- 5. Zhang C, Forlano PM, Cone RD. AgRP and POMC neurons are hypophysiotropic and coordinately regulate multiple endocrine axes in a larval teleost. Cell Metab. 2012;15(2):256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88(1):131–141. [DOI] [PubMed] [Google Scholar]
- 7. Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278(5335):135–138. [DOI] [PubMed] [Google Scholar]
- 8. Lu D, Willard D, Patel IR, Kadwell S, Overton L, Kost T, Luther M, Chen W, Woychik RP, Wilkison WO, et al. . Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature. 1994;371(6500):799–802. [DOI] [PubMed] [Google Scholar]
- 9. Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385(6612):165–168. [DOI] [PubMed] [Google Scholar]
- 10. Metherell LA, Chapple JP, Cooray S, David A, Becker C, Rüschendorf F, Naville D, Begeot M, Khoo B, Nürnberg P, Huebner A, Cheetham ME, Clark AJ. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat Genet. 2005;37(2):166–170. [DOI] [PubMed] [Google Scholar]
- 11. Roy S, Rached M, Gallo-Payet N. Differential regulation of the human adrenocorticotropin receptor [melanocortin-2 receptor (MC2R)] by human MC2R accessory protein isoforms alpha and beta in isogenic human embryonic kidney 293 cells. Mol Endocrinol. 2007;21(7):1656–1669. [DOI] [PubMed] [Google Scholar]
- 12. Webb TR, Chan L, Cooray SN, Cheetham ME, Chapple JP, Clark AJ. Distinct melanocortin 2 receptor accessory protein domains are required for melanocortin 2 receptor interaction and promotion of receptor trafficking. Endocrinology. 2009;150(2):720–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fridmanis D, Petrovska R, Kalnina I, Slaidina M, Peculis R, Schiöth HB, Klovins J. Identification of domains responsible for specific membrane transport and ligand specificity of the ACTH receptor (MC2R). Mol Cell Endocrinol. 2010;321(2):175–183. [DOI] [PubMed] [Google Scholar]
- 14. Hinkle PM, Serasinghe MN, Jakabowski A, Sebag JA, Wilson KR, Haskell-Luevano C. Use of chimeric melanocortin-2 and -4 receptors to identify regions responsible for ligand specificity and dependence on melanocortin 2 receptor accessory protein. Eur J Pharmacol. 2011;660(1):94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sebag JA, Hinkle PM. Regions of melanocortin 2 (MC2) receptor accessory protein necessary for dual topology and MC2 receptor trafficking and signaling. J Biol Chem. 2009;284(1):610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cerdá-Reverter JM, Agulleiro MJ, Cortés R, Sánchez E, Guillot R, Leal E, Fernández-Durán B, Puchol S, Eley M. Involvement of melanocortin receptor accessory proteins (MRAPs) in the function of melanocortin receptors. Gen Comp Endocrinol. 2013;188:133–136. [DOI] [PubMed] [Google Scholar]
- 17. Chan LF, Webb TR, Chung TT, Meimaridou E, Cooray SN, Guasti L, Chapple JP, Egertová M, Elphick MR, Cheetham ME, Metherell LA, Clark AJ. MRAP and MRAP2 are bidirectional regulators of the melanocortin receptor family. Proc Natl Acad Sci USA. 2009;106(15):6146–6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Asai M, Ramachandrappa S, Joachim M, Shen Y, Zhang R, Nuthalapati N, Ramanathan V, Strochlic DE, Ferket P, Linhart K, Ho C, Novoselova TV, Garg S, Ridderstråle M, Marcus C, Hirschhorn JN, Keogh JM, O’Rahilly S, Chan LF, Clark AJ, Farooqi IS, Majzoub JA. Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science. 2013;341(6143):275–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Agulleiro MJ, Roy S, Sánchez E, Puchol S, Gallo-Payet N, Cerdá-Reverter JM. Role of melanocortin receptor accessory proteins in the function of zebrafish melanocortin receptor type 2. Mol Cell Endocrinol. 2010;320:145–152. [DOI] [PubMed] [Google Scholar]
- 20. Josep Agulleiro M, Cortés R, Fernández-Durán B, Navarro S, Guillot R, Meimaridou E, Clark AJ, Cerdá-Reverter JM. Melanocortin 4 receptor becomes an ACTH receptor by coexpression of melanocortin receptor accessory protein 2. Mol Endocrinol. 2013;27(11):1934–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang J, Li X, Zhou Y, Cui L, Li J, Wu C, Wan Y, Li J, Wang Y. The interaction of MC3R and MC4R with MRAP2, ACTH, α-MSH and AgRP in chickens. J Endocrinol. 2017;234(2):155–174. [DOI] [PubMed] [Google Scholar]
- 22. Sánchez E, Rubio VC, Thompson D, Metz J, Flik G, Millhauser GL, Cerdá-Reverter JM. Phosphodiesterase inhibitor-dependent inverse agonism of agouti-related protein on melanocortin 4 receptor in sea bass (Dicentrarchus labrax). Am J Physiol Regul Integr Comp Physiol. 2009;296(5):R1293–R1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soletto L, Hernández Balfagó S, Rocha A, Kleinau G, Scheerer P, Cerda-Reverter JM. Data from: Melanocortin receptor accessory protein 2-induced adrenocorticotropic hormone response of human melanocortin 4 receptor. Mendeley Data 2018. Deposited 21 November 2018. 10.17632/4wpb5rjn76.1. [DOI] [PMC free article] [PubMed]
- 24. Bangol D, Lu X-Y, Kaelin CB, Day HEW, Ollmann M, Gantz I, Akil H, Barsh GS, Watson SJ. Anatomy of an endogenous antagonist: Relationship between Agouti-related protein and proopiomelanocortin in the brain J Neurosci 1999;19(18):RC26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Castro MG, Morrison E. Post-translational processing of proopiomelanocortin in the pituitary and in the brain. Crit Rev Neurobiol. 1997;11(1):35–57. [DOI] [PubMed] [Google Scholar]
- 26. Smith AI, Funder JW. Proopiomelanocortin processing in the pituitary, central nervous system, and peripheral tissues. Endocr Rev. 1988;9(1):159–179. [DOI] [PubMed] [Google Scholar]
- 27. Hentges ST, Otero-Corchon V, Pennock RL, King CM, Low MJ. Proopiomelanocortin expression in both GABA and glutamate neurons. J Neurosci. 2009;29(43):13684–13690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iacobaş DA, Iacobas S, Chachua T, Goletiani C, Sidyelyeva G, Velíšková J, Velíšek L. Prenatal corticosteroids modify glutamatergic and GABAergic synapse genomic fabric: insights from a novel animal model of infantile spasms. J Neuroendocrinol. 2013;25(11):964–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brunson KL, Eghbal-Ahmadi M, Baram TZ. How do the many etiologies of West syndrome lead to excitability and seizures? The corticotropin releasing hormone excess hypothesis. Brain Dev. 2001;23(7):533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brunson KL, Avishai-Eliner S, Baram TZ. ACTH treatment of infantile spasms: mechanisms of its effects in modulation of neuronal excitability. Int Rev Neurobiol. 2002;49:185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iacobaş DA, Chachua T, Iacobaş S, Benson MJ, Borges K, Velíšková J, Velíšek L. ACTH and PMX53 recover synaptic transcriptome alterations in a rat model of infantile spasms. Sci Rep. 2018;8(1):5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shipp SL, Yi J, Dridi S, Gilbert ER, Cline MA. The central anorexigenic mechanism of adrenocorticotropic hormone involves the caudal hypothalamus in chicks. Neuropeptides. 2015;53:29–35. [DOI] [PubMed] [Google Scholar]
- 33. Vergoni AV, Poggioli R, Bertolini A. Corticotropin inhibits food intake in rats. Neuropeptides. 1986;7(2):153–158. [DOI] [PubMed] [Google Scholar]
- 34. Liang J, Li L, Jin X, Xu B, Pi L, Liu S, Zhu W, Zhang C, Luan B, Gong L, Zhang C. Pharmacological effect of human melanocortin-2 receptor accessory protein 2 variants on hypothalamic melanocortin receptors. Endocrine. 2018;61(1):94–104. [DOI] [PubMed] [Google Scholar]
- 35. Rouault AAJ, Lee AA, Sebag JA. Regions of MRAP2 required for the inhibition of orexin and prokineticin receptor signaling. Biochim Biophys Acta Mol Cell Res. 2017;1864(12):2322–2329. [DOI] [PubMed] [Google Scholar]
- 36. Srisai D, Yin TC, Lee AA, Rouault AAJ, Pearson NA, Grobe JL, Sebag JA. MRAP2 regulates ghrelin receptor signaling and hunger sensing. Nat Commun. 2017;8(1):713. [DOI] [PMC free article] [PubMed] [Google Scholar]