Abstract
Males are known to have profound adipose tissue macrophage (ATM) accumulation in gonadal white adipose tissue (GWAT) during obesity, whereas females are protected from such an inflammatory response even with increased adiposity. The inflammatory tone in males is linked to insulin resistance and might be the underlying cause for sex differences in metabolic disease. Factors regulating the meta-inflammatory response remain unclear but enhanced lipid storage in females may explain the reduced inflammatory response to high-fat diets. In this study, we evaluated lean and obese females with stimulated lipolysis to understand whether a stress release of free fatty acids (FFAs) could induce female ATMs. We demonstrate that in both lean and obese females, GWAT CD11c− resident ATMs accumulate with β-3 adrenergic receptor–stimulated lipolysis. Lipolysis elevated serum FFA, triglyceride, and IL-6 levels in females that corresponded to significant phosphorylated hormone-sensitive lipase and adipose triglyceride lipase protein expression in obese female GWAT compared with males. Increased lipolytic response in obese females was associated with crown-like structures and induced Il6, Mcp1, Arg1, and Mgl1 expression in obese female GWAT, suggesting an environment of lipid clearance and adipose remodeling. With this finding we next investigated whether lipid storage and lipolytic mediators differed by sex. Diacylglycerol, ceramides, phospholipids, and certain fatty acid species associated with inflammation were elevated in male GWAT compared with obese female GWAT. Overall, our data demonstrate a role for GWAT lipid storage and lipolytic metabolites to induce inflammation in males and induce remodeling in females that might explain sex differences in overall metabolic health.
Obesity-induced chronic inflammation is associated with a broad range of metabolic consequences such as type 2 diabetes, cardiovascular diseases, hypertension, and atherosclerosis (1–4). A major source for the chronic inflammation is an enhanced inflammatory state in visceral adipose tissue (VAT). However, such an inflammatory response is primarily seen in males only with high-fat diet (HFD) exposure. Our prior research has demonstrated that saturated fatty acids (FAs) are a direct activator of myeloid inflammation in obese males whereas females remain protected (5, 6). Young males fed an HFD have an increase in monocyte production and recruitment, leading to the accumulation of proinflammatory CD11c+ adipose tissue macrophages (ATMs) (5–7). Females, however, are protected from HFD-induced reprogramming of myeloid progenitors, proinflammatory monocytes, and tissue ATMs with minimal change in insulin resistance, even with gains in adiposity and weight (5, 6, 8).
The primary cause for an inflammatory response in males is thought to be rapid expansion of adipose tissue and a release of lipid mediators prompting a macrophage response. Although studies show that a sex dimorphism exists in lipid storage with more fat accumulation in VAT in men and more storage in subcutaneous adipose tissue in women (9, 10), it is not yet clear whether there is a sex dimorphic response in inflammation to an induced lipolysis. In males with HFD exposure, adipose tissue expands and stores the excess lipids as triglycerides (TGs). TG hydrolysis through β-adrenergic receptor (ADRB) stimulation generates free FAs (FFAs) and glycerol in times of elevated energy demands. The lipolytic cascade includes adipose TG lipase (ATGL) that converts TG to diacylglycerol (DG) (11). DG is hydrolyzed to monoacylglycerol (MG) by hormone-sensitive lipase (HSL) (12), and monoglyceride lipase cleaves MG into glycerol and FFAs (13). The FFAs released in circulation are shuttled to peripheral organs for β-oxidation or re-esterified back into TG for storage. These FFAs are either synthesized directly within the adipocyte via de novo lipogenesis or obtained through lipoprotein lipase–mediated hydrolysis of TG-enriched particles, such as chylomicrons or hepatic-derived lipoproteins (14–16). The activation of lipolytic enzymes is classically regulated by adrenergic stimulation via catecholamines and the antilipolytic action of insulin (17). Other key regulators include lipid droplet–associated proteins such as peripilin 1 and α/β-hydrolase domain containing protein 5, cytokines such as IL-1β, IL-6, IL-15, and TNFα, adipokines, and atrial natriuretic peptides (17).
In obesity, the subtle balance between lipid storage and utilization is critical to prevent lipotoxicity from leading to reduced mitochondrial function, increased endoplasmic reticulum stress, systemic insulin resistance, and increased basal lipolysis (18–20). Secondary adipocyte hypertrophy and death also lead to ATM activation and recruitment of a CD11c+ population of ATMs that is directly associated with the metabolic syndrome (3, 4, 7, 21, 22). ATMs along with hypertrophied adipocytes produce high levels of proinflammatory cytokines, including TNFα, IL-6, PAI-1, and chemokines such as MCP1 that exaggerate the inflammatory response (7). This recruitment of CD11c+ ATMs is low in females, and the metabolic factors that regulate an immune response to obesity and the accumulation of macrophages and other immune cells in adipose tissue remain poorly defined, especially in females.
Prior studies have shown that acute activation of ADRB3 induces lipolysis and triggers expression of proinflammatory genes, including MCP1, IL-6, and PAI-1, among others, suggesting a link between lipolysis and inflammation (23–25). Owing to sex differences in adipocyte hypertrophy, it is possible that obese males have enhanced basal lipolysis, leading to activation of chemokines and cytokines that leads to inflammation, whereas females are protected from inflammation due to low levels of lipolysis in obesity. However, these outcomes cannot be extrapolated in females because these studies have been done in male models only. Given that there is improved lipid storage in females, it is critical to evaluate whether induced lipolysis triggers the same type of proinflammatory response. We hypothesized that if lipolysis was induced, an inflammatory response could be elicited, even in female mice. To determine sex-dependent responses in lipolysis-induced inflammation, we stimulated FFA release in a model of HFD-induced obesity in mice and investigated the effects on adipocyte lipid metabolism and inflammation compared with their lean counterparts.
Our data show that adipocyte lipolysis upregulates CD11c− ATM accumulation in lean and obese female gonadal white adipose tissue (GWAT) and is driven by sex-specific lipolytic mediators. To further understand sex differences in GWAT lipid composition and generation of lipolytic mediators that might drive inflammation differences between sexes, we also performed an untargeted metabolomics analysis of lipid metabolites in GWAT. Lipidomics assays of obese GWAT from male and female mice led to identification of a high incidence of proinflammatory mediators in obese males such as DG, ceramides (Cers), and FAs such as arachidic acid and arachidonic acid. Conversely, females presented an attenuated inflammatory lipid profile with accumulation of linoleic acid and oleic acid after ADRB3 stimulation. Thus, lipid metabolic pathways are a likely contributor to sex differences in metabolic disease and represent potential therapeutic targets to prevent or delay the onset and progression of obesity-induced inflammation and secondary metabolic disease in male obesity.
Methods
Animals and CL-316,243 treatment
Mice used in the experiments were male and female C57BL/6J purchased from The Jackson Laboratory. Mice were fed ad libitum either a control normal diet (ND) consisting of 13% fat (5001; Laboratory Diet, St. Louis, MO) or an HFD of 60% of calories from fat (D12492; Research Diets, New Brunswick, NJ) starting at 6 weeks of age for 16 weeks in duration. Pubertal mice were used to capture the effects of the changes in sex hormones on metabolic development with an HFD. Animals were housed in a specific pathogen-free facility with a 12-hour light/12-hour dark cycle and given free access to food and water. All animal use was in compliance with the Institute of Laboratory Animal Research Guide for the Care and Use of Laboratory Animals and approved by the University Committee on Use and Care of Animals at the University of Michigan (animal welfare assurance no. A3114-01). For stimulation of lipolysis, mice were injected IP with 1 mg/kg CL-316,243 (CL; Tocris Bioscience, Minneapolis, MN), an ADRB3 selective agonist, or saline twice (4 hours between each injection). Mice were euthanized 14 hours after the second injection. GWAT and inguinal white adipose tissue (IWAT) were excised and weighed for each animal. Tissue samples were frozen in liquid nitrogen and stored at −80°C prior to RNA extraction. Adipose tissues were collected and immediately processed for stromal vascular fraction (SVF) preparation and flow cytometry staining. For chronic CL experiments, 1 mg/kg/d CL was injected IP for 3 days in 12-week mice with 6 weeks of HFD starting at 6 weeks of age.
Immunohistochemistry and immunofluorescence staining
Antibodies used for immunofluorescence included polyclonal anti-caveolin (26) and anti-Mac2 (27). For histology, tissues were formalin fixed, paraffin embedded, sectioned at 5 μm and stained with hematoxylin and eosin (H&E) or immunostained with antibodies directed against Mac-2 and Ki67 (28). H&E staining was performed by the University of Michigan’s Comprehensive Cancer Center Histology Core. Quantification of crown-like structures (CLSs) was performed with ImageJ software (National Institutes of Health, Bethesda, MD).
Western blotting analysis
Cell extracts were prepared in RIPA buffer [10 mM Tris-HCl (pH 7.4), 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS] containing protease and phosphatase inhibitors (Roche, Indianapolis, IN). After centrifugation at 1000g for 5 minutes, the supernatant was collected and protein was measured using a Bio-Rad Protein Assay Dye reagent kit with BSA as the standard. Thirty micrograms of protein extracts was dissolved in 2× Laemmli sample buffer, heated at 95°C for 5 minutes, and resolved on a 4% to 20% SDS-PAGE gel. After electrophoresis, gels were transferred to nitrocellulose membranes. Subsequently, membranes were blocked in 5% BSA in TBST buffer [20 mM Tris-HCl (pH 7.4), 500 mM NaCl, 0.05% Tween 20]. Membranes were washed and incubated with the indicated primary antibodies (1:1000 dilution) on a rotary shaker at 4°C overnight. The blots were then incubated with peroxidase-conjugated goat anti-rabbit secondary antibody for 1 hour at room temperature and developed with enhanced chemiluminescent reagent (Thermo Fisher Scientific). The following antibodies were used: phosphorylated HSL (p-HSL) (29), HSL (30), ATGL (31), and glyceraldehyde 3-phosphate dehydrogenase (32).
Real-time PCR
RNA was extracted from adipose tissue using TRIzol LS (Life Technologies), and cDNA was generated using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). SYBR Green PCR Master Mix (Applied Biosystems) and the StepOnePlus system (Applied Biosystems) were used for real-time quantitative PCR. Gapdh expression was used as an internal control for data normalization. Samples were assayed in duplicate, and relative expression was determined using the 2−ΔΔCT method. All primers used are listed in an online repository (33).
Serum insulin, FA, glycerol, and TG estimation
Serum insulin levels were determined with an ultrasensitive insulin ELISA kit (Crystal Chem, Elk Grove Village, IL). For in vivo lipolysis measurement, serum FFA was measured using the HR NEFA series (Wako Diagnostics, Richmond, VA). Serum free glycerol was measured using free glycerol determination reagent (Sigma-Aldrich, St. Louis, MO). Serum TG was measured with an Infinity TG determination kit (Thermo Fisher Scientific, Waltham, MA) following the manufacturer’s instructions. Liver TG was assayed after extraction.
Explant lipolysis
For ex vivo lipolysis, gonadal, inguinal, and brown fat pads isolated from 16-week HFD-fed and chow-fed control mice were cut into ∼50-mg pieces and incubated at 37°C in 200 μL of Krebs-Ringer buffer with 1% FA-free BSA with or without 10 nM CL for 2 hours. FFA (Wako Diagnostics) and glycerol release (Sigma-Aldrich) was measured in aliquots from incubation media, and tissue weight was used to normalize the lipolytic signals.
SVF isolation and flow cytometry
Adipose tissue fractionation and flow cytometry analysis were performed as described previously (34). SVF cells were stained with CD64-PE (35), CD45.2-eFluor 450 (36), and CD11c-allophycocyanin-Cy7 (37), and gating was performed for macrophage populations and by CD45 gates to determine ATMs (38, 39).
Liquid chromatography with tandem mass spectrometry
Reagents and internal standards
HPLC-grade acetonitrile and dichloromethane were purchased from Sigma-Aldrich, isopropanol [Optima; liquid chromatography with mass spectrometry (LC-MS) grade] was purchased from Thermo Fisher Scientific, and methanol (LC-MS grade) was from J.T. Baker. Water was obtained from a high-purity water dispenser (EMD Millipore, Billerica, MA). The following MS–grade lipid standards were obtained from Sigma-Aldrich: 1-heptadecanoyl-2-hydroxy-sn-glycero-3-phosphocholine (17:0/0:0), 1,2-diheptadecanoyl-sn-glycero-3-phosphocholine (17:0/17:0), 1,2-diheptadecanoyl-sn-glycero-3-phosphoethanolamine (17:0/17:0), 1,2-diheptadecanoyl-sn-glycero-3-phospho-l-serine (sodium salt) (17:0/17:0), N-heptadecanoyl-d-erythro-sphingosylphosphorylcholine 17:0 (d18:1/17:0), cholest-5-en-3β-yl heptadecanoate 17:0 cholesteryl ester, 1-palmitoyl-2-oleoyl-sn-glycerol 16:0-18:1, 1-heptadecanoyl-rac-glycerol 17:0, 1,2,3-triheptadecanoyl-glycerol triheptadecanoate 17:0, N-heptadecanoyl-d-erythro-sphingosine C17 (d18:1/17:0), 1,2-diheptadecanoyl-sn-glycero-3-phosphate (sodium salt) 17:0, 1,2-diheptadecanoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (sodium salt) 17:0, 1-heptadecanoyl-2-(5Z,8Z,11Z,14Z-eicosatetraenoyl)-sn-glycero-3-phospho-(1′-myo-inositol) (ammonium salt) 17:0-20:4, 1,3(d5)-dinonadecanoyl-2-hydroxy-glycerol d5-(19:0/0:0/19:0), and glyceryl tri(palmitate-d31) d31.
Sample preparation
The tissues were accurately weighed and then homogenized. Lipids were extracted using a modified Bligh-Dyer method (40) using a 2:2:2 ratio volume of methanol/water/dichloromethane at room temperature after spiking internal standards (described above) The organic layer was collected and completely dried under nitrogen. Before MS analysis, the dried lipid extract was reconstituted in 100 μL of buffer B (10:85:5 acetonitrile/isopropyl alcohol/water) containing 10 mM ammonium acetate and subjected to LC-MS.
Internal standards and quality controls
Quality control samples were prepared by pooling equal volumes of each sample and injected at the beginning and the end of each analysis and after every 10 sample injections to provide a measurement of the system’s stability and performance as well as reproducibility of the sample preparation method (41).
Two kinds of controls were used to monitor the sample preparation and MS. To monitor instrument performance, 10 μL of a dried matrix-free mixture of the internal standards reconstituted in 100 μL of buffer B (85% isopropyl alcohol/10% acetonitrile/5% water in 10 mM NH4OAc) was analyzed. As additional controls to monitor the profiling process, an equimolar mixture of 13 authentic internal standards and a characterized pool of human plasma and test pool (a small aliquot from the all-diet plasma used in this study; extracted in tandem with diet plasma) were analyzed along with the diet plasma samples. Each of these controls was included several times into the randomization scheme such that sample preparation and analytical variability could be monitored constantly.
Data-dependent LC-MS/MS for measurements of lipids
Chromatographic separation was performed on a Shimadzu CTO-20A Nexera X2 UHPLC system equipped with a degasser, binary pump, thermostat autosampler, and column oven [all components manufactured by Shimadzu (Canby, OR)]. The column heater temperature was maintained at 55°C and an injection volume of 5 μL was used for all analyses. For lipid separation, the lipid extract was injected onto a 1.8-μm particle diameter, 50-mm × 2.1-mm inner diameter Waters Acquity HSS T3 column (Waters, Milford, MA). Elution was performed using acetonitrile/water (40:60, v/v) with 10 mM ammonium acetate as solvent A and acetonitrile/water/isopropanol alcohol (10:5:85, v/v/v) with 10 mM ammonium acetate as solvent B. For chromatographic elution we used a linear gradient during a 20-minute total run time, with a 60% solvent A and 40% solvent B gradient in the first 10 minutes. Then the gradient was ramped in a linear fashion to 100% solvent B, which was maintained for 7 minutes. Thereafter, the system was switched back to 60% solvent B and 40% solvent A for 3 minutes. The flow rate used for these experiments was 0.4 mL/min and the injection volume was 5 μL. The column was equilibrated for 3 minutes before the next injection and run at a flow rate of 0.400 μL/min for a total run time of 20 minutes.
MS data acquisition for each sample was performed in both positive and negative ionization modes using a TripleTOF 5600 equipped with a DuoSpray ion source (AB Sciex, Concord, ON, Canada). Column effluent was directed to the electrospray ionization source and voltage was set to 5500 V for positive ionization and 4500 V for the negative ionization mode. The declustering potential was 60 V and the source temperature was 450°C for both modes. The curtain gas flow, nebulizer, and heater gas were set to 30, 40, and 45, respectively (arbitrary units). The instrument was set to perform one time of flight MS survey scan (150 ms) and 15 MS/MS scans with a total duty cycle time of 2.4 seconds. The mass range of both modes was 50 to 1200 m/z. Acquisition of MS/MS spectra was controlled by the data-dependent acquisition function of the Analyst TF software (AB Sciex) with application of the following parameters: dynamic background subtraction, charge monitoring to exclude multiply charged ions and isotopes, and dynamic exclusion of former target ions for 9 seconds. Collision energy spread of 20 V was set whereby the software calculated the collision energy value to be applied as a function of m/z.
A DuoSpray source coupled with automated calibration system (AB Sciex) was used to maintain mass accuracy during data acquisition. Calibrations were performed at the initiation of each new batch or polarity change.
Data processing
The raw data were converted to mgf data format using ProteoWizard software (42). The NIST MS PepSearch program was used to search the converted files against LipidBlast (43, 44) libraries in batch mode. We optimized the search parameters using the NIST11 library and LipidBlast libraries and comparing them against our lipid standards. The m/z width was determined by the mass accuracy of internal standards and was set at 0.001 for the positive mode and 0.005 for the negative mode. The minimum match factor used in the PepSearch Program was set to 200. The MS/MS identification results from all of the files were combined using an in-house software tool to create a library for quantification. All raw data files were searched against this library of identified lipids with mass and retention time using MultiQuant 1.1.0.26 (AB Sciex) (45). Quantification was done using MS1 data. The quality control samples were also used to remove technical outliers and lipid species that were detected below the lipid class–based lower limit of quantification. Quality control samples evenly distributed along analytical runs of the study were analyzed. The average coefficient of variation of all the lipids detected in the study samples was 25%.
Statistical analyses
All values are reported as the mean ± SEM unless otherwise stated. One-way or two-way analysis of variance was performed with factors of sex and diet. When there was a main effect for either factor, then statistical significance of differences between controls and other diet groups as well as treatments were determined using the unpaired two-tailed Student t test.
Results
Obese females have an increased induced lipolytic response compared with obese males
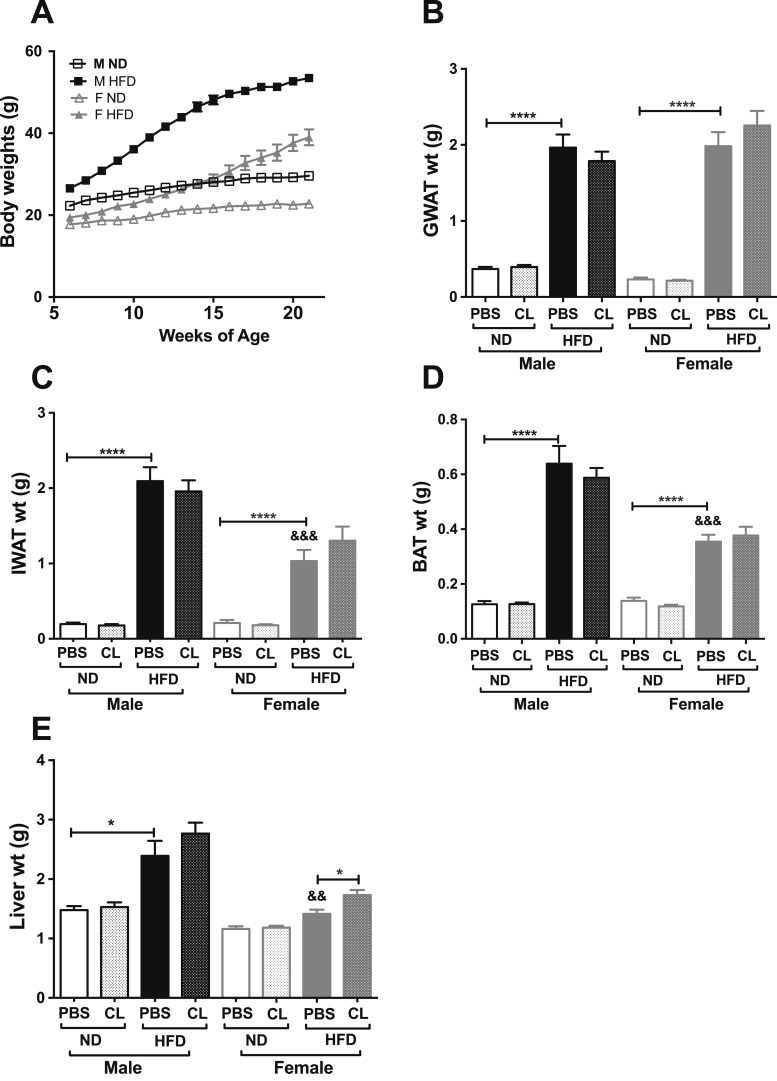
Earlier studies with ADRB3 knockout and transgenic mice demonstrated that ADRB3 is the exclusive target for the agonist CL (46, 47). Acute activation of ADRB3 induces an efflux of excessive FFA and has been used as a tractable model to investigate induced adipose tissue inflammation in male mice (23–25, 46). To determine whether there are sex differences in stimulated lipolysis, we used the ADRB3 selective agonist CL in male and female C57BL6/J mice placed on an HFD at 6 weeks of age for 16 weeks (60% HFD) to induce obesity. We then examined body adiposity and adipose tissue weights. As anticipated, male mice on an HFD gained more weight compared with females (Fig. 1A), and short-term CL treatment showed no effect on body weights in obese males and females. Both obese male and female mice had increased visceral GWAT, but adiposity as a percentage was higher in females (Fig. 1B) (33). However, fat accumulation in IWAT, brown adipose tissue (BAT), and livers was significantly lower in the obese females (Fig. 1C–1E) (33). Chronic CL treatment in lean male mice has been shown to reduce only visceral adipose tissue weights (48). In our short-term lipolysis model of 18 hours, ADRB3 stimulation did not alter adipose tissue weights (Fig. 1B–1D) (33) but significantly increased liver weights in obese females, suggesting lipid accumulation in the liver (Fig. 1E) (33) after lipolysis is induced.
Figure 1.
Sex differences in total body adiposity and tissue weights in response to HFD feeding and CL treatment. (A) Weekly body weights of C57BL6/J male and female on ND or 60% HFD starting at 6 wk of age up to 16 wk. (B) GWAT weight. (C) IWAT weight. (D) BAT weight. (E) Liver weight. n = 7 to 12 per group; error bars are SEM. *P < 0.05; ****P < 0.0001. Comparisons of male HFD PBS vs female HFD PBS are shown as &&P < 0.01 and &&&P < 0.005. F, female; M, male.
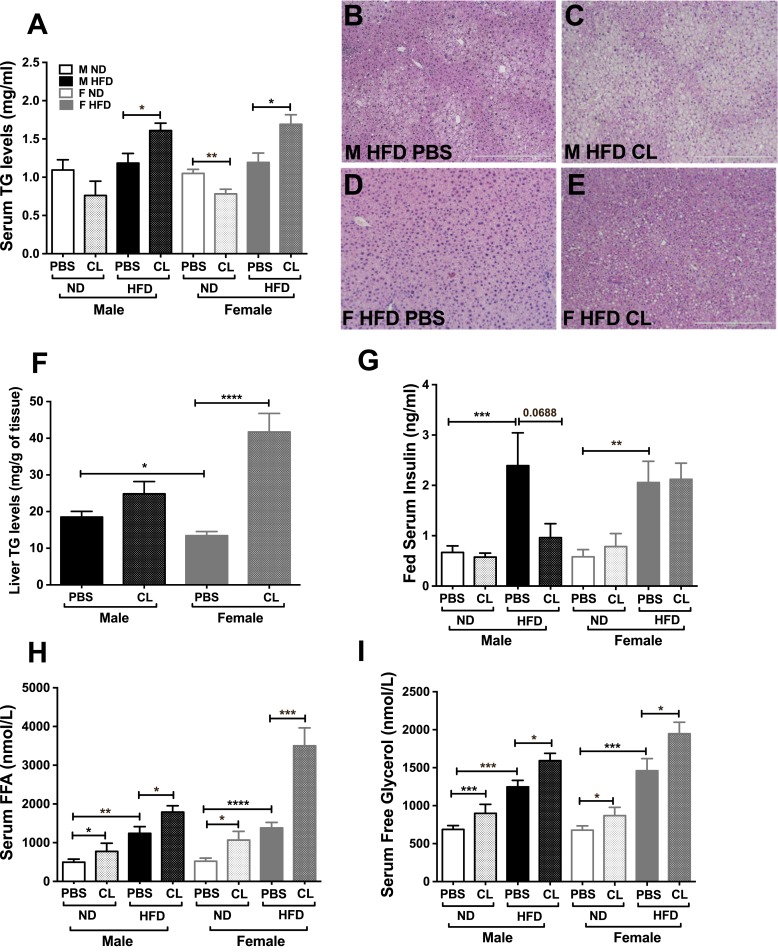
To next understand the response on lipid trafficking, we evaluated serum and liver lipids. Serum TGs significantly increased in both sexes upon lipolytic stimulation, but only in HFD mice (Fig. 2A). Concurrently, there was lipid accumulation in both male and female livers in the stimulated condition as seen in H&E-stained sections compared with controls (Fig. 2B–2E). Although obese male livers showed mostly microsteatosis (Fig. 2C), the female livers depicted both microsteatosis and macrosteatosis in response to CL (Fig. 2E). ADRB3 stimulation increased liver TG content in the livers of both sexes. However, obese female livers showed greater TG accumulation compared with males after CL by almost twofold (Fig. 2F). Serum insulin levels were significantly higher in both obese males and females (Fig. 2G), but CL treatment only suppressed obese male insulin levels (Fig. 2G). Serum FFA and free glycerol levels were assessed next to determine overall lipolytic response. ADRB3 stimulation increased the serum FFA (Fig. 2H) and glycerol levels (Fig. 2I) in lean mice, but during obesity basal lipolysis in both sexes was already increased with nonesterified FFA and glycerol in serum. However, upon lipolytic stimulation, obese female mice responded with a drastic increase in serum FFA and glycerol levels compared with males (Fig. 2H and 2I).
Figure 2.
Sex differences in ADRB3 induced lipolysis. (A) Serum TG levels in male and female obese mice after PBS and 18 h of CL injection. (B–E) H&E images of 16-wk HFD livers from male and female mice with and without CL treatment. Scale bars, 500 μm. (F) Liver TG, (G) fed serum insulin, (H) serum FFA, and (I) serum free glycerol released in male and female obese mice after CL treatment. n = 7 to 12 per group; error bars are SEM. *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.0001. F, female; M, male.
Overall, this demonstrates that short-term ADBR3 agonist stimulation induces lipolysis in both sexes in lean and obese states, but during obesity females have an enhanced response with liver TGs and serum FFAs and glycerol.
Sexual dimorphism in adipose depot-specific lipolytic responses
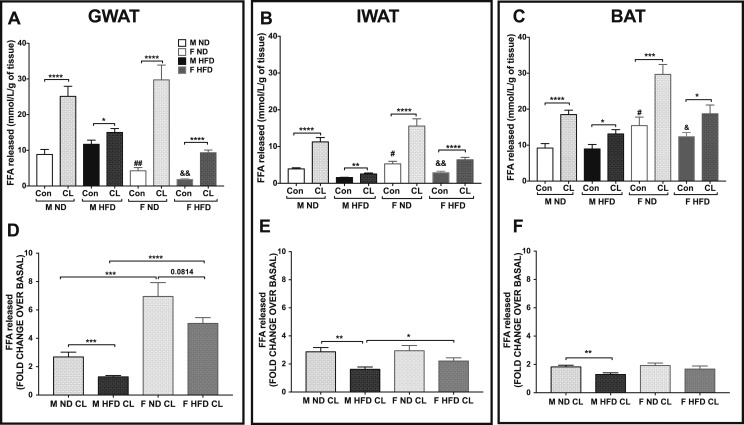
Given that the systemic FFAs, glycerol, and liver TGs may be enhanced through release of FFAs from many fat depots, we next isolated representative fat depots and evaluated their individual contribution in lipolytic response. To determine sex differences in fat depot–specific lipolytic responses, we assessed tissue lipolytic capacity with GWAT, IWAT, and BAT explants. After 2 hours of stimulation with CL, FFA release and free glycerol release were assessed. Raw values of FFA from GWAT, IWAT, and BAT are depicted in Fig. 3A, 3B, and 3C, respectively. Basal FFA released was higher in lean and obese male GWAT compared with female GWAT (Fig. 3A). Conversely, in the IWAT and BAT, basal FFA levels were higher in lean and obese female explants, although similarly induced with CL (Fig. 3B and 3C). To better compare the differences, we calculated the fold changes for ADRB3-stimulated lipolysis over basal FFA levels in each condition. Obesity decreased the lipolytic response in all of the male fat depots (Fig. 3D–3F). In the lean and obese state, female GWAT had the highest lipolytic response from baseline upon ADRB3 stimulation compared with obese male GWAT (Fig. 3D). Glycerol responses matched FFA responses in all depots (33).
Figure 3.
Comparison of ADRB3-stimulated lipolysis in lean and obese male and female WAT and BAT depot explants. (A–C) FFA determination in lean and obese (A) GWAT, (B) IWAT, and (C) BAT explants. (D–F) FFA release calculated as fold change over basal conditions in lean and obese (D) GWAT, (E) IWAT, and (F) BAT explant tissues. n = 8 per group; error bars are SEM. *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.0001. Comparisons of male ND PBS vs female ND PBS are shown as #P < 0.05 and ##P < 0.01, and male HFD PBS vs female HFD PBS are shown as &P < 0.05 and &&P < 0.01. Con, control; F, female; M, male.
Overall, we observed that the GWAT and IWAT from lean and obese female mice are more responsive to ADRB3 stimulation. However, note that the highest lipolytic response with stimulated lipolysis was from female GWAT mice in both nutritional states. Our results suggest overall impairments in the lipolytic pathway in the male obese fat depots and exaggerated responses in females.
ATGL and HSL lipolytic protein levels contribute to enhanced female lipolytic responses in obesity
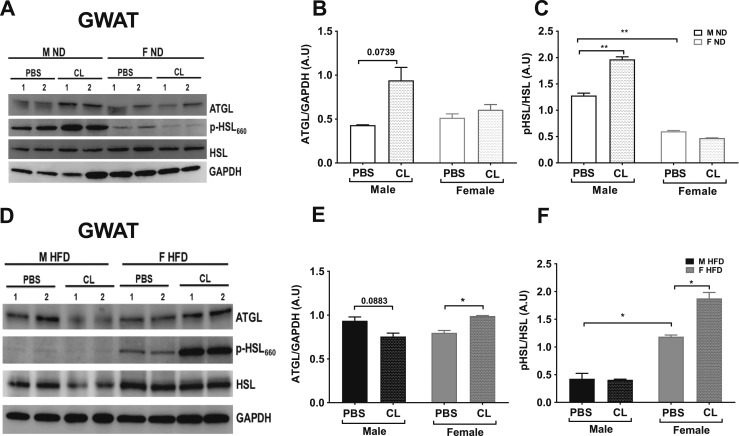
The varied lipolytic response to ADRB3 stimulation in obese females compared with obese males suggests differential levels of lipolytic proteins in male and female fat depots. The current literature suggests that in lean rodents ATGL and HSL are the major lipases for TG and DG, respectively, and account for 95% of lipase activity in murine male WAT (49). Therefore, these molecular steps that regulate lipolysis can be potentially affected by obesity and also be distinctly regulated in both sexes. Because our data indicated higher lipolytic levels in GWAT than in IWAT, we performed immunoblotting of key lipolytic proteins, ATGL and HSL, in lean and obese GWAT (Fig. 4A and 4D). ATGL content was lower in lean GWAT compared with obese GWAT (Fig. 4A, 4B, 4D, and 4E). ATGL protein content was increased with ADRB3 stimulation in lean male GWAT, but no changes were observed in lean female GWAT upon stimulation (Fig. 4A and 4B). Upon ADRB3 stimulation, ATGL protein content was notably increased in the lean male GWAT (Fig. 4A and 4B), but conversely in the HFD mice, it was increased in the obese female GWAT (Fig. 4D and 4E). p-HSL (Ser660) content was significantly suppressed in the GWAT of lean female mice in basal and stimulated conditions, despite no change in total HSL protein content in both sexes (Fig. 4A and 4C). Upon ADRB3 stimulation, p-HSL was upregulated in lean males (Fig. 4A and 4C). However, in the obese state and upon ADRB3 stimulation, p-HSL was upregulated in obese females significantly compared with obese male GWAT (Fig. 4D and 4F). Overall, these results imply that in a lean state, lipolysis is not favored in females but in the males. However, in the obese state with excess storage of fat, the lipolytic response is higher than in males.
Figure 4.
Lipolytic protein expression in GWAT. (A) Western blot of lean male and female GWAT with and without CL treatment. (B) ATGL protein, (C) p-HSL/HSL protein levels assessed after ADRB3 stimulation, (D) Western blot of 16-week HFD male and female GWAT with and without CL treatment, (E) ATGL protein, (F) p-HSL/HSL protein levels assessed after ADRB3 stimulation. Representative blots with summarized data are from n = 5 per group. *P < 0.05; **P < 0.01. F, female; M, male.
ADRB3 stimulation impairs lipid metabolism genes and activates inflammatory genes in both males and females
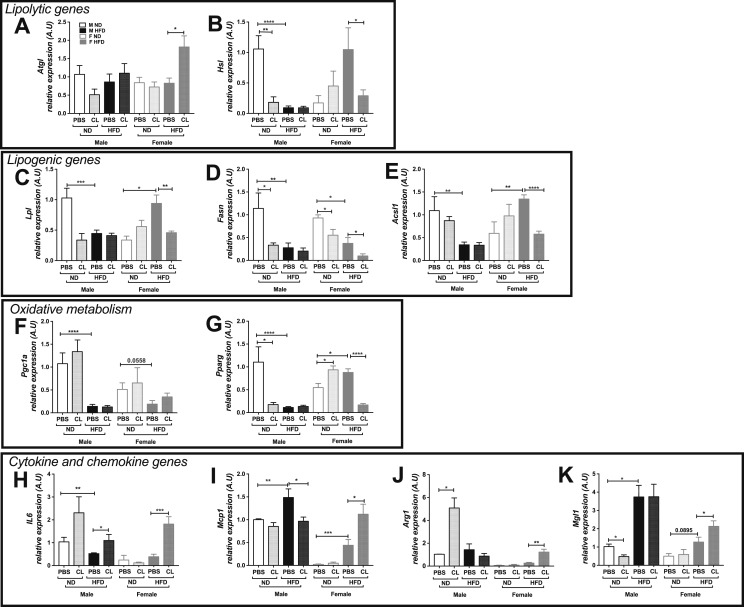
Although basal and stimulated protein levels of lipolytic enzymes were altered with sex and nutritional status, we wanted to evaluate whether the expression of genes involved in the regulation of lipolysis and lipid metabolism was altered. ADRB3 stimulation did not alter lipolytic gene expression levels of Atgl in male GWAT; however, there was a significant increase in Atgl expression with ADRB3 stimulation in the obese female GWAT (Fig. 5A). In contrast, Hsl expression was downregulated significantly with obesity and ADRB3 stimulation in male GWAT (Fig. 5B). Hsl mRNA levels increased significantly with obesity in females, similar to the changes in protein levels (Fig. 5B), but was repressed during obesity with ADRB3 stimulation. Genes involved in glucose uptake (Glut4) and insulin signaling (Akt) gene expression levels remained unchanged in both sexes in lean and obese conditions (33). Lipogenic gene expression levels of Lpl, Fasn, and Acsl were significantly repressed with an HFD in obese male GWAT (Fig. 5C–5E). Interestingly, in the female GWAT, Acsl1 and Lpl mRNA levels increased significantly with an HFD and were downregulated only after ADRB3 stimulation in obesity (Fig. 5D and 5E). Also, in the female GWAT Fasn expression levels decreased with obesity and also with ADRB3 stimulation in the lean as well as obese conditions (Fig. 5D). In the male GWAT, the mRNA levels of Pgc1a and Pparg involved in oxidative metabolism were downregulated with obesity (Fig. 5F and 5G). In female GWAT, obesity led to decreased Pgc1a expression, which was not affected by ADRB3 stimulation (Fig. 5F). Alternatively, Pparg expression in female GWAT significantly increased upon ADRB3 stimulation in the lean state and was suppressed with an HFD (Fig. 5G). It is likely that the excess FFA levels upon ADRB3 stimulation repressed the Pparg mRNA expression.
Figure 5.
Lipolytic, lipogenesis, oxidative metabolism, and inflammatory gene expression in GWAT. Lipolytic genes, (A) Atgl and (B) Hsl expression; lipogenic genes, (C) Lpl, (D) Fasn, and (E) Acsl1 expression; oxidative metabolic genes, (F) Pgc1a and (G) Pparg expression; cytokine and chemokine genes, (H) Il6, (I) Mcp1, (J) Arg1, and (K) Mgl1 expression in lean and obese male and female GWAT with and without ADRB3 stimulation. n = 5 to 8. *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.0001. A.U., arbitrary units normalized to Gapdh; F, female; M, male.
Obesity is well known to trigger inflammatory cytokine production, specifically in male GWAT (6, 7). Acute activation of ADRB3 in lean animals has been shown to trigger expression of proinflammatory genes such as Il6, Mcp1, and Pai1 (24). To determine the effects of short-term ADRB3 stimulation on inflammatory cytokines in obese mice, we performed gene expression for Il6 and Mcp1. In males, Il6 was increased with ADRB3 stimulation in lean and obese GWAT (Fig. 5H). However, in females, upregulated Il6 expression was observed with ADRB3 stimulation in the obese GWAT only (Fig. 5H), suggesting an effect of excess FFA efflux. An HFD significantly increased Mcp1 expression in male and female GWAT but comparatively the expression was lower in females (Fig. 5I), suggesting lower recruitment signals. However, ADRB3 stimulation significantly reduced Mcp1 expression in obese male GWAT but showed an elevated expression in obese female GWAT (Fig. 5I). To determine the effects of stimulated lipolysis on the nature of the inflammatory response as being M1 or M2, we assessed markers for M2 macrophages. Arg1 and Mgl1 were highly expressed in the obese female GWAT upon ADRB3 stimulation (Fig. 5J and 5K), suggesting a state of tissue remodeling through resident macrophage activation in response to lipolysis.
Short-term lipolysis stimulation promotes CLS accumulation in obese female GWAT
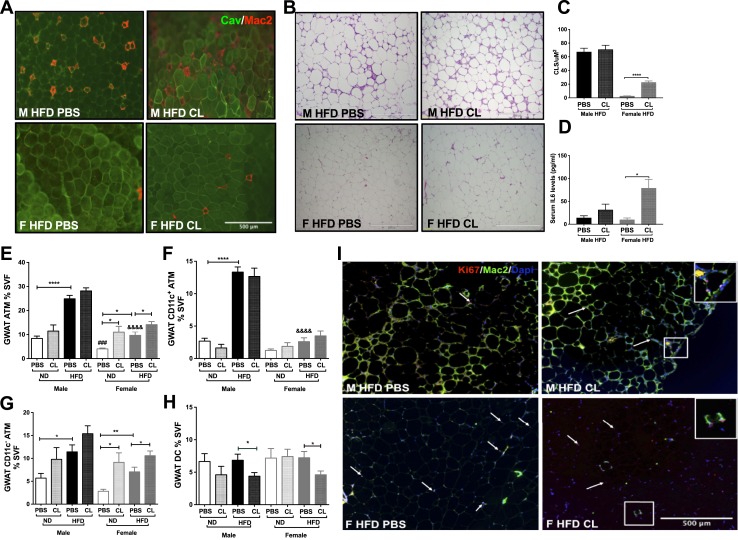
In rodent models of obesity, CLSs of macrophage accumulation are observed in large numbers in the male GWAT with very few in the male IWAT after HFD exposure. In contrast, female GWAT shows very few proinflammatory macrophages with HFD (5–7). Prior studies show that FFAs are potential ligands for inflammatory pathways, suggesting a link between lipolysis-induced FFAs and inflammation (23, 24). To investigate the effects of FFAs on adipose tissue inflammation, we first performed histological examination of GWAT, which showed prominent differences between the sexes. As expected, the untreated obese male GWAT showed increased CLSs (Fig. 6A and 6B, top left), whereas the obese female GWAT showed few macrophages (Fig. 6A and 6B, bottom left). Given the large number of macrophages in obesity, the CLS numbers in male obese GWAT were not altered with ADRB3 stimulation (Fig. 6A and 6B, top right). ADRB3 stimulation in the female obese GWAT led to the appearance of CLSs in obese female GWAT depicted by Mac-2 staining for macrophages and caveolin-1 for adipocytes (Fig. 6A, bottom right) and by H&E (Fig. 6B, bottom right). Figure 6C shows the quantification of CLS numbers in H&E images. Previous studies have reported increased IL-6 production with acute CL-stimulated lipolysis in male mice (25, 50). In our study, serum IL-6 levels were significantly increased in females after acute ADRB3 stimulation (Fig. 6D; analysis of variance, P < 0.05). Increased IL-6 cytokine production suggests underlying tissue remodeling through macrophage action predominantly in obese females with induced lipolysis.
Figure 6.
Imaging of ATMs and flow cytometry assessment of ATMs in lean and obese GWAT SVF. (A) Immunofluorescence images depicting CLS labeled for caveolin (Cav; green) and macrophages (Mac2; red) in obese male and female GWAT with and without CL treatment. Scale bar, 500 μm. (B) H&E images of obese male and female GWAT before and after ADRB3 stimulated lipolysis. Scale bars, 500 μm. (C) Graph depicting quantification of CLS numbers per standard unit area and (D) serum IL-6 cytokine levels in mice treated with and without CL. Quantitation of (E) GWAT percentage ATMs, (F) GWAT CD11c+ ATMs, (G) GWAT CD11c− ATMs, and (H) GWAT dendritic cells (DC) numbers. n = 7 to 12 per group. *P < 0.05; **P < 0.01; ****P < 0.0001. Comparisons of M ND PBS vs F ND PBS are shown as ###P < 0.005, and those of M HFD PBS vs F HFD PBS are shown as &&&&P < 0.0001. (I) Immunofluorescence images of male and female obese GWAT depicting Ki67+ proliferating cell nuclei (in red indicated by arrows) and Mac2 labeling of CLS (green) and 4′,6-diamidino-2-phenylindole (DAPI) nuclear stain (blue). Scale bar, 500 μm. F, female; M, male.
ADRB3 induces GWAT ATM accumulation in obese females and reduced dendritic cell populations in male and female GWAT
To our knowledge, sex differences in lipolysis induced ATM profiles have not been previously studied. To assess these differences, we studied ADRB3-mediated ATM induction in SVF of GWAT and IWAT from 16-week HFD-fed male and female mice by flow cytometry. ATMs were identified using CD64, a specific marker for murine macrophages (51). As expected, obese males had profoundly more CD64+ ATMs, specifically CD11c+ ATMs, in the GWAT SVF compared with obese females (Fig. 6E and 6F) as well as in the IWAT SVF (33). Given the hypothesis that CL would induce changes in IWAT and GWAT, we examined both fat depots. However, consistent with our ex vivo studies we found more robust changes within the GWAT compartment. Interestingly, female mice with the increased lipolytic response demonstrated an increase in CD11c− ATMs with ADRB3 stimulation in the lean as well as obese GWAT, which is primarily a resident macrophage population (Fig. 6G). This induction of CD11c− ATMs was seen only in the obese male GWAT, and CL treatment showed no effect (Fig. 6G) (33). A similar response of increased CD11c− ATMs was seen in lean females (Fig. 6G). Dendritic cell populations were reduced in obese male and female GWAT with stimulated lipolysis (Fig. 6H), but IWAT showed reduction only in obese female GWAT with CL treatment (33).
We further performed immunofluorescence studies with Ki67 to determine whether the enhanced ATMs in response to ADRB3 stimulation were due to proliferation. GWAT immunofluorescence studies showed fewer proliferating Ki67+ cells in CL-treated obese male GWAT (Fig. 6I, top right) compared with obese female GWAT (Fig. 6I, bottom). Interestingly, in obese female GWAT, Ki67+ cells were observed both in the Mac2+ CLS as well as in between adipocytes, suggesting resident cell proliferation or adipogenesis (Fig. 6I, bottom). These changes depict an effect of excess FFA efflux on proliferation of CD11c− ATM populations that form CLSs in obese females. Overall, these results demonstrate that adipocyte lipolysis leads to different patterns of ATM accumulation specifically in GWAT in males and females.
Adipose tissue expansion is essential to induce ADRB3 lipolysis-mediated GWAT ATM accumulation in HFD-fed females
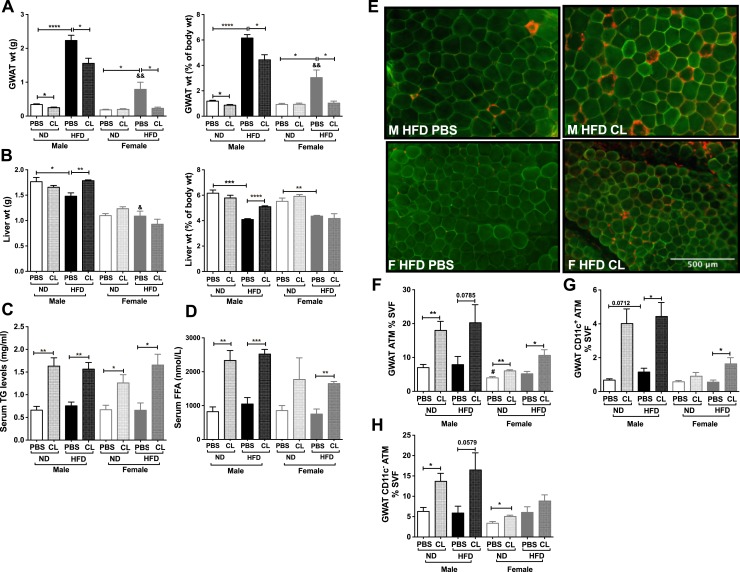
To determine whether sex differences in inflammatory response are dependent on stimulated lipolysis and significant obesity, we performed a more chronic CL injection model (52) in male and female C57BL6/J mice placed on 6 weeks of an HFD starting at 6 weeks of age. In this short-term HFD and chronic CL model we examined body adiposity and adipose tissue weights. As seen with 16-week HFD mice, male mice on an HFD gained more weight compared with females. However, chronic CL treatment failed to decrease body weights in lean or obese males and females. GWAT (mean weight, 0.78 g; Fig. 7A) and IWAT (mean weight, 0.59 g) fat depots expanded in both male and female mice even with 6 weeks of HFD but fat pads were still much smaller than in the 16-week HFD (mean GWAT weight, 1.98 g; mean IWAT weight, 1.03 g; Fig. 1B and 1C). On a 6-week HFD, BAT expanded only in the male obese mice, but liver weights were reduced in both sexes as a percentage of total body weight (Fig. 7B). Lean males and females did not show a reduction in visceral adipose tissue weights with chronic CL treatment as observed in prior studies in males (48) (Fig. 7B). After 3 days of CL, both obese male and female mice showed a decrease in visceral GWAT (Fig. 7B), but loss of adiposity in the IWAT was observed only in the obese females. Chronic CL treatment led to an increase in liver weights in obese male mice with no significant effect in the females (Fig. 7B). Chronic CL led to elevated serum TG levels and increased serum FFA levels (Fig. 7C and 7D) and accumulation of CLSs predominantly in obese male GWAT but not in the obese female GWAT (Fig. 7E). Flow cytometry analysis showed ATM accumulation in lean male and female GWAT and in the obese male GWAT with CL (Fig. 7F). Both CD11c+ ATMs and CD11c− ATMs were observed to increase with HFD and CL treatment in the obese male GWAT (Fig. 7G and 7H). However, in 6-week HFD females GWAT CD11c+ ATMs increased with 3-day CL whereas CD11c− ATMs increased in lean female GWAT (Fig. 7G and 7H). These results suggest that in short-term HFD feeding both female and male CD11c+ ATMs respond to lipolysis. However, in the females, CD11c− ATMs failed to expand, possibly due to the lack of GWAT adipocyte expansion and thereby limited FFA toxicity upon lipolysis stimulation within the short-term 6-week HFD challenge as compared with a prolonged HFD exposure.
Figure 7.
Adiposity and inflammation in response to chronic CL treatment and short-term HFD. (A) GWAT weight as percentage of body weight. (B) Liver weight as percentage of body weight. n = 4 per group. *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.0001. (C) Serum TG and (D) serum FFA in male and female mice after PBS and 3-d CL injection and 6-wk HFD. (E) Immunofluorescence images depicting CLS labeled for caveolin (green) and Mac2 (macrophages, red) in 6-wk HFD-fed male and female GWAT with and without CL treatment. Scale bar, 500 μm. (F–H) Quantitation of (F) GWAT percentage ATMs, (G) GWAT CD11c+ ATMs, and (H) GWAT CD11c− ATMs. n = 4 per group. *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.0001. Male HFD PBS vs female HFD PBS are shown as &P < 0.05 and &&P < 0.01. Comparisons of M ND PBS vs F ND PBS are shown as #P < 0.05. F, female; M, male.
Changes in lipid mediators link inflammation to lipolysis in a sex-dependent manner
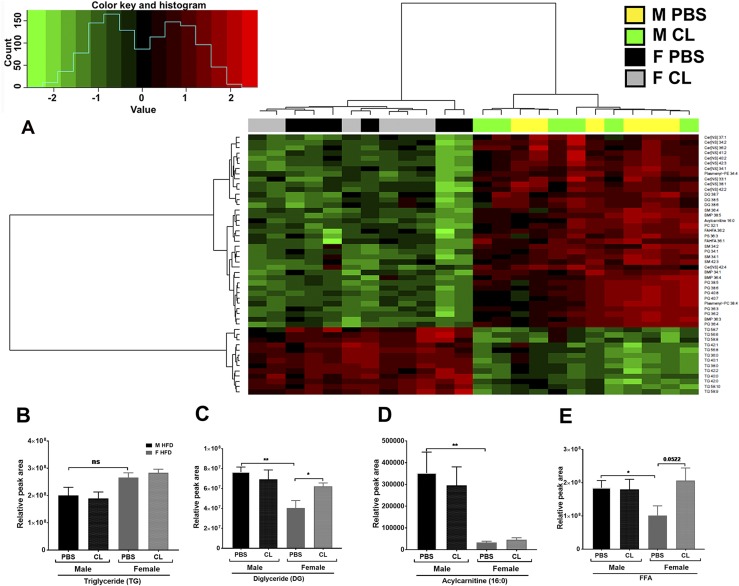
Lipolytic products and intermediates such as DG, MG, and FFA (and metabolites derived from these) play essential roles in multiple signaling pathways, both at the systemic level and at the intracellular level (53). When present in excess, several of these lipid intermediates have been suggested to independently induce insulin resistance in ectopic tissues (54, 55). Adrenergic activation of lipolysis upregulates COX-2, in vitro and in vivo, leading to the increased production of lipid metabolites of the cyclooxygenase pathway in male mice (56). Given that males and females show sexual dimorphism in fat accumulation, it is quite likely that a sex-dependent lipid metabolite profile would provide insight into the varied inflammatory response in both sexes. We performed untargeted lipidomics in obese male and female GWAT with and without ADRB3 stimulation. GWAT was chosen because this depot was observed to have more meta-inflammatory changes with an HFD and subsequent CL treatment in both sexes. Interestingly, the lipidomics data showed a very distinct lipid profile differential by sex in obese male and female GWAT irrespective of lipolysis stimulation (Fig. 8A). Clustering analysis showed 336 lipid species different by sex, demonstrating that males and females store lipids very differently when exposed to the same HFD. Figure 8A depicts the top 50 lipid species that are significantly different by sex. The order of the metabolites was arranged on the basis of clustering analysis. These included several species of TG, DG, Cer, sphingomyelin (SM), bis(monoacylglycero)phosphate, phosphatidylglycerol (PG), phosphatidylcholine (PC), acylcarnitine, and FA ester of hydroxy FA (FAHFA).
Figure 8.
Lipidomic evaluations of obese GWAT with and without ADRB3 stimulation. (A) Heat map and cluster analysis of top 50 lipid metabolites by untargeted lipidomics differential by sex in obese male and female GWAT. (B) Relative TG content, (C) DG content, (D) acylcarnitine content, and (E) FFA content in obese male and female GWAT. n = 6 per group. *P < 0.05; **P < 0.01. F, female; M, male; ns, not significant.
Several TG species were significantly lower in obese males than in females. The total TG content was not significantly different (Fig. 8B). Among the DG species, 38:7, 38:5, and 38:6 were differential by sex and were most abundant in the obese male GWAT compared with female GWAT (Fig. 8C). However, the DG content in the obese female GWAT increased upon ADRB3 stimulation (Fig. 8C). Acylcarnitines and specifically the long-chain acylcarnitine 16:0 was significantly higher in obese male GWAT, suggesting insufficient β-oxidation compared with females (Fig. 8A and 8D). In line with elevated FFA levels in obese females upon ADRB3 stimulation, FFA species were found to be significantly higher in females with CL treatment (Fig. 8E). However, the obese male GWAT showed increased FAHFA content (33). The total content of phosphatidylserine (PS), PC, lyso-PC, phosphatidylethanolamine, PG, phosphatidylinositol (PI) were also higher in obese male GWAT than female (33). These phospholipid species have been associated with obesity, inflammation and apoptosis (57) suggesting that changes in lipid metabolism is linked to inflammatory mediators in obese males.
Impaired β-oxidation of FFAs may further lead to accumulation of Cers, known mediators of proinflammatory responses (58, 59). Several Cer species (Fig. 9A) and the total Cer content (Fig. 9B) were highly upregulated in the obese male GWAT compared with females and remained so even with the CL treatment. FA species such as arachidic acid (22:0) (eicosanoic acid, saturated) and arachidonic acid (20:4) [polyunsaturated FA (PUFA)] (Fig. 9C) were also upregulated in the male obese GWAT with no further effect after CL treatment.
Figure 9.
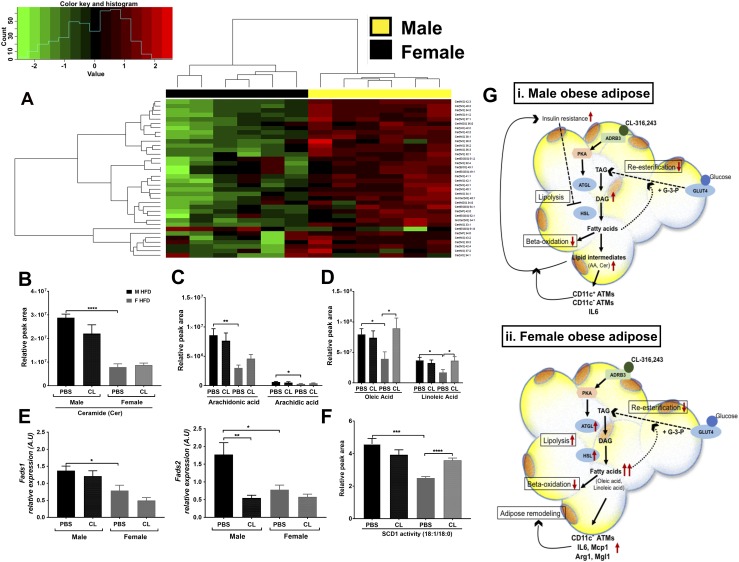
Lipidomic assessment of proinflammatory lipid mediators and proposed model. (A) Heat map and cluster analysis of Cer species differential by sex in obese male and female GWAT. (B) Relative Cer content, (C) arachidonic acid and arachidic acid content, (D) oleic and linoleic acid content, (E) Fads1 gene expression and Fads2 gene expression, and (F) SCD1 desaturase activity in obese male and female GWAT with and without CL treatment. n = 6 per group. *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.0001. (G) Proposed model for ADRB3-stimulated lipolysis-induced inflammation. (i) In obese male GWAT, increased insulin levels in obese males leads to inhibition of HSL phosphorylation/activity that impairs the breakdown of DG, thereby facilitating its accumulation. Excess FFA from ADRB3 stimulation may generate aberrant Cer, arachidonic acid (AA) accumulation, causing decreased β-oxidation, and increased inflammatory cytokine (IL-6) production, leading to persistent CD11c+ ATM and CD11c− ATM accumulation and thereby creating a feedback loop between lipid mediators and inflammation. (ii) In obese female GWAT, excess FFA from induced lipolysis leads to elevated p-HSL activity and IL-6 and MCP1 production, but giving rise to less inflammatory lipid mediators such as oleic acid and linoleic acid, leading to CD11c− ATM accumulation and initiating adipose tissue remodeling. DAG, diacylglycerol; F, female; M, male; TAG, triacylglycerol.
Interestingly, oleic acid [monounsaturated FA (MUFA)] and linoleic acid (PUFA) were upregulated upon lipolysis stimulation in the obese female GWAT (Fig. 9D), consistent with a more regulatory inflammatory response.
Fads1 and Fads2 genes mediate the conversion of MUFAs to PUFAs such as arachidonic acid, which is used for the synthesis of eicosanoids and is a potential trigger for inflammation in obesity (60, 61). Obese male GWAT exhibited relatively higher expression of both Fads1 and Fads2 genes compared with female GWAT. However, upon lipolysis stimulation, Fads2 expression was significantly reduced in the obese male GWAT, suggesting changes in lipid metabolism upon lipolysis stimulation (Fig. 9E). A key enzyme involved in TG synthesis is stearoyl–coenzyme A desaturase 1 (SCD1). The desaturase enzyme SCD1 catalyzes the conversion of palmitic acid to the MUFA oleic acid. The desaturase activity index of SCD1 calculated by the ratio of stearic acid/oleic acid (18:1/18:0) depicted increased SCD1 activity in the obese male GWAT compared with female GWAT (Fig. 9F). However, upon increased FFA efflux by ADRB3 stimulation, the obese female GWAT responded with increased SCD1 activity (Fig. 9F). These results suggest that pathways required for excess lipid handling get activated upon ADRB3 stimulation in the obese female GWAT, but in the obese male GWAT the pathways are blunted possibly due to high lipotoxicity leading to increased proinflammatory lipid mediators.
Discussion
Inflammation arising from obese AT is strongly linked with metabolic diseases. The factors that regulate ATM accumulation in obesity are not well defined, especially in females. The effects of metabolic perturbations on ATM accumulation and function also remain largely unexplored. Because there is sexual dimorphism in fat accumulation, it is important to understand the underlying variables contributing to differences between health outcomes seen in women and men. We hypothesized that metabolic changes in the form of increased FFA concentrations might drive the accumulation of ATMs within female adipose tissue depots. In this study, we provide evidence for sex differences in inflammation mediated by sex differences in lipolytic metabolites. Compared with males, obese female mice were more responsive to ADRB3-induced lipolysis. Although we hypothesized that females would have more robust lipid changes in IWAT, our studies in this mouse model demonstrated significant changes in the visceral GWAT compartment in terms of CLSs. Lipolysis promoted the appearance of CLS and induced Il6, Mcp1, Arg1, and Mgl1 gene expression in obese female GWAT, giving rise to an accumulation of CD11c− ATMs. We also show sex differences in the GWAT lipolytic metabolites with the obese male GWAT having more proinflammatory mediators compared with female GWAT (Fig. 9G).
A major cause of inflammation is impairment in the adipocyte energy storing system, which results in lipotoxicity (3, 4). Excess FFAs and high circulating serum IL-6 can both elicit insulin resistance (62, 63). CL treatment in obese male mice decreased fed serum insulin levels but not in the obese females. Obese females had both elevated FFA and serum IL-6 levels that might have contributed to an insulin resistance scenario during the CL treatment and induced further inflammation. Alternatively, lipotoxicity can be dampened by diverting FFAs from inflammation-inducing pathways into TG droplets for storage (64), which seems to be the favored mechanism in obese female GWAT. Unlike the female GWAT, TG synthesis and storage genes such as Lpl, Fasn, and Acsl1 showed impairment early on in male GWAT owing to obesity and/or ADRB3 stimulation, suggesting that female GWAT preferentially stores fat compared with male GWAT during HFD exposure. Previous studies in 8- to 12-week-old lean males show a role for HSL in AT plasticity during acute and chronic activation of ADRB3 (24, 65), and with obesity, male mice showed suppressed lipolysis (66). However, studies in female mice are lacking. In our study, ADRB3 stimulation elicited a significantly higher lipolytic response in the obese female mice. Our explant studies showed that even with obesity and excess FFA supply, the female GWAT and IWAT were more responsive to CL stimulation compared with male GWAT and IWAT. Also, in the female, the GWAT showed higher lipolytic levels than did IWAT. Our data from in vivo and in vitro studies hence suggest sex differences in fat depots in their response to nutritional and pharmacological signals.
Fat storage in male GWAT aggravated impairments in the lipolytic pathway, but the effects were less pronounced in obese female GWAT. Suppression of p-HSL expression during epinephrine-stimulated lipolysis in obese male GWAT and IWAT has been reported earlier (66) and was also observed in our study. In contrast, ADRB3 stimulation in the obese female GWAT was not impaired and showed elevated expression of p-HSL and ATGL. However, a limitation of our study was the disconnect between protein and gene expression levels that make the time course of response events to lipolysis difficult to interpret. For example, unlike protein levels, the Hsl mRNA levels were significantly decreased in obese female GWAT when lipolysis was stimulated. Collectively, these results suggest sex differences in regulation of transcription and translation processes in the lipolytic cascade prompted by nutritional status that may play key roles in modulating regional fat distribution. Sex hormones clearly influence adipose tissue function and deposition, determining how to capture and use their function in a time of caloric surplus (10). However, the existing VAT gene expression profiling data from children with and without obesity demonstrate a relative paucity of differentially expressed inflammatory genes unlike those in obese adult adipose tissue of men and women (67, 68). Estrogen is known to facilitate and prioritize fat storage because lipids energetically support reproductive processes in women (69). After menopause, fat deposition occurs in the visceral depot, and this shift is accompanied by a parallel increase in metabolic risk reminiscent of that seen in men (70). This places a clinically relevant context to our findings of increased storage of FFAs in female adipose depots with exaggerated CL responses in females. Overall, fat depot distribution and lipolysis are more likely to be tightly regulated in females than in males to promote lipid and glucose homeostasis and prevent the metabolic syndrome in reproductive years.
Lipolysis per se is not detrimental provided that FA oxidation is increased to counter the increased FFA flux (71, 72). Preventing accumulation of intracellular FFAs within adipocytes by promoting FFA oxidation might be one of the means of preventing inflammation. Male rodent studies have shown that chronic activation of ADRB3 dramatically expands mitochondrial FA oxidation in WAT, and this expansion appears to be critical for limiting inflammation and improving insulin action (46). In our studies, the gene expression of Pgc1a and Pparg, which are critical regulators of mitochondrial biogenesis and energy expenditure, were significantly reduced in GWAT of obese males but remained unaffected in obese female GWAT. However, upon ADRB3 stimulation, a markedly decreased expression of Pgc1a, Pparg, Lpl, Fasn, and Acsl1 was seen in obese female GWAT, implying a direct effect of the excess lipid load on FA oxidation and lipogenesis. Taken together, our study shows sex differences in lipogenesis, oxidation, and the lipolytic capacity of fat depots that might explain the sexually dimorphic inflammatory response. Lipid overload and overflow may thus provoke counterregulatory effects in the adipose tissue. Earlier studies in obese human omental or visceral fat have also shown a decrease in lipogenic markers and enzymes that correlated with an increase in inflammatory markers such as IL-18 (73, 74). In addition to the role of sex hormones in the regulation of adipose tissue function, the regulatory effects of adipostatic hormones, such as leptin (75) and resistin (76), and other mediators, such as caveolin-1, have been implicated in regulation of adipose tissue expansion (77).
Studies in male mice show that M2-type ATMs exist in lean AT in a lean state (78), but in obesity they are marked by the profound accumulation of proinflammatory M1-type macrophages that make up as much as 60% to 75% of all GWAT ATMs (7, 79). These proinflammatory cells release large amounts of tissue cytokines and chemokines that can cause insulin resistance through paracrine mechanisms. FFA and other lipids have been found to regulate the activation state and immune function of myeloid cells and macrophages (5). In contrast, female mice are generally protected from AT inflammation (6). Hormonal factors may play a role in this protection because ovariectomy induces partial changes to a female obese phenotype (80) and increased inflammation. Inflammatory factors, such as altered MCP1, have also been shown to play a role in sex differences in myelopoiesis (81), as observed with our obese female mice having lower Mcp1 and Il6 gene expression than did obese male GWAT. The idea that regulation of lipolytic responses between males and females may lead to a sex difference in metabolic inflammation is relatively unexplored. In our studies, ADRB3 stimulation increased Il6 and Mcp1 mRNA levels in the obese female GWAT as well as serum IL-6 levels that corresponded to appearance of CLS in GWAT. Additionally, accumulation of CD11c− ATMs was observed with stimulated lipolysis in the obese female GWAT, corresponding to an increase in expression of the M2 macrophage markers Arg1 and Mgl1. ATMs may be derived locally as a result of proliferation or other resident progenitor during normal physiology (82). Our proliferation studies showed the presence of Ki67+ cells in CLSs of CL-treated obese female GWAT as well as in male GWAT ATMs, but Ki67+ staining in regions in between adipocytes was observed only in obese female GWAT. It was also recently shown that rapid AT expansion with 8 weeks of an HFD led to local macrophage proliferation in male GWAT (83). In our short-term HFD model, chronic CL induced ATM accumulation in both lean males and females but significantly more in the lean males, implying a role for FFAs and sex hormones in CLS formation. In the 6-week HFD males, a robust ATM response was observed with stimulated lipolysis, but after 16 weeks of an HFD, the response tapered off. Alternatively, in the 6-week HFD state, lipolysis stimulated females to induce CD11c+ ATMs and not CD11c- ATMs, but overall compared with the males the inflammatory response was still weaker. However, there is a heightened inflammatory response with stimulated lipolysis in the 16-week HFD females, but in the form of regulatory CD11c− ATMs. Previous studies from our group have shown that the average adipocyte size and degree of adipocyte hypertrophy were similar between sexes after 16 weeks of an HFD (5). No significant changes in adipocyte size were observed with CL treatment in our studies. Our findings thus demonstrate a distinct role for increased FFA efflux through lipolysis stimulation that promotes resident and local macrophage accumulation specifically in obese females.
Our studies overall show that females exhibit a dampened and protective inflammatory response that is exaggerated only with stimulated lipolysis in a profoundly obese condition owing to excessive fat storage and therefore high lipotoxicity. We speculate that recruitment of CD11c+ ATMs might occur with a longer HFD duration and/or more chronic ADRB3 activation in female mice. Additionally, although it is possible that the initial lipolysis response with FFA release in adipose tissue leads to direct local proliferation of CD11c− ATMs within an adipose depot, it cannot be ruled out that the robust systemic release of FFAs leads to a systemic inflammatory response that is identified with indirect expansion of ATMs.
Factors mediating CD11c− ATM accumulation may include IL-6 production or extracellular signals of saturated FAs through activation of classical inflammatory pathways such as Toll-like receptors (84–86). ADRB3-mediated inflammation depends on activation of HSL and ATGL, and therefore we alternatively hypothesized that lipolytic products may act as proinflammatory mediators. From our lipidomics studies, we identified elevated levels of TG, DG, acylcarnitine, Cer, oleic acid, linoleic acid, arachidic acid, and arachidonic acid in obese male GWAT. DG accumulation is a direct effect of suppressed HSL activity in the obese GWAT. Acylcarnitine, Cer, arachidic acid, and arachidonic acid have been associated with metabolic disorders (60, 87, 88) and thus are potential triggers for the chronic inflammatory response in obese males. Other potential metabolites such as FAHFA, phosphatidylserine, PC, lyso-PC, phosphatidylethanolamine, PG, and phosphatidylinositol that were highly elevated in the obese male GWAT are implicated in diabetes and obesity-associated diseases (57). A limitation of our untargeted lipidomics approach is a one-time point assessment of a dynamic process such as lipolysis. Therefore, this approach did not fully allow us to understand the storage and kinetics of lipid metabolism or identify a specific lipid metabolite responsible for this process. Also, a chronic lipolysis model in the female mice may have led to even greater induction of inflammatory mediators downstream of arachidonic acid such as eicosanoids and prostaglandins that are direct mediators of inflammation.
In summary, our study shows that ADRB3 stimulation induces sexually dimorphic lipolytic and inflammatory responses that are linked owing to altered lipid metabolism even with the same HFD exposure. Because obese males already have a high threshold of inflammation, ADRB3 stimulation fails to enhance the proinflammatory profile in the GWAT (Fig. 9Gi). In obese females that have a lower inflammatory tone with CD11c− ATM profiles, there is an ability to induce the necessary inflammation to manage and promote healthy tissue function even with the higher lipolytic response (Fig. 9Gii). Our results suggest a potential role for increased FA storage in females as a regulator of lower inflammation in female obesity. It is highly likely that the controlled release of polyunsaturated FFAs from adipocytes regulates the synthesis and biological action of eicosanoids and prostaglandins in the inflammatory response. However, any stressors that induce lipolytic responses are likely to enhance inflammatory responses, even in females.
Acknowledgments
We thank Dr. Carey Lumeng and Dr. David Bridges for advice on the study design and data analysis and interpretation.
Financial Support: This work was supported by Pediatrics Elizabeth E. Kennedy Children’s Research Award, the Claude D. Pepper Older Americans Independence Center/Michigan Biology of Cardiovascular Aging pilot award (National Institute on Aging Grant AG024824/UL1TR002240), National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grants K08DK101755 and R01 DK115583, and by Edith Briskin/SKS Foundation Taubman Emerging Scholar support (to K.S.). This work used Core Services from the Michigan Nutrition and Obesity Research Center supported by National Institutes of Health Grant DK089503 to the University of Michigan.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ADRB
β-adrenergic receptor
- ATGL
adipose triglyceride lipase
- ATM
adipose tissue macrophage
- BAT
brown adipose tissue
- Cer
ceramide
- CL
CL-316,243
- CLS
crown-like structure
- DG
diacylglycerol
- FA
fatty acid
- FAHFA
fatty acid ester of hydroxyl fatty acid
- FFA
free fatty acid
- GWAT
gonadal white adipose tissue
- H&E
hematoxylin and eosin
- HFD
high-fat diet
- HSL
hormone-sensitive lipase
- IWAT
inguinal white adipose tissue
- LC-MS
liquid chromatography with mass spectrometry
- MG
monoacylglycerol
- MS
mass spectrometry
- MUFA
monounsaturated fatty acid
- ND
normal diet
- PC
phosphatidylcholine
- PG
phosphatidylglycerol
- p-HSL
phosphorylated hormone-sensitive lipase
- PUFA
polyunsaturated fatty acid
- SCD1
stearoyl–coenzyme A desaturase 1
- SVF
stromal vascular fraction
- TG
triglyceride
- VAT
visceral adipose tissue
References
- 1. Stokes A, Preston SH. Deaths attributable to diabetes in the United States: comparison of data sources and estimation approaches. PLoS One. 2017;12(1):e0170219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314(1):1–16. [DOI] [PubMed] [Google Scholar]
- 3. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. [DOI] [PubMed] [Google Scholar]
- 4. Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83(2):461S–465S. [DOI] [PubMed] [Google Scholar]
- 5. Singer K, DelProposto J, Morris DL, Zamarron B, Mergian T, Maley N, Cho KW, Geletka L, Subbaiah P, Muir L, Martinez-Santibanez G, Lumeng CN. Diet-induced obesity promotes myelopoiesis in hematopoietic stem cells. Mol Metab. 2014;3(6):664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singer K, Maley N, Mergian T, DelProposto J, Cho KW, Zamarron BF, Martinez-Santibanez G, Geletka L, Muir L, Wachowiak P, Demirjian C, Lumeng CN. Differences in hematopoietic stem cells contribute to sexually dimorphic inflammatory responses to high fat diet-induced obesity. J Biol Chem. 2015;290(21):13250–13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56(1):16–23. [DOI] [PubMed] [Google Scholar]
- 8. Griffin C, Lanzetta N, Eter L, Singer K. Sexually dimorphic myeloid inflammatory and metabolic responses to diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2016;311(2):R211–R216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med. 2009;6(Suppl 1):60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol. 2015;402:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306(5700):1383–1386. [DOI] [PubMed] [Google Scholar]
- 12. Haemmerle G, Zimmermann R, Hayn M, Theussl C, Waeg G, Wagner E, Sattler W, Magin TM, Wagner EF, Zechner R. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J Biol Chem. 2002;277(7):4806–4815. [DOI] [PubMed] [Google Scholar]
- 13. Fredrikson G, Tornqvist H, Belfrage P. Hormone-sensitive lipase and monoacylglycerol lipase are both required for complete degradation of adipocyte triacylglycerol. Biochim Biophys Acta. 1986;876(2):288–293. [DOI] [PubMed] [Google Scholar]
- 14. Forest C, Tordjman J, Glorian M, Duplus E, Chauvet G, Quette J, Beale EG, Antoine B. Fatty acid recycling in adipocytes: a role for glyceroneogenesis and phosphoenolpyruvate carboxykinase. Biochem Soc Trans. 2003;31(Pt 6):1125–1129. [DOI] [PubMed] [Google Scholar]
- 15. Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res. 2009;48(5):275–297. [DOI] [PubMed] [Google Scholar]
- 16. Proença AR, Sertié RA, Oliveira AC, Campaña AB, Caminhotto RO, Chimin P, Lima FB. New concepts in white adipose tissue physiology. Braz J Med Biol Res. 2014;47(3):192–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frühbeck G, Méndez-Giménez L, Fernández-Formoso JA, Fernández S, Rodríguez A. Regulation of adipocyte lipolysis. Nutr Res Rev. 2014;27(1):63–93. [DOI] [PubMed] [Google Scholar]
- 18. Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS. Regulation of lipolysis in adipocytes. Annu Rev Nutr. 2007;27(1):79–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–461. [DOI] [PubMed] [Google Scholar]
- 20. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. [DOI] [PubMed] [Google Scholar]
- 21. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meyer MR, Clegg DJ, Prossnitz ER, Barton M. Obesity, insulin resistance and diabetes: sex differences and role of oestrogen receptors. Acta Physiol (Oxf). 2011;203(1):259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, Ferrante AW Jr. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120(10):3466–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mottillo EP, Shen XJ, Granneman JG. β3-Adrenergic receptor induction of adipocyte inflammation requires lipolytic activation of stress kinases p38 and JNK. Biochim Biophys Acta. 2010;1801(9):1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang W, Mottillo EP, Zhao J, Gartung A, VanHecke GC, Lee JF, Maddipati KR, Xu H, Ahn YH, Proia RL, Granneman JG, Lee MJ. Adipocyte lipolysis-stimulated interleukin-6 production requires sphingosine kinase 1 activity. J Biol Chem. 2014;289(46):32178–32185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. RRID: AB_10699017.
- 27. RRID: AB_837133.
- 28. RRID: AB_2687446.
- 29. RRID: AB_490997.
- 30. RRID: AB_1503425.
- 31. RRID: AB_2167955.
- 32. RRID: AB_350715.
- 33. Varghese M, Griffin C, McKernan K, Eter L, Lanzetta N, Agarwal D, Abrishami S, Singer K. Data from: Sex differences in inflammatory responses to adipose tissue lipolysis in diet-induced obesity. Deep Blue Data 2018. Deposited 14 November 2018. https://deepblue.lib.umich.edu/data/concern/generic_works/1c18dg65s?locale=en. [DOI] [PMC free article] [PubMed]
- 34. Morris DL, Oatmen KE, Wang T, DelProposto JL, Lumeng CN. CX3CR1 deficiency does not influence trafficking of adipose tissue macrophages in mice with diet-induced obesity. Obesity (Silver Spring). 2012;20(6):1189–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. RRID: AB_647241.
- 36. RRID: AB_1518806.
- 37. RRID: AB_1548652.
- 38. Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57(12):3239–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Griffin C, Eter L, Lanzetta N, Abrishami S, Varghese M, McKernan K, Muir L, Lane J, Lumeng CN, Singer K. TLR4, TRIF, and MyD88 are essential for myelopoiesis and CD11c+ adipose tissue macrophage production in obese mice. J Biol Chem. 2018;293(23):8775–8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911–917. [DOI] [PubMed] [Google Scholar]
- 41. Gika HG, Macpherson E, Theodoridis GA, Wilson ID. Evaluation of the repeatability of ultra-performance liquid chromatography-TOF-MS for global metabolic profiling of human urine samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;871(2):299–305. [DOI] [PubMed] [Google Scholar]
- 42. Chambers MC, Maclean B, Burke R, Amodei D, Ruderman DL, Neumann S, Gatto L, Fischer B, Pratt B, Egertson J, Hoff K, Kessner D, Tasman N, Shulman N, Frewen B, Baker TA, Brusniak MY, Paulse C, Creasy D, Flashner L, Kani K, Moulding C, Seymour SL, Nuwaysir LM, Lefebvre B, Kuhlmann F, Roark J, Rainer P, Detlev S, Hemenway T, Huhmer A, Langridge J, Connolly B, Chadick T, Holly K, Eckels J, Deutsch EW, Moritz RL, Katz JE, Agus DB, MacCoss M, Tabb DL, Mallick P. A cross-platform toolkit for mass spectrometry and proteomics. Nat Biotechnol. 2012;30(10):918–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kind T, Meissen JK, Yang D, Nocito F, Vaniya A, Cheng YS, Vandergheynst JS, Fiehn O. Qualitative analysis of algal secretions with multiple mass spectrometric platforms. J Chromatogr A. 2012;1244:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meissen JK, Yuen BT, Kind T, Riggs JW, Barupal DK, Knoepfler PS, Fiehn O. Induced pluripotent stem cells show metabolomic differences to embryonic stem cells in polyunsaturated phosphatidylcholines and primary metabolism. PLoS One. 2012;7(10):e46770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ejsing CS, Duchoslav E, Sampaio J, Simons K, Bonner R, Thiele C, Ekroos K, Shevchenko A. Automated identification and quantification of glycerophospholipid molecular species by multiple precursor ion scanning. Anal Chem. 2006;78(17):6202–6214. [DOI] [PubMed] [Google Scholar]
- 46. Grujic D, Susulic VS, Harper ME, Himms-Hagen J, Cunningham BA, Corkey BE, Lowell BB. β3-Adrenergic receptors on white and brown adipocytes mediate β3-selective agonist-induced effects on energy expenditure, insulin secretion, and food intake. A study using transgenic and gene knockout mice. J Biol Chem. 1997;272(28):17686–17693. [DOI] [PubMed] [Google Scholar]
- 47. Preitner F, Muzzin P, Revelli JP, Seydoux J, Galitzky J, Berlan M, Lafontan M, Giacobino JP. Metabolic response to various β-adrenoceptor agonists in β3-adrenoceptor knockout mice: evidence for a new β-adrenergic receptor in brown adipose tissue. Br J Pharmacol. 1998;124(8):1684–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mottillo EP, Balasubramanian P, Lee Y-H, Weng C, Kershaw EE, Granneman JG. Coupling of lipolysis and de novo lipogenesis in brown, beige, and white adipose tissues during chronic β3-adrenergic receptor activation. J Lipid Res. 2014;55(11):2276–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledelius C, Jacobsen P, Tornqvist H, Zechner R, Zimmermann R. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem. 2006;281(52):40236–40241. [DOI] [PubMed] [Google Scholar]
- 50. Buzelle SL, MacPherson REK, Peppler WT, Castellani L, Wright DC. The contribution of IL-6 to beta 3 adrenergic receptor mediated adipose tissue remodeling. Physiol Rep. 2015;3(2):e12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma’ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ; Immunological Genome Consortium . Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13(11):1118–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Guirguis E, Hockman S, Chung YW, Ahmad F, Gavrilova O, Raghavachari N, Yang Y, Niu G, Chen X, Yu ZX, Liu S, Degerman E, Manganiello V. A role for phosphodiesterase 3B in acquisition of brown fat characteristics by white adipose tissue in male mice. Endocrinology. 2013;154(9):3152–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zechner R, Zimmermann R, Eichmann TO, Kohlwein SD, Haemmerle G, Lass A, Madeo F. Fat signals—lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012;15(3):279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Boura-Halfon S, Zick Y. Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am J Physiol Endocrinol Metab. 2009;296(4):E581–E591. [DOI] [PubMed] [Google Scholar]
- 55. Copps KD, Hancer NJ, Opare-Ado L, Qiu W, Walsh C, White MF. Irs1 serine 307 promotes insulin sensitivity in mice. Cell Metab. 2010;11(1):84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gartung A, Zhao J, Chen S, Mottillo E, VanHecke GC, Ahn YH, Maddipati KR, Sorokin A, Granneman J, Lee MJ. Characterization of eicosanoids produced by adipocyte lipolysis: implication of cyclooxygenase-2 in adipose inflammation. J Biol Chem. 2016;291(31):16001–16010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rauschert S, Uhl O, Koletzko B, Kirchberg F, Mori TA, Huang RC, Beilin LJ, Hellmuth C, Oddy WH. Lipidomics reveals associations of phospholipids with obesity and insulin resistance in young adults. J Clin Endocrinol Metab. 2016;101(3):871–879. [DOI] [PubMed] [Google Scholar]
- 58. Chaurasia B, Kaddai VA, Lancaster GI, Henstridge DC, Sriram S, Galam DL, Gopalan V, Prakash KN, Velan SS, Bulchand S, Tsong TJ, Wang M, Siddique MM, Yuguang G, Sigmundsson K, Mellet NA, Weir JM, Meikle PJ, Bin M Yassin MS, Shabbir A, Shayman JA, Hirabayashi Y, Shiow ST, Sugii S, Summers SA. Adipocyte ceramides regulate subcutaneous adipose browning, inflammation, and metabolism. Cell Metab. 2016;24(6):820–834. [DOI] [PubMed] [Google Scholar]
- 59. Chiurchiù V, Leuti A, Maccarrone M. Bioactive lipids and chronic inflammation: managing the fire within. Front Immunol. 2018;9:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lottenberg AM, Afonso MS, Lavrador MS, Machado RM, Nakandakare ER. The role of dietary fatty acids in the pathology of metabolic syndrome. J Nutr Biochem. 2012;23(9):1027–1040. [DOI] [PubMed] [Google Scholar]
- 61. Pickens CA, Sordillo LM, Zhang C, Fenton JI. Obesity is positively associated with arachidonic acid-derived 5- and 11-hydroxyeicosatetraenoic acid (HETE). Metabolism. 2017;70:177–191. [DOI] [PubMed] [Google Scholar]
- 62. Kim JH, Bachmann RA, Chen J. Interleukin-6 and insulin resistance. Vitam Horm. 2009;80:613–633. [DOI] [PubMed] [Google Scholar]
- 63. Sears B, Perry M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015;14(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117(9):2621–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mottillo EP, Shen XJ, Granneman JG. Role of hormone-sensitive lipase in beta-adrenergic remodeling of white adipose tissue. Am J Physiol Endocrinol Metab. 2007;293(5):E1188–E1197. [DOI] [PubMed] [Google Scholar]
- 66. Gaidhu MP, Anthony NM, Patel P, Hawke TJ, Ceddia RB. Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: role of ATGL, HSL, and AMPK. Am J Physiol Cell Physiol. 2010;298(4):C961–C971. [DOI] [PubMed] [Google Scholar]
- 67. Aguilera CM, Gomez-Llorente C, Tofe I, Gil-Campos M, Cañete R, Gil Á. Genome-wide expression in visceral adipose tissue from obese prepubertal children. Int J Mol Sci. 2015;16(4):7723–7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Singer K, Lumeng CN. The initiation of metabolic inflammation in childhood obesity. J Clin Invest. 2017;127(1):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Varlamov O, Bethea CL, Roberts CT Jr. Sex-specific differences in lipid and glucose metabolism. Front Endocrinol (Lausanne). 2015;5:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pfeilschifter J, Köditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23(1):90–119. [DOI] [PubMed] [Google Scholar]
- 71. Tansey JT, Sztalryd C, Gruia-Gray J, Roush DL, Zee JV, Gavrilova O, Reitman ML, Deng CX, Li C, Kimmel AR, Londos C. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci USA. 2001;98(11):6494–6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nishino N, Tamori Y, Tateya S, Kawaguchi T, Shibakusa T, Mizunoya W, Inoue K, Kitazawa R, Kitazawa S, Matsuki Y, Hiramatsu R, Masubuchi S, Omachi A, Kimura K, Saito M, Amo T, Ohta S, Yamaguchi T, Osumi T, Cheng J, Fujimoto T, Nakao H, Nakao K, Aiba A, Okamura H, Fushiki T, Kasuga M. FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J Clin Invest. 2008;118(8):2808–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Poulain-Godefroy O, Lecoeur C, Pattou F, Frühbeck G, Froguel P. Inflammation is associated with a decrease of lipogenic factors in omental fat in women. Am J Physiol Regul Integr Comp Physiol. 2008;295(1):R1–R7. [DOI] [PubMed] [Google Scholar]
- 74. Ortega FJ, Mayas D, Moreno-Navarrete JM, Catalán V, Gómez-Ambrosi J, Esteve E, Rodriguez-Hermosa JI, Ruiz B, Ricart W, Peral B, Fruhbeck G, Tinahones FJ, Fernández-Real JM. The gene expression of the main lipogenic enzymes is downregulated in visceral adipose tissue of obese subjects. Obesity (Silver Spring). 2010;18(1):13–20. [DOI] [PubMed] [Google Scholar]
- 75. Frühbeck G, Gómez-Ambrosi J. Rationale for the existence of additional adipostatic hormones. FASEB J. 2001;15(11):1996–2006. [DOI] [PubMed] [Google Scholar]
- 76. Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–312. [DOI] [PubMed] [Google Scholar]
- 77. Catalán V, Gómez-Ambrosi J, Rodríguez A, Silva C, Rotellar F, Gil MJ, Cienfuegos JA, Salvador J, Frühbeck G. Expression of caveolin-1 in human adipose tissue is upregulated in obesity and obesity-associated type 2 diabetes mellitus and related to inflammation. Clin Endocrinol (Oxf). 2008;68(2):213–219. [DOI] [PubMed] [Google Scholar]
- 78. Morris DL, Singer K, Lumeng CN. Adipose tissue macrophages: phenotypic plasticity and diversity in lean and obese states. Curr Opin Clin Nutr Metab Care. 2011;14(4):341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Grove KL, Fried SK, Greenberg AS, Xiao XQ, Clegg DJ. A microarray analysis of sexual dimorphism of adipose tissues in high-fat-diet-induced obese mice. Int J Obes. 2010;34(6):989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kim WK, Choi EK, Sul OJ, Park YK, Kim ES, Yu R, Suh JH, Choi HS. Monocyte chemoattractant protein-1 deficiency attenuates oxidative stress and protects against ovariectomy-induced chronic inflammation in mice. PLoS One. 2013;8(8):e72108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Amano SU, Cohen JL, Vangala P, Tencerova M, Nicoloro SM, Yawe JC, Shen Y, Czech MP, Aouadi M. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 2014;19(1):162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Muir LA, Kiridena S, Griffin C, DelProposto JB, Geletka L, Martinez-Santibañez G, Zamarron BF, Lucas H, Singer K, O’ Rourke RW, Lumeng CN. Frontline science: rapid adipose tissue expansion triggers unique proliferation and lipid accumulation profiles in adipose tissue macrophages. J Leukoc Biol. 2018;103(4):615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276(20):16683–16689. [DOI] [PubMed] [Google Scholar]
- 85. Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S, Kotani H, Yamaoka S, Miyake K, Aoe S, Kamei Y, Ogawa Y. Role of the Toll-like receptor 4/NF-κB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27(1):84–91. [DOI] [PubMed] [Google Scholar]
- 86. Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116(11):3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Meikle PJ, Summers SA. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat Rev Endocrinol. 2017;13(2):79–91. [DOI] [PubMed] [Google Scholar]
- 88. Chaurasia B, Summers SA. Ceramides—lipotoxic inducers of metabolic disorders [published correction appears in Trends Endocrinol Metab. 2018;29(1):66–67] Trends Endocrinol Metab. 2015;26(10):538–550. [DOI] [PubMed] [Google Scholar]