Abstract
Owing to the high incidence of multi-drug resistance and challenges posed by the complex and long duration of treatments, Mycobacterium tuberculosis (Mtb) infections remain a significant clinical burden, which would benefit from development of novel immuno-therapeutic-based treatment strategies. Among early immune effectors, invariant or innate-like (i)T cells are attracting attention because of their potential regulatory activity, which can shape anti-mycobacterial immune responses. Unlike conventional T cells, iT cells express a semi-invariant T cell receptor, and respond rapidly and robustly to molecular patterns presented by MHC class I-like molecules. To date, functional studies of iT cells in vivo has been problematic and the role of iT cells in anti-Mtb responses remains unclear. Here, after reviewing the recent literature on anti-mycobacterial iT cell immunity, we describe a novel alternative model system in the amphibian Xenopus laevis tadpoles during infection with Mycobacterium marinum (Mm). X. laevis tadpoles rely mostly on a few distinct prominent innate-like (i)T cell subsets, whose development and function are governed by distinct MHC class I-like molecules. Thus, X. laevis tadpoles provide a convenient and cost-effective in vivo model uniquely suited to investigate the roles of iT cells during mycobacterial infections. We have developed reverse genetics and MHC tetramer technology to characterize this MHC-like/iT system in tadpoles. Our study in X. laevis provides evidence of a conserved convergent function of iT cells in host defenses against mycobacteria between mammals and amphibians.
Introduction
Mycobacterium tuberculosis (Mtb), the causative agent for human pulmonary tuberculosis (TB), still infects approximately one third of the human population worldwide (WHO: http://www.who.int/tb/en/). Mtb undergoes an actively replicating stage followed by a metabolic dormant stage, leading to its latency in the infected hosts (reviewed in [1]). Due to this latency, the current treatment requires multi-antibiotic regimens that are subject to multi-drug resistance. While the current vaccine for tuberculosis disease using Mycobacterium bovis (BCG) has shown protection against pulmonary TB in children, its efficiency is more variable among adolescents, presumably due to the latency of TB [2]. Since BCG can elicit conventional CD4 and CD8 responses [3], its limited protection against TB has renewed interest in better understanding the role of unconventional immune cell effectors, such as innate-like T (iT) cells, for novel immunotherapeutic approaches.
To date, two iT cell populations, invariant natural killer T (iNKT) cells and mucosal associated innate T (MAIT) cells, have been implicated in host defenses against mycobacteria. Studies in humans and rodents suggest that these iT cell subsets are early responders with protective potential against mycobacterial infections (reviewed in [4, 5]). However, the specific functions of these iT cells in immune response to mycobacteria in general, and Mtb in particular, are still not fully understood. Further difficulty in studying iT cell function comes from some limitations of current mammalian models, including the relative low frequency of these cells and the compensatory effects exerted by conventional T cells in knockout mice deficient for specific MHC class I-like genes or lacking iT cell subsets. The field would benefit from an alternative animal model to circumvent these limitations. While iT cells were thought to be mainly a mammalian attribute, their characterization in the amphibian Xenopus laevis has changed this perception and provided strong evolutionary evidence of their biological relevance. Moreover, X. laevis and particularly its tadpole stage presents several useful features for investigating iT cell function. Notably, tadpoles develop an adaptive immune system free of maternal influence within a few weeks following fertilization, which is fundamentally similar to that of mammals. However, unlike murine models, tadpoles rely predominantly on iT cells. Concomitant with a suboptimal classical MHC class I function and a diversification of MHC class I-like genes, there is a preponderance of six distinct invariant TCRα rearrangements that implies the overrepresentation of six putative iT cell subsets represented in tadpoles (Table 1). In fact, one of these six iT cell subsets expressing the rearrangement Vα45-Jα1.14 has recently been shown to be critical for host defense against Mycobacterium marinum (Mm) [6]. In addition, large genetic and genomic resources, MHC-defined inbred strains of frogs and the amenability to reverse genetic loss-of-function by transgenesis further position the X. laevis tadpole as an attractive model for investigating MHC class I-like and iT cell function during mycobacterial infection. Lastly, X. laevis tadpoles’ transparency is convenient for intravital microscopy, which permits investigators to visualize the dynamic process of mycobacterial infections in the host in real time.
Table 1.
Amino acid sequence of the six invariant TCRa rearrangement with their MHC class I-like interacting elements in Xenopus laevis tadpoles. CDR3 sequences are in bold.
| iTCR rearrangement |
Deduced Amino Acid Sequence | interacting MHC class I-like molecule |
|---|---|---|
| Va45-Ja1.14 | SGSVFGDSVKEKDSQLFAEEGISVDLACSYSTSFSTTYNLYWYRQYTYGGPEYILFKANQGSLK NTAPFAEKKFQSEVKTNSTTLTITNVKPEDSATYRCALQRAYSGSGWELNFGSGTQLIVQPNIKDVEPSMYRLKACTR |
XNC4 |
| Va6-Ja1.43 | LALMDRMFSNPSPIFQYYRGNMYRLNCTHSGADYLYWYVQYPNKPLELLVNNLGQKSNGEFTVKIE KKDFHLCKGEAKVTDSAAYFCAASDTGGSTKLTFGKGTKLTVLPNIKDVEPSMYRLKACQS |
XNC10 |
| Va22-Ja1.32 | TCDHYQSVYLVGGHTASLPCSYKDSAISNLKWYRQYPGEKPNELMTIFTDG NRTEGRFTACATNAGAGSKLIFGTGTKLSVFPHIKDVEPSMYRLKACQS |
unknown |
| Va23-Ja1.3 | CVVGNAINSKEEYISRRIGENVTLTCEYSTSSTTPYLFWYRQYPNQIEYLLYRGAKGYSNLKHDGNYEKGKFDSI TNDTSTQLIIFSLTVEDSALYLCALSDTGIGYGKNIFGMATKLTVKHDIKDVEPSMYRLKACQS |
unknown |
| Va40-Ja1.22 | SLCQANVIQPTMEEVFAGANLTLQCKHPSITTSDYIHWYKQTPDQQPKFLIRALKDTTSDLLTIIF SKDRKSSELHIQNVKAEESGVYLCAVSTGGYGNMIFGQGTQLKVNPNIKDVEPSMYRLKACQS |
unknown |
Here, we describe a model in the X. laevis tadpole for studying MHC class I-like/iT cell function in host defense to Mm. We review the previously published work to introduce an MHC class I-tetramer approach and an intravital imaging platform to further study recruitment and intracellular interactions of important immune effector cells such as iT cells.
1. Innate-like T cells and MHC class I-like antigen recognition system in mammals
iT cells represent a distinct type of T lymphocyte that has features of both innate and adaptive immune cell effectors. They express a dimeric αβ T-cell receptor (TCR) as conventional T cells, but with more limited TCR rearrangement diversity (i.e., invariant TCRα chain paired with a limited β chain diversity). Unlike conventional T cells recognizing peptide presented by classical MHC class-Ia, iT cells recognize MHC class I-like molecules that are encoded by genes outside the MHC locus and that present unusual molecular products such as lipids and vitamin B derivatives. In addition, iT cells are in a “poised effector” state permitting the rapid activation of effector function within few hours post-stimulation [7]. Given that iT cells utilize a semi-invariant TCR repertoire and engage with oligomorphic molecules, it is speculated that iT cells recognize conserved microbial ligands or pathogen-associated molecular pattern (PAMPs).
Currently, the most extensively studied iT cell subsets in human and mouse are invariant natural killer T (iNKT) and mucosal associated invariant T (MAIT), which are restricted by MHC class I-like molecules CD1d, and MR1, respectively. iNKT cells recognize different lipids molecules either derived from self or from pathogens that are presented by CD1d molecules (reviewed in [8]). These CD1d-restricted iNKT cells were first defined in 1997 by Kawano T and collaborators [9, 10]. Interestingly, the study showed that α-galactosylceramide (αGalCer), a lipid molecule originally derived from a marine sponge Agelas mauritanus, strongly activated iNKT cells [11]. Other lipids with similar structures isolated from bacteria (e.g., Borrelia burgdorferi or Streptococcus pneumonia) were later identified as ligands for CD1d (reviewed in [12]). The ability to recognize ligands derived from genetically distant bacterial and multicellular species is consistent with the hypothesis that iNKT cells respond to conserved molecules or molecular patterns.
MAIT cells recognize ligands presented by MR1, which is highly conserved among mammalian species [13, 14]. MAIT cells recognize vitamin B byproducts derived from microbial biosynthesis of riboflavin [15]. The low frequency of MAIT cells in mouse (less than 1% of total peripheral T cells) makes functional studies difficult in this species. In contrast, MAIT cells are abundant in human, accounting for up to 10% of T cell population in the blood circulation [16]. To circumvent the problem, genetically modified mice enriched for MAIT cells were generated by over-expressing the mouse MAIT invariant (mVα19-Jα33) TCRα transgene [17]. However, several reports indicate that normal T cell ontogeny, especially γδ T cells, is perturbated in these transgenic (tg) mice [18, 19]. An alternative to artificially increase the number of iT cells in mouse would be to take advantage of an animal model with a natural predominance of iT cells, which is the case of X. laevis tadpoles as we will discuss in more detail in section 3.
2. Relevance of MHC class I-like/iT cells system in host defenses during mycobacterial infection.
Due to their relative high precursor frequency, their binding to non-polymorphic MHC class I-like molecules with conserved ligands, and their preset effector functions, iT cells are attractive candidates as immune therapeutic targets for TB disease. Several in vitro and in vivo studies suggest an involvement of both iNKT and MAIT cell subsets during Mtb infections (reviewed in [4]). CD1d-restricted iNKT cells can kill Mtb-infected murine macrophages in vitro [20]. Similarly, human MAIT cells are activated in response to Mtb infected epithelial cells in vitro [21]. Upon interaction with MR1, MAIT cells release pro-inflammatory cytokines such as interferon γ (IFNγ) and tumor necrosis factor α (TNFα). Although these in vitro studies are consistent with an anti-mycobacterial activity of iT cells, the respective role of MAIT and iNKT cells in host response is still poorly understood.
For example, CD1d surface expression is upregulated by macrophages adoptively transferred into the Mtb-infected lung of mice but not in uninfected recipient control [22]. CD1d upregulation by activated macrophages in turn induces the release of IFNγ by splenic iNKT cells [22]. Although this study suggests the activation of iNKT cell during Mtb infections in vivo, studies using CD1d−/− mice are more ambiguous since they show a similar susceptibility as wild type mice to Mtb infection [23]. This could be due to a compensatory response of conventional T cells. Overlapping responses of conventional CD4 T and iNKT cells are also documented in humans in which GM-CSF is not only released by iNKT cells co-incubated with Mtb-infected macrophages, but is also produced by CD4+ T cells from blood of TB patients [24]. With regard to clinical studies, additional limitations are encountered. IFNγ response of iNKT cells was detected from both peritoneal fluid and peripheral blood of TB patients following stimulation with PMA plus ionomycin [25]. However, the age of the patients tested was relatively wide (23-71 years old), and the human samples were collected only if Mtb bacteria were detected in the peritoneal fluids by acid fast staining. Thus, it is probable that the study has revealed the function of iNKT cells not only during the early stage but also during latent TB stages of infection. In addition, MAIT cell frequencies are highly variable among TB infected patients as well as in the healthy donors, which makes a general conclusion of the study difficult.
Experimental and clinical studies have also implicated MAIT cells in host defense to Mtb. A study has found a decreased number of MAIT cells in the blood circulation of active TB patient compared to latent TB patients [26]. Presumably, this might be due to the recruitment of TB into the Mtb infected lung. Also, the MAIT cells from active TB patient showed high expression of program death-1 (PD-1), which further implied an activation of MAIT cells in TB patients [27]. Consistent with clinical studies, MAIT cells isolated from peripheral blood of rhesus macaques were activated after BCG vaccination and TB infection, although the frequency of these cells in the blood did not change compared to healthy control. [28]. Whether MAIT cells are recruited, undergo local expansion, or retention at the site of active mycobacterial infection remain to be determined, which is not easy with non-human primate and human patients. Thus, in order to investigate MAIT cells activation and function at the tissue level, a pre-clinical murine model is crucial. However, again the relative low frequency of MAIT cells in mice poses a challenge and the data obtained with MR1-deficient tg mice are difficult to interpret. For example, when either MR1−/− or MR1+/+ tg mice were intravenously infected with M. abscessus, the bacterial burden in the spleen was not different [29]. In contrast, tg mice overexpressing MAIT cells specific invariant TCR transgenes Vα19 or Vβ6 had significantly higher burden of M. abscessus [29]. However, expressing these TCRαβ transgenes in mice resulted in aberrant T cell development, [18].
In that regard, X. laevis and more particularly X. laevis tadpoles represent an attractive new alternative model due to their predominant iT mediated immune response, and relatively easy accessibility compared to clinical and pre-clinical model including nonhuman primate, which is convenient for experimentation.
3. The MHC class I-like system of X. laevis
From the analysis of its annotated genome assembly, X. laevis possess at least 23 MHC class I-like genes, initially designated as Xenopus nonclassical (XNCs) [30]. However, since these genes are grouped in a locus outside the MHC proper, they should be considered as MHC class I-like genes. Compared to the limited number of MHC class I-like genes in mammals, the large number of XNC genes presented in X. laevis suggests a diversification of their immune functions. XNC genes exhibit no to low polymorphism and most of them have a limited expression pattern, which are the hallmarks of MHC class I-like molecules. While the phylogenetic relationships among MHC class I genes remains to be elucidated, it is noteworthy that neither CD1 nor MR1 gene homologs have been identified in ectothermic vertebrates so far. As such, XNC genes are most likely serving immune functions analogous or convergent to those of MHC-like genes in mammals [33].
Notably, XNC genes are expressed in tadpoles, whereas optimal expression and function of classical MHC class I molecules does not occur until the onset of metamorphosis [31]. The diversification of MHC class I-like genes in Xenopus and their preponderance in tadpoles has stimulated the search for iT cells. Indeed, analysis of the TCRα repertoire by deep sequencing has revealed the predominance (>80% of the sequences) of 6 rearrangements in germinal configuration among CD8negative and CD8low splenic T lymphocytes of 3-weeks old tadpoles, which is a hallmark of invariant TCR rearrangements [32, 33]. These data imply that T cell immunity in tadpoles is predominantly governed by iT cells [32]. In support of this hypothesis, two distinct iT cell subsets, iVα6 and iVα45 T cells, requiring distinct XNC molecules for their developments and functions have been characterized to date using reverse genetics approaches and MHC tetramer technology [6, 32].
4. A first non-mammalian iT cell/class I-like system in X. laevis based on XNC10-restricted iVa6T cells
The first evidence of a critical role of iT cells in immune defenses to pathogens in X. laevis was obtained by an RNAi-based loss-of-function approach combined with transgenesis to target XNC10 gene, and by using XNC10 tetramers to identify iVα6 T cells [32]. Notably, XNC10 deficient tg tadpoles had reduced transcript levels of the invariant rearrangement iVa6-J1.43, whereas the occurrence of the 5 other invariant rearrangements was unaltered, therefore indicating that XNC10 is required for the specific development of iVα6 T cells. The XNC10 and concomitant iVα6 T cell deficiency had a notable consequence on antiviral immune defenses: XNC10 knockdown (KD) tg tadpoles were markedly more susceptibility to infection with the ranavirus FV3 than the wild type (WT) infected controls. In the absence of the early recruitment of iVα6 T cells at the site of infection, pro-inflammatory and antiviral responses were blunted followed by increased FV3 viral loads. These data imply that iVα6 T cells are critical for early control of viral infection in tadpoles.
5. A second MHC class I-like/iT cells system in X. laevis critical during Mycobacterium marinum (Mm) infection.
By assessing the effect of silencing different XNCs on the expressions of other iTCRα, we found that XNC4 deficiency abrogated iT cells expressing iVα45-Jα1.14 (iVα45 T cells) [6]. This led us to postulate that iVα45 T cells are restricted to, or interact with, XNC4 for their development and function similarly to XNC10-restricted iVα6 T cells. However, the impact of XNC4 loss-of-function on immune function has revealed to be more complex. XNC4 KD tg tadpoles exhibited impaired immune defense against both viral (FV3) and mycobacterial (Mm) pathogens. Yet, when we targeted directly iVα45 T cells either by disrupting the Jα1.14 by CRISPR-Cas9 mutagenesis or the expression of the rearrangement by RNAi (KD), both methods impaired more specifically tadpoles’ immune defense against Mm and not against FV3. This suggests that although iVα45 T cells are specific anti-Mm cell effectors, XNC4 may also interact with other immune cell types important for antiviral immunity. As in mammals, candidate immune effectors interacting with XNC4 to consider may include other iT cell subsets, γδT and NK cells.
6. Further developing the X. laevis tadpole model to study mycobacteria host immunity
Due to the predominance of iT cells over conventional T cells, the X. laevis tadpole is an attractive alternative model for investigating the function of iT cells in vivo at the whole organism level. It is noteworthy that, iVα45-deficiency obtained by targeting directly the iVα45 TCR rearrangement results in increased susceptibility to Mm but not to FV3 pathogens as observed with XNC4 deficiency [6]. This suggests a specialization of iVα45 T cells toward mycobacterial rather than viral pathogens. Considering that these two pathogens are unrelated, we postulate that iVα45 T cells recognize distinct conserved motifs or patterns derived from mycobacteria but not from ranavirus, and which are presented by XNC4. However, the presence and the identity of such ligand(s) remain to be determined.
To begin to characterize the iVα45 T cells interacting with XNC4, we have followed the same approach used for XNC10 by generating XNC4 tetramers [32]. These tetramers are recombinant proteins consisting of the X. laevis beta-2 microglobulin linked to three alpha domains of XNC4, lacking the transmembrane and cytoplasmic domains but containing a BirA site for biotinylation (Figure 1A). The XNC4 tetramer construct was then transfected into SF9 insect cell lines, followed by purification of the XNC4 monomers that were assembled into tetravalent with fluorescently conjugated biotin. Although XNC4 tetramers were not loaded with any exogenous ligands, our rationale was that the conserved molecular patterns present in insect cells should allow an appropriate folding similar to XNC10 [32]. Our preliminary data show that XNC4 tetramers (XNC4-T) bind to a distinct lymphocyte population among tadpoles’ splenocytes with a mean frequency of 9% and a heterogeneous CD8 cell surface expression at steady state (Figure 1). To substantiate the involvement of these XNC4 interacting iVα45 T cells in anti-mycobacterial immune response, we infected tadpoles by intraperitoneal injection with Mm, then monitored the occurrence of XNC4-T+ lymphocytes in the liver by flow cytometry (Fig. 2A, B). In parallel, we determined the Mm genome copy number in the tadpoles’ liver using real time PCR with primers specific to Mm 16srRNA gene (Figure 2C). Although our preliminary flow cytometry study needs more data points to reach statistical significance, it suggests a rapid recruitment of XNC4-T+ cells in the liver of Mm infected tadpoles within 3 days post-infection (dpi), which persists at 6 dpi. These findings need also to be substantiated by the characterizing TCR rearrangements of XNC4-T+ binding cells. It will also be important to define in more details the kinetics of the response and the possible expansion of XNC4-T+ cells in the spleen or at the infection site, as well as the effector function of these XNC4-T+ cells during Mm infection.
Figure 1: Generation of XNC4-tetramers (XNC4-T) for identifying XNC4-interacting iT cells.
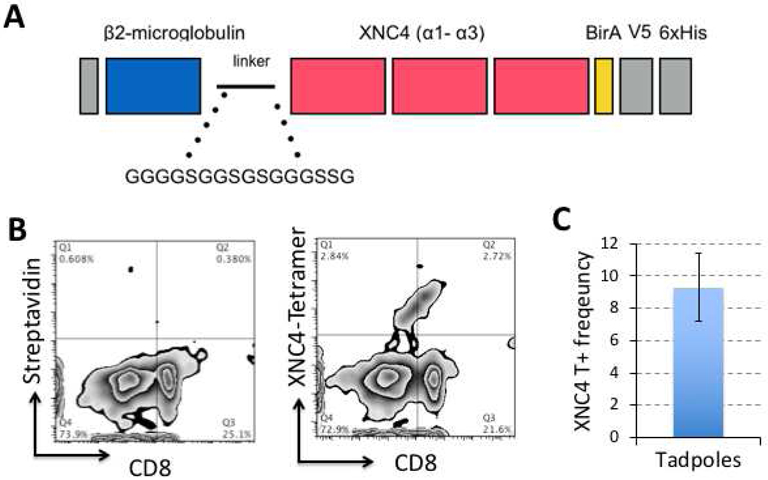
(A) Schematic view of the cDNA construct consisting of the X. laevis β2-microglobulin fused to the three alpha domains of XNC4, and followed by a BirA biotinylation site. The construct also contains a V5 and 6x Histidine tags for purification and identification of the recombinant protein. (B) Representative flow plot of splenocytes from outbred three weeks-old tadpoles (stage 53-55) co-stained with biotinylated, and tetramerized XNC4 with fluorescent (APC)-conjugated streptavidin and X. laevis-specific anti-CD8 monoclonal antibody. For negative control, only APC-conjugated streptavidin was used (C) Average frequency of the XNC4-tetramer binding T cell population in 5 individual tadpoles determined by adding the events in quadrans Q1 and Q2.
Figure 2. Detection of XNC4-T+ iT cells during Mm infection in tadpoles’ liver.
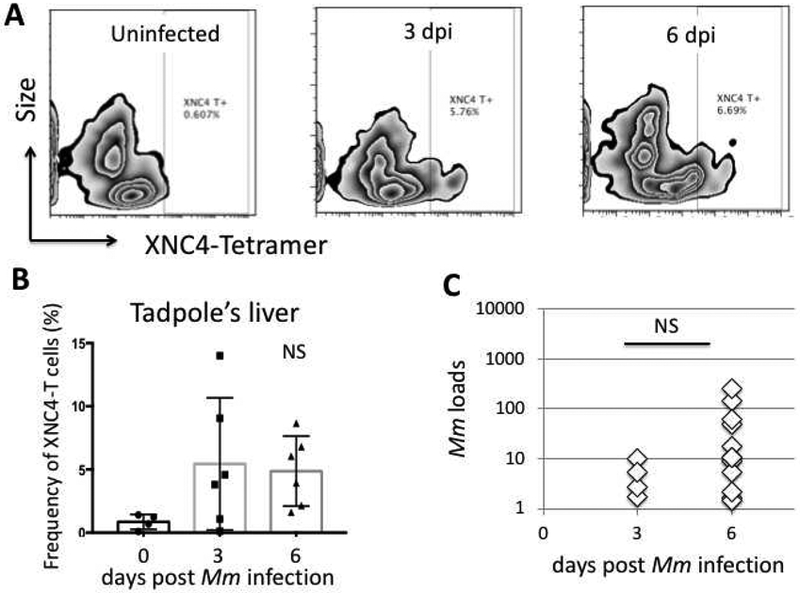
(A) Representative flow plot of lymphocytes isolated from liver of uninfected or intraperitoneally infected tadpoles at 3 and 6 days post-infection (dpi) and stained with XNC4-Tetramers conjugated with streptavidin-APC. The Y-axis represents forward scatter intensity (cell size), the X-axis represents the fluorescent intensity. For each sample, 10,000 events gated on live cells, were collected with Accuri C6 (BD Biosciences). Data was analyzed with FlowJo (TreeStar). (B) Average frequency of XNC4-T+ lymphocytes detected the liver of uninfected and infected tadpoles (N=6). (C) Mm loads determined by real time PCR with Mm specific 16srRNA genes. The Mann-Whitney U and ANOVA test were used for statistical analysis of flow cytometry and viral load data.
While reliable, intraperitoneal injection is likely to result in wide and rapid Mm dissemination. Additionally, to investigate the cellular interactions of iT cells in vivo, the peritoneal cavity is not easily accessible for intravital microscopy observation. Thus, to better examine immune cell recruitment as well as cells infected by Mm and the dissemination of these infected cells, we have implemented a more spatially localized route of infection suitable for intravital real-time microscopy. The system takes advantage of different X. laevis tg strains expressing fluorescent reporters in immune cell populations including cells of the myeloid lineages (GFP controlled by the lurp or Ly-6/uPAR-related protein promoter), macrophages (mCherry or GFP controlled by the zebrafish macrophage-specific mpeg1 promoter), or both lurp:gfp/mpeg1:mCherry [34]. This system is complemented by a stable Mm transfectant bacteria expressing dsRED as a fluorescent reporter. The use of fluorescent Mm has already been valuable to identify infected cells by flow cytometry [6]. With regard to our system, we infected tadpoles by microinjecting 6×105 colony forming units (CFU) of dsRED Mm (1μl of volume) into the tail muscle of mpeg1:gfp Tg tadpoles (Figure 3A). By using confocal microscopy, we detected mpeg+ macrophages infected with dsRED Mm within 1 day post-infection (dpi). At 15 dpi, the infected mpeg1+ cells were localized in the muscles as well as in neighboring blood vessels suggesting the systemic Mm dissemination through blood circulation.
Figure 3. Mm-Tg infection model to visualize immune cells during Mm infection in X. laevis tadpoles.
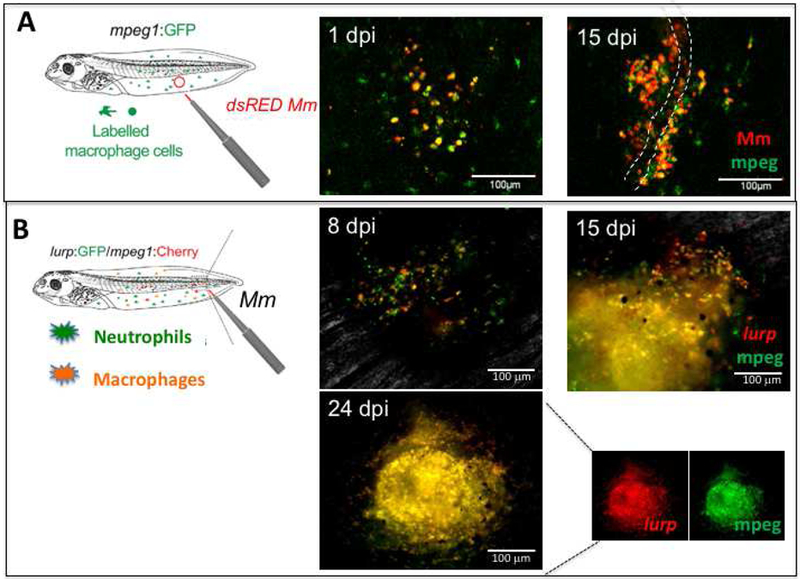
(A) dsRED Mm (6 ×105 CFU in 1 μl volume) was injected into the muscle tail of mpeg1:GFP tg tadpoles, then infiltration of mpeg1+ macrophage (green) and Mm-infected macrophages (red+ green+) were monitored in live tadpoles at 1 and 15 dpi by confocal microscopy (stage 53). The white dashed line indicates a lining of blood vessels. (B) Granuloma-like structure visualized by using double tg tadpoles (lurp:gfp/mpeg1:mCherry). The tg tadpoles visualize the two subsets of immune cells, neutrophils as green (mpeg1+) and macrophages as yellow or orange (lurp+ mpeg+). The intravital images were taken at 8, 15, and 24 dpi under a conventional fluorescent microscope. An unmerged picture of the granuloma-like structure at 24 dpi in a single channel to visualize mCherry only and GFP only signals are shown. Scale bars are presented as a white solid line. Image were taken under FV1000 Olympus Laser Scanning Confocal Microscope for (A), and Axiovert 200 inverted fluorescent microscope for (B).
To determine whether Mm infection results in a local granuloma structure similar to that of human infected with mTB, we utilized double tg (lurp:gfp/mpeg1:mCherry) tadpoles, which permits us to visualize not only macrophages but also neutrophils (Figure 3B). An accumulation of macrophages and neutrophils was observed by fluorescent microscopy on the same tadpole at different time points. Both macrophages and neutrophils accumulated at the site of infection within a few days and increased in numbers from 8 to 24 dpi, at which time the infection site exhibited a structure similar to a granuloma. The dual fluorescence signals of lurp:gfp/mpeg1:mCherry suggest that this granuloma-like structure consists of mostly macrophages. Collectively, these intravital images suggest that Mm readily infects mpeg1+ macrophages, and induces granuloma-like structure mostly consisting of mpeg1+/lurp+ macrophages. In future experiments we will use three-dimensional confocal imaging to further analyze in real time the cellular interactions of important immune cells including Mm infected macrophages and different iT cell populations with the tetramer technology.
Concluding remarks
Evidence from mammalian models convincingly show that iT cells act as early responders able to prime the microenvironment for an upcoming adaptive immune response and thus serve as “bridges” between innate and adaptive immune cell effectors. Furthermore, clinical and pre-clinical studies, as well as findings using our comparative X. laevis model suggest that iT cells have a protective function during mycobacterial infections. In X. laevis, while XNC10-restricted iVα6 T cells are considered to be a functional analog to iNKT cells, iVα45 T cells show more ambiguous characteristics similar to both iNKT cells and MAIT cells, especially during Mm infection. For example, iVα45 T-deficient tg tadpoles exhibit reduced gene expressions of IFNγ during Mm infection [6]. This implies that iVα45 T cells are critical for a robust IFNγ response against Mm, which is also found for murine iNKT cells and human MAIT cells during mycobacterial infections [35, 36]. Interestingly, the release of IFNγ by human intrahepatic MAIT cells is induced by IL-12 and IL-18 via intrahepatic monocytes [37]. In X. laevis, the ablated expressions of IL-12, and IL-18 resulting from iVα45 T deficiency is also consistent with a similar regulation loop, although the source of these cytokines in the liver of X. laevis is unknown. An interaction between iVα45 T cells and Mm-infected macrophages at the site of infection in the tail muscle is plausible according to our intravital microscopy observations in mpeg1:gfp tg tadpoles. One can only speculate at this stage whether similar interactions occur in the liver.
Based on these different findings, we have developed a working model to guide our future investigation where at steady state iVα45 T cells would maintain immune homeostasis by interacting with resident innate immune cells such as Kupffer cells (Figure 4, left panel). This would explain our result showing deregulated expression of multiple cytokine genes (TGFβ, iNOS, IL-1β and IL-10 [6]) in iVα45 T cell-deficient tadpoles. We further postulate that this homeostatic activity may require the presentation of a self- or commensal-derived ligand by XNC4. During Mm infection, immune homeostasis is disrupted by the infiltration of Mm-infected monocytes/macrophages, which results in the stimulation of a pro-inflammatory response by iVα45 T cells (Figure 4, right panel). The impaired expression response of IFNγ and TNFα as well as IL-12 and IL-18 in iVα45 T-deficient tg tadpoles is consistent with this [6]. To trigger the iVα45 T cell response, we hypothesize the presentation of Mm-derived ligand by XNC4. The overall inflammatory response would allow more leukocyte infiltration and ultimately the formation of granuloma-like structure.
Figure 4. Working model of iVα45 T cells involvement at steady state and during Mm infection.
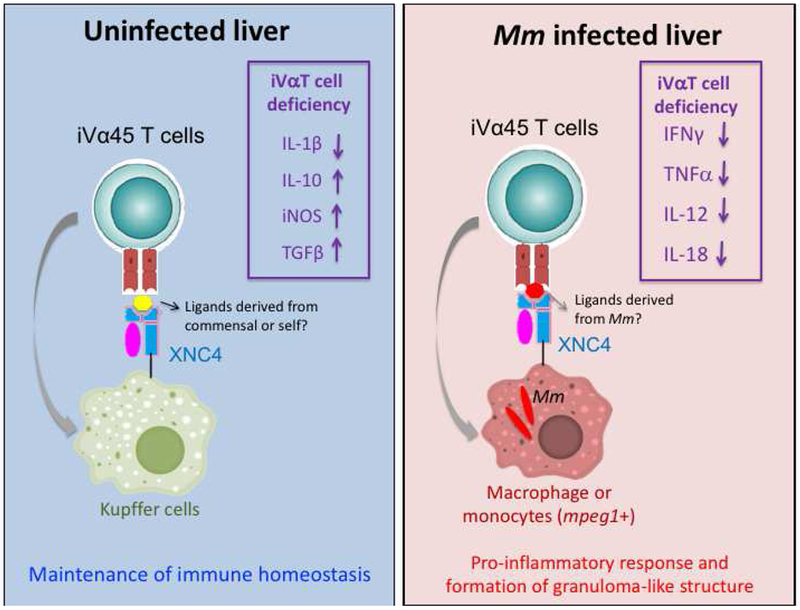
(Left) Putative role of iVα45 T cells in maintaining immune homeostasis is illustrated. The model postulates the interactions of iVα45 T cells with resident leukocytes such as Kupffer cells through the presentation of ligands self or commensal-derived by XNC4. The deregulation of multiple cytokine genes listed resulting from impairing iVα45 T cell function (boxed) is consistent with this model. (Right) Putative role of iVα45 T cells during Mm infection. The model postulates that immune homeostasis is disrupted by the infiltration of Mm-infected monocytes/macrophages presenting Mm-derived ligands in the context of XNC4 that activate iVα45 T cells and induce a pro-inflammatory response. The impaired gene expressions of IFNγ and TNFα as well as IL-12 and IL-18 in iVα45 T-deficient tadpoles (boxed) are consistent with this hypothesis.
We are actively pursuing the identification of XNC4 ligands. The ability to generate folded and reactive XNC4 tetramer using insect cell lines suggests the presence of some type of conserved ligands in the system. It is also noteworthy that the CDR3 regions of Vα45-Jα1.14 TCRα unlike the CDR3 of the other invariant TCRα in X. laevis contain an arginine (R) amino acid residue, which is postulated to interact with lipid ligands based on a crystal structure of TCR chimera from TB patients using CD1b tetramer [38]. Thus, it is tempting to speculate that iVα45 T cells interact with ligands potentially derived from Mm, self, or commensal microbes via a TCR-dependent pathway. In parallel, the combination of tg tadpole strains expressing fluorescent reporters, fluorescently labeled Mm and loss-of-function by transgenesis will allow us to obtain visual insights into the role of iT cells in altering other immune cell function such as macrophages in controlling Mm infection. As such, the model system we have established in X. laevis tadpoles will contribute to a better understanding of specific and direct functions of iT cells during mycobacterial infection and improve new vaccine development for TB.
Highlights.
X. laevis tadpoles rely on prominent MHC class I-like iT cell-mediated immunity
Tadpoles are uniquely suited to investigate anti-mycobacterial iT cell immunity
Tadpoles serve as an alternative model for study of immunity against Mtb
Acknowledgments
Funding information
We would like to thank Tina Martin for the expert animal husbandry, and Anne Benraiss PhD and Connor McGuire for critical reading of the manuscript. This research was supported by a predoctoral fellowship to RKH (T32AI118689), as well as R21AI139718 and R24AI059830 from the National Institute of Allergy and Infectious Diseases (NIH/NIAID) and IOS-1456213 from the National Science Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Flynn JL and Chan J, Immunology of tuberculosis. Annu Rev Immunol, 2001. 19: p. 93–129. [DOI] [PubMed] [Google Scholar]
- 2.Andersen P and Kaufmann SH, Novel vaccination strategies against tuberculosis. Cold Spring Harb Perspect Med, 2014. 4(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mittrucker HW, et al. , Poor correlation between BCG vaccination-induced T cell responses and protection against tuberculosis. Proc Natl Acad Sci U S A, 2007. 104(30): p. 12434–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang S, Targeting Innate-Like T Cells in Tuberculosis. Front Immunol, 2016. 7: p. 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Rhijn I and Moody DB, CD1 and mycobacterial lipids activate human T cells. Immunol Rev, 2015. 264(1): p. 138–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edholm ES, et al. , Distinct MHC class I-like interacting invariant T cell lineage at the forefront of mycobacterial immunity uncovered in Xenopus. Proc Natl Acad Sci U S A, 2018. 115(17): p. E4023–E4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brennan PJ, Brigl M, and Brenner MB, Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol, 2013. 13(2): p. 101–17. [DOI] [PubMed] [Google Scholar]
- 8.Joyce S, Girardi E, and Zajonc DM, NKT cell ligand recognition logic: molecular basis for a synaptic duet and transmission of inflammatory effectors. J Immunol, 2011. 187(3): p. 1081–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Exley M, et al. , Requirements for CD1d recognition by human invariant Valpha24+ CD4-CD8- T cells. J Exp Med, 1997. 186(1): p. 109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porcelli S, et al. , Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med, 1993. 178(1): p. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawano T, et al. , CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science, 1997. 278(5343): p. 1626–9. [DOI] [PubMed] [Google Scholar]
- 12.Kinjo Y, et al. , Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol, 2006. 7(9): p. 978–86. [DOI] [PubMed] [Google Scholar]
- 13.Huang S, et al. , MR1 antigen presentation to mucosal-associated invariant T cells was highly conserved in evolution. Proc Natl Acad Sci U S A, 2009. 106(20): p. 8290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldfinch N, et al. , Conservation of mucosal associated invariant T (MAIT) cells and the MR1 restriction element in ruminants, and abundance of MAIT cells in spleen. Vet Res, 2010. 41(5): p. 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corbett AJ, et al. , T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature, 2014. 509(7500): p. 361–5. [DOI] [PubMed] [Google Scholar]
- 16.Martin E, et al. , Stepwise development of MAIT cells in mouse and human. PLoS Biol, 2009. 7(3): p. e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawachi I, et al. , MR1-restricted V alpha 19i mucosal-associated invariant T cells are innate T cells in the gut lamina propria that provide a rapid and diverse cytokine response. J Immunol, 2006. 176(3): p. 1618–27. [DOI] [PubMed] [Google Scholar]
- 18.Terrence K, et al. , Premature expression of T cell receptor (TCR)alphabeta suppresses TCRgammadelta gene rearrangement but permits development of gammadelta lineage T cells. J Exp Med, 2000. 192(4): p. 537–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erman B, et al. , Early TCRalpha expression generates TCRalphagamma complexes that signal the DN-to-DP transition and impair development. Nat Immunol, 2002. 3(6): p. 564–9. [DOI] [PubMed] [Google Scholar]
- 20.Rothchild AC, et al. , iNKT cell production of GM-CSF controls Mycobacterium tuberculosis. PLoS Pathog, 2014. 10(1): p. e1003805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gold MC, et al. , Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol, 2010. 8(6): p. e1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skold M, et al. , Interplay of cytokines and microbial signals in regulation of CD1d expression and NKT cell activation. J Immunol, 2005. 175(6): p. 3584–93. [DOI] [PubMed] [Google Scholar]
- 23.Behar SM, et al. , Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J Exp Med, 1999. 189(12): p. 1973–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothchild AC, et al. , Role of Granulocyte-Macrophage Colony-Stimulating Factor Production by T Cells during Mycobacterium tuberculosis Infection. MBio, 2017. 8(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu C, et al. , Antigen-specific human NKT cells from tuberculosis patients produce IL-21 to help B cells for the production of immunoglobulins. Oncotarget, 2015. 6(30): p. 28633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwon YS, et al. , Mucosal-associated invariant T cells are numerically and functionally deficient in patients with mycobacterial infection and reflect disease activity. Tuberculosis (Edinb), 2015. 95(3): p. 267–74. [DOI] [PubMed] [Google Scholar]
- 27.Jiang J, et al. , Mucosal-associated invariant T-cell function is modulated by programmed death-1 signaling in patients with active tuberculosis. Am J Respir Crit Care Med, 2014. 190(3): p. 329–39. [DOI] [PubMed] [Google Scholar]
- 28.Greene JM, et al. , MR1-restricted mucosal-associated invariant T (MAIT) cells respond to mycobacterial vaccination and infection in nonhuman primates. Mucosal Immunol, 2017. 10(3): p. 802–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Bourhis L, et al. , Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol, 2010. 11(8): p. 701–8. [DOI] [PubMed] [Google Scholar]
- 30.Flajnik MF, et al. , A novel type of class I gene organization in vertebrates: a large family of non-MHC-linked class I genes is expressed at the RNA level in the amphibian Xenopus. EMBO J, 1993. 12(11): p. 4385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salter-Cid L, Nonaka M, and Flajnik MF, Expression of MHC class Ia and class Ib during ontogeny: high expression in epithelia and coregulation of class Ia and lmp7 genes. J Immunol, 1998. 160(6): p. 2853–61. [PubMed] [Google Scholar]
- 32.Edholm ES, et al. , Nonclassical MHC class I-dependent invariant T cells are evolutionarily conserved and prominent from early development in amphibians. Proc Natl Acad Sci U S A, 2013. 110(35): p. 14342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edholm ES, Banach M, and Robert J, Evolution of innate-like T cells and their selection by MHC class I-like molecules. Immunogenetics, 2016. 68(8): p. 525–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paredes R, et al. , Xenopus: An in vivo model for imaging the inflammatory response following injury and bacterial infection. Dev Biol, 2015. 408(2): p. 213–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang J, et al. , Enhanced immune response of MAIT cells in tuberculous pleural effusions depends on cytokine signaling. Sci Rep, 2016. 6: p. 32320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sada-Ovalle I, et al. , Innate invariant NKT cells recognize Mycobacterium tuberculosis-infected macrophages, produce interferon-gamma, and kill intracellular bacteria. PLoS Pathog, 2008. 4(12): p. e1000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jo J, et al. , Toll-like receptor 8 agonist and bacteria trigger potent activation of innate immune cells in human liver. PLoS Pathog, 2014. 10(6): p. e1004210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Rhijn I, et al. , A conserved human T cell population targets mycobacterial antigens presented by CD1b. Nat Immunol, 2013. 14(7): p. 706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]


