Abstract
Background:
A treat-to-target therapeutic approach is emerging as the new standard of care for treating inflammatory bowel disease (IBD), Crohn’s disease (CD) and ulcerative colitis (UC).
Aims:
We aimed to investigate the association of serum adalimumab concentrations during maintenance therapy with biochemical, endoscopic and histologic remission in IBD.
Methods:
This retrospective multi-center study included consecutive IBD patients on adalimumab maintenance therapy who had a C-reactive protein (CRP) within one week and/or endoscopic evaluation within twelve weeks of therapeutic drug monitoring between July 2013 and December 2016. Biochemical remission was defined as a normal CRP (≤5 mg/L). Endoscopic remission was defined as absence of any ulceration/erosion or a Rutgeerts score of ≤i1 for patients with an ileocolonic resection for CD and a Mayo endoscopic score of ≤1 for UC. Histologic remission was defined as absence of any sign of active inflammation. Adalimumab concentrations were measured using the homogeneous mobility shift assay.
Results:
Ninety-one CRP levels and 72 colonoscopies from 98 IBD patients [CD: n=72 (73%)] were evaluated. Based on receiver operating characteristic analyses we identified an adalimumab concentration threshold of 11.8, 12 and 12.2 μg/ml in CD and 10.5, 16.2 and 16.2 μg/ml in UC to stratify patients with or without biochemical, endoscopic or histologic remission, respectively. Adalimumab concentrations ≥12 μg/ml (OR: 8; 95%CI: 2–31.9; p=0.003) and ≥ 12.2 μg/ml (OR: 9.6; 95%CI: 1.7–56.1; p=0.012) were independently associated with endoscopic and histologic remission in CD, respectively.
Conclusions:
This study demonstrates that higher maintenance adalimumab concentrations are associated with objective therapeutic outcomes in IBD.
Keywords: Anti-TNF therapy, Crohn’s disease, ulcerative colitis, antibodies to adalimumab, therapeutic drug monitoring
INTRODUCTION
Anti-tumor necrosis factor (anti-TNF) therapies are a cornerstone of the medical management for inflammatory bowel disease (IBD).1 Goals of care for Crohn’s disease (CD) and ulcerative colitis (UC) are now moving towards more strict and objective therapeutic outcomes, such as endoscopic, histologic or composite remission.2
Therapeutic drug monitoring (TDM) is effective for optimizing anti-TNF therapy in IBD, and numerous studies have demonstrated that higher serum drug concentrations correlate with favorable therapeutic outcomes.3–18 Nevertheless, most of the data refer to infliximab and the enzyme-linked immunosorbent assay (ELISA), and typically focus on clinical response or remission.
The primary goal of the study was to investigate the association of serum adalimumab concentrations during maintenance therapy with biochemical, endoscopic or histologic remission in patients with IBD, using the homogeneous mobility shift assay (HMSA), while a secondary aim was to identify factors associated with these objective therapeutic outcomes.
MATERIALS AND METHODS
Study design, patient population and definitions
This was a retrospective multi-center study. Consecutive patients with IBD on adalimumab maintenance therapy, who had a C-reactive protein (CRP) within one week and/or endoscopic evaluation within twelve weeks of therapeutic drug monitoring (TDM) between July 2013 and December 2016, were eligible for the study. Patients with IBD unclassified, an ostomy or ileal pouch-anal anastomosis were excluded. Patients underwent colonoscopy either for assessment of mucosal healing, screening for dysplasia (surveillance colonscopies), or for IBD-related symptoms and evaluation of disease activity. A per event analysis was performed. A patient could be analyzed more than once provided that at least 6 months elapsed between CRP or colonoscopy and that each test correlated with an adjacent (and unique) serum sample. Biochemical remission was defined as a normal CRP (≤5 mg/L). Endoscopic remission was defined as absence of a mucosal break (ulceration or erosion) for CD, a Rutgeerts score of ≤i1 for CD patients with an ileocolonic resection, and a Mayo endoscopic score of ≤1 for patients with UC. Histologic remission was defined as absence of any sign of active inflammation including erosions, abscesses, or neutrophil infiltration. All demographic, biochemical, endoscopic and histological data were obtained from the electronic medical records. The study was approved by the Institutional Review Boards of the Beth Israel Deaconess Medical Center, Boston, Massachusetts and the University of Minnesota, Minneapolis.
Adalimumab concentration and antibodies to adalimumab
All serum adalimumab trough concentrations and antibodies to adalimumab (ATA) during maintenance therapy were measured by Prometheus Laboratories (San Diego, CA) using the HMSA.21 Adalimumab concentrations of < 1.6 μg/mL and ATA <1.7 U/mL were considered as undetectable.
Statistical analysis
Descriptive statistics were presented as median with interquartile range (IQR) for continuous variables and frequencies/percentages for categorical variables. Receiver-operating characteristic (ROC) analysis was performed to identify adalimumab concentration thresholds associated with biochemical, endoscopic or histologic remission. Optimal thresholds were chosen using the Youden index, which maximizes the sum of the specificity (SP) and sensitivity (SN) of the ROC curve.22 Adalimumab concentrations were compared between groups by a Mann-Whitney U-test. Univariate and multivariate binary logistic regression analyses were performed to determine the independent effects of variables associated with biochemical, endoscopic or histologic remission both for CD and UC. These included gender, age at start of adalimumab, disease duration, CD location and behaviour (for CD), upper gastrointestinal CD (for CD), perianal fistulizing CD (for CD), UC extension (for UC), smoking, adalimumab as first-line treatment, ADM dosing other than 40mg every other week at time of TDM, concomitant immunomodulators, ATA and adalimumab concentrations as a categorical variable using as cut-offs the ones derived from ROC analyses. Only variables with a p-value less than 0.1 from univariable analyses entered the multivariate analyses which were then performed based on the Backward Wald selection method. The results were expressed as odds ratio (OR) with 95% confidence intervals (CI) followed by the corresponding p-value. An incremental gain analysis was performed using 4 μg/mL increments of adalimumab concentration vis-à-vis their respective rate of biochemical, endoscopic or histologic remission. Results were considered statistically significant when p <0.05. All statistical analyses were performed using the SPSS 24.0 software (SPSS, Chicago, Illinois, USA) and GraphPad Prism version 5.03 for Windows (GraphPad Software, San Diego CA, USA).
RESULTS
Study population
Ninety-one CRP levels and 72 colonoscopies [(surveillance colonoscopies, n=28 (39%)] from 98 IBD patients [CD: n=72 (73%)] were evaluated. Patients’ demographic and clinical characteristics are depicted in Table 1.
Table 1.
Patient demographic and clinical characteristics.
| Patient characteristics | N=98 |
|---|---|
| Male, (%) | 38 (39) |
| Age at start of ADM, median (IQR), years | 33 (26–47) |
| Disease durationa, median (IQR), years | 7 (2–14) |
| CD, (%) | 72 (73) |
| CD locationb, (%): L1;L2;L3 | 20/72 (28); 13/72 (18); 39/72 (54) |
| Upper GI CD (L4)b, (%) | 3/72 (4) |
| CD behaviorb, (%): B1;B2;B3 | 33/72 (46); 10/72 (14); 29/72 (40) |
| UC extentb, (%): E1;E2;E3 | 0/26 (0); 16/26 (62); 10/26 (28) |
| Perianal fistulising CD, (%) | 32/72 (44) |
| Prior ileocolonic resection, (%) | 28/72 (39) |
| Smoking ever, (%) | 34 (35) |
| ADM as first-line treatment, (%) | 55 (56) |
| Concomitant IMM, (%) | 26 (27) |
| ATA at first TDM (%) | 12 (12) |
| ADM dosing other than 40mg eow at first TDM, (%) | 36/95 (38) |
| Reactive TDM | 56 (57) |
From diagnosis to start of ADM
Montreal classification.
ATA: antibodies to adalimumab; CD: Crohn’s disease; UC: ulcerative colitis; ADM: adalimumab; TDM: therapeutic drug monitoring; IMM: immunomodulators; IQR: interquartile range; GI: gastrointestinal; eow: every other week.
Crohn’s disease
Biochemical remission was found in 39/68 (57%) of CRP evaluations. Serum adalimumab concentration was statistically significant higher in patients with biochemical remission compared to those without (14.5 [(10–21.2] μg/mL vs. 9.4 [(4.8–13.9] μg/mL, p=0.007) (Figure 1A). ROC analysis identified an adalimumab concentration threshold of 11.8 μg/mL to be statistically significantly associated with biochemical remission (SN: 69%; SP: 66%) (Figure 2A). Univariable analysis identified adalimumab concentration ≥11.8 μg/mL [p=0.007, OR: 4.3 (95% CI: 1.5– 11.9)] as the only variable associated with biochemical remission (Table 2A). Multivariate analysis was not feasible as adalimumab concentration ≥11.8 μg/mL was the only variable associated with biochemical remission with a p-value less than 0.1 from univariable analysis. Based on incremental gain analysis, we identified an adalimumab concentration therapeutic window of 12–16 μg/mL to be associated with a biochemical remission rate over 65%, although the rate continued to increase beyond this range (Figure 3A).
Figure 1.
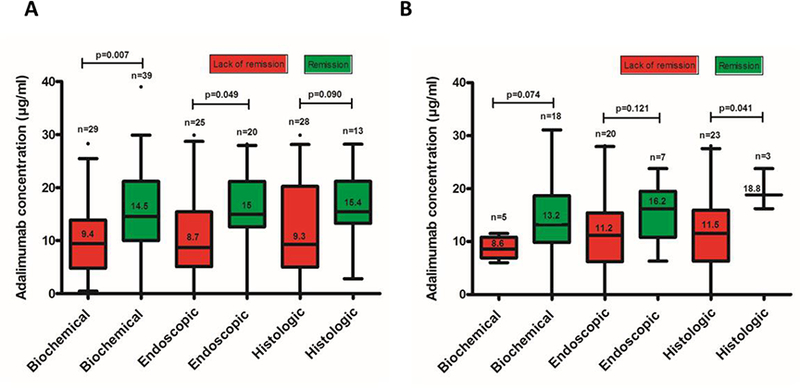
Distribution of serum adalimumab concentrations during maintenance therapy based on biochemical, endoscopic or histologic remission in patients with Crohn’s disease (A) and ulcerative colitis (B). Box plots (5–95%) show the median (solid line within box), interquartile range (upper and lower box boundaries), standard deviation (whiskers) and outliers (black dot).
Figure 2.
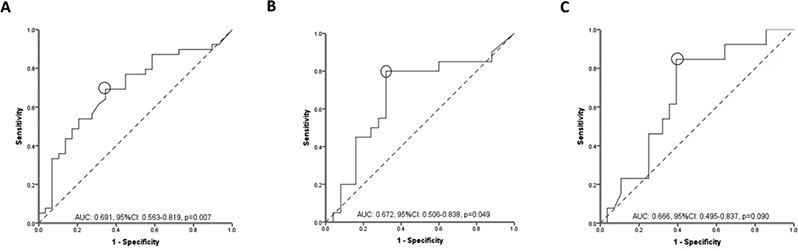
Receiver operator curve (ROC) analysis for serum adalimumab concentrations during maintenance therapy stratifying patients with Crohn’s disease with and without biochemical (A), endoscopic (B) or histologic (C) remission.
AUC: area under the curve, CI: confidence intervals.
Table 2.
Variables associated (p<0.1) with biochemical, endoscopic and histologic remission in Crohn’s disease.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variables | P | OR | 95%CI | P | OR | 95%CI |
| A. Biochemical remission | ||||||
| ADM concentration ≥11.8 μg/ml | 0.007 | 4.3 | 1.5–11.9 | |||
| B. Endoscopic remission | ||||||
| Disease duration | 0.073 | |||||
| ADM dosing other than 40mg eow at time of TDM | 0.006 | 16 | 1.8–143.2 | |||
| ADM concentration ≥12 μg/rnL | 0.002 | 8.5 | 2.1–33.8 | 0.003 | 8 | 2–31.9 |
| C. Histologic remission | ||||||
| CD location | 0.064 | |||||
| Disease duration | 0.052 | |||||
| ADM dosing other than 40mg eow at time of TDM | 0.048 | 5.2 | 1.01–26.8 | |||
| ADM concentration ≥12.2 μg/ml | 0.009 | 8.5 | 1.6–45.9 | 0.012 | 9.6 | 1.7–56.1 |
OR: odds ratio; CI: confidence interval; ADM: adalimumab; CD: Crohn’s disease; eow: every other week; TDM: therapeutic drug monitoring.
Figure 3.
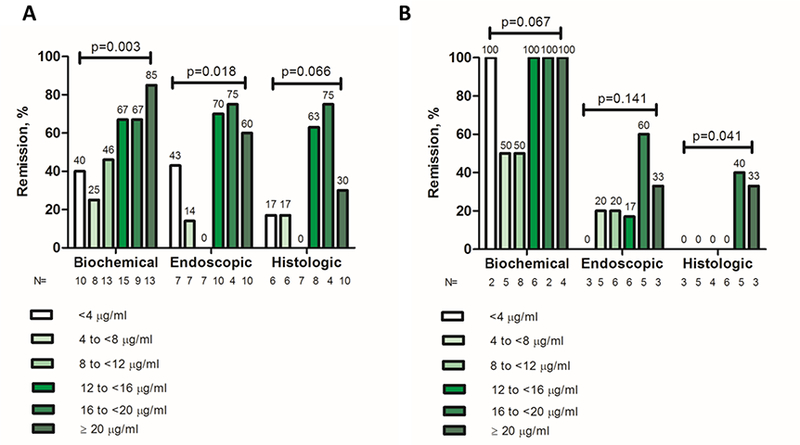
Incremental gain in biochemical, endoscopic and histologic remission rates in relation to serum adalimumab concentrations during maintenance therapy in patients with Crohn’s disease (A) and ulcerative colitis (B). Increments of 4 μg/mL adalimumab concentration were used to define the range of maximal increase in the rate of biochemical, endoscopic or histologic remission with any increase in drug concentration during maintenance therapy.
P-value (chi-square test, linear-by-linear association).
Endoscopic remission was noted in 20/45 (44%) of colonoscopies. Serum adalimumab concentration was statistically significantly higher in patients with endoscopic remission compared to those without (15 [12.6–21.2] μg/mL vs 8.7 [5.1– 15.4] μg/mL, p=0.049) (Figure 1A). ROC analysis identified an adalimumab concentration threshold of 12 μg/mL to be statistically significantly associated with endoscopic remission (SN: 80%; SP: 68%) (Figure 2B). In multivariate analysis, adalimumab ≥12 μg/mL was the only variable independently associated with endoscopic remission [p=0.003, OR: 8 (95% CI: 2–31.9)] (Table 2B). Based on incremental gain analysis, we identified an adalimumab concentration therapeutic window of 12–16 μg/mL to be associated with an endoscopic remission rate over 60% (reaching a plateau after an adalimumab concentration of ≥12 μg/mL) (Figure 3A).
Histologic remission was seen in 13/41 (32%) of colonoscopies. Serum adalimumab concentration was numerically higher in patients with histologic remission compared to those without (15.4 [13.3–21.2] μg/mL vs. 9.3 [5–20.3], μg/mL, p=0.090) (Figure 1A). ROC analysis identified an adalimumab concentration threshold of 12.2 μg/mL to be statistically significantly associated with histologic remission (SN 85%; SP 61%) (Figure 2C). In multivariate analysis, adalimumab concentration ≥12.2 μg/mL was identified as the only variable independently associated with histologic remission [p=0.012, OR 9.6 (95% CI 1.7–56.1)] (Table 2C). An incremental gain analysis of adalimumab concentrations associated with histologic remission in CD is shown in Figure 3A.
Ulcerative colitis
Biochemical remission was demonstrated in 18/23 (78%) of CRP evaluations. Serum adalimumab concentration was numerically higher in patients with biochemical remission compared to those without (13.2 [(9.8–18.7] μg/mL vs 8.6 [(6.9–10.8] μg/mL, p=0.074) (Figure 1B). ROC analysis identified an adalimumab concentration threshold of 10.5 μg/mL to be statistically significantly associated with biochemical remission (SN: 78%; SP: 80%) (Figure 4A). Univariable analysis identified adalimumab concentration ≥10.5 μg/mL [p=0.033, OR: 14 (95% CI: 1.2–163.4)] and age at start of adalimumab [p=0.044, OR: 0.92 (95% CI: 0.85–0.99)] as the only variables associated with biochemical remission. Following multivariate analysis none of these variables were independently associated with biochemical remission (Table 3A). An incremental gain analysis of adalimumab concentrations associated with biochemical remission in UC is shown in Figure 3B.
Figure 4.
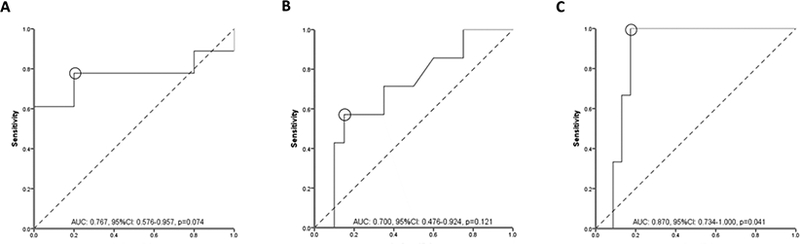
Receiver operator curve (ROC) analysis for serum adalimumab concentrations during maintenance therapy stratifying patients with ulcerative colitis with and without biochemical (A), endoscopic (B) or histologic (C) remission.
AUC: area under the curve, CI: confidence intervals.
Table 3.
Variables associated (p<0.1) with biochemical, endoscopic and histologic remission in ulcerative colitis.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variables | P | OR | 95%CI | P | OR | 95%CI |
| A. Biochemical remission | ||||||
| ADM concentration ≥10.5 μg/ml | 0.033 | 14 | 1.2–163.4 | |||
| Male | 0.056 | |||||
| Age at start of ADM | 0.044 | 0.92 | 0.85–0.99 | |||
| B. Endoscopic remission | ||||||
| ADM concentration ≥ 16.2 μg/rnL | 0.050 | 7.6 | 1.1–52.4 | |||
| C. Histologic remission | ||||||
| ADM concentration ≥ 16.2 μg/ml | 0.013 | N/A | N/A | |||
OR: odds ratio; CI: confidence interval; ADM: adalimumab; N/A: not applicable.
Endoscopic remission was found in 7/27 (26%) of colonoscopies. Serum adalimumab concentration was numerically higher in patients with endoscopic remission compared to those without (16.2 [10.8–19.5] μg/mL vs 11.2 [6.2–15.4] μg/mL, p=0.121) (Figure 1B). ROC analysis identified an adalimumab concentration threshold of 16.2 μg/mL associated with endoscopic remission (SN: 57%; SP: 85%) (Figure 4B). Univariable analysis identified adalimumab concentration ≥16.2 μg/mL [p=0.050, OR: 7.6 (95% CI: 1.1–52.4)] as the only variable associated with biochemical remission (Table 3B). Multivariate analysis was not feasible as adalimumab concentration ≥16.2 μg/mL was the only variable associated with biochemical remission with a p-value less than 0.1 from univariable analysis. An incremental gain analysis of adalimumab concentrations associated with endoscopic remission in UC is shown in Figure 3B.
Histologic remission was seen in 3/26 (12%) of colonoscopies. Serum adalimumab concentration was statistically significantly higher in patients with histologic remission compared to those without (18.8 [range: 16.2–23.8] μg/mL vs. 11.5 [6.3–15.9], μg/mL, p=0.041) (Figure 1B). ROC analysis identified an adalimumab concentration threshold of 16.2 μg/mL to be statistically significantly associated with histologic remission (SN 100%; SP 83%) (Figure 4C). Based on incremental gain analysis, we identified an adalimumab concentration therapeutic window of 16–20 μg/mL to be associated with a histologic remission rate over 30% (reaching a plateau after an adalimumab concentration of ≥16 μg/mL) (Figure 3B).
DISCUSSION
This multi-center, retrospective study demonstates that higher adalimumab concentrations during maintenance therapy are associated with objectibe therapeutic outcomes in IBD. We identified an adalimumab concentration threshold of 11.8, 12 and 12.2 μg/ml in CD and 10.5, 16.2 and 16.2 μg/ml in UC to stratify patients with or without biochemical, endoscopic or histologic remission, respectively. Moreover, we found adalimumab concentrations ≥ 12 μg/ml (OR: 8; 95%CI: 2–31.9; p=0.003) and ≥ 12.2 μg/ml (OR: 9.6; 95%CI: 1.7–56.1; p=0.012) to be independently associated with endoscopic and histologic remission in CD, respectively.
Besides the relatively large sample size and the exclusive use of the HMSA, our study is the first to investigate the association of adalimumab concentrations with biochemical, endoscopic and histologic remission in UC. Previous studies, typically referring to CD and using ELISA as the TDM assay, demonstrated that maintenance adalimumab concentration thresholds of 5.6–6.6 μg/ml and 4.9–10.3 μg/ml were associated with CRP normalization and mucosal healing, respectively. 6, 8, 11–18, 23 Yarur et al. showed that the adalimumab concentration that was best associated with histologic healing in patients with IBD was 7.8 μg/ml.7 Ungar et al. identified an adalimumab therapeutic drug window of 8–12 μg/mL as being associated with a mucosal healing rate of 80–90%.6 In our study, we actually showed that higher thresholds may be necessary for achieving the desired outcomes, especially in UC patients typically representing an IBD population with a higher inflammatory burden possibly requiring more drug on board to achieve higher rates of stringent objective therapeutic outcomes.
While our study demonstrates a therapeutic adalimumab window of 12–16 μg/ml in the maintenance phase for biochemical or endoscopic remission in CD, some patients may require even higher concentrations to achieve these outcomes. In fact, adalimumab concentrations ≥ 20μg/ml were associated with the highest rates of biochemical remission (85%), although due to the nature of our study we cannot prove causality. A similar dose reponse between a clinical outcome and anti-TNF concentration was noted by Yarur et al. with infliximab and fistula healing in CD.24 Consequently, in everyday clinical practice, each clinician can use a different therapeutic drug range depending on the therapeutic outcome of interest, as ‘one size cannot fit all.25
Limitations of this study include its retrospective nature and consequemtly the lack of central reading of endoscopies, and the lack of fecal calprotectin and a histologic score for defining histologic remission. Moreover, due to the retrospective design, this study can only establish association and not causality and higher serum drug concentrations may just reflect a lower inflammatory burden disease state related to reduced drug clearance.26
In conclusion, this study demonstrates that higher adalimumab concentrations during maintenance therapy are associated with objective therapeutic outcomes in IBD and provides first preliminary evidence that adalimumab concentration thresholds may vary depending on therapeutic outcome of interest and disease phenotype. Large, prospective studies are warranted to confirm these results and to identify clinically relevant thresholds.
Acknowledgments
K.P.: Ruth L. Kirschstein NRSA Institutional Research Training Grant (5T32DK007760–18). The content of this project is solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Author contributions: K.P.: study concept and design, data acquisition, analysis and interpretation, statistical analysis and manuscript writing; A.J.: data acquisition and interpretation and manuscript writing; B.P.V.: data acquisition and interpretation and manuscript critical revision; A.S.C: study concept and design, data analysis and interpretation and manuscript critical revision. All the authors approved of the final draft.
Conflict of interest: A.S.C: received consultancy fees from AbbVie, Janssen, Takeda, Ferring, Miraca, AMAG, and Pfizer; B.P.V.: receives research support from Takeda, Genentech, and Celgene and has received compensation from Janssen and AbbVie for speaking and advisory boards; The remaining authors disclose no conflicts of interest.
Ethical standards All procedures performed in the study were in accordance with the International Standard of Good Clinical Practice procedures and with the principles of the Declaration of Helsinki (1964) and relevant amendments. The protocol was approved by all institutional review boards and ethics committees at participating sites.
REFERENCES
- 1.Miligkos M, Papamichael K, Vande Casteele N, et al. efficacy and safety profile of anti-tumor necrosis factor-α versus anti-integrin agents for the treatment of Crohn’s disease: A network meta-analysis of indirect comparisons. Clin Ther 2016; 38: 1342– 58. [DOI] [PubMed] [Google Scholar]
- 2.Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015; 110: 1324–38. [DOI] [PubMed] [Google Scholar]
- 3.Papamichael K, Cheifetz AS. Therapeutic drug monitoring in IBD: the new standard-of-care for anti-TNF therapy. Am J Gastroenterol 2017; 112:673–6. [DOI] [PubMed] [Google Scholar]
- 4.Papamichael K, Cheifetz AS. Therapeutic drug monitoring in IBD: the new standard-of-care for anti-TNF therapy. Am J Gastroenterol 2017; 112:673–6. [DOI] [PubMed] [Google Scholar]
- 5.Papamichael K, Chachu KA, Vajravelu RK, et al. Improved long-term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin Gastroenterol Hepatol 2017;15:1580–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ungar B, Levy I, Yavne Y, et al. Optimizing anti-TNF-α therapy: serum levels of infliximab and adalimumab are associated with mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2016;14:550–7. [DOI] [PubMed] [Google Scholar]
- 7.Yarur AJ, Jain A, Hauenstein SI, et al. Higher adalimumab levels are associated with histologic and endoscopic remission in patients with Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis 2016;22:409–15. [DOI] [PubMed] [Google Scholar]
- 8.Ward MG, Warner B, Unsworth N, et al. Infliximab and adalimumab drug levels in Crohn’s disease: contrasting associations with disease activity and influencing factors. Aliment Pharmacol Ther 2017;46:150–61. [DOI] [PubMed] [Google Scholar]
- 9.Papamichael K, Baert F, Tops S, et al. Post-induction adalimumab concentration is associated with short-term mucosal healing in patients with ulcerative colitis. J Crohns Colitis 2017;11:53–9. [DOI] [PubMed] [Google Scholar]
- 10.Papamichael K, Cheifetz AS. Higher adalimumab drug levels are associated with mucosal healing in patients with Crohn’s disease. J Crohns Colitis 2016;10:507–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baert F, Vande Casteele N, Tops S, et al. Prior response to infliximab and early serum drug concentrations predict effects of adalimumab in ulcerative colitis. Aliment Pharmacol Ther 2014;40:1324–32. [DOI] [PubMed] [Google Scholar]
- 12.Mazor Y, Almog R, Kopylov U, et al. Adalimumab drug and antibody levels as predictors of clinical and laboratory response in patients with Crohn’s disease. Aliment Pharmacol Ther 2014;40:620–8. [DOI] [PubMed] [Google Scholar]
- 13.Morita Y, Imaeda H, Nishida A, et al. Association between serum adalimumab concentrations and endoscopic disease activity in patients with Crohn’s disease. J Gastroenterol Hepatol 2016;31:1831–6. [DOI] [PubMed] [Google Scholar]
- 14.Morita Y, Bamba S, Takahashi K, et al. Prediction of clinical and endoscopic responses to anti-tumor necrosis factor-α antibodies in ulcerative colitis. Scand J Gastroenterol 2016;51:934–41. [DOI] [PubMed] [Google Scholar]
- 15.Zittan E, Kabakchiev B, Milgrom R, et al. Higher adalimumab drug levels are associated with mucosal healing in patients with Crohn’s disease. J Crohns Colitis 2016;10:510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roblin X, Marotte H, Rinaudo M, et al. Association between pharmacokinetics of adalimumab and mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2014;12:80–4. [DOI] [PubMed] [Google Scholar]
- 17.Roblin X, Rinaudo M, Tedesco E, et al. Development of an algorithm incorporating pharmacokinetics of adalimumab in inflammatory bowel diseases. Am J Gastroenterol 2014;109:1250–6. [DOI] [PubMed] [Google Scholar]
- 18.Aguas Peris M, Bosò V, Navarro B, et al. Serum adalimumab levels predict successful remission and safe deintensification in inflammatory bowel disease patients in clinical practice. Inflamm Bowel Dis 2017;23:1454–60. [DOI] [PubMed] [Google Scholar]
- 19.Vaughn BP, Martinez-Vazquez M, Patwardhan VR, et al. Proactive therapeutic concentration monitoring of infliximab may improve outcomes for patients with inflammatory bowel disease: results from a pilot observational study. Inflamm Bowel Dis 2014;20:1996–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaughn BP, Sandborn WJ, Cheifetz AS. Biologic concentration testing in inflammatory bowel disease. Inflamm Bowel Dis 2015;21:1435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang SL, Hauenstein S, Ohrmund L, et al. Monitoring of adalimumab and antibodies-to-adalimumab levels in patient serum by the homogeneous mobility shift assay. J Pharm Biomed Anal 2013;78–79:39–44. [DOI] [PubMed] [Google Scholar]
- 22.Papamichael K, Van Stappen T, Vande Casteele N, et al. Infliximab concentration thresholds during induction therapy are associated with short-term mucosal healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2016;14:543–9. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe K, Matsumoto T, Hisamatsu T, et al. Clinical and pharmacokinetic factors associated with adalimumab-induced mucosal healing in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2018;16:542–9. [DOI] [PubMed] [Google Scholar]
- 24.Yarur AJ, Kanagala V, Stein DJ, et al. Higher infliximab trough levels are associated with perianal fistula healing in patients with Crohn’s disease. Aliment Pharmacol Ther 2017;45:933–40. [DOI] [PubMed] [Google Scholar]
- 25.Papamichael K, Cheifetz AS. Letter: infliximab concentrations during induction therapy-one size does not fit all. Aliment Pharmacol Ther 2018;47:1334–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brandse JF, van den Brink GR, Wildenberg ME, et al. Loss of infliximab into feces is associated with lack of response to therapy in patients with severe ulcerative colitis. Gastroenterology 2015;149:350–5. [DOI] [PubMed] [Google Scholar]


