Abstract
Copper 8-hydroxyquinoline-2-carboxaldehyde-thiosemicarbazide complex (CuHQTS) is a copper complex with strong anticancer activity against cisplatin-resistant neuroblastoma and prostate cancer cells in vitro by cell proliferation assay or fluorescent microscopic imaging. This study aimed to evaluate anti-prostate cancer activity of CuHQTS in vivo by bioluminescence Imaging (BLI) and tumor size measurement, using athymic nu/nu mice implanted with prostate cancer cells carrying luciferase reporter gene (Luc-PC3). Growth of Luc-PC3 cells (1× 105 cells) implanted in athymic nu/nu mice treated with CuHQTS for 2 weeks was suppressed by measurement of luciferase signals (6.18 × 107 to 5.36 × 107 p/sec/cm2/sr) with BLI, compared with luciferase signals of Luc-PC3 cells (4.66 × 107 to1.51 × 108 p/sec/cm2/sr, p < 0.05) in the mice treated with normal saline of placebo control. Moreover, the size of PC-3 xenograft tumor (126.5 ± 34.2 mm3) in athymic nu/nu mice treated with CuHQTS was significantly smaller than the size of PC-3 xenograft tumor (218.6 ± 48.0 mm3, p < 0.05) in athymic nu/nu mice treated with normal saline of placebo control, suggesting in vivo tumor growth inhibition activity of CuHQTS on prostate cancer. The findings of this study support further investigation of CuHQTS as a promising new anticancer agent for treatment of metastatic prostate cancer refractory to anticancer drugs currently available.
Keywords: Prostate cancer, anticancer copper complex, CuHQTS, Bioluminescence Imaging
Introduction
Prostate cancer is one of leading causes of death in men worldwide [1, 2]. Effective drugs are urgently needed for treatment of the patients diagnosed with metastatic prostate cancer refractory to therapies currently available. Cisplatin is a potent metallotherapeutic drug, but clinical application of cisplatin for treatment of prostate cancer is limited by side effects and acquired cisplatin-resistance of metastatic prostate cancer [3–6]. In addition to synthesizing and testing new organic molecules or compounds for anti-prostate cancer activities, there are continued efforts to develop new metallotherapeutic drugs for treatment of patients suffered from metastatic prostate cancer that are resistant to cisplatin or other anticancer drugs currently available [7–9].
Strong anticancer activity of many copper complexes were reported early since 1960s [10, 11]. Copper-pyrrolidine dithiocarbamate complexes [Cu(PDTC)2, Fig 1] suppressed proliferation of cisplatin-resistant BE2C human neuroblastoma cells with an IC50 of 8.0 µM, compared to cisplatin IC50 of 80 µM [12]. Based on Schiff-base copper coordination to form copper complex, copper complexes of 8-hydroxyquinoline-2-carboxaldehyde-thiosemicarbazide (CuHQTS, Fig 1) and 8-hydroxyquinoline-2-carboxaldehyde-4,4-dimethyl-3-thiosemicarbazide (CuHQDMTS, Fig 1) were synthesized, which showed strong anticancer activity on cisplatin-resistant neuroblastoma cells [13]. Many of copper anticancer complexes containing S, N, O-Donor system, Schiff base system, polydentate or macrocyclic system, N-N di-imine system were cytotoxic against prostate cancer cells or cells of other types of cancer [14, 15]. Recently, anti-prostate cancer activity of CuHQTS and CuHQDMTS were demonstrated in vitro by Cell Counting Kits-8 (CCK8) assay and fluorescent microscopic imaging [16]. The findings suggest that CuHQTS and CuHQDMTS are promising anticancer drugs for treatment of prostate cancer refractory to cisplatin chemotherapy.
Figure 1.
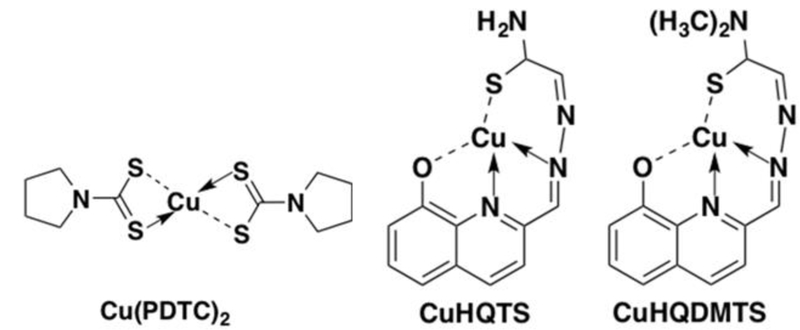
The structure of pyrrolidine dithiocarbamate copper complexes [Cu(PDTC)2], 8-hydroxyquinoline-2-carboxaldehyde–thiosemicarbazide copper complexes (CuHQTS), and 8-hydroxyquinoline-2-carboxaldehyde–4,4-dimethyl-3-thiosemicarbazide copper complexes (CuHQDMTS).
The purpose of this study was to test anti-prostate cancer activity of CuHQTS in vivo by treatment of mice implanted with prostate cancer cells with CuHQTS, using Bioluminescence Imaging (BLI). Tumor growth inhibition activity of CuHQTS in vivo was assessed by comparing the size of xenograft prostate tumor in mice treated with CuHQTS with the size of xenograft tumor in mice treated with normal saline (placebo control). The data from this study are expected to provide useful information for further study of potential application of CuHQTS for treatment of metastatic prostate cancer in humans.
Materials and Methods
Chemicals, reagents, and cells
Ammonium tetrathionolybdate (TM), a copper chelator, was purchased from Sigma-Aldrich (St. Louis, MO). 8-hydroxyquinoline-2-carboxaldehyde-thiosemicarbazide and 8-hydroxyquinoline-2-carboxaldehyde-4,4-dimethyl-3-thiosemicarbazide were purchased from Acros (New Jersy, NJ). The other reagents used in this study include matrigel matrix (corning, NY) and D-luciferin sodium (Gold Biotechnology, St. Louis, MO) were used without further purification. CuHQTS was synthesize and characterized as reported previously [13]. MSCV-Luciferase-EF1a-copGFP-T2A-Puro Pre-packaged Virus, a lentivirus containing a luciferase reporter gene, was purchased from System Biosciences (Palo Alto, CA). Prostate cancer cells was purchased from ATCC and cultured in RPMI 1640 (Sigma-Aldrich, St. Louis, MO) and Dulbecco’s Modified Eagle Medium (DMEM, Sigma-Aldrich, St. Louis, MO) medium containing 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO) and 1% penicillin and streptomycin (Gibco, Auckland, NZ). PC-3 cell line carrying luciferase reporter gene (Luc-PC-3) was established by infection of PC-3 cells with MSCV-Luciferase-EF1a-copGFP-T2A-Puro Lenti-virus in a protocol from the Vendor (System Biosciences, Palo Alto, CA).
Cell growth assay in vivo by Bioluminescence Imaging (BLI)
To evaluate prostate cancer cell growth in vivo, Luc-PC-3 cells (1× 104 or 5× 104 cells) suspended in 100 µL of 2:1 of medium and matrigel matrix were implanted subcutaneously in bilateral upper legs of athymic nu/nu mice (Taconic, Hudson, NY). Bioluminescence Imaging (BLI) was performed in a method as previously reported [17]. Immediately after subcutaneous injection of D-luciferin (in 80 µL of PBS) in the back region, the mice implanted with Luc-PC-3 cells were placed on a warming pad in the imaging box and a series of images were acquired for 35 minutes with auto exposure time (f stop = 1, stage setting = D) using an IVIS® Spectrum system (Perkin-Elmer (Xenogen), Alameda, CA). The mice were anesthetized by inhalation of 2% isoflurane (Butler animal health supply, Dublin, OH) with 2 L/min oxygen. Upon completion of imaging, elliptical regions of interests (ROIs) were draw over cell implantation site on the mice and the luminescence signals in the ROI (total flux: p/sec/cm2/sr) were quantified using the Living Imaging, V 4.3.1 (PerkinElmer). The same ROIs were applied to all images acquired sequentially in a single imaging session for a given mouse. The total signal intensity was plotted against time after D-luciferin injection to generate a time-intensity curve. Peak time and peak signal were determined for assessment of signals from implanted Luc-PC-3 cells. The growth of Luc-PC-3 cells implanted in mice were monitored every week by BLI for 4 weeks. All procedures using rodents for this study were conducted in a small animal study protocol approved by Institutional Animal Care and Use Committee (IACUC) of the University of Texas Southwestern Medical Center, Dallas, TX, USA.
Cell growth inhibition assay in vivo by BLI
To test feasibility of assessing effect of anticancer agents on cell growth in vivo by BLI, the mice implanted with Lu-PC3 cells were orally administered with copper chelator TM (10, 20, 30, 40 µg/g body weight) daily, followed by BLI every week to monitor growth of Luc-PC-3 cells for 4 weeks. To evaluate cell growth inhibition activity of CuHQTS, Luc-PC-3 cells (1× 104 cells) suspended in 100 µL of 2:1 of medium and matrigel matrix were implanted subcutaneously in bilateral upper legs of athymic nu/nu mice. Luc-PC-3 cells (1× 105 cells) suspended in 100 µL of 2:1 of medium and matrigel matrix were implanted subcutaneously in bilateral upper legs of athymic nu/nu mice and growth of Luc-PC-3 cells was tracked with Bioluminescence Imaging (BLI). When luminescence signals of Luc-PC-3 cells in mice (n = 6) reached about 5 × 107 p/sec/cm2/sr (about 2 weeks after implantation), CuHQTS (15 µg/g body weight/100 μL of 0.1% DMSO) was injected intravenously every other day to the mice bearing Luc-PC-3 cells, followed by BLI every other 2 to 3 days to monitor growth of Luc-PC-3 cells.
Tumor growth inhibition Assay in vivo
To evaluate tumor growth inhibition activity of CuHQTS in vivo, athymic nu/nu mice were implanted with PC-3 cells (5 × 106/injection) subcutaneously in the left and right flanks. Three days after implantation of PC-3 cells, the athymic nu/nu mice implanted with PC-3 cells were treated with intra-peritoneal injection of CuHQTS (15 µg/g body weight, dissolved in100 µL saline containing 1% DMSO) every other day. Another group of athymic nu/nu mice implanted with PC-3 cells were treated with normal saline as a group of placebo control. Tumor growth in mice implanted with PC-3 cells was evaluated by visual assessment of tumor growth every 2 days for two weeks and the size of tumor was measured with a ruler and recorded by length and width in centimeters. The volume of the tumor by a formula: V = 1/2ab2.
Statistical Analysis
A paired T-test was conducted for difference of luminescent signals from Luc-PC-3 cells in mice treated with CuHQTS and the luminescent signals from Luc-PC-3 cells in mice treated with normal saline. Difference of the PC-3 prostate xenograft tumor size in the mice treated with CuHQTS and the tumor size in the mice treated with normal saline (placebo control) was also tested by a paired student t test. A p value < 0.05 was considered statistically significant.
Results
Growth of Luc-PC-3 cells implanted in mice tracked by BLI
To determine whether growth of prostate cancer cells could be monitored with BLI, athymic nu/nu mice (n = 3) were implanted with Luc-PC3 cells (1 × 104 cells on the left leg and 5× 104 cells on the right leg). Establishment and viability of Luc-PC3 cells implanted in mice were tested by BLI at 3 days post implantation of the cells. Luciferase activity signals of Luc-PC3 cells carrying luciferase reporter gene were detected at implantation site on the mice, indicating successful stay and viability of Luc-PC3 cells. Growth of Luc-PC3 cells implanted in mice was tracked by BLI imaging every week for 4 weeks (Figure 2). Constant growth rate of Luc-PC3 cells was detected by BLI when 1 × 104 cells were implanted (Figure 2A). In contrast, variable growth rate of Luc-PC3 cells by BLI was observed when a larger number of Luc-PC3 cells (5× 104 cells) were implanted to an athymic nu/nu mice (Figure 2B). This suggested that implantation of a small number of 1 × 104 luc-PC3 cells will allow accurate assessment of cell growth inhibition activity of anticancer drugs, such as copper lowering drugs or anticancer copper complexes, in vivo by BLI.
Figure 2.
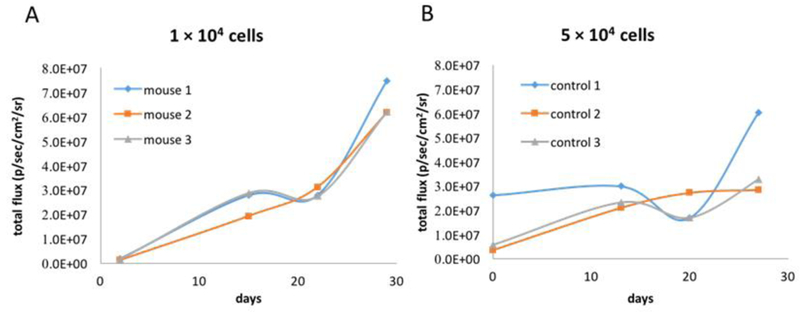
Growth of Luc-PC3 Prostate cancer cells in mice tracked by BLI. (A) Gradual increase of luminescence signals (total flux: p/sec/cm2/sr.) from Luc-PC3 cells was detected in mice implanted with 1 × 104 Luc-PC3 cells by BLI, with stead increase until 4 weeks post implantation. (B) Variable quantity of luminescence signals were detected in athymic mice implanted with 5 × 104 luc-PC3 cells by BLI. The findings suggested that implantation of 1 × 104 Luc-PC3 cells in mice was desirable for experiments of assessing cell growth inhibition activity of CuHQTS or other anticancer agents on prostate cancer cells in mice using BLI.
Cell growth inhibition activity of TM on Luc-PC3 cells in vivo by BLI
Copper is required for proliferation of prostate cancer cells and proliferation of prostate cancer cells could be suppressed by treatment with a copper chelator such as TM. To test feasibility of using BLI to track effects of anticancer agents on cell growth in vivo, athymic nu/nu mice implanted with Luc-PC-3 cells (1 × 104 luc-PC3 cells/site) were treated with oral administration of TM at a different dose (10, 20, 30, or 40 µg/g body weight). Suppressed growth of implanted Luc-PC-3 cells in the mice treated with TM was detected by BLI, compared with growth of Luc-PC-3 cells implanted in the control mice treated with normal saline (Figure 3). Luminescence signals of Luc-PC-3 cells implanted in the mice treated with TM (1.21 × 106, 4.12 × 105, 9.58 × 106, 8.67 × 106 p/sec/cm2/sr for 10, 20, 30, 40 µg/g body weight, respectively) were significantly lower than the luminescence signals of Luc-PC-3 cells in the control mice (6.61 × 107 p/sec/cm2/sr, p < 0.05). For unknown reasons, there was almost complete suppression of Luc-PC-3 cells growth when the mice were treated with 10, 20, or 40 µg TM/g body weight for 4 weeks. In contrast, incomplete suppression of Luc-PC-3 cell growth was detected by BLI when the mice implanted with Luc-PC-3 cells were treated with a dose of 30 µg/g body weight of TM for 4 weeks (Figure 3).
Figure 3.
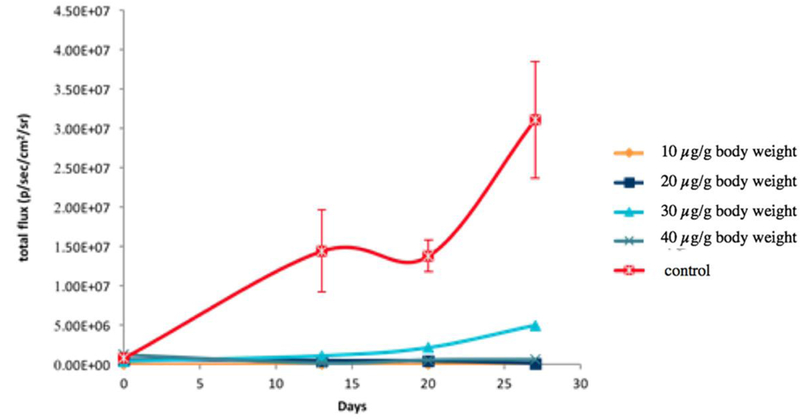
Cell growth inhibition activity of TM on prostate cancer cells in vivo by BLI. Luminescence signals measured from Luc-PC3 cells implanted in mice treated with TM were significantly lower than the luminescence signals of Luc-PC3 cells implanted in the mice treated with normal saline of placebo control. The findings demonstrated feasibility of using BLI to track cell growth inhibition activity of anticancer agents on prostate cancer cells implanted in athymic nu/nu mice.
Cell growth inhibition activity of CuHQTS on Luc-PC-3 cells in vivo by BLI
Cell growth inhibition activity of CuHQTS on Luc-PC3 cells implanted in athymic nu/nu mice (n = 6) was assessed by BLI, based on the feasibility of using BLI to track cell growth inhibition activity of TM on prostate cancer cells in vivo. Initially, 1 × 105 cells were implanted in bilateral upper legs of athymic nu/nu mice. At 2 weeks post implantation of Luc-PC3 cells, when luminescence signals of Luc-PC-3 cells reached about 5 × 107 p/sec/cm2/sr, the mice implanted with Luc-PC3 cells were treated with intravenous injection of CuHQTS (15 µg/g body weight/ 100 µL of 0.1% DMSO), once every other day. On 6th day post administration of CuHQTS, luminescence signals of Luc-PC-3 cells in mice treated with CuHQTS were found to be lower than the luminescence signals of Luc-PC-3 cells detected in the mice of placebo control (Figure 3, 4). By 13th day post treatment, luminescence signals of Luc-PC-3 cells implanted in the mice treated with CuHQTS (5.36 × 107 p/sec/cm2/sr) were significantly lower than the luminescence signals of Luc-PC-3 cells in the mice of placebo control (1.51 × 108 p/sec/cm2/sr, p < 0.05).
Figure 4:
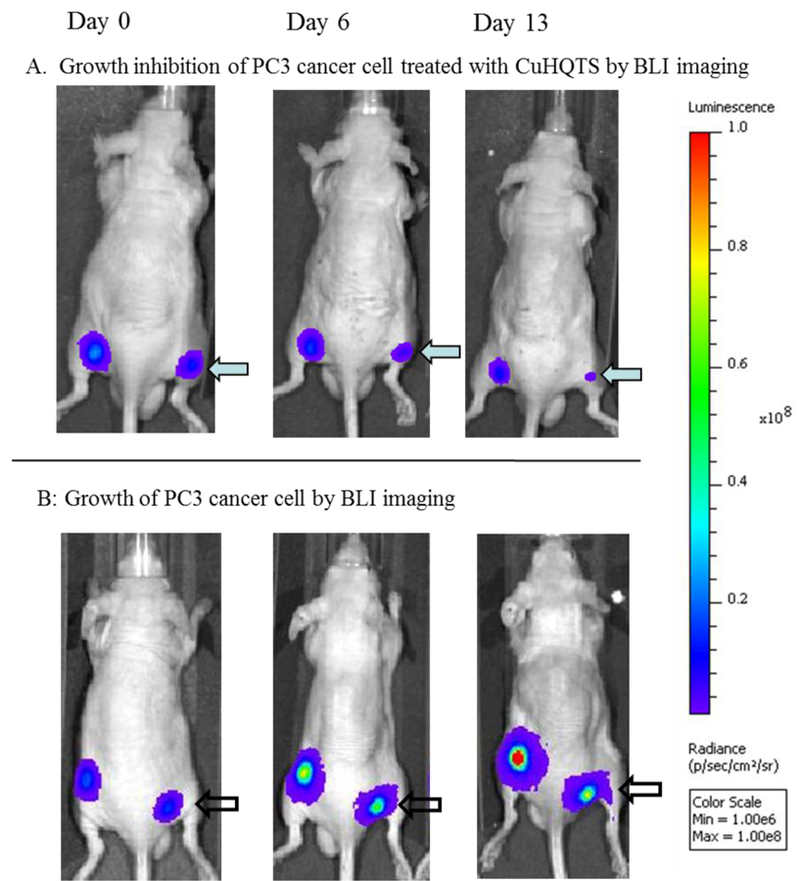
Representative BLI images showing Cell growth inhibition activity of CuHQTS on Luc-PC3 cells in mice. Luminescence signals of Luc-PC3 cells visualized in mice treated with CuHQTS (A) were significantly lower than the luminescence signals of Luc-PC3 cells visualized in mice treated with normal saline of placebo control (B) at 13 days post treatment with CuHQTS (15 µg/g body weight, once every other day). Day 0: day when injection of CuHQTS started (14 days post implantation of Luc-PC3 cells); Day 6 and Day 13: 6th and 13th day when injection of CuHQTS started.
Tumor growth inhibition activity of CuHQTS on prostate cancer xenograft tumors in mice
Anti-prostate cancer activity of CuHQTS was further evaluated in vivo by assessment of effects of CuHQTS on growth of PC3 xenograft tumors in athymic nu/nu mice (n=6, 3 in treatment group and 3 in placebo control. Starting 3 days post implantation of PC-3 cells (5 × 106 cells/site), the mice implanted with PC-3 cells were treated with peritoneal injection of CuHQTS (15 µg/g body weight, dissolved in100 μL saline containing 1% DMSO), once every other day. Growth of PC3 xenograft tumors in mice treated with CuHQTS was examined every day and size of tumor was measured and compared with the PC3 xenograft tumors in mice of placebo control. Growth of PC3 tumors in mice treated with CuHQTS was suppressed, compared with growth of PC3 tumors in mice treated with normal saline of placebo control (Figure 6). On 14th days post treatment, the average volume of PC3 xenograft tumors in athymic nu/nu mice treated with CuHQTS (126.5 ± 34.2 mm3 ) was significantly smaller than the volume of PC3 xenograft tumors in the mice of placebo control (218.6 ± 48.0 mm3, p < 0.05).
Figure 6.
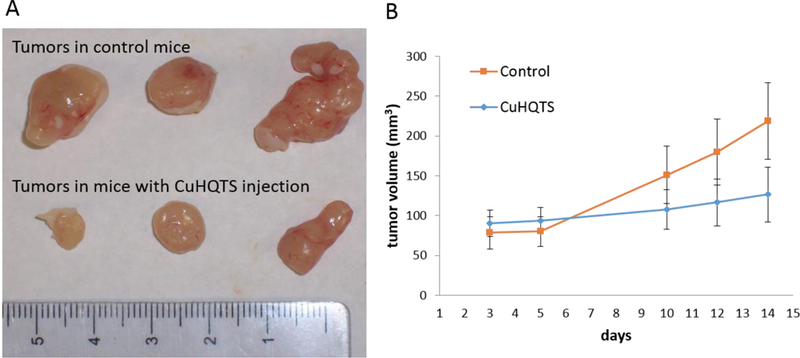
PC-3 Xenograft Tumor growth inhibition by treatment of tumor-bearing mice with CuHQTS. (A) PC-3 xenograft tumors from the mice treated with peritoneal injection of CuHQTS (15 µg/g body weight, once every other day, starting at 3 days post implantation of PC-3 cells) were smaller than PC-3 xenograft tumors in the mice treated with normal saline of placebo control at 14 days post implantation of PC3 cells. (B) Quantitatively, the volume of PC3 xenograft tumors in the mice treated with CuHQTS were smaller than the PC3 tumors of control mice treated with normal saline, as calculated from measurements on day 10, 12, and 14 post implantation of PC3 cells onto athymic mice (n=3).
Discussion
Copper complexes are promising new anticancer agents which may be used for treatment of cancer resistant to cisplatin [7–9, 18]. CuHQTS is a new copper complex with anticancer activity on prostate cancer cells in vitro [16], in addition to potent anticancer activity on cisplatin-resistant neuroblastoma cells [12]. In this study, we tested in vivo anti-prostate cancer activity of CuHQTS in mice for its potential application in treatment of metastatic prostate cancer refractory to anticancer drugs currently available.
We attempted to establish BLI method to assess cell growth inhibition activity of CuHQTS on small number of cells implanted in mice for its potential use for treatment of minimal residual disease of metastatic prostate cancer, because early treatment of minimal residual disease with CuHQTS may be more effective than treatment of late stage, large solid tumor of prostate cancer. Bioluminescence imaging (BLI) is a useful optical imaging tool for detection of cancer metastasis and assessment of therapeutic effects of anticancer drugs in preclinical anticancer drug development using small animal models [19–23]. Using cancer cells expressing luciferase reporter gene, BLI could be used to monitor therapeutic effect of anticancer drugs such as topotecan, on cancer cells with high sensitivity [20–21]. Although its low spatial resolution and depth sensitivity may limit its application in humans, BLI allows noninvasive, rapid preclinical testing of new anticancer drugs with high sensitivity and throughput [23–25].
In this study, we attempted to establish animal model for tracking effects of anticancer drugs on growth of cancer cell carrying luciferase reporter gene implanted in mice by BLI. We observed constant increase of luminescence signals along with growth of Luc-PC3 cells in mice by BLI, after implantation of 1 × 104 Luc-PC-3 cells subcutaneously. The findings demonstrated feasibility of tracking growth of Luc-PC-3 cells in mice with BLI. To determine feasibility of using BLI to assess effects of anticancer drugs on growth of prostate cancer cells in vivo, we tested effects of TM, a copper chelator, on growth of Luc-PC3 cells implanted in mice by BLI. TM is a copper chelator with known anticancer activity against prostate cancer and many types of other tumors in vivo [26–30]. Molecular mechanism of anticancer activity of TM is not well-defined, likely related to formation of a complex blocking the absorption of copper, the other one is formation of tripartite complex with copper and albumin in blood, blocking the cellular uptake of copper [26]. Marked decrease of luminescence signals of Luc-PC3 cells was detected in mice treated with TM, compared with luminescence signals of Luc-PC3 cells in mice of placebo control (Figure 3). These findings proved BLI is a useful tool to track effect of anticancer drugs on growth of cancer cells implanted in mice. The luminescence signals of Luc-PC3 cells detected in the mice treated with 10 or 20 μg/g body weight of TM were lower than the luminescence signals of Luc-PC3 cells in the mice treated with 30 μg/g body weight of TM (Fig. 3). The cause of this atypical dose response remained to be determined, which may be related to different microenvironment or other in vivo confounding factors present in the body of different mice.
Using BLI as a tool, we evaluated in vivo cell growth inhibition activity of CuHQTS on Luc-PC3 cells implanted in mice. Low luminescence signals of Luc-PC3 cells were visualized in the mice treated with CuHQTS, compared with the luminescence signals of Luc-PC3 cells in the mice treated with normal saline of placebo control (Fig 4, 5). In addition to cell growth inhibition demonstrated by BLI, the size of PC-3 xenograft tumors in the mice treated with CuHQTS was also smaller than the size of PC-3 xenograft tumors in the mice treated with normal saline (Fig 6). These findings demonstrated in vivoin vivo anti-prostate cancer activity of CuHQTS, supporting further investigation of potential use of CuHQTS for treatment of metastatic prostate cancer in humans.
Figure 5:
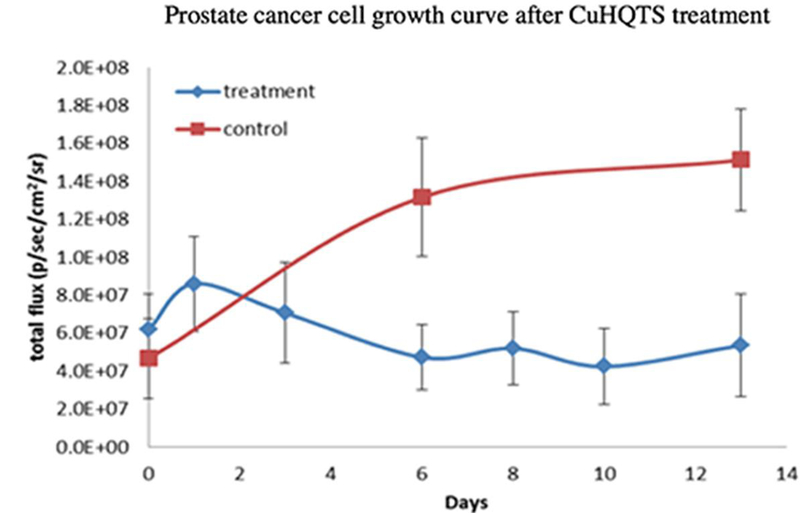
Quantitative comparison of luminescence signals of Luc-PC3 cells in mice treated with CuHQTS with luminescence signals in mice of placebo control by BLI. There was continuous increase of luminescence signals of Luc-PC3 cells in mice of placebo control starting day 0 when the mice of treatment group were treated with CuHQTS. In contrast, there was no increase or slight decrease of luminescence signals of Luc-PC3 cells in mice treated with intravenous injection of CuHQTS (15 µg/g body weight, once every other day). Treatment: a group of mice (n=3) treated with intravenous injection of CuHQTS (15 µg/g body weight, once every other day). Day 0: day when CuHQTS treatment started (14 days post implantation of Luc-PC3 cells). BLI was performed on days 0, 6 and 13 for control group, and on day 0, 3, 6, 8, 10, 13 for treatment group.
No death of mice was detected in the experiments of this study, until one month following intravenous injection of CuHQTS at a dose of 15 µg/g body weight contained in 100 µl of 0.1% DMSA. However, significant body weight loss (> 10% body weight) was noted starting 3 days post treatment with CuHQTS. Molecular mechanism of significant body weight loss in mice treated with CuHQTS remains to be elucidated, that may be related to toxic effect of thiosemicarbazone [31] or side effects of CuHQTS due to lack of cancer cell specificity. It is significant to continue synthesis of new thiosemicarbazone copper complexes with different molecular structures with selective cytotoxicity on prostate cancer cells. In addition to targeted delivery with polymer-based delivery vehicles [32, 33] and nano-particles [34, 35], targeted delivery of CuHQTS to prostate cancer lesions in vivo with small molecules of high prostate-specific membrane antigen (PSMA) binding affinity [36, 37] represent another promising approach to maximize anti-prostate cancer activity and minimize side effects from non-specific toxicity of CuHQTS on normal tissue or organs.
Conclusion
CuHQTS is a promising new anti-prostate cancer agent with strong anticancer activity in vivo, as demonstrated by cell growth inhibition activity in vivo demonstrated by BLI and tumor growth inhibition activity by tumor sized measurement. BLI was successfully used for assessing cell growth inhibition activity of CuHQTS against Luc-PC3 prostate cancer cells implanted in mice, suggesting that BLI may be a useful tool for development of anticancer drugs targeting minimal residual disease in metastatic prostate cancer, and potentially other cancers.
Acknowledgment
The authors thank Haiyuan Zhang for synthesis of CuHQTS used for this study. This study was partly funded by a faculty research development fund awarded to F. P by Harold C. Simmons Comprehensive Cancer Center, UT Southwestern Medical Center for financial support. The study was supported in part with infrastructure of the Southwestern Small Animal Imaging Resource provided by 1P30 CA142543 and P41-EB015908.
References
- 1.Berger MF, Lawrence MS, Demichelis F, Drier Y, Cibulskis K, Sivachenko AY, Sboner A, Esgueva R, Pflueger D, Sougnez C, Onofrio R, Carter SL, Park K, Habegger L, Ambrogio L, Fennell T, Parkin M, Saksena G, Voet D, Ramos AH, Pugh TJ, Wilkinson J, Fisher S, Winckler W, Mahan S, Ardlie K, Baldwin J, Simons JW, Kitabayashi N, MacDonald TY, Kantoff PW, Chin L, Gabriel SB, Gerstein MB, Golub TR, Meyerson M, Tewari A, Lander ES, Getz G, Rubin MA and Garraway LA (2011) Nature 470:214–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD and Jemal A (2017) CA Cancer J Clin 67:7–30 [DOI] [PubMed] [Google Scholar]
- 3.Muhammad N and Guo Z (2014) Curr Opin Chem Biol 19:144–153 [DOI] [PubMed] [Google Scholar]
- 4.Florea AM and Busselberg D (2011) Cancers 3:1351–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dasari S and Tchounwou PB (2014) Eur J Pharmacol 740:364–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siddik ZH (2003) Oncogene 22:7265–7279 [DOI] [PubMed] [Google Scholar]
- 7.Tabti R, Tounsi N, Gaiddon C, Bentouhami E and Desaubry L (2017) Med Chem 7:875–879 [Google Scholar]
- 8.Chaudhary A and Singh A (2016) Int J Curr Res Chem Pharm Sci 3:57–67 [Google Scholar]
- 9.Santini C, Pellei M, Gandin V, Porchia M, Tisato F and Marzano C (2014) Chem Rev 114:815–862 [DOI] [PubMed] [Google Scholar]
- 10.French FA and Freedlander BL (1958), Cancer Res, 18:1290. [PubMed] [Google Scholar]
- 11.Petering HG, Buskirk HH and Underwood GE (1964), Cancer Res, 24:367. [PubMed] [Google Scholar]
- 12.Zhang H, Wu JS and Peng F (2008) Anticancer Drugs 19:125–132. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Thomas R, Oupicky D and Peng F (2008) J Biolo Inorg Chem 13:47–55 [DOI] [PubMed] [Google Scholar]
- 14.Cater MA, Pearson HB, Wolyniec K, et al. (2013) ACS Chemistry Biol, 8,:1621–1631 [DOI] [PubMed] [Google Scholar]
- 15.Palanimuthu D, Shinde SV, Somasundaram K, Samuelson AG (2013). J. Med. Chem, 56:722–734. [DOI] [PubMed] [Google Scholar]
- 16.Xie F and Peng F (2017) J Fluoresc 27:1937–1941 [DOI] [PubMed] [Google Scholar]
- 17.Paroo Z, Bollinger RA, Braasch DA, Richer E, Corey DR, Antich PP and Mason RP (2004) Mol Imaging 3:117–124 [DOI] [PubMed] [Google Scholar]
- 18.Tisato F, Marzano C, Porchia M, Pellei M and Santini C (2010) Med Res Rev 30:708–749 [DOI] [PubMed] [Google Scholar]
- 19.Luker GD and Luker KE (2008) J Nucl Med 49:1–4 [DOI] [PubMed] [Google Scholar]
- 20.Edinger M, Sweeney TJ, Tucker AA, Olomu AB, Negrin RS and Contag CH (1999) Neoplasia 1:303–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Contag CH, Jenkins D, Contag PR and Negrin RS (2000) Neoplasia 2:41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaijzel EL, van der Pluijm G and Lowik CW (2007) Clin Cancer Res 13:3490–3497 [DOI] [PubMed] [Google Scholar]
- 23.Licha K and Olbrich C (2005) Adv Drug Deliver Rev 57:1087–1108 [DOI] [PubMed] [Google Scholar]
- 24.Willmann JK, van Bruggen N, Dinkelborg LM and Gambhir SS (2008) Nat Rev Drug Discov 7:591–607 [DOI] [PubMed] [Google Scholar]
- 25.Rudin M and Weissleder R (2003) Nat Rev Drug Discov 2:123–131 [DOI] [PubMed] [Google Scholar]
- 26.Cox C, Teknos TN, Barrios M, Brewer GJ, Dick RD and Merajver SD (2001) Laryngoscope 111:696–701 [DOI] [PubMed] [Google Scholar]
- 27.Redman BG, Esper P, Pan Q, Dunn RL, Hussain HK, Chenevert T, Brewer GJ and Merajver SD (2003) Clin Cancer Res 9:1666–1672 [PubMed] [Google Scholar]
- 28.Brewer GJ, Dick RD, Grover DK, LeClaire V, Tseng M, Wicha M, Pienta K, Redman BG, Jahan T and Sondak VK (2000) Clin Cancer Res 6:1–10 [PubMed] [Google Scholar]
- 29.Hassouneh B, Islam M, Nagel T, Pan Q, Merajver SD and Teknos TN (2007) Mol Cancer Ther 6:1039–1045 [DOI] [PubMed] [Google Scholar]
- 30.Pan Q, Kleer CG, Van Golen KL, Irani J, Bottema KM, Bias C, De Carvalho M, Mesri EA, Robins DM and Dick RD (2002) Cancer Res 62:4854–4859 [PubMed] [Google Scholar]
- 31.Mihich E, Simpson CL, Mulhern AI (1965) Cancer Res 25_1417–1431. [PubMed] [Google Scholar]
- 32.Yuan J, You Y, Lu X, Muzik O, Oupicky D and Peng F (2007) Mol Imaging 6:10–17 [PubMed] [Google Scholar]
- 33.Yuan J, Zhang H, Kaur H, Oupicky D, Peng F (2013) Mol Imaging 12:203–212. [PubMed] [Google Scholar]
- 34.Hrkach J, Hoff DV, Ali MM, et al. (2012). Sci Transl Med 4:128–39. [Google Scholar]
- 35.Xie Cai, F., Mulgaonkar A, Chen L, Sun X, Hsieh JT, Peng F, Tian R, Li L, Wu C and Ai H (2018) Nanomedicine DOI 10.2217/nnm-2018-0062 [DOI] [PubMed]
- 36.TKularatne SA, Wang K, Santhapuram HK, Low PS (2009). Mol Pharm 6:780–789. [DOI] [PubMed] [Google Scholar]
- 37.Tykvart J, Schimer J, Bařinková J, et al. (2014). Bioorg Med Chem 22:4099–108. [DOI] [PubMed] [Google Scholar]


