Abstract
Background
Elderly patients with acute coronary syndromes (ACS) are at higher risk both for ischemic and bleeding complications. Current guidelines recommend the PRECISE-DAPT score for bleeding risk stratification in this setting, but no study assessed its applicability in elderly patients. This study aimed to assess the performance of the PRECISE-DAPT score in a series of non-selected elderly patients with ACS from routine clinical practice.
Methods
The IFFANIAM registry included prospectively patients aged ≥ 75 years with ST segment elevation myocardial infarction (STEMI). Main outcome measured was the incidence of relevant bleeding after discharge (bleeding leading to hospital readmission, need for transfusion, intervention, stop of antithrombotic drugs or death). Bleeding risk was classified: (A) according to PRECISE-DAPT values above or not the recommended cut-off point (≥ 25); and (B) according to the quartiles of PRECISE-DAPT values observed in the IFFANIAM series (Q1: < 30; Q2: 30–35; Q3: 36–44; Q4: ≥ 45).
Results
A total of 208 patients were included. Mean age was 81.9 ± 4.5 years. Most patients (92.6%) had a PRECISE-DAPT value > 25. A total of 25 patients (12.0%) had bleeding events and 49 patients (23.6%) died. No significant differences regarding the incidence of bleeding were observed according to the recommended cutt of point ≥ 25. However, a progressive increase in the incidence of bleeding was observed across PRECISE-DAPT quartiles observed in this series (P = 0.038).
Conclusions
The vast majority of elderly patients have PRECISE-DAPT values above the recommended cut-off point for bleeding risk. Using different cut-off points could be a more rational approach for predicting bleeding risk in these complex patients.
Keywords: Acute coronary syndromes, Bleeding, Prognosis, The elderly
1. Introduction
The ageing of population is leading to a progressive increase in the number of elderly patents admitted with acute coronary syndromes (ACS).[1] These patients are at higher risk both for ischemic and bleeding complications, mortality and consumption of healthcare resources.[2] Therefore, the care of patients with ACS at older ages is becoming a major healthcare problem.[3]
On the other hand, there is strong evidence about the association between bleeding complications and increased mortality in this scenario.[4] Post-discharge bleeding episodes in patients with ACS have also been associated to poorer outcomes.[5] Bleeding risk is one of the main limiting factors for the choice of antithrombotic therapy in these patients.[6]
Current guidelines[7] recommend the use of the PRECISE-DAPT score[8] for bleeding risk stratification in this setting, suggesting a more conservative antithrombotic approach in patients with a PRECISE-DAPT value ≥ 25. Age accounts for a significant proportion of points of this score, and elderly patients have commonly high score values. To our knowledge, no study assessed the applicability of the PRECISE-DAPT score in patients at older ages. Therefore, the aim of this study was to assess the performance of the PRECISE-DAPT score in a series of non-selected elderly patients with ACS from routine clinical practice.
2. Methods
The IFFANIAM registry[9] included prospectively patients aged 75 years or older with ST segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention. A comprehensive geriatric assessment was performed during the admission, assessing clinical outcomes at mid-term follow up.
For the purpose of this subject analysis, we assessed 208 consecutive patients from this registry admitted in a tertiary care hospital and surviving after the admission. We registered baseline clinical characteristics, biochemistry, echocardiographic and angiographic data. PRECISE-DAPT score value was calculated for each patient.
A clinical follow up was performed by phone contact or review of medical records. Main outcome measured was the incidence of clinically relevant bleeding episodes after discharge, defined as those bleeding episodes leading to hospital readmission, need for transfusion, intervention, stop of antithrombotic drugs or death. Patients were classified according to their bleeding risk: (A) according to if PRECISE-DAPT score values were or not above the recommended cut-off point (≥ 25); and (B) according to the quartiles of PRECISE-DAPT score values observed in the IFFANIAM series (Q1: < 30; Q2: 30–35; Q3: 36–44; Q4: ≥ 45).
The association between PRECISE-DAPT score (as a continuous variable) and the incidence of post-discharge bleeding was assessed by a Fine and Gray competing risks regression method, considering the incidence of clinically relevant bleeding as dependent variable and mortality not due to bleeding as competing event. The discriminative ability of the model was assessed by calculating Receiver Operating Chaacteristics (ROC) curves and their corresponding area under the curve (AUC).
An additional analysis was performed in order to explore the specific contribution of each of the components of the score to the incidence of post-discharge bleeding in patients form the IFFANIAM registry. This analysis was also performed by Fine and Gray competing risks regression method, considering the incidence of clinically relevant bleeding as dependent variable and mortality not due to bleeding as competing event. All the components of the PRECISE-DAPT score (age, haemoglobin, creatinine clearance, white blood cell count and previous bleeding) were included in the model as independent variables. All analyses were performed by STATA 14.
3. Results
A total of 208 patients were included, of whom 115 (55.3%) were male. Mean age was 81.9 ± 4.5 years. Mean PRECISE-DAPT score value was 38 (range 15–83). Clinical characteristics for the whole cohort are shown in Table 1.
Table 1. Clinical characteristics and management according to the occurrence of post discharge bleeding.
| Overall cohort (n = 208) | Bleeding (n = 25) | No bleeding (n = 183) | P-value | |
| Baseline characteristics | ||||
| Age, yrs | 81.9 ± 5 | 82.6 ± 6 | 81.9 ± 4 | 0.538 |
| Male | 115 (55.3%) | 16 (64%) | 99 (54.1%) | 0.380 |
| Diabetes mellitus | 67 (32.2%) | 5 (20%) | 62 (33.9%) | 0.154 |
| Hypertension | 157 (75.5%) | 19 (76%) | 138 (75.4%) | 0.979 |
| Dislypidemia | 135 (64.9%) | 13 (52%) | 122 (66.7%) | 0.129 |
| Peripheral artery disease | 26 (12.5%) | 5 (20%) | 21 (11.5%) | 0.189 |
| Prior stroke | 27 (13%) | 2 (8%) | 25 (13.7%) | 0.390 |
| Prior bleeding | 25 (12%) | 4 (16%) | 21 (11.5%) | 0.359 |
| Prior myocardial infarction | 30 (14.4%) | 6 (24%) | 24 (13.1%) | 0.154 |
| Killip class at admission ≥ 2 | 66 (31.7%) | 6 (24%) | 60 (32.8%) | 0.422 |
| Haemoglobin, g/dL | 13.2 ± 6 | 11.6 ± 2 | 13.4 ± 7 | 0.184 |
| Creatinine clearance, mL/min | 65 ± 25 | 52 ± 20 | 66 ± 25 | 0.422 |
| White blood cell count | 12040 ± 8189 | 11526 ± 3511 | 12111 ± 8690 | 0.740 |
| PRECISE-DAPT score | 38 ± 11 | 41 ± 12 | 37 ± 11 | 0.088 |
| LVEF | 47 ± 10 | 45 ± 11 | 48 ± 10 | 0.313 |
| Multivessel disease | 105 (50.5%) | 14 (56%) | 91 (49.7%) | 0.726 |
| Left main disease | 9 (4.3%) | 3 (12%) | 6 (3.3%) | 0.081 |
| Geriatric syndromes | ||||
| Comorbidity (CI#) | 1.6 ± 1.3 | 1.8 ± 1.6 | 1.6 ± 1.3 | 0.601 |
| Disability (BIΔ) | ||||
| Independient | 139 (66.8%) | 16 (64%) | 123 (67.2%) | 0.444 |
| Mild disability | 50 (24%) | 7 (28%) | 43 (23.5%) | |
| Moderate disability | 7 (3.4%) | 1 (4%) | 6 (3.3%) | |
| Severe disability | 2 (1%) | 1 (4%) | 1 (0.5%) | |
| Completely dependent | 6 (2.9%) | 0(0%) | 6 (3.3%) | |
| Frailty (FRAIL scale) | ||||
| No | 80 (38.5%) | 8 (32%) | 72 (39.3%) | 0.741 |
| Prefrail | 90 (43.3%) | 12 (48%) | 78 (42.6%) | |
| Frail | 38 (18.3%) | 5 (20%) | 33 (18%) | |
| Cognitive impairment (Pfeiffer test) | ||||
| No | 144 (69.2%) | 18 (72%) | 126 (68.9%) | 0.402 |
| Mild | 56 (26.9%) | 5 (20%) | 51 (27.9%) | |
| Severe | 8 (3.8%) | 2 (8%) | 6 (3.3%) | |
| Risk of malnutrition (MNA-SF) | 94 (45.2%) | 13 (52%) | 81 (44.3%) | 0.495 |
| Antithrombotic treatment at discharge | ||||
| Acetilsalicilic acid | 205 (98.6%) | 25 (100%) | 180 (98.4%) | 0.517 |
| Clopidogrel | 183 (88%) | 24 (96%) | 159 (86.9%) | 0.157 |
| Ticagrelor | 15 (7.2%) | 1 (4%) | 14 (7.7%) | 0.501 |
| Prasugrel | 2 (1%) | 0(0%) | 2 (1.1%) | 0.771 |
| Novel oral anticoagulants | 2 (1%) | 0(0%) | 2 (1.1%) | 0.879 |
| Vitamin K antagonists | 4 (1.9%) | 0(0%) | 4 (2.2%) | 0.594 |
Data are presented as means ± SD or n (%). LVEF: left ventricle ejection fraction; MNA-SF: mini-nutritional assessment (short form) test. CI: charlson index; BI: barthel index.
Clinical follow up was performed in 200 patients (96.1%). Median follow up time was 861 days (interquartile range 743), a total of 25 patients (12.0%) had bleeding events and 49 patients (23.6%) died. Most common causes of death were of non-cardiac origin (34/49, 69.4%), especially infections (12, 35.3%) and malignancy (3, 8.8%). Median time to occurrence of bleeding was 231 days, and 24% of bleeding events occurred within the first three months.
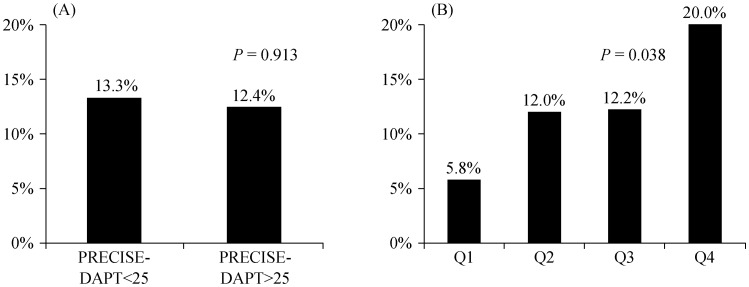
Patients who suffered bleeding events had slightly higher PRECISE-DAPT score values, without significant differences regarding age, sex, clinical characteristcs or geriatric syndromes. No significant differences regarding the incidence of bleeding were observed according to the recommended cut of point PRECISE-DAPT ≥ 25. However, a progressive increase in the incidence of bleeding was observed across PRECISE-DAPT quartiles observed in this series (Figure 1).
Figure 1. Proportion of patients suffering post-discharge bleeding events.
(A): According to PRECISE-DAPT values above or not the recommended cut-off point (≥ 25); and (B): according to the quartiles of PRECISE-DAPT values observed in the IFFANIAM series.
The PRECISE-DAPT score as a continuous variable was significanty associated with a higher incidence of post discharge bleeding [Sub-hazard ratios (SHR) = 1.03, 95% CI: 1.01–1.05; P = 0.049]. Overall, the discriminative ability of the model was modest (AUC = 0.621, 95% CI: 0.501–0.741; P = 0.062). Using the quartiles of PRECISE-DAPT significantly improved the predictive ability of the recommended cut-off point [net reclassification improvement (NRI) = 0.174, index discrimination improvement (IDI) = 0.182; P = 0.021].
The specific contribution of each of the components of the PRECISE-DAPT score for predicting bleeding in these patients is shown in Table 2. The only variable with a significant association with post discharge bleeding was baseline haemoglobin (SHR = 0.77, 95% CI: 0.71–0.83; P = 0.001).
Table 2. Specific contribution of each of the components of the PRECISE-DAPT score for the prediction of clinically relevant bleeding in the IFFANIAM series.
| Variable | Sub-hazard ratios (95% CI) | P-value |
| Age (for each increase 10 yrs) | 0.91 (0.32–2.57) | 0.853 |
| Previous bleeding | 1.34 (0.48–3.79) | 0.582 |
| White blood cell count | 0.99 (0.99–1.00) | 0.562 |
| Haemoglobin (for each increase of 1 g/dL) | 0.77 (0.71–0.83) | 0.001 |
| Creatinine clearance (for each increase 10 mL/min) | 0.95 (0.82–1.09) | 0.445 |
4. Discussion
Recommended bleeding risk scores in patients with ACS were developed from series with a low representation of patients at older ages.[10],[11] This is probably one of the main reasons for the lower ability of these scores for predicting bleeding in elderly patients. Likewise, the PRECISE-DAPT score was developed in a series from 8 randomized clinical trials with a mean age about 60–65 years. Age is one of the main predictors of bleeding in the PRECISE-DAPT series, accounting for a significant proportion of the score. In fact, an age of 75 years is equal to 12 points of the score. Therefore, the vast majority of elderly patients will have PRECISE-DAPT score values above the recommended cut-off point ≥ 25. In fact, 92.6% of patients from this series had values ≥ 25), in contrast to the original PRECISE-DAPT series, in whom these patients were only about 25% of case.
The role of a comprehensive geriatric assessment for predicting bleeding in elderly patients with ACS remains poorly understood. While a significant association between frailty and in-hospital bleeding has been suggested,[12] other reports described a modest predictive role of ageing related variables, mainly driven by the effect of comorbidity.[13] No study assessed the role of frailty and other ageing variables for the prediction of post-discharge bleeding in patients with ACS. Data from our study revealed no significant association with bleeding for any of the different components of the geriatric assessment.
On the other hand, baseline haemoglobin was the only variable significantly associated with bleeding in our patients. The loss of effect of age might be due to the selection of patients aged 75 years or older, thus reducing the variability of patients values regarding this component of the score. This fact might have lead to a reduction of the overall predictive ability of the PRECISE-DAPT score in this setting. On the other hand, significant changes of the magnitude of the association between known risk factors and bleeding in elderly patients with ACS have been previously been described.[14] However, these data should be cautiously interpreted due to the low number of events observed in this study.
Current guidelines suggest a more conservative approach in patients with a PRECISE-DAPT score value ≥ 25. In our opinion, this may not be the optimal approach in elderly patients, which in turn have higher prevalence of diabetes mellitus, previous revascularization, and more extensive coronary artery disease or left main disease, and therefore are usually at higher risk for recurrent ischemic events.[15] Using different cut-off points in patients at older ages could probably be a more rational approach for predicting bleeding risk in these complex patients.
This study has some limitations, such as its moderate sample size, the relativey low number of events and the low utilisation of ticagrelor. In addition, information about the DAPT duration was not available, and the bleeding definition used was different form the TIMI bleding classification used in the original publication. Finally, these data come from a single center, so our findings should be validated in larger series with different clinical profile and management.
Despite these limitations, in our opinion these data reasonably show the need for adapting of the PRECISE-DAPT score in non-selected elderly patients with ACS from routine clinical practice. Optimizing risk sratification and clinical management in these age subgroup may lead to a more rational healthcare resources management.
Acknowledgments
All authors report no conflicts of interest.
References
- 1.Degano IR, Elosua R, Marrugat J. Epidemiology of acute coronary syndromes in Spain: estimation of the number of cases and trends from 2005 to 2049. Rev Esp Cardiol (Engl Ed) 2013;66:472–481. doi: 10.1016/j.rec.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 2.Khandelwal D, Goel A, Kumar U, et al. Frailty is associated with longer hospital stay and increased mortality in hospitalizad older patients. J Nutr Health Aging. 2012;16:732–735. doi: 10.1007/s12603-012-0369-5. [DOI] [PubMed] [Google Scholar]
- 3.Saunderson CE, Brogan RA, Simms AD, et al. Acute coronary syndrome management in older adults: guidelines, temporal changes and challenges. Age Ageing. 2014;43:450–455. doi: 10.1093/ageing/afu034. [DOI] [PubMed] [Google Scholar]
- 4.Eikelboom JW, Mehta SR, Anand SS, et al. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114:774–782. doi: 10.1161/CIRCULATIONAHA.106.612812. [DOI] [PubMed] [Google Scholar]
- 5.Caneiro-Queija B, Abu-Assi E, Raposeiras-Roubín S, et al. Differential prognostic impact on mortality of myocardial infarction compared with bleeding severity in contemporary acute coronary syndrome patients. Rev Esp Cardiol (Engl Ed) 2018;71:829–836. doi: 10.1016/j.rec.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Almendro-Delia M, García-Alcántara Á, de la Torre-Prados MV, et al. Safety and efficacy of prasugrel and ticagrelor in acute coronary syndrome. Results of a “real world” multicenter registry. Rev Esp Cardiol (Engl Ed) 2017;70:952–959. doi: 10.1016/j.rec.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2018;39:213–260. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 8.Costa F, van Klaveren D, James S, et al. PRECISE-DAPT Study Investigators. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389:1025–1034. doi: 10.1016/S0140-6736(17)30397-5. [DOI] [PubMed] [Google Scholar]
- 9.Ariza-Solé A, Formiga F, Vidán MT, et al. Impact of frailty and functional status on outcomes in elderly patients with ST-segment elevation myocardial infarction undergoing primary angioplasty: rationale and design of the IFFANIAM study. Clin Cardiol. 2013;36:565–569. doi: 10.1002/clc.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ariza-Solé A, Formiga F, Lorente V, et al. Efficacy of bleeding risk scores in elderly patients with acute coronary syndromes. Rev Esp Cardiol (Engl Ed) 2014;67:463–470. doi: 10.1016/j.rec.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Faustino A, Mota P, Silva J. Non-ST-elevation acute coronary syndromes in octogenarians: applicability of the GRACE and CRUSADE scores. Rev Port Cardiol. 2014;33:617–627. doi: 10.1016/j.repc.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 12.Alonso Salinas GL, Sanmartín Fernández M, Pascual Izco M, et al. Frailty predicts major bleeding within 30 days in elderly patients with acute coronary syndrome. Int J Cardiol. 2016;222:590–593. doi: 10.1016/j.ijcard.2016.07.268. [DOI] [PubMed] [Google Scholar]
- 13.Ariza-Solé A, Guerrero C, Formiga F, et al. Global geriatric assessment and in-hospital bleeding risk in elderly patients with acute coronary syndromes: insights from the LONGEVO-SCA registry. Thromb Haemost. 2018;118:581–590. doi: 10.1055/s-0038-1623532. [DOI] [PubMed] [Google Scholar]
- 14.Garay A, Ariza-Solé A, Formiga F, et al. Prediction of post-discharge bleeding in elderly patients with acute coronary syndromes: insights from the BleeMACS registry. Thromb Haemost. 2018;118:929–938. doi: 10.1055/s-0038-1635259. [DOI] [PubMed] [Google Scholar]
- 15.Jokhadar M, Wenger NK. Review of the treatment of acute coronary syndrome in elderly patients. Clin Interv Aging. 2009;4:435–444. doi: 10.2147/cia.s3035. [DOI] [PMC free article] [PubMed] [Google Scholar]