Abstract
Host disease resistance is the most desirable strategy for control of citrus canker, a disease caused by a gram-negative bacterium Xanthomonas citri subsp. citri. However, no resistant commercial citrus cultivar has been identified. Cybridization, a somatic hybridization approach that combines the organelle and nuclear genomes from different species, was used to create cybrids between citrus canker resistant ‘Meiwa’ kumquat (Fortunella crassifolia Swingle snym. Citrus japonica Thunb.) and susceptible grapefruit (Citrus paradisi Macfad) cultivars. From these fusions, cybrids with grapefruit nucleus, kumquat mitochondria and kumquat chloroplasts and cybrids with grapefruit nucleus, kumquat mitochondria and grapefruit chloroplasts were generated. These cybrids showed a range of citrus canker response, but all cybrids with kumquat chloroplasts had a significantly lower number of lesions and lower Xanthomonas citri subsp. citri populations than the grapefruit controls. Cybrids with grapefruit chloroplasts had a significantly higher number of lesions than those with kumquat chloroplasts. To understand the role of chloroplasts in the cybrid disease defense, quantitative PCR was performed on both cybrid types and their parents to examine changes in gene expression during Xanthomonas citri subsp. citri infection. The results revealed chloroplast influences on nuclear gene expression, since isonuclear cybrids and ‘Marsh’ grapefruit had different gene expression profiles. In addition, only genotypes with kumquat chloroplasts showed an early up-regulation of reactive oxygen species genes upon Xanthomonas citri subsp. citri infection. These cybrids have the potential to enhance citrus canker resistance in commercial grapefruit orchards. They also serve as models for understanding the contribution of chloroplasts to plant disease response and raise the question of whether other alien chloroplast genotypes would condition similar results.
Keywords: cybridization, plastid, disease resistance, Xanthomonas citri subsp. citri, Citrus
Introduction
Citrus canker, caused by Xanthomonas citri subsp. citri, is a serious disease in terms of economic losses to the citrus industry and is present in most citrus-producing areas of the wet subtropics worldwide (Graham et al., 2004; Pruvost et al., 2014). The financial impact is strongly associated with the biological and epidemiological characteristics of the disease. The causal agent is easily disseminated, and once the bacterium is established in an orchard, the disease can cause defoliation, blemished fruit, premature fruit drop and general tree decline (Gottwald et al., 2002; Graham et al., 2004). Citrus canker is a quarantine disease. In Florida, citrus plants and fruits moving into and out of the state need to be inspected and certified by the USDA (Dewdney and Graham, 2012). Besides these regulatory obstacles, control of citrus canker is costly for growers. In canker-free areas, it is important to prevent bacterial entry into the orchard through decontamination of equipment, vehicles and personnel and to inspect and remove symptomatic trees (Graham et al., 2004; Gottwald et al., 2001). In areas where citrus canker is endemic, use of windbreaks, protection of fruits and leaves with copper bactericidal sprays, control of leaf miner and use of tolerant citrus cultivars are recommended (Graham et al., 2004; Dewdney and Graham, 2012).
The use of host resistance is the most effective way to manage various plant diseases, including citrus canker, but no commercial citrus resistant to this disease has been identified (Deng et al., 2010). Nevertheless, commercial citrus cultivars are not equally susceptible to Xanthomonas citri subsp. citri. Grapefruits, navel oranges, and some early sweet oranges are considered highly susceptible. ‘Hamlin’ oranges and tangelos are less susceptible, but suffer severe loss of fruit under the most conducive conditions for Xanthomonas citri subsp. citri infection. ‘Valencia’ oranges, tangerines and tangors are tolerant to citrus canker (Gottwald et al., 2002; Fu et al., 2012). Approaches ranging from conventional breeding methods to production of transgenic plants are being utilized in order to produce resistant plants (Viloria et al., 2004; Grosser et al., 2005; Liu and Deng, 2007; Mendes et al., 2010; Zhang et al., 2010; Machado et al., 2011; Yang et al., 2011; Guo et al., 2013; Omar et al., 2018).
Transgenic citrus plants that express antimicrobial proteins (Boscariol et al., 2006; Stover et al., 2013), harpin proteins (Barbosa-Mendes et al., 2009) and resistance genes from other plant species (Mendes et al., 2010; Zhang et al., 2010; Dutt et al., 2015; Omar et al., 2018) have been produced. Most of these transformed plants have reduced citrus canker severity associated with activation of resistance pathways. However, there is no prediction for when these transgenic plants will be available for growers since many regulatory steps need to be approved until the release of a genetically modified (GM) fruit crop (Potrykus, 2013). Even if the transgenic plants are released, there is still the issue of public acceptance for GM products (Lucht, 2015), and therefore other alternatives should be considered for developing citrus canker resistance in desirable cultivars.
For conventional breeding approaches, the source of resistance comes from citrus germplasm. Some non-commercial citrus and citrus-related species possess field resistance to citrus canker. Calamondin (Citrus mitis) and kumquats (Fortunella spp.) are considered highly resistant (Khalaf et al., 2007, 2011; Deng et al., 2010). Even though these species are sexually compatible with commercial citrus cultivars, including sweet oranges and grapefruits, few commercial outcomes from these crosses have been released (Viloria et al., 2004). Several factors contribute to the lack of conventional breeding success in citrus, especially those associated with botanical and biological characteristics, such as apomixis, high heterozygosity and a long juvenility period (Grosser et al., 2000; Navarro et al., 2004; Machado et al., 2011). Finally, the lack of genetic knowledge for the inheritance of important horticultural traits makes it difficult to identify a promising cross (Navarro et al., 2004).
By comparison, somatic hybridization has been widely used in citrus breeding because this approach overcomes the main obstacles of conventional breeding (Grosser and Gmitter, 1990; Grosser et al., 2000, 2005). This technique allows production of hybrids with genomes of two parents avoiding the problems associated with heterozygosity and incompatibility (Navarro et al., 2004). Citrus is one of a few commodities where the potential of somatic hybridization has been extensively used for scion and rootstock improvement (Grosser and Gmitter, 1990; Grosser et al., 2000). One interesting outcome from this technique is the production of cybrids by asymmetric protoplast fusion procedures in which the nucleus of one species is combined with the cytoplasm of another species (Moreira et al., 2000; Cai et al., 2009; Bassene et al., 2011; Guo et al., 2013; Omar et al., 2017). Although the precise mechanisms that govern this outcome are unknown, it is likely due to the behavior of organelles as discussed by Greiner et al. (2015). Given a condition of heteroplasmy (more than one organelle genotype in a cell), organelles sort to homogeneity through subsequent cell division. This allows investigation of nuclear-cytoplasmic genome interactions in citrus breeding. Following this approach, protoplast fusions of ‘Meiwa’ kumquat with three different grapefruit cultivars (‘Marsh,’ ‘Flame,’ and ‘N11-11’ somaclone of ‘Ruby Red’ grapefruit) were performed with the goal of producing grapefruit cultivars with the potential for citrus canker resistance. Grapefruit mesophyll protoplasts were used as the nuclear donor and protoplasts from embryogenic suspension culture cells of kumquat served as the cytoplasmic donor. Protoplast somatic fusion mediated by the polyethylene glycol (PEG) method was used to successfully regenerate over 100 diploid grapefruit cybrids (Omar et al., 2017).
Even though the production is not as large as that of oranges, grapefruit is very important for the fresh fruit market and juice industry in Florida. During the 2012–2013 production season, 18 million boxes of grapefruit were harvested (Hodges et al., 2014). However, as mentioned previously, grapefruits are highly susceptible to citrus canker and several disease management strategies are required to successfully produce grapefruit in endemic citrus canker areas (Dewdney and Graham, 2012). Based on this, grapefruit was chosen as a nuclear donor to keep the important horticultural traits, and kumquat was chosen as a cytoplasmic donor due to citrus canker resistance observed in orchards (Khalaf et al., 2007). These new cybrids were produced in an effort to create grapefruit cultivars morphologically equivalent to standard commercial grapefruit but with enhanced citrus canker resistance (Omar et al., 2017). These were genotyped with two plastid and two mitochondrial markers and two different classes of cybrids were identified: one carrying both kumquat organelle genomes (Cybrid-K) and another one with kumquat mitochondria and grapefruit plastid genomes (Cybrid-G) (Omar et al., 2017).
This study aims to characterize the citrus canker response in these two different grapefruit cybrid types and compare their disease phenotypes to those of ‘Meiwa’ kumquat and ‘Flame,’ ‘Marsh,’ and ‘Ruby Red’ grapefruit. These studies will provide insight into the role of cytoplasmic genomes in host–pathogen interactions and identify novel genetic strategies to develop important disease response traits. Moreover, this study may lead to the development of important commercial grapefruit cultivars with resistance to citrus canker.
Materials and Methods
Plant Material
The present study used 22 diploid grapefruit cybrids regenerated from protoplast fusions between ‘Meiwa’ kumquat and three grapefruit cultivars ‘Marsh,’ ‘Flame,’ and ‘N11-11’ somaclone of ‘Ruby Red.’ Protoplasts of embryogenic suspension culture of ‘Meiwa’ kumquat were derived from the citrus embryogenic callus collection of the University of Florida’s Citrus Research and Education Center (UF-CREC) and mesophyll protoplasts were isolated from fully expanded leaves of the three grapefruit cultivars. The production and confirmation of these cybrids are fully described in a previous study (Omar et al., 2017).
The regenerated grapefruit cybrids have two different genotypes: 18 Cybrid-K types with grapefruit nucleus, kumquat mitochondria, and kumquat chloroplast and 4 Cybrid-G types with grapefruit nucleus, kumquat mitochondria and grapefruit chloroplast (Table 1). Ten plants from each genotype were T-bud grafted onto US-802 rootstocks, a cross between ‘Siamese’ pummelo (Citrus grandis) and ‘Gotha Road’ trifoliate orange (Poncirus trifoliata). Plants were cultivated in soilless medium (The Scotts Co., Marysville, OH, United States) contained in 3.8 L pots and maintained in the greenhouse between 20 and 30°C. Plants were fertilized twice a year with slow release fertilizer containing NPK (12–3–9) plus minor elements (Harrell’s LCC, Lakeland, FL, United States). After 1 year, these replicates were moved to a quarantine greenhouse used exclusively for citrus canker inoculation assays.
Table 1.
List of genotypes used on citrus canker inoculation assays.
| Genotypes | Fusion parental genotype | Organelle genome | ||
|---|---|---|---|---|
| Parent A | Parent B | Mitochondria | Chloroplast | |
| Parents | ||||
| ‘Marsh’ grapefruit | – | – | Grapefruit | Grapefruit |
| ‘Flame’ grapefruit | – | – | Grapefruit | Grapefruit |
| ‘N11-11’ grapefruit∗ | – | – | Grapefruit | Grapefruit |
| ‘Meiwa’ kumquat | – | – | Kumquat | Kumquat |
| Cybrids | ||||
| M-9 | ‘Meiwa’ | ‘Marsh’ | Kumquat | Kumquat |
| M-10 | ‘Meiwa’ | ‘Marsh’ | Kumquat | Kumquat |
| M-11 | ‘Meiwa’ | ‘Marsh’ | Kumquat | Kumquat |
| M-13 | ‘Meiwa’ | ‘Marsh’ | Kumquat | Kumquat |
| M-31 | ‘Meiwa’ | ‘Marsh’ | Kumquat | Kumquat |
| M-78 | ‘Meiwa’ | ‘Marsh’ | Kumquat | Grapefruit |
| M-81 | ‘Meiwa’ | ‘Marsh’ | Kumquat | Grapefruit |
| M-102 | ‘Meiwa’ | ‘Marsh’ | Kumquat | Grapefruit |
| F-2 | ‘Meiwa’ | ‘Flame’ | Kumquat | Kumquat |
| F-3 | ‘Meiwa’ | ‘Flame’ | Kumquat | Kumquat |
| F-5 | ‘Meiwa’ | ‘Flame’ | Kumquat | Kumquat |
| F-6 | ‘Meiwa’ | ‘Flame’ | Kumquat | Kumquat |
| F-10 | ‘Meiwa’ | ‘Flame’ | Kumquat | Kumquat |
| F-13 | ‘Meiwa’ | ‘Flame’ | Kumquat | Kumquat |
| F-15 | ‘Meiwa’ | ‘Flame’ | Kumquat | Kumquat |
| F-20 | ‘Meiwa’ | ‘Flame’ | Kumquat | Kumquat |
| N-4 | ‘Meiwa’ | ‘N11-11’ | Kumquat | Grapefruit |
| N-6 | ‘Meiwa’ | ‘N11-11’ | Kumquat | Kumquat |
| N-8 | ‘Meiwa’ | ‘N11-11’ | Kumquat | Kumquat |
| N-10 | ‘Meiwa’ | ‘N11-11’ | Kumquat | Kumquat |
| N-12 | ‘Meiwa’ | ‘N11-11’ | Kumquat | Kumquat |
| N-18 | ‘Meiwa’ | ‘N11-11’ | Kumquat | Kumquat |
∗‘N11-11’ is somaclone of ‘Ruby Red’ grapefruit.
Bacterial Strain and Culture Conditions
The Xanthomonas citri subsp. citri strain 2004-00054 was used for all inoculation assays. This strain was isolated in 2004 from sweet orange (C. sinensis) in Dade County, FL, United States. The strain was stored in glycerol under -80°C conditions in an ultra-low freezer. For inoculation assay, the Xanthomonas citri subsp. citri culture stored in glycerol was thawed and streaked on BD DifcoTM Nutrient Agar (0.3% beef extract, 0.5% peptone, 1.5% agar) (Fisher Scientific, Pittsburgh, PA, United States). The plate was kept at 28°C for 48–72 h. A single bacterial colony was seeded into 25 mL of sterile BD DifcoTM Nutrient Broth (0.3% beef extract, 0.5% peptone) (Fisher Scientific) and grown at 28°C for 24 h and shaken at 150 rpm. This condition is required for Xanthomonas citri subsp. citri to reach the log phase, a stage at which the bacterium is actively growing. The bacterial suspension was centrifuged at 5,000 × g for 10 min at 4°C, re-suspended in sterile saline phosphate buffer (PBS; 40 mM Na2HPO4 + 25 mM KH2PO4) and kept on ice. The bacterial suspension was adjusted to 0.3 OD at 600 nm, equivalent to 108 colony-forming units (cfu) mL-1 for gene expression studies. For disease resistance screening, the suspension was adjusted to 104 cfu mL-1. To confirm viability of the bacterium, the suspension was serially diluted and 50 μL of the final dilution was spread on each of two nutrient agar plates.
Attached Leaf Assay
The attached leaf assay was conducted with the genotypes listed in Table 1. The plants were pruned to stimulate the growth of new flushes. The most susceptible leaf stage for citrus canker inoculation was reached 2–3 weeks after pruning when immature leaves were 75% expanded. Bacterial suspension was pressure infiltrated in young leaves using a 1 cm3 needle-less tuberculin syringe. The syringe tip was pressed against the abaxial surface of the leaf. The bacterial suspension (approximately 2 μL) was infiltrated into the leaf until the water-soaked area reached about 6 mm in diameter (Francis et al., 2010). Three areas on each side of the leaf mid-vein were infiltrated. Excess inoculum was wiped from the leaf surface with KIMTECH delicate task wipes (Kimberly-Clark, Koblenz, Germany). Six leaves per plant and six plants per genotype were inoculated. The inoculated flushes were covered with clear plastic bags for 24 h to maintain high humidity. The plants were completely randomized and kept in the greenhouse at a temperature range from 20 to 30°C. Leaves inoculated with sterile PBS buffer were used as the control following the same protocol for infiltration and observation. Development of symptoms on leaves was observed weekly up to 21 days post inoculation (dpi). The number of lesions per leaf was counted at 14 dpi. The experiment was repeated three times. The sum of number of lesions per leaf was analyzed using the General Linear Models procedure (SAS Institute, Cary, NC, United States). Mean separation was performed according to the Student–Newman–Keuls test (P ≤ 0.05).
Bacterial Growth Curve in vivo
Bacterial growth curves were performed on selected genotypes. The genotypes chosen for comparison of resistance to Xanthomonas citri subsp. citri were ‘Meiwa’ kumquat, ‘Marsh’ grapefruit and two cybrids, M-9 (Cybrid-K type) and M-102 (Cybrid-G type). The leaves were inoculated as described previously in the attached leaf assay (Francis et al., 2010). Three 1-cm leaf disks per genotype, circumscribing the infiltrated area, were excised using a 1-cm cork borer immediately after inoculation and then at days 3, 8, 10, and 14. Leaf disks were surface disinfested by dipping in 0.5% sodium hypochlorite for 1 min, followed by immersion in 70% ethanol for 1 min and rinsed twice with sterile distilled water. Each disk was macerated individually in 1.0 mL of PBS buffer using a sterile mortar and pestle under aseptic conditions. Tenfold serial dilutions of the leaf suspension were made and dilutions were plated in duplicate on nutrient agar medium. One disk per plant and three plants per genotype were assayed for each time point. Total bacterial colonies per inoculation site were expressed as log10 cfu per inoculation site. The experiment was repeated three times. The log transformation of bacterial population per inoculation sites was analyzed using the ANOVA procedure (SAS Institute). Mean separation was performed according to Tukey’s Studentized Range (HSD) Test (P ≤ 0.05).
Total RNA Isolation
To identify genes that were differentially expressed between the four genotypes, a time-course experiment was designed utilizing the citrus/Xanthomonas citri subsp. citri pathosystem. Five Xanthomonas citri subsp. citri or mock-inoculated plants of each genotype were used and each plant was considered an independent biological replicate. Attached leaves were washed with distilled water and followed by pressure infiltration inoculation on the abaxial side using a 1 cm3 needleless tuberculin syringe. Three inoculations, each composed of 2 μL of bacterial suspension, were made on both sides of the mid-vein. Six 1-cm leaf disks per leaf, circumscribing the infiltrated area, were excised using a 1-cm cork borer. The disks were placed in sterile RNase-free 2-mL tube, immediately immersed in liquid nitrogen and stored at -80°C until RNA extraction. Leaves inoculated with sterile PBS buffer were used as the control following the same protocol for inoculation and sample collection.
Leaf disks were harvested from untreated, inoculated and mock-inoculated plants before inoculation (time point 0) and 4, 24, and 96 h after inoculation. Leaf disks (100 mg) after freezing in liquid nitrogen were ground using Tissue Lyser II system (Qiagen, Valencia, CA, United States) for disruption and homogenization of leaf tissue through high-speed shaking in 2-mL plastic tubes containing one stainless steel bead. The tubes were shaken twice at 30 rps for 30 s. Total RNA was isolated from macerated leaf samples using RNeasy Plant Mini Kit (Qiagen) according to the manufacturer’s recommendations. The RNA samples were treated with RNase-free DNAse (Qiagen) to remove any contaminant genomic DNA. To ensure the quality of RNA samples, RNA concentration and integrity was measured by NanoDrop® spectrophotometer, 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, United States) and bleach agarose gel (Aranda et al., 2012).
Quantitative Polymerase Chain Reaction (qPCR)
Independent RNA samples extracted from five untreated plants, five plants inoculated with Xanthomonas citri subsp. citri and five plants inoculated with PBS buffer for each genotype (‘Marsh’ grapefruit, ‘Meiwa’ kumquat, M-9 cybrid and M-102 cybrid) per each time point (0, 4, 24, and 96 hai) were used for quantitative PCR. From the RNA isolated as described above, cDNAs were synthesized from 1 μg of total RNA using QuantiTect Reverse Transcription Kit (Qiagen, Valencia, CA, United States) following the manufacturer’s instructions.
Quantitative PCR was performed using QuantiTech SYBR®Green PCR Kit (Qiagen). The reaction consisted of 1.0 μL of cDNA and 500 nM of each gene-specific primer in a final volume of 20 μL. Amplification was carried out for two technical replicates for each sample, including negative controls. Reactions were performed in a ABI Prism7500 sequence detector (Applied Biosystems, Foster City, CA, United States) with the following thermal cycles: 50°C for 2 min, 95°C for 10 min; 40 cycles of 95°C for 15 s, and 60°C for 1 min. Expression levels were assessed based on the number of amplification cycles needed to reach a fixed threshold (cycle threshold – Ct) in the exponential phase of PCR. Ct data were analyzed using the 7500 software v.2.0.6. (Applied Biosystems). For relative quantification, change in gene expression was calculated by the ΔΔCt method (Livak and Schmittgen, 2001) comparing both Xanthomonas citri subsp. citri-inoculated samples and mock-inoculated controls to non-inoculated sample for each time point. The relative expression is presented as log2-fold change.
Primers specific to 10 selected genes were designed using the PerlPrimer software (Marshall, 2004) based on the mRNA sequences (Table 2). GAPC2 and FBOX were selected as internal reference genes (Mafra et al., 2012; Yan et al., 2012). The sequences of endogenous gene primers are listed in Table 2. For a trustworthy analysis, relative quantitative methods assume that the target and endogenous genes amplify with similar efficiency. The amplification rate was calculated on the basis of a linear regression slope of a dilution row and efficiency (E) was determined based on the equation E = 10[-1/slope] (Pfaffl, 2002).
Table 2.
q-PCR primer sequences of the selected defense genes and internal control genes.
| Gene | Gene ID | Gene annotation | Forward 5′–3′ | Reverse 5′–3′ |
|---|---|---|---|---|
| Internal control genes | ||||
| FBOX | 102626717 | F-box/kelch-repeat protein | TTGGAAACTCTTTCGCCACT | CAGCAACAAAATACCCGTCT |
| GAPC2 | 837904 | Glyceraldehyde-3-phosphate dehydrogenase | TCTTGCCTGCTTTGAATGGA | TGTGAGGTCAACCACTGCGACAT |
| Target genes | ||||
| CAO | 818849 | Copper amine oxidase | AACACTTCTTCATTGCCCGT | GTTCTCGTATTCCTCACAATCCAG |
| APX | 107826841 | Ascorbate oxidase | AGCAGTTCCCTACCATCTCC | CTCAGCCTTGTCATCTCTTCC |
| CAT | 18641273 | Catalase | GGACCCAACTACTTGATGCT | GTAATCGACCTCCTCATCCC |
| COI | 732662 | Coronatine-insensitive | GGCTCCTTCAATCATCCACC | ACATTCCTCGTCTCAAGAATTTCC |
| LOB | 102614340 | Lateral organ boundaries | TCCACCAACCGAACCATACA | GGCACTTGCTTCATAGACCAT |
| OPR2 | 102631049 | 12-Oxophytodienoate reductase 2 | GGGCAGCAAACTGTGAGGAC | CAGATAGGGTTGGGATAAG |
| ICS1 | 102630235 | Isochorismate synthase | GGAGGAGGAGAGAGTGAATTTG | GGGTTGCTTCCTTCTACTATCC |
| PAL | 102620173 | Phenylalanine ammonia lyase | CACATTCTTGGTAGCGCTTTG | AGCTACTTGGCTGACAGTATTC |
| NPR1 | 102617188 | Non-expressor of PR genes 1 | AACTCGCCTCAAGACTACCT | TGCAACTGTGTCGTTCCATA |
| PR2 | 102623685 | β-1,3-Glucanases | TTCCACTGCCATCGAAACTG | TGTAATCTTGTTTAAATGAGCCTCTTG |
| PR5 | 102621661 | Thaumatin-like protein | TACCTCCACCTCTCTCATTCTT | GTGCGAGAGAAGGTTAGCTATG |
Results
Citrus Canker Response in Grapefruit Cybrids
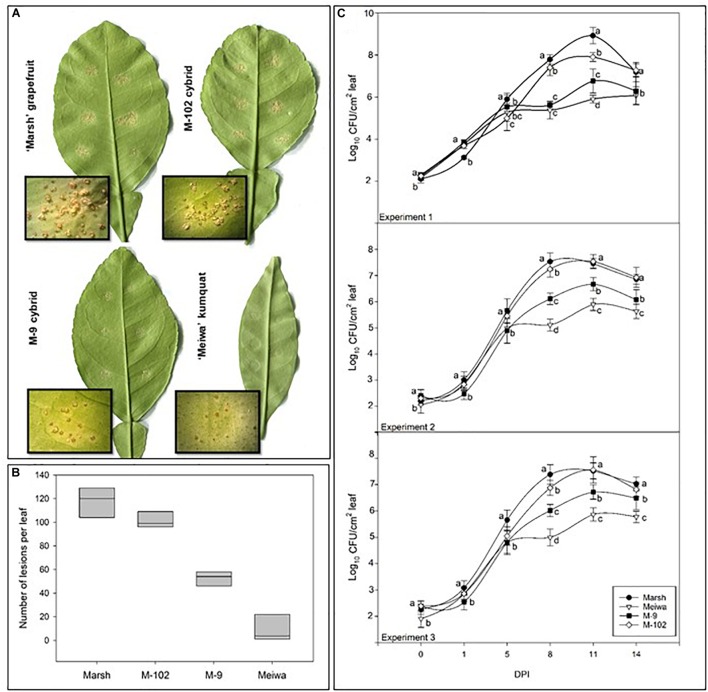
To examine the effect of cybridization on canker response, 22 independently derived cybrids (4 Cybrid-G and 18 Cybrid-K) along with grapefruit and kumquat controls were examined for response to the pathogen by an attached leaf assay. Citrus canker symptoms appeared on grapefruit cultivars about 7 days post inoculation (dpi) with a suspension of Xanthomonas citri subsp. citri at 104 cfu mL-1. The lesions were characterized by raised, light brown, blister-like pustules typical of the compatible host reaction (Figure 1A). Cybrids showed the same typical canker lesions; however, the number of lesions varied (Figure 1B). Most of the ‘Meiwa’ kumquat leaves did not show any symptoms, but a few inoculation sites showed slightly raised, dark brown pustules (Figure 1A). No sign of a hypersensitive response (HR) was observed.
FIGURE 1.
Characterization of citrus canker resistance in grapefruit cybrids. (A) Citrus canker lesions in ‘Marsh’ grapefruit, ‘Meiwa’ kumquat, cybrid M-9 and cybrid M-102. Photos were taken 14 days post-inoculation with Xanthomonas citri subsp. citri suspension at 104 cfu mL-1. (B) Mean of total number of lesions per leaf on attached leaves of ‘Marsh’ grapefruit, ‘Meiwa’ kumquat, as well as the cybrids M-9 and M-102 14 days after pressure infiltration inoculation of Xanthomonas citri subsp. citri suspension at 104 cfu mL-1. Data represent the average of six leaves from five biological replicates with SD. (C) Growth curves for Xanthomonas citri subsp. citri on attached leaves of ‘Marsh’ grapefruit, ‘Meiwa’ kumquat, cybrid M-9 and cybrid M-102 during 14-day period after pressure infiltration inoculation with Xanthomonas citri subsp. citri suspension at 104 cfu mL-1. Xanthomonas citri subsp. citri population was estimated by counting the number of Xanthomonas citri subsp. citri colonies and expressed as log10 of bacteria per leaf per time point. Data followed by the same letter for each time point are not significantly different at P ≤ 0.05 according to Tukey’s Studentized Range (HSD) test. The experiment was repeated three times with similar results.
All cybrids with kumquat chloroplasts (Cybrid-K) had significantly fewer lesions (46–85 lesions per leaf), compared to their grapefruit parent cultivars (Table 3). ‘Marsh,’ ‘Flame,’ and ‘Ruby Red’ grapefruit cultivars had consistently higher numbers of lesions in all experiments (>104 lesions per leaf) (Table 3 and Figure 1B). ‘Flame’ grapefruit had the highest number of lesions (138 lesions per leaf). Cybrids with grapefruit chloroplasts (Cybrid-G) had the highest number of lesions among all cybrids (86–109 lesions per leaf) and did not significantly differ in number of lesions compared to ‘Ruby Red’ grapefruit in Experiment 2 or 3 (Table 3). ‘Meiwa’ kumquat had the lowest number of lesions in all experiments (1–20 lesions per leaf) (Table 3 and Figure 1B). Based on performance in the attached leaf assay, two cybrids were selected for detailed studies: M-102 (a Cybrid-G type) and M-9 (a Cybrid-K type).
Table 3.
Mean of total number of citrus canker lesions on attached leaves in three independent experiments on three grapefruit cultivars, 22 cybrid clones and ‘Meiwa’ kumquat inoculated with Xanthomonas citri subsp. citri by using the pressure infiltration method.
| Genotype | Mean of total number of lesion per leaf | ||
|---|---|---|---|
| Experiment 1x | Experiment 2x | Experiment 3x | |
| ‘Flame’ grapefruit | -y | 138.16a | 111.13b |
| ‘Marsh’ grapefruit | 129.25a | 104.73b | 119.93a |
| N-4z | 86.620c | 103.10b | 77.63d |
| ‘Ruby Red’ grapefruit | - | 101.70b | 90.36c |
| M-102z | 109.92b | 99.36b | 96.96c |
| M-78z | 106.33b | 98.13b | 90.33c |
| M-81z | - | 98.03b | 89.66c |
| M-13 | 60.25fgh | 85.26b | 57.60ghi |
| M-31 | 83.33cd | 78.53c | - |
| N-10 | 73.62def | 78.36cd | 52.90hi |
| N-8 | 57.00ghi | 76.13cd | - |
| N-18 | 53.66hi | 72.70cd | 57.06ghi |
| F-2 | 73.37def | 70.23de | 65.00efg |
| F-5 | 70.87defg | 64.33ef | 56.62ghi |
| M-10 | - | 63.26efg | 58.73fghi |
| F-13 | - | 61.86efg | 51.33i |
| F-3 | 53.25hi | 58.13fgh | 65.83efg |
| M-11 | 52.08hi | 56.73fgh | 62.06fghi |
| F-15 | - | 55.83fgh | 57.96ghi |
| M-9 | 46.41i | 53.90fgh | 58.60fghi |
| N-6 | 63.58fgh | 50.93gh | 74.43de |
| F-10 | 64.62efgh | 48.56h | 70.03def |
| F-6 | 78.16cde | 46.10h | 64.30efgh |
| N-12 | 70.50defg | - | 65.36efg |
| F-20 | 65.79efgh | - | - |
| ‘Meiwa’ kumquat | 1.20j | 3.86i | 22.23j |
The number of lesions was counted 14 days post-inoculation and six plants per genotype were inoculated.xMeans in the same column followed by different letters are significantly different based on Student-Newman–Keuls test (α = 0.05).yMissing values. Some genotypes did not grow young vegetative flushes in time for inoculation.zCybrids with grapefruit chloroplast (Cybrid-G).
Bacterial growth curves were examined in three independent experiments (Figure 1C). Around 100 cfu of Xanthomonas citri subsp. citri were detected from leaf disks of all genotypes immediately after inoculation. All genotypes showed similar bacterial growth until 5 dpi (∼105 cfu). At 8 and 11 dpi, significant differences in bacterial populations were observed among the genotypes. ‘Marsh’ grapefruit showed a rapid increase in Xanthomonas citri subsp. citri multiplication, reaching 108 cfu per disk at 11 dpi. ‘Meiwa’ kumquat showed the lowest bacteria population of 105 cfu per disk at 14 dpi. Interestingly, the cybrids followed the same bacterial growth pattern as their chloroplast donor parent. Bacteria multiplied rapidly in the M-102 cybrid, which carries grapefruit chloroplasts, and at 5 dpi in experiments 2 and 3, the Xanthomonas citri subsp. citri populations did not significantly differ from those of ‘Marsh’ grapefruit. In all experiments, the M-9 cybrid with kumquat chloroplasts had populations one to two log units lower than ‘Marsh’ grapefruit at 11 dpi. At 14 dpi, Xanthomonas citri subsp. citri populations declined in all genotypes (Figure 1C).
Gene Expression Features of Cybrid and Parent Genotypes
To gain insight into the role of chloroplasts in plant defense, expression of some defense-related genes in response to Xanthomonas citri subsp. citri inoculation was analyzed. To identify differences in the Xanthomonas citri subsp. citri response between ‘Meiwa’ kumquat, ‘Marsh’ grapefruit, M-9 cybrid (Cybrid-K) and M-102 cybrid (Cybrid-G), leaves of these four genotypes were pressure infiltrated with Xanthomonas citri subsp. citri at 108 cfu mL-1 and kept under greenhouse conditions. At 4 hours post inoculation (hpi), only ‘Marsh’ leaves started to show water-soaking symptoms with the darkening of the inoculation site (Figure 2). At 24 hpi, the water-soaking progressed to tissue hyperplasia in ‘Marsh’ as well as M-9 and M-102 cybrids, whereas ‘Meiwa’ kumquat showed a slight water-soaking and necrosis at the center of the inoculation site. At 96 hpi, ‘Marsh’ grapefruit and the cybrids M-9 and M-102 showed similar hyperplasia and hypertrophy symptoms typical of canker lesions, while in ‘Meiwa’ kumquat, the necrotic spot resembled an HR (Figure 2). Thus, although M-9 was exhibited resistance to citrus canker when inoculated with Xanthomonas citri subsp. citri at 104 cfu mL-1, under high bacteria concentration ‘Marsh’ and M-9 expressed a similar compatible interaction, while ‘Meiwa’ showed signs of necrosis, corresponding to an incompatible interaction.
FIGURE 2.
Citrus canker symptoms after pressure infiltration with Xanthomonas citri subsp. citri suspension at 108 cfu mL-1. Comparison of canker disease development in leaves of ‘Meiwa’ kumquat, M-9 cybrid, M-102 cybrid and ‘Marsh’ grapefruit after pressure infiltration with Xanthomonas citri subsp. citri suspension at high concentration. Pictures were taken at 4, 24, and 96 hours post inoculation (hpi).
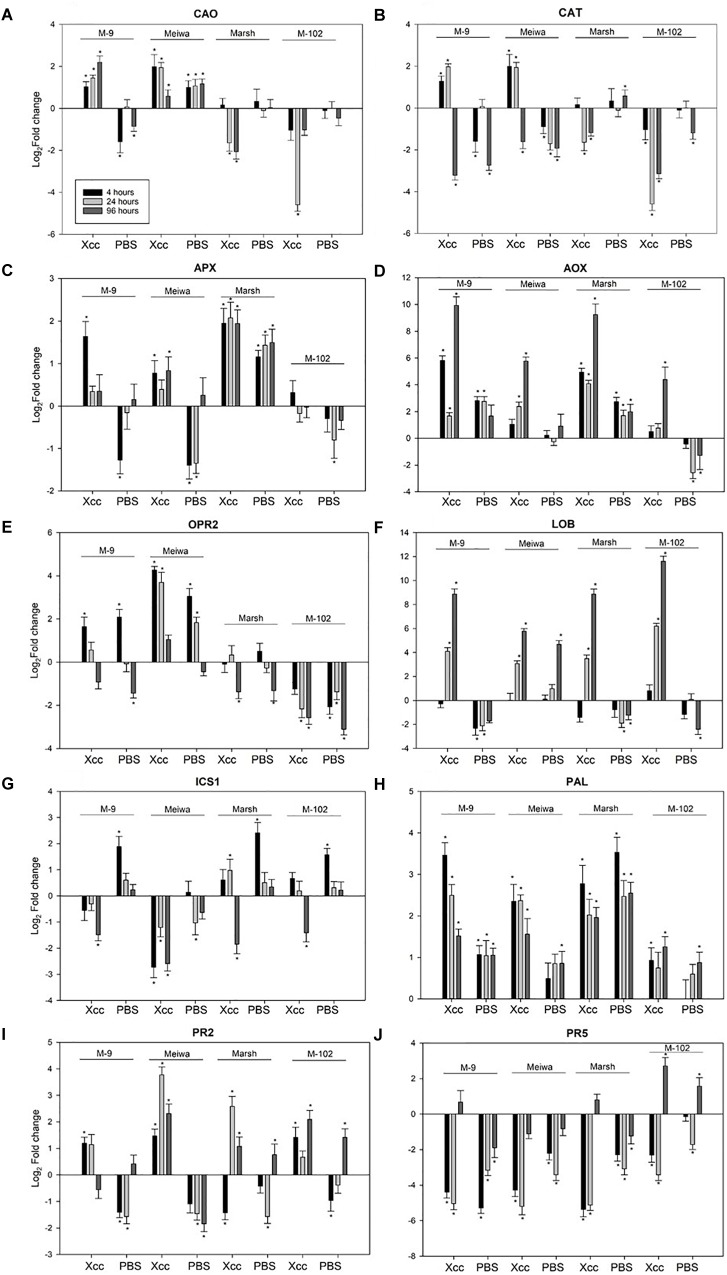
The four genotypes differed not only with respect to their inoculation phenotypes. The expression levels of selected defense-related genes were significantly different among the four genotypes evaluated. These 10 genes were divided into sub-categories according to their function. Among genes related to reactive oxygen species (ROS) pathways, copper amine oxidase (CAO) was upregulated in ‘Meiwa’ and M-9 cybrid at all time points after Xanthomonas citri subsp. citri inoculation. Under the same conditions, CAO was downregulated in ‘Marsh’ and M-102 cybrid (Figure 3A). Similar results were observed for catalase (CAT). CAT was upregulated in ‘Meiwa’ kumquat and M-9 cybrid and downregulated in ‘Marsh’ and M-102 cybrid in the first 24 h (Figure 3B). Ascorbate peroxidase (APX) was upregulated in all genotypes, except for the M-102 cybrid, but with a higher expression in ‘Marsh’ (Figure 3C). However, APX was also upregulated in ‘Marsh’ treated with PBS buffer. Alternative oxidase (AOX) is known to be associated with mitochondrial ROS production. Both cybrids have the same nuclear and mitochondrial genotypes, but they showed different AOX expression patterns. The M-9 cybrid and ‘Marsh’ grapefruit share the same nuclear genotype and have different mitochondrial genotypes, but AOX expression was similar in these genotypes (Figure 3D).
FIGURE 3.
Expression profiles of ROS, JA, SA-related genes and PR genes in ‘Meiwa’ kumquat, M-9 cybrid, M-102 cybrid and ‘Marsh’ grapefruit in response to Xanthomonas citri subsp. citri inoculation and mock-treatment with PBS buffer. Each graphic represents one gene and each bar represents one time point. Copper amine oxide (A), catalase (B), ascorbate peroxidase (C), alternative oxidase (D), 12-oxophytodienoate reductase 2 (E), and lateral organ boundaries (F), isochorismate synthase (G), phenylalanine ammonia lyase (H), endo-β-1,3-glucanases (I) and thaumatin protein (J). Gene expression columns followed by an asterisk are significant at P ≤ 0.05 according to Student’s t-test.
The 12-oxophytodienoate reductase 2 (OPR2) gene, encoding a key enzyme for jasmonic acid (JA) synthesis, was upregulated in canker-resistant genotype ‘Meiwa’ kumquat at all time points after Xanthomonas citri subsp. citri inoculation (Figure 3E). This upregulation was also observed in the canker moderate resistant M-9 cybrid at 4 and 24 hpi, but absent or downregulated in susceptible ‘Marsh’ grapefruit and the M-102 cybrid. In ‘Marsh,’ OPR2 was downregulated at 96 hpi, and in M-102 cybrid, it was downregulated at all time points. However, in all genotypes, both Xanthomonas citri subsp. citri and mock treatments induced similar expression pattern for this gene. The lateral organ boundaries (LOB) gene, encoding a transcription factor that acts as a repressor of JA-regulated defense genes, was upregulated in all genotypes at 24 and 96 hpi (Figure 3F). After PBS treatment, LOB was upregulated in ‘Meiwa’ while it was downregulated in the other genotypes.
Genes encoding the enzymes responsible for salicylic acid (SA) synthesis, isochorismate synthase (ICS1) and phenylalanine ammonia lyase (PAL) were also evaluated. Overall, ‘Meiwa’ and the M-9 cybrid showed a downregulation of ICS1 whereas ‘Marsh’ and the M-102 cybrid showed an upregulation after Xanthomonas citri subsp. citri inoculation (Figure 3G). After mock inoculation treatment, ICS1 was upregulated in ‘Marsh’ grapefruit and both cybrids. PAL was upregulated in all genotypes at all time points under both conditions, but ‘Meiwa’ and the M-9 cybrid showed a higher expression of PAL when treated with Xanthomonas citri subsp. citri compared to water (Figure 3H).
Pathogenesis-related (PR) genes encoding endo-β-1,3-glucanase (PR-2) and thaumatin protein (PR-5) were investigated (Figures 3I,J). PR-2 was upregulated in ‘Meiwa’ at all-time points, showing a high expression at 24 hpi. ‘Marsh’ grapefruit showed an upregulation of this gene only at 24 hpi. In both cybrids, PR-2 was upregulated under Xanthomonas citri subsp. citri inoculation. In contrast, PR-5 was downregulated in all genotypes under both treatment conditions with the exception of 96 hpi in M-102, a time point at which this gene was upregulated.
Discussion
The hypothesis of this study is that kumquat chloroplasts and/or mitochondria, when combined with the grapefruit nucleus, may enhance grapefruit resistance to citrus canker. Kumquats are considered highly resistant to citrus canker (Khalaf et al., 2007). Although this species is in a different genus than commercial citrus, kumquats can be used to study inheritance of canker resistance due to their sexual compatibility with citrus (Khalaf et al., 2007). Kumquat resistance to citrus canker has been characterized as an HR reaction to Xanthomonas citri subsp. citri infection, which blocks the bacterial proliferation in the plants (Trivedi and Wang, 2014). The new combinations of kumquat organelles and grapefruit nucleus opened a door to investigate the influence of the kumquat chloroplasts and/or mitochondria in citrus canker response (Omar et al., 2017).
Attached leaf assays by pressure infiltration inoculation with Xanthomonas citri subsp. citri suspension of 104 cfu mL-1 enable differentiation of citrus genotype response based on the number of disease lesions (Francis et al., 2009). Our results (Figure 1) confirmed that ‘Marsh,’ ‘Flame,’ and ‘Ruby Red’ grapefruit are highly susceptible to citrus canker (Gottwald et al., 2002), because these cultivars produced raised blister-like leaf lesions typical of a compatible interaction. ‘Meiwa’ kumquat showed contrasting reaction to Xanthomonas citri subsp. citri inoculation and no canker lesions were observed on most of the inoculated leaves. Few leaves showed slightly raised dark brown lesions at the inoculation site; but no sign of an HR reaction was observed. Grapefruits had an average of 117 lesions per leaf compared to nine lesions observed in kumquat based on three independent experiments (Table 3). In addition, at 8 dpi, the bacterial population in the kumquat leaves was about 100-fold less than grapefruit leaves (Figure 1C). These results substantiate the use of grapefruit cultivars and ‘Meiwa’ kumquat as susceptible and resistant controls, respectively.
The response of grapefruit cybrids to Xanthomonas citri subsp. citri inoculation was previously unknown. The attached leaf assay was further used to investigate the reaction of four independent Cybrid-G and 18 independent Cybrid-K clones to citrus canker. The total number of lesions per inoculation site varied among the cybrids; however, all Cybrid-K types had a significantly lower number of lesions compared to grapefruit cultivars and to Cybrid-G types (Table 3 and Figure 1B). In the genetic background comprised of the grapefruit nuclear and mitochondrial genotypes, the kumquat chloroplast genotype clearly co-segregated with increased resistance to Xanthomonas citri subsp. citri.
In addition, the bacterial populations in a selected Cybrid-K (M-9) were 10- to 100-fold lower than those in the ‘Marsh’ grapefruit or in a selected Cybrid-G (M-102) (Figure 1C). These results indicate that even though both cybrids have the same grapefruit nucleus and mitochondria, their responses against artificial Xanthomonas citri subsp. citri inoculation were significantly different. Cybrids with kumquat chloroplasts (Cybrid-K) were more resistant to citrus canker than cybrids with grapefruit chloroplasts (Cybrid-G) with respect to both lesion number and bacteria multiplication (Table 3 and Figure 1C). These data clearly demonstrate the role of kumquat chloroplasts in the reduction of citrus canker severity in grapefruit cybrids, since the only genetic difference between both cybrids is the chloroplast genome. These findings demonstrate an important role of chloroplast in the resistance of citrus cybrids to citrus canker.
Several studies have demonstrated the role of chloroplasts in plant resistance against biotic stress, but most of these experiments were done in Arabidopsis thaliana or tobacco (Nicotiana benthamiana) (Nomura et al., 2012; De Torres Zabala et al., 2015; Serrano et al., 2016). So far, no research on perennial trees such as citrus has been conducted to establish the function of chloroplasts in disease resistance. Chloroplasts are vital organelles for plants and are well known to be an active metabolic center by converting solar energy to carbohydrates through the process of photosynthesis (Daniell et al., 2016). In addition to this primary function, chloroplasts play a role in other vital physiological processes in plants including synthesis of amino acids, nucleotides and vitamins. Chloroplasts also host a variety of intermediate metabolic pathways, such as biosynthesis of plant hormones including SA, JA, abscisic acid (ABA), indole-3-acetic acid (IAA), and cytokinins (CKs) and generation of ROS (Nomura et al., 2012; Pfannschmidt and Yang, 2012). Therefore, chloroplasts have a central role in sensing and signaling cellular responses to various stimuli (Pfannschmidt and Yang, 2012), including pathogen attack.
To further investigate how plastid genotype might affect the Xanthomonas citri subsp. citri response, quantitative PCR analysis targeting chloroplast-related genes was performed to compare the gene expression of four genotypes, canker susceptible ‘Marsh’ grapefruit and M-102 cybrid (Cybrid-G type), canker moderate resistant M-9 cybrid (Cybrid-K type) and canker resistant ‘Meiwa’ kumquat. M-9 and M-102 cybrids showed a strong contrasting response to Xanthomonas citri subsp. citri inoculation (Table 3). These genotypes were chosen to test candidate genes for possible involvement in the differential pathogen response. Because only one cybrid of each type was tested, the responses identified and discussed below must be further tested to confirm their association with disease phenotypes.
Reactive oxygen species generated in chloroplasts is responsible for triggering signaling pathways that influence nuclear encoded genes (Balazadeh et al., 2012). Hydrogen peroxide (H2O2) is produced in response to a variety of different stress conditions, including biotic stress, and is scavenged by different antioxidant/enzyme reactions in the ascorbate and glutathione cycles involving APX, catalase (CAT), and peroxiredoxin (PRX) (Tripathi et al., 2009). CAO catalyzes the degradation of cellular polyamines, producing H2O2 derived from polyamine oxidation. This process has been correlated with wound-healing and cell wall reinforcement during pathogen invasion (Liu et al., 2015). Notably, both CAO and CAT were upregulated in M-9 and ‘Meiwa’ and downregulated in M-102 and ‘Marsh’ at 4 and 24 hpi (Figures 3A,B). These results indicate a clear correlation among the ROS gene expression, canker resistance, and chloroplasts in the ‘Meiwa’ kumquat and M-9 cybrid in ROS gene expression, suggesting that ROS pathway in citrus depends on the chloroplast since ‘Meiwa’ and M-9 share the same chloroplast genome. Previous studies have shown that expression profiles of genes related to ROS differ in resistant and susceptible genotypes under biotic stress (Coram and Pang, 2006; Jain et al., 2016); however, this is the first time that this function has been linked to the chloroplast alone. The gene expression of APX was similar in the M-9 cybrid and ‘Meiwa’ where the gene is upregulated only after Xanthomonas citri subsp. citri inoculation. On the other hand, in ‘Marsh,’ APX is upregulated after Xanthomonas citri subsp. citri and water infiltration of leaves, suggesting that the activation of ROS in the susceptible genotype is a response to wounding and not specifically to Xanthomonas citri subsp. citri.
We also evaluated expression of the gene encoding mitochondrial AOX, proposed to prevent ROS formation when the cytochrome respiration pathway is compromised. In citrus plants, AOX is described as having an important role during biotic stress in non-host plant pathogen interaction (Daurelio et al., 2009). Kumquat and all cybrids have the same mitochondrial genotype but AOX showed a different gene expression profile for each genotype (Figure 3D). This contrasting expression between the cybrids can be explained by the cross talk of mitochondria and chloroplasts. Even though both organelles have been considered autonomous, they are not completely independent and researches have shown several metabolic, functional and physical inter-connections between mitochondria and chloroplasts (Shapiguzov et al., 2012; Trotta et al., 2014). The M-102 cybrid is the only genotype that has chloroplast (grapefruit) and mitochondria (kumquat) from different parents and this new combination could have caused a change in the functioning of both organelles.
Salicylic acid and JA are phytohormones that play an important role in different signaling pathways involved in plant defense against a variety of biotic and abiotic stresses (Hu et al., 2009; Lu, 2009; War et al., 2011). SA is synthesized in chloroplasts from chorismic acid by ICS1 (Serrano et al., 2013), but can also be synthesized from phenylalanine by PAL (Kim and Hwang, 2014). Our results suggest that, after pathogen attack, early biosynthesis of SA is controlled only by PAL in the M-9 cybrid and ‘Meiwa’ kumquat, because the expression of ICS1 was downregulated after Xanthomonas citri subsp. citri inoculation of both genotypes (Figure 3H). On the other hand, in ‘Marsh’ grapefruit, genes of both SA pathways were upregulated, but the higher expression in leaves inoculated with PBS suggests that SA is not used as a defense signaling pathway in this susceptible genotype. For the M-9 cybrid and ‘Meiwa,’ the early chloroplast-dependent SA biosynthesis by PAL may be involved in the resistance to Xanthomonas citri subsp. citri since the upregulation of this gene was higher after Xanthomonas citri subsp. citri inoculation than mock inoculation (Figure 3H). PAL has been demonstrated to be required for the induction of SA-dependent signaling in pepper defense against Xanthomonas campestris pv. vesicatoria (Kim and Hwang, 2014).
Jasmonic acid is a plant hormone whose biosynthesis begins in the chloroplast but is completed in the peroxisome (Serrano et al., 2016). The enzyme 12-oxophytodienoate reductase 2 (OPR2) is involved in the biosynthesis of JA. ‘Meiwa’ kumquat and M-9 cybrids showed a contrasting OPR2 gene expression compared to ‘Marsh’ grapefruit and M-102 cybrids (Figure 3E). OPR2 was induced by both Xanthomonas citri subsp. citri and mock inoculation at 4 hpi in the ‘Meiwa’ kumquat and the M-9 cybrid, but was not expressed or downregulated in the ‘Marsh’ grapefruit and M-102 cybrid. Therefore, biosynthesis of JA appears to be important for citrus defense against Xanthomonas citri subsp. citri, but it is not Xanthomonas citri subsp. citri specific. Another gene evaluated encodes the transcription factor LOB. This gene represses a subset of jasmonate-mediated defenses and is considered a critical canker disease susceptibility gene in citrus (Hu et al., 2014). LOB was upregulated in all genotypes at 24 and 96 hpi; however, in ‘Meiwa,’ this gene was upregulated after mock inoculation as well, while in the other genotypes, LOB was downregulated after mock inoculation (Figure 3F). This result suggests that the canker susceptibility gene LOB does not play a role in ‘Meiwa’ kumquat, which agrees with its resistant phenotype. In addition, LOB expression was higher in ‘Marsh’ and both cybrids at 96 hpi, a time point at which the bacteria are successfully multiplying inside the host.
Besides chloroplast-related genes, two defense-related genes were selected for qPCR. Production and accumulation of pathogenesis-related (PR) proteins in plants in response to the invading pathogen are very important for plant defense. PR proteins accumulate locally in the infected area and in remote uninfected tissues, where they can prevent the affected plants from further infection (Durrant and Dong, 2004). ‘Marsh’ grapefruit exhibited upregulation of PR-2 at 24 hpi, however this gene activation did not prevent citrus canker development. Transcriptomic analysis with kumquat and grapefruit showed that PR-2 is upregulated in both susceptible and resistant genotypes after Xanthomonas citri subsp. citri infection, therefore this gene is considered a basic plant defense gene but not a key for citrus canker resistance (Khalaf et al., 2007; Fu et al., 2012). PR-5 seems to not be involved in citrus canker host–parasite interaction since it was downregulated under all treatments (Figure 3J). The summary of our results is described in Figure 4.
FIGURE 4.
Gene expression overview of ‘Marsh’ grapefruit, M-102 cybrid (Cybrid-G), M-9 cybrid (Cybrid-K) and ‘Meiwa’ kumquat in response to Xanthomonas citri subsp. citri infection. Green arrows indicate upregulation and orange arrows indicate downregulation.
Our results strongly support that kumquat chloroplast transfer to grapefruit cybrids enhanced resistance to citrus canker. Plastid-nuclear genome incompatibilities potentially elicit or enhance the defense responses uncovered here. The assembly and function of photosynthetic electron transfer complexes is achieved through interaction of plastid and nuclear genetic systems. Hundreds of nuclear genes mediate transcription, transcript stability, intron splicing, RNA editing, translation, protein complex assembly and protein turnover in the plastid (Barkan, 2011; Belcher et al., 2015; Ichinose and Sugita, 2016). The co-evolution of these interactions creates species-specific differences such that novel nuclear-plastid genome combinations can produce subtle or significant differences in plastid function (Herrmann et al., 2002; Greiner and Bock, 2013). Failure to correctly edit the Arabidopsis thaliana plastid ndhD transcript impairs cyclic electron flow around photosystem I with a concomitant increase in resistance to the fungal pathogen Plectospherella cucumerina (Garcia-Andrade et al., 2013). While the RNA editing defect was conditioned by a nuclear mutation in this example, the co-evolution of plastid RNA editing sites and their nuclear-encoded recognition factors is known to create species-specific editing requirements (Tillich et al., 2005). This might also be the situation for other types of plastid gene expression factors. The citrus cybrids and their parents described here lay the groundwork for mechanistic investigations of plastid signaling in plant defense. They raise the question of whether other alien plastid genomes, even those originating from susceptible genotypes, might condition reduced susceptibility to citrus canker and other diseases, leading to new avenues toward plant disease control.
It must be noted that all the canker assays discussed in this report were conducted in a greenhouse, and the improved response of the grapefruit cybrids containing kumquat chloroplasts against citrus canker must still be validated under real-world conditions in the field. Thus, the entire population of cybrid grapefruit plants, along with replicates of some of the more interesting clones, has been planted at a field site with heavy canker pressure near Fort Pierce, FL, United States. Citrus greening disease or huanglongbing (HLB) is also now endemic in this area and grapefruit is highly susceptible. It will be interesting to see if any of the cybrid grapefruit show a different response to HLB.
Author Contributions
MM, AO, ZM, CC, JWG, and JHG contributed conception and design of the study. AO produced and regenerated all the cybrid materials used in this study. MM performed the experiments, statistical analyses, and wrote the first draft of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer FW declared a shared affiliation, with no collaboration, with several of the authors, ZM and CC, to the handling Editor at the time of review.
Footnotes
Funding. The authors would like to thank the Brazilian institution CAPES (Science without Borders Program) and Hunt Brothers Fellowship which provided funding for a Graduate Scholarship for the first author at University of Florida.
References
- Aranda P. S., LaJoie D. M., Jorcyk C. L. (2012). Bleach gel: a simple agarose gel for analyzing RNA quality. Electrophoresis 33 366–369. 10.1002/elps.201100335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazadeh S., Jaspert N., Arif M., Mueller-Roeber B., Maurino V. G. (2012). Expression of ROS-responsive genes and transcription factors after metabolic formation of H2O2 in chloroplasts. Front. Plant. Sci. 3:234. 10.3389/fpls.2012.00234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa-Mendes J. M., Mourao Filho Fd A. A., Bergamin Filho A., Harakava R., Beer S. V., et al. (2009). Genetic transformation of Citrus sinensis cv. Hamlin with hrpN gene from Erwinia amylovora and evaluation of the transgenic lines for resistance to citrus canker. Sci. Hortic. 122 109–115. 10.1016/j.scienta.2009.04.001 [DOI] [Google Scholar]
- Barkan A. (2011). Expression of plastid genes: organelle-specific elaborations on a prokaryotic scaffold. Plant Physiol. 155 1520–1532. 10.1104/pp.110.171231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassene J., Froelicher Y., Navarro L., Ollitrault P., Ancillo G. (2011). Influence of mitochondria on gene expression in a citrus cybrid. Plant Cell Rep. 30 1077–1085. 10.1007/s00299-011-1014-1 [DOI] [PubMed] [Google Scholar]
- Belcher S., Williams-Carrier R., Stiffler N., Barkan A. (2015). Large-scale genetic analysis of chloroplast biogenesis in maize. Biochim. Biophys. Acta 1847 1004–1016. 10.1016/j.bbabio.2015.02.014 [DOI] [PubMed] [Google Scholar]
- Boscariol R. L., Monteiro M., Takahashi E. K., Chabregas S. M., Vieira M. L. C., Vieira L. G., et al. (2006). Attacin a gene from Tricloplusia ni reduces susceptibility to Xanthomonas axonopodis pv. citri in transgenic Citrus sinensis Hamlin. J. Am. Soc. Hortic. Sci. 131 530–536. [Google Scholar]
- Cai X., Fu J., Chen C., Guo W. (2009). Cybrid/hybrid plants regenerated from somatic fusions between male sterile Satsuma mandarin and seedy tangelos. Sci. Hortic. 122 323–327. 10.1016/j.scienta.2009.05.017 [DOI] [Google Scholar]
- Coram T. E., Pang E. C. (2006). Expression profiling of chickpea genes differentially regulated during a resistance response to Ascochyta rabiei. Plant Biotechnol. J. 4 647–666. 10.1111/j.1467-7652.2006.00208.x [DOI] [PubMed] [Google Scholar]
- Daniell H., Lin C., Yu M., Chang W. (2016). Chloroplast genomes: diversity, evolution, and applications in genetic engineering. Genome Biol. 17:134. 10.1186/s13059-016-1004-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daurelio L. D., Checa S. K., Barrio J. M., Ottado J., Orellano E. G. (2009). Characterization of Citrus sinensis type 1 mitochondrial alternative oxidase and expression analysis in biotic stress. Biosci. Rep. 30 59–71. 10.1042/BSR20080180 [DOI] [PubMed] [Google Scholar]
- De Torres Zabala M., Littlejohn G., Jayaraman S., Studholme D., Bailey T., Lawson T., et al. (2015). Chloroplasts play a central role in plant defence and are targeted by pathogen effectors. Nat. Plants 1:15074. 10.1038/nplants.2015.74 [DOI] [PubMed] [Google Scholar]
- Deng Z. N., Xu L., Li D. Z., Long G. Y., Liu L. P., Fang F., et al. (2010). Screening citrus genotypes for resistance to canker disease (Xanthomonas axonopodis pv. citri). Plant Breed. 129 341–345. 10.1111/j.1439-0523.2009.01695.x [DOI] [Google Scholar]
- Dewdney M., Graham J. (2012). “Citrus canker,” in Florida Citrus Pest Management Guide, eds Rogers M. E., Dewdeny M. M. (Gainesville, FL: University of Florida; ), 93–96. [Google Scholar]
- Durrant W. E., Dong X. (2004). Systemic acquired resistance. Annu. Rev. Phytopathol. 42 185–209. 10.1146/annurev.phyto.42.040803.140421 [DOI] [PubMed] [Google Scholar]
- Dutt M., Barthe G., Irey M., Grosser J. (2015). Transgenic citrus expressing an Arabidopsis NPR1 gene exhibit enhanced resistance against Huanglongbing (HLB; Citrus Greening). PLoS One 10:e0137134. 10.1371/journal.pone.0137134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis M. I., Peña A., Graham J. H. (2010). Detached leaf inoculation of germplasm for rapid screening of resistance to citrus canker and citrus bacterial spot. Eur. J. Plant Pathol. 127 571–578. 10.1007/s10658-010-9620-2 [DOI] [Google Scholar]
- Francis M. I., Redondo A., Burns J. K., Graham J. H. (2009). Soil application of imidacloprid and related SAR-inducing compounds produces effective and persistent control of citrus canker. Eur. J. Plant Pathol. 124 283–292. 10.1007/s10658-008-9415-x [DOI] [Google Scholar]
- Fu X., Gong X., Zhang Y., Wang Y., Liu J. (2012). Different transcriptional response to Xanthomonas citri subsp. citri between kumquat and sweet orange with contrasting canker tolerance. PLoS One 7:e41790. 10.1371/journal.pone.0041790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Andrade J., Ramirex V., Lopez A., Vera P. (2013). Mediated plastid RNA editing in plant immunity. PLoS Pathog. 9:e1003713. 10.1371/journal.ppat.1003713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwald T. R., Graham J. H., Schubert T. S. (2002). Citrus canker: the pathogen and its impact. Plant Health Prog. 10:32 10.1094/PHP-2002-0812-01-RV [DOI] [Google Scholar]
- Gottwald T. R., Hughes G., Graham J. H., Sun X., Riley T. (2001). The citrus canker epidemic in Florida: the scientific basis of regulatory eradication policy for an invasive species. Phytopathology 91 30–34. 10.1094/PHYTO.2001.91.1.30 [DOI] [PubMed] [Google Scholar]
- Graham J. H., Gottwald T. R., Cubero J., Achor D. S. (2004). Xanthomonas axonopodis pv. citri: factors affecting successful eradication of citrus canker. Mol. Plant Pathol. 5 1–15. 10.1046/j.1364-3703.2004.00197.x [DOI] [PubMed] [Google Scholar]
- Greiner S., Bock R. (2013). Tuning a menage a trois: co-evolution and co-adaptation of nuclear and organellar genomes in plants. Bioessays 35 354–365. 10.1002/bies.201200137 [DOI] [PubMed] [Google Scholar]
- Greiner S., Sobanski J., Bock R. (2015). Why are most organelle genomes transmitted maternally? Bioessays 37 80–94. 10.1002/bies.201400110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosser J., Ananthakrishnan G., Calovic M., Serrano P., Chandler J., Gmitter F. G., et al. (2005). Applications of somatic hybridization and cybridization in scion and rootstock improvement with focus on citrus. In Vitro Cell. Dev. Biol. Plant. 40 73–81. [Google Scholar]
- Grosser J. W., Gmitter F. G. (1990). Protoplast fusion and citrus improvement. Plant Breed. Rev. 8 339–374. 10.1002/9781118061053.ch10 [DOI] [Google Scholar]
- Grosser J. W., Ollitrault P., Olivares-Fuster O. (2000). Somatic hybridization in citrus: an effective tool to facilitate variety improvement. In Vitro Cell. Dev. Biol. Plant. 36 434–449. 10.1007/s11627-000-0080-9 [DOI] [Google Scholar]
- Guo W., Xiao S., Deng X. (2013). Somatic cybrid production via protoplast fusion for citrus improvement. Sci. Hortic. 163 20–26. 10.1016/j.scienta.2013.07.018 [DOI] [Google Scholar]
- Herrmann R. G., Maier R. M., Schmitz-Linneweber C. (2002). Eukaryotic genome evolution: rearrangement and coevolution of compartmentalized genetic information. Philos. Trans. R. Soc. Lond. Biol. 358 87–97. 10.1098/rstb.2002.1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges A. W., Rahmani M., Steven T. J., Spreen T. H. (2014). Economic impacts of the florida citrus industry in 2012-13. Food Resour. Econ. Dep. 07:2010. [Google Scholar]
- Hu X., Wansha L., Chen Q., Yang Y. (2009). Early signals transduction linking the synthesis of jasmonic acid in plant. Plant Signal. Behav. 4 696–697. 10.4161/psb.4.8.9181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Zhang J., Jia H., Sosso D., Li T., Frommer W. B., et al. (2014). Lateral organ boundaries 1 is a disease susceptibility gene for citrus bacterial canker disease. Proc. Natl. Acad. Sci. U.S.A. 111 e521–e529. 10.1073/pnas.1313271111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose M., Sugita M. (2016). RNA editing and its molecular mechanism in plant organelles. Genes 8:5. 10.3390/genes8010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S., Chittem K., Brueggeman R., Osorno J. M., Richards J., Nelson B. D., Jr. (2016). Comparative transcriptome analysis of resistant and susceptible common bean genotypes in response to soybean cyst nematode infection. PLoS One 11:e0159338. 10.1371/journal.pone.0159338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalaf A. A., Gmitter F. G., Jr., Conesa A. F., Dopazo J., Moore, Moore G. A. (2011). Fortunella margarita transcriptional reprogramming triggered by Xanthomonas citri subsp. citri. BMC Plant Biol. 11:159. 10.1186/1471-2229-11-159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalaf A. A., Moore G. A., Jones J. B., Gmitter F. G., Jr. (2007). New insights into the resistance of Nagami kumquat to canker disease. Physiol. Mol. Plant Pathol. 71 240–250. 10.1016/j.pmpp.2008.03.001 [DOI] [Google Scholar]
- Kim D. S., Hwang B. K. (2014). An important role of the pepper phenylalanine ammonia-lyase gene (PAL1) in salicylic acid-dependent signalling of the defence response to microbial pathogens. J. Exp. Bot. 65 2295–2306. 10.1093/jxb/eru109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Wang W., Wu H., Gong X., Moriguchi T. (2015). Polyamines function in stress tolerance: from synthesis to regulation. Front. Plant Sci. 6:827. 10.3389/fpls.2015.00827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Deng X. (2007). Citrus breeding and genetics in China. Asian Aust. J. Sci. Biotechnol. 1 23–28. [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2- ΔΔCT method. Methods 25 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lu H. (2009). Dissection of salicylic acid-mediated defense signaling networks. Plant Signal. Behav. 4 713–717. 10.4161/psb.4.8.9173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucht J. M. (2015). Public acceptance of plant biotechnology and GM crops. Viruses 7 4254–4281. 10.3390/v7082819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado M. A., Cristofani-Yaly M., Bastianel M. (2011). Breeding, genetic and genomic of citrus for disease resistance. Rev. Bras. Frutic. 33 158–172. 10.1590/S0100-29452011000500019 [DOI] [Google Scholar]
- Mafra V., Kubo K. S., Alves-Ferreira M., Ribeiro-Alves M., Stuart R. M., Boava L. P., et al. (2012). Reference genes for accurate transcript normalization in citrus genotypes under different experimental conditions. PLoS One 7:e31263. 10.1371/journal.pone.0031263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall O. J. (2004). PerlPrimer: cross-platform, graphical primer design for standard, bisulphite and real-time PCR. Bioinformatics 20 2471–2472. 10.1093/bioinformatics/bth254 [DOI] [PubMed] [Google Scholar]
- Mendes B., Cardoso S., Boscariol-Camargo R., Cruz R., Mourão Filho F., Bergamin A. (2010). Reduction in susceptibility to Xanthomonas axonopodis pv. citri in transgenic Citrus sinensis expressing the rice Xa21 gene. Plant Pathol. 59 68–75. 10.1111/j.1365-3059.2009.02148.x [DOI] [Google Scholar]
- Moreira C. D., Chase C. D., Gmitter F. G., Grosser J. W. (2000). Inheritance of organelle genomes in citrus somatic cybrids. Mol. Breed. 6 401–405. 10.1023/A:1009632223708 [DOI] [Google Scholar]
- Navarro L., Olivares-Fuster O., Juarez J., Aleza P., Pina J., Ballester-Olmos J., et al. (2004). Applications of biotechnology to citrus improvement in Spain. Acta Hortic. 632 221–234. 10.17660/ActaHortic.2004.632.28 [DOI] [Google Scholar]
- Nomura H., Komori T., Uemura S., Kanda Y., Shimotani K., Nakai K., et al. (2012). Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nature 3:926. 10.1038/ncomms1926 [DOI] [PubMed] [Google Scholar]
- Omar A. A., Murata M., Yu Q., Gmitter F. G., Chase C. D., Graham J. H., et al. (2017). Production of three new grapefruit cybrids with potential for improved citrus canker resistance. In Vitro Cell. Dev. Biol. Plant. 53 1–14. 10.1007/s11627-017-9816-7 [DOI] [Google Scholar]
- Omar A. A., Murata M. M., El-Shamy H., Graham J. H., Grosser J. W. (2018). Enhanced resistance to citrus canker in transgenic mandarin expressing Xa21 from rice. Transgenic Res. 27 179–191. 10.1007/s11248-018-0065-2 [DOI] [PubMed] [Google Scholar]
- Pfaffl M. (2002). “Relative quantification in book,” in Real Time PCR, ed. Dorak M. T. (Oxford: Taylor & Francis; ). [Google Scholar]
- Pfannschmidt T., Yang C. (2012). The hidden function of photosynthesis: a sensing system for environmental conditions that regulates plant acclimation responses. Protoplasma 249 125–136. 10.1007/s00709-012-0398-2 [DOI] [PubMed] [Google Scholar]
- Potrykus I. (2013). Unjustified regulation prevents use of GMO technology for public good. Trends Biotechnol. 31 131–133. 10.1016/j.tibtech.2012.11.008 [DOI] [PubMed] [Google Scholar]
- Pruvost O., Magne M., Boyer K., Leduc A., Tourterel C., Drevet C., et al. (2014). A MLVA genotyping scheme for global surveillance of the citrus pathogen Xanthomonas citri pv. citri suggests a worldwide geographical expansion of a single genetic lineage. PLoS One 9:e98129. 10.1371/journal.pone.0098129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano I., Audran C., Rivas S. (2016). Chloroplasts at work during plant innate immunity. J. Exp. Bot. 67 3845–3854. 10.1093/jxb/erw088 [DOI] [PubMed] [Google Scholar]
- Serrano M., Wang B., Aryal B., Garcion C., Abou-Mansour E., Heck S., et al. (2013). Export of salicylic acid from the chloroplast requires the multidrug and toxin extrusion-like transporter EDS5. Plant Physiol. 162 1815–1821. 10.1104/pp.113.218156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiguzov A., Vainonen J., Wrzaczek M., Kangasjärvi J. (2012). ROS-talk–how the apoplast, the chloroplast, and the nucleus get the message through. Front. Plant Sci. 3:292. 10.3389/fpls.2012.00292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover E., Stange R. R., McCollum T. G., Jaynes J., Irey M., Mirkov E. (2013). Screening antimicrobial peptides in vitro for use in developing transgenic citrus resistant to Huanglongbing and citrus canker. J. Am. Soc. Hortic. Sci. 138 142–148. [Google Scholar]
- Tillich M., Funk H. T., Schmitz-Linneweber C., Poltnigg P., Sabater B., Martin M., et al. (2005). Editing of plastid RNA in Arabidopsis thaliana ecotypes. Plant J. 43 708–715. 10.1111/j.1365-313X.2005.02484.x [DOI] [PubMed] [Google Scholar]
- Tripathi B. N., Bhatt I., Dietz K. (2009). Peroxiredoxins: a less studied component of hydrogen peroxide detoxification in photosynthetic organisms. Protoplasma 235 3–15. 10.1007/s00709-009-0032-0 [DOI] [PubMed] [Google Scholar]
- Trivedi P., Wang N. (2014). Host immune responses accelerate pathogen evolution. ISME J. 8 727–731. 10.1038/ismej.2013.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta A., Rahikainen M., Konert G., Finazzi G., Kangasjarvi S. (2014). Signalling crosstalk in light stress and immune reactions in plants. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369:20130235. 10.1098/rstb.2013.0235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viloria Z., Drouillard D., Graham J., Grosser J. (2004). Screening triploid hybrids of ‘Lakeland’limequat for resistance to citrus canker. Plant Dis. 88 1056–1060. 10.1094/PDIS.2004.88.10.1056 [DOI] [PubMed] [Google Scholar]
- War A. R., Paulraj M. G., War M. Y., Ignacimuthu S. (2011). Role of salicylic acid in induction of plant defense system in chickpea (Cicer arietinum L.). Plant Signal. Behav. 6 1787–1792. 10.4161/psb.6.11.17685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Yuan F., Long G., Qin L., Deng Z. (2012). Selection of reference genes for quantitative real-time RT-PCR analysis in citrus. Mol. Biol. Rep. 39 1831–1838. 10.1007/s11033-011-0925-9 [DOI] [PubMed] [Google Scholar]
- Yang L., Hu C., Li N., Zhang J., Yan J., Deng Z. (2011). Transformation of sweet orange Citrus sinensis (L.) Osbeck] with pthA-nls for acquiring resistance to citrus canker disease. Plant Mol. Biol. 75 11–23. 10.1007/s11103-010-9699-z [DOI] [PubMed] [Google Scholar]
- Zhang X., Francis M. I., Dawson W. O., Graham J. H., Orbovic V., Triplett E. W., et al. (2010). Over-expression of the Arabidopsis NPR1 gene in citrus increases resistance to citrus canker. Eur. J. Plant Pathol. 128 91–100. 10.1007/s10658-010-9633-x [DOI] [Google Scholar]