Introduction
Leukemia is a malignancy of the hematopoietic system clinically characterized by nonspecific systemic symptoms; however, many leukemia subtypes exhibit cutaneous manifestations.1 These findings may be classified as either unspecific, indicating associated nonleukemic dermatologic disease, or specific, denoting infiltration of leukemic cells into the epidermis, dermis, or subcutaneous tissue. These specific findings are known as leukemia cutis (LC), and may precede, follow, or occur concomitantly with systemic leukemia.2 LC may present as single or multiple lesions, most commonly on the trunk and extremities, but may also be disseminated.3 LC can present as a variety of morphologies3 but is most commonly described as pink, red-brown, or violaceous papules, nodules, and plaques.
Erythroderma is an inflammatory reaction characterized by erythema, pruritus, and scaling of the skin, typically presenting diffusely and covering most or all of the body, commonly defined as greater than 90% body surface area (BSA),4 although some references define it as at least 70% BSA.5 The most common causes include progression of existing dermatoses, drug reactions, underlying malignancy, or idiopathic causes.4 We present a case of a patient with newly diagnosed acute myelogenous leukemia (AML) who had erythrodermic LC shortly after induction chemotherapy.
Case report
A 50-year-old woman with no significant medical history had flu-like symptoms, including body aches, nausea, and sore throat, and was found to have a white blood cell count of 200,600/μL. Further clinical workup, including bone marrow biopsy, cytogenetic analysis, and flow cytometry, led to the diagnosis of FLT3 D835 mutation-positive AML. Immediately after diagnosis, she underwent induction treatment with 7 days of cytarabine and 3 days of daunorubicin. After completion of induction therapy, a bone marrow biopsy was negative for residual disease.
Three days after completing chemotherapy, she presented to the dermatology department with a diffuse erythematous and violaceous eruption across the arms, chest, trunk, and legs, consistent with erythroderma (Fig 1). Punch biopsy of the lesion found a monomorphous infiltrate beneath a grenz zone composed of intermediate size mononuclear cells with abundant cytoplasm and oval nuclei, consistent with LC (Fig 2). Given these findings, she was admitted for reinduction treatment, and the dermatology department was consulted for re-evaluation of her skin findings given her atypical clinical presentation for LC.
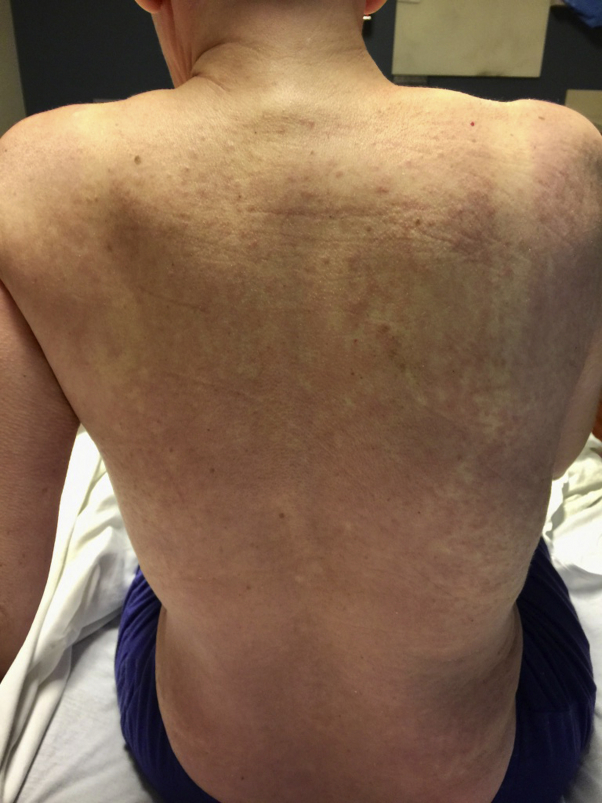
Fig 1.
Clinical presentation. On the scalp, back, abdomen, and chest, there were numerous 3- to 5-mm pink papules coalescent into confluent plaques on the abdomen and back.
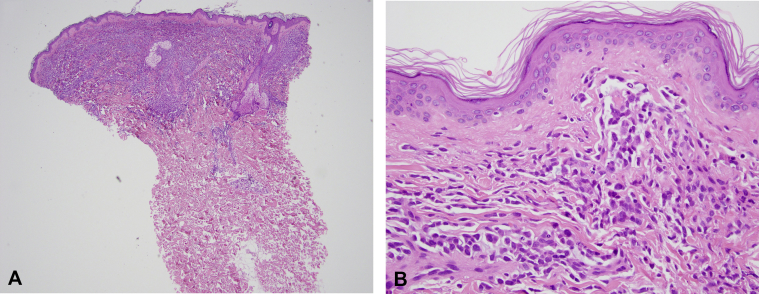
Fig 2.
Histopathology of LC. A, Punch biopsy shows a monomorphous infiltrate that fills the papillary dermis beneath a Grenz zone. B, Higher magnification shows intermediate size mononuclear cells with abundant cytoplasm and oval nuclei that are intercalating between the collagen bundles; a Grenz zone is again noted. (A and B, Hematoxylin-eosin stain; original magnifications: A, ×40; B, ×400.)
Physical examination found numerous erythematous to violaceous papules on the scalp, chest, abdomen, and upper back, which coalesced into confluent plaques and patches on the back and abdomen (Fig 1). Morbilliform violaceous, confluent, blanchable patches were seen on her bilateral upper and lower extremities (Fig 3). Overall, the eruption involved approximately 70% of her body surface area. There was no mucocutaneous involvement. Repeat punch biopsy of a papule on the right breast found a similar inflammatory reaction pattern to that of the previous biopsy with immunohistochemistry showing tumor cells positive for lysozyme and CD68 and negative for myeloperoxidase, CD56, and TCL1 (Fig 2, A and B). This finding was consistent with a diagnosis of LC associated with AML with monocytic differentiation. Reinduction therapy with high-dose cytarabine for 7 days led to complete resolution of the eruption within 2 weeks, and a repeat bone marrow biopsy was negative for residual disease.
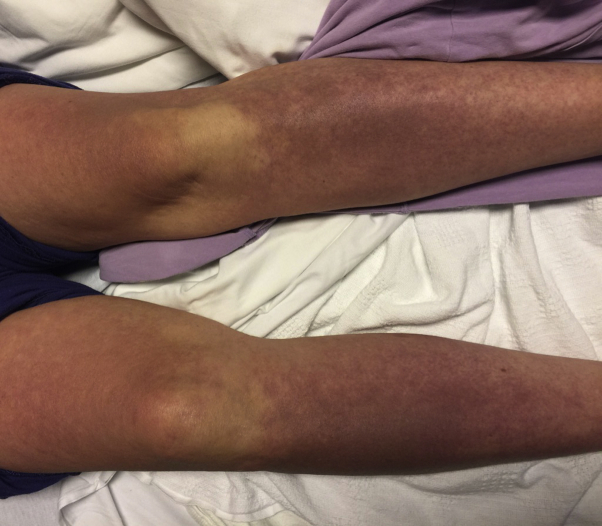
Fig 3.
Confluent deeply erythematous to violaceous patches covered the bilateral arms and legs. Overall, these patches and plaques covered 70% BSA, consistent with erythroderma.
Three months after initial diagnosis, she underwent an allogeneic unrelated donor hematopoietic stem cell transplant. She has not had any recurrence of cutaneous or systemic leukemia for the last 2 years and continues to be in remission.
Discussion
Of leukemia subtypes, AML is the most likely to develop LC,1 and the prevalence of biopsy-proven LC in patients with this subtype has been reported as 2.9%.6 Patients with different leukemia subtypes may have a wide variety of cutaneous manifestations, but only 4 other cases have been reported in the literature of LC presenting as erythroderma (Table I).5, 7, 8, 9 Of the cases reported, there is an equal ratio of male to female patients, and both acute and chronic subtypes of leukemia are represented. The prognosis for these patients ranges from death to full resolution of the leukemia after treatment.
Table I.
Erythrodermic LC reported in the literature
| Study | Patient age (y), sex | Leukemia subtype | Treatment | Erythroderma at presentation | BSA involved | Patient outcome |
|---|---|---|---|---|---|---|
| Su et al, 19849∗ | NR | Chronic lymphocytic leukemia | NR | Yes | NR | NR |
| Jeong et al, 20097 | 82, male | Small cell variant of T-cell prolymphocytic leukemia | Oral fludarabine and prednisone | Yes | 100% † | Death within 2 wks of chemotherapy initiation |
| Raj et al, 20118 | 75, male | Chronic myeloid leukemia | Imatinib | Yes | NR | Resolution within 1 mo of imatinib treatment |
| Novoa et al, 20155 | 65, female | Pre–B-cell acute lymphoblastic leukemia | Hospice care | No | 70% | Death |
| Current case, 2018 | 50, female | AML | Cytarabine, HSCT | No | 70% | Resolution within 2 wks of cytarabine reinduction |
HSCT, Hematopoietic stem cell transplant; NR, not reported.
Su et al describe 2 cases of erythroderma in patients with CLL as a part of a case series. It is not explicitly described whether the erythroderma was caused by leukemic infiltration of the skin or if it was related to the underlying leukemia.
Jeong et al describe involvement of the whole body although do not explicitly quantify the BSA involvement.
Prognosis remains poor in patients with LC. Treatment is aimed at addressing the underlying leukemia, which is dictated by subtype and the patient's tolerance of systemic therapy. Radiation therapy, specifically total skin electron beam therapy, is found to be an effective treatment for cutaneous disease, but systemic chemotherapy remains the treatment modality of choice.10
To our knowledge, this is the first reported case of a patient with AML subsequently having erythrodermic LC. Erythroderma represents a rare, but important presentation of LC that should be included in the differential diagnosis of erythroderma. LC may manifest as a variety of morphologies, and a high level of suspicion may be necessary to facilitate early recognition. Accurate diagnosis with skin biopsies to provide histopathologic and immunohistochemical information is crucial for subsequent treatment, prognosis, and overall patient quality of life.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Wagner G., Fenchel K., Back W., Schulz A., Sachse M.M. Leukemia cutis - epidemiology, clinical presentation, and differential diagnoses. J German Soc Dermatol. 2011;10:27–36. doi: 10.1111/j.1610-0387.2011.07842.x. [DOI] [PubMed] [Google Scholar]
- 2.Wagner L.I., Berg S.R., Gandhi M. The development of a Functional Assessment of Cancer Therapy (FACT) questionnaire to assess dermatologic symptoms associated with epidermal growth factor receptor inhibitors (FACT-EGFRI-18) Support Care Cancer. 2013;21:1033–1041. doi: 10.1007/s00520-012-1623-4. [DOI] [PubMed] [Google Scholar]
- 3.Kang Y.S., Kim H.S., Park H.J. Clinical characteristics of 75 patients with leukemia cutis. J Korean Med Sci. 2013;28:614–619. doi: 10.3346/jkms.2013.28.4.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan G.F., Kong Y.L., Tan A.S., Tey H.L. Causes and features of erythroderma. Ann Acad Med. 2014;43:391–394. [PubMed] [Google Scholar]
- 5.Novoa RA, Wanat K.A., Rosenbach M., Frey N., Frank D.M., Elenitsas R. Erythrodermic leukemia cutis in a patient with pre-B-cell acute lymphoblastic leukemia. Am J Dermatopathol. 2015;37:650–652. doi: 10.1097/DAD.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 6.Agis H., Weltermann A., Fonatsch C. A comparative study on demographic, hematological, and cytogenetic findings and prognosis in acute myeloid leukemia with and without leukemia cutis. Ann Hematol. 2002;81:90–95. doi: 10.1007/s00277-001-0412-9. [DOI] [PubMed] [Google Scholar]
- 7.Jeong K.H., Lew B.L., Sim W.Y. Generalized leukaemia cutis from a small cell variant of T-cell prolymphocytic leukaemia presenting with exfoliative dermatitis. Acta Dermato-venereologica. 2009;89:509–512. doi: 10.2340/00015555-0672. [DOI] [PubMed] [Google Scholar]
- 8.Raj A., Rai R., Rangarajan B. Exfoliative dermatitis with leukemia cutis in a patient with chronic myeloid leukemia: A rare association. Indian J Dermatol Venereol Leprol. 2011;77:208–210. doi: 10.4103/0378-6323.77471. [DOI] [PubMed] [Google Scholar]
- 9.Su W.P., Buechner S., Li C.Y. Clinicopathologic correlations on leukemia cutis. J Am Acad Dermatol. 1984;11:121–128. doi: 10.1016/s0190-9622(84)70145-9. [DOI] [PubMed] [Google Scholar]
- 10.Elsayad K., Oertel M., Haverkamp U., Eich H.T. The effectiveness of radiotherapy for leukemia cutis. J Cancer Res Clin Oncol. 2017;143:851–859. doi: 10.1007/s00432-016-2338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]