Abstract
T cells engineered with chimeric antigen receptors (CARs) have emerged as a potent new class of therapeutics for cancer, based on their remarkable potency in blood cancers. Since the first clinical reports of their efficacy emerged 7 years ago, investigators have focused on the mechanisms and properties that make CARs effective or toxic, and their effects on T cell biology. Novel CAR designs coupled with improvements in gene transfer technology, incorporating advances in gene editing, have the potential to increase access to engineered cell therapies, as well as improve their potency in solid tumors.
Keywords: CAR T cells, immunotherapy, chimeric antigen receptors, cancer
Main Text
Adoptive transfer of T cells collected from autologous peripheral blood and engineered to express chimeric antigen receptors (CARs) has produced impressive clinical responses in patients with hematologic malignancies.1 Together with checkpoint blockade therapy,2 CAR T cells are revolutionizing the field of cancer therapies, providing hope to patients with previously refractory cancers.3, 4, 5, 6, 7, 8, 9 CAR T cells targeting CD19 were recently approved by the US Food and Drug Administration (FDA) and the European Commission (EC) for the treatment of relapsed or refractory acute lymphoblastic leukemia (ALL) in pediatric and young adults (Kymriah), and for the treatment of relapsed or refractory diffuse large B cell lymphoma in adults (Kymriah and Yescarta). Emerging clinical data are proving the potential of CD22 and B cell maturation antigen (BCMA)-directed CAR T cells to eradicate leukemia and multiple myeloma, respectively, heralding a new era for the treatment of blood cancers.10, 11
Although the human T cell is a highly potent anti-tumor agent, several challenges remain for successfully applying CAR T cells to solid tumors. First, the potency of these “living drugs” can lead to lethal on-target toxicity if the target is expressed on a life-sustaining tissue.12 Second, active proliferation of the CAR T cells in the body can itself lead to potentially serious side effects, such as cytokine release syndrome13 and neurologic toxicity,14 although these are typically transient and reversible. Third, although finding a single uniquely expressed tumor antigen is already a challenge,15 targeting only one antigen may be insufficient to obtain sustained responses, because antigen loss has been identified as a frequent cause of tumor resistance to CAR T cell therapies.3, 10, 16 Fourth, in order to be effective in solid tumors, CAR T cells will have to overcome multiple challenges, including an immunosuppressive tumor microenvironment. Recent clinical reports suggest that CAR T cells can engraft in the peripheral blood, traffic to solid tumors, and respond to antigen.17, 18, 19, 20 We define engraftment as the ability for CAR T cells to be detectable in peripheral blood at any time after infusion. Moreover, evidence of transient antitumor activity has been observed in patients with difficult-to-treat tumors.21, 22 However, with rare exceptions, clinical responses in patients with solid tumors have been minor and transient, highlighting the importance of optimizing T cell therapeutics to tackle the solid tumor challenge. Finally, simplified methods to culture and engineer T cells are required to lower the costs, accelerate development, and improve access to this novel treatment.
In this review, we discuss the importance of fine-tuning each of the modules that form a CAR as an initial step to improve T cell specificity, antigen recognition, and T cell function. Second, we will summarize the current and novel tools that are being used to engineer CAR T cells, including recent developments in gene-editing technology that allow researchers to edit specific genes within the human genome, and even knock-in transgenes in specific integration sites. Finally, we will provide a comprehensive overview of exciting strategies that are being developed to generate new synthetic receptors with enhanced function and reduced toxicities.
For simplicity, this review focuses on modification of αβ-T cells with CARs. Substantial effort has been invested in identifying the best T cell subsets for adoptive cell transfer. Moreover, strategies to introduce CARs into other immune cell types, including γδ-T cells, natural killer cells, natural killer T cells, and macrophages have garnered growing attention. CAR design will need to be tailored to each cell type. The pursuit of the ideal cell type for redirection with CARs is beyond the scope of this review and has been covered elsewhere recently.23, 24, 25
Design and Optimization of CARs
CARs are designed in a modular fashion that typically consists of an extracellular target-binding domain, a hinge region, a transmembrane domain that anchors the CAR to the cell membrane, and one or more intracellular domains that transmit activation signals. Depending on the number of costimulatory domains, CARs can be classified into first (CD3z only), second (one costimulatory domain + CD3z), or third generation CARs (more than one costimulatory domain + CD3z). Introduction of CAR molecules into a T cell successfully redirects the T cell with additional antigen specificity and provides the necessary signals to drive full T cell activation. Because antigen recognition by CAR T cells is based on the binding of the target-binding single-chain variable fragment (scFv) to intact surface antigens, targeting of tumor cells is not MHC restricted, co-receptor dependent, or dependent on processing and effective presentation of target epitopes. In this section, we describe the properties and function of each of the modules that compose CARs. Despite intensive research efforts to define optimal CAR design, a universally improved CAR structure has not yet been discovered. As of now, each CAR construct needs empirical testing for evaluation, and several studies indicate that small modifications can have major consequences on the therapeutic outcome.
scFv
The antigen-binding capability of the CAR is defined by the extracellular scFv, not the targeted antigen. For instance, the scFv of both of the approved anti-CD19-targeted CARs, Kymriah and Yescarta, is derived from the murine anti-human CD19 antibody FMC63, which targets a specific epitope of CD19 found in exon 4 of the CD19 gene.26 Other anti-CD19 CARs may recognize different epitopes of CD19, or the same immunodominant epitope but with differential affinities, such as the scFv derived from SJ25C1,14 which is in the JCAR015 product. Investigators targeting MUC1 with CARs have used scFvs derived from antibody clones HMFG2, SM3, and 5E5, among others. HMFG2 and SM3 both recognize the PDTR epitope of the MUC1 variable tandem repeat; however, MUC1 is a highly glycosylated protein, especially within these tandem repeats, and SM3 only recognizes the deglycosylated protein, whereas HMFG2 can recognize deglycosylated or fully glycosylated protein.27 CAR T cells targeting MUC1, either utilizing SM3 scFv and co-expressing interleukin-12 (IL-12) or utilizing an HMFG2-based scFv, were employed in a phase I clinical trial to treat metastatic seminal vesicle cancer. HMFG2-based CAR T cells caused tumor necrosis, whereas SM3-based CAR T cells did not seem to have efficacy.28 In contrast, CARs using the 5E5 scFv recognize the GSTA epitope of the MUC1 repeat only when glycosylated with Tn antigen, an abnormal glycoform found in many epithelial cancers.29 Because glycosylation of tumor-associated antigens can be modulated by cell type or oncogenic transformation, this difference in epitope binding can define the target of CAR T cells and potentially discriminate between normal tissue expressing a TAA and a tumor cell expressing the same TAA. In fact, changes to the tumor cell surface glycome can affect cell signaling, affect cell-matrix and cell-cell interactions, and influence immune modulation and the cellular metastatic potential.30 Investigating the changes of the tumor membrane glycolipids and glycoproteins, which are encompassed in a broader view of the tumor “surfaceome,” can provide additional targetable avenues for cancer immunotherapy.
The format of an scFv is generally two variable domains linked by a flexible peptide sequence, either in the orientation VH-linker-VL or VL-linker-VH. The orientation of the variable domains within the scFv, depending on the structure of the scFv, may contribute to whether a CAR will be expressed on the T cell surface or whether the CAR T cells target the antigen and signal.31 In addition, the length and/or composition of the variable domain linker may be an important contribution to the stability of the scFv; in studies generating scFvs from a TAG72 antibody (clone B72.3), linkers up to 6xGGGGS demonstrated higher molecular weight dimers and multimers, with clustering decreasing with increasing linker length.32 If these scFvs are utilized in CAR molecules, this clustering may lead to antigen-independent signaling, or “tonic signaling.” Another linker of a TAG72-based scFv (clone CC49) designed to enhance proteolytic stability (Whitlow “218” linker: GSTGSGSKPGSGEGSTKG) had the effect of enhancing scFv affinity,33 which may alter CAR T cell behavior as well.
Two studies have demonstrated that variance of CAR scFv affinities, either through mutagenesis of complementary-determining regions while holding the epitope constant, or through CAR development with scFvs derived from therapeutic antibodies against the same target, but not the same epitope, can change the strength of the T cell signal and allow CAR T cells to differentiate overexpressed antigens from normally expressed antigens.34, 35 In fact, researchers demonstrated that trastuzumab-based CAR T cells with reduced scFv affinity could degranulate in response to HER2+ breast cancer cells without reactivity against normal primary human cells that express HER2.34 These results suggest that the scFv, a critical component of a CAR molecule, can be carefully designed and manipulated to influence specificity and differential targeting of tumors versus normal tissues. Given that these differences may only be measurable with CAR T cells (as opposed to soluble antibodies), pre-clinical testing of normal tissues for expression of the target, and susceptibility to on-target toxicities, requires live-cell assays rather than immunohistochemistry on fixed tissues.
Hinge
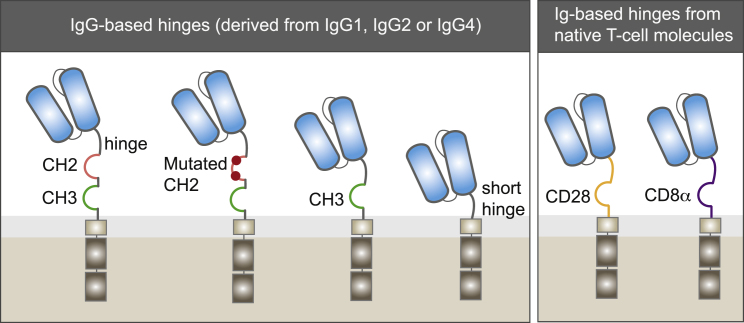
The hinge, also referred to as a spacer, is the extracellular structural region of the CAR that separates the binding units from the transmembrane domain (Figure 1). With the exception of a few CARs based on the entire extracellular moiety of a receptor, such as NKG2D,36 the majority of CAR T cells are designed with immunoglobulin (Ig)-like domain hinges. These spacers generally supply stability for efficient CAR expression and activity.
Figure 1.
Two Families of Hinges
IgG-based hinges, derived from IgG1, IgG2, or IgG4, provide flexibility to the scFv to target membrane-proximal epitopes. However, binding of the CH2 domain to Fc receptors in myeloid cells can impair functionality. Modification or deletion of the CH2 domain can restore CAR T cell functionality. CARs with shorter hinges, including an IgG-derived hinge lacking the CH2-CH3 regions, or hinges derived from native CD28 and CD8 hinges, can be used to target membrane-distal epitopes.
The hinge also provides flexibility to access the targeted antigen. Several studies have demonstrated that the optimal spacer length of a given CAR depends on the position of the targeted epitope.37 Long spacers provide extra flexibility to the CAR and allow for better access to membrane-proximal epitopes37, 38 or complex glycosylated antigens.39 By contrast, CARs bearing short hinges are more effective at binding membrane-distal epitopes.40, 41 It has been proposed that the length of the spacer is crucial to provide adequate intercellular distance for immunological synapse formation,42 highlighting the need to optimize spacers for individual epitopes accordingly.
Hinges can affect the overall performance of CAR T cells. Human IgG-derived spacers consisting of two Ig-like domains, CH2 and CH3, have been used for efficient antigen recognition and are especially useful in assessing the level of CAR expression at the surface of the T cell. However, several studies indicate that IgG-based spacers retain their capacity to bind to Fc gamma receptor (FcγR) through their CH2 domain even after they are incorporated into CARs. This feature leads to off-target activation by myeloid and lymphoid cells expressing FcγRs,43 and correlates with off-tumor localization and poor engraftment in animal models.41, 44, 45 Point mutations to ablate the FcγR binding domain and spacers trimmed down to a single CH3 domain have improved CAR T cell engraftment and anti-tumor efficacy in in vivo experiments.41, 45, 46 Alternatively, extracellular domains naturally lacking FcγR binding activity, such as CD28 and CD8α, have been extensively used as spacers.47, 48 In fact, these two spacers are found in Yescarta and Kymriah, respectively.
Transmembrane Domain
The transmembrane domain consists of a hydrophobic α helix that spans the cell membrane and is probably the least characterized region of the CAR. Although the main function of the transmembrane is to anchor the CAR in the T cell membrane, some evidence suggests that the transmembrane can be relevant for CAR T cell function.
The nature of the CAR signaling is not well understood; however, if CARs behave similar to TCRs, it is likely that CARs need to dimerize or associate with other accessory molecules for proper signaling initiation. In that regard, one study demonstrated that first generation CARs need specific regions within the CD3 transmembrane for CAR dimerization or incorporation into endogenous TCR clusters.49
Second and third generation CARs have been designed with numerous single-span transmembranes, mainly derived from CD4, CD8α, or CD28.47, 50, 51 A recent study demonstrated that when using CARs containing the inducible T cell costimulator (ICOS) intracellular domain, incorporation of the ICOS transmembrane, instead of the extensively used CD8α transmembrane, was required for increased CAR T cell persistence and overall anti-tumor efficacy.52 In another study, Morin et al.53 analyzed the function of the extracellular and transmembrane domains of the endogenous CD28 molecule using a transgenic mouse that lacks the intracellular tail of CD28. Interestingly, the authors show that the extracellular and transmembrane domains of CD28 can partially induce T cell activation.53
Altogether, these results suggest that the transmembrane domain of certain costimulatory molecules can be involved in synapse formation or T cell signaling. Linking the proximal intracellular domain to its corresponding transmembrane domain may enable proper CAR T cell signaling, while using the widely used CD8α or CD28 transmembrane domains may favor CAR expression or stability.
Costimulatory Domains: The Art of Signaling
Substantial effort at CAR T cell engineering has been invested in understanding the effects of CAR costimulation with the aim of identifying an optimal endodomain to include in CAR constructs. CAR endodomains, or intracellular domains, are usually derived from costimulatory molecules from the CD28 family (including CD28 and ICOS) or the tumor necrosis factor receptor (TNFR) family of genes (including 4-1BB, OX40, or CD27). The CD28 family signals through the phosphatidylinositol 3-kinase (PI3K)-Akt pathway, with ICOS inducing a stronger PI3K activation than CD28.52, 54 Unique to CD28 is the recruitment of Grb2 and Lck to its intracellular tail, which results in high levels of IL-2 production,55 as well as the recruitment of Itk. The TNFR family members signal through recruitment of TRAF proteins56 and are implicated in modulating T cell proliferation, differentiation, and survival.57, 58, 59
CD28 and 4-1BB are the most widely used costimulatory endodomains in CARs. Clinical trials with CARs incorporating CD28 or 4-1BB intracellular domains showed similar response rates in patients with hematologic malignancies. However, the persistence of T cells engineered with these two CAR designs is strikingly different. Pre-clinical studies identified these T cell persistence differences in head-to-head comparisons of CD28- and 4-1BB-based CAR T cells in animal models.48, 60 Clinical trials for B cell malignancies have shown that CD28-based CAR T cells are typically undetectable beyond 3 months,6, 61 whereas 4-1BB-based CAR T cells can persist in patients for several years after treatment.62 Exhaustive studies indicate that signaling through CD28-based CARs results in more rapid T cell activation, proliferation, cytolysis, and increased glycolysis, but shorter T cell persistence. By contrast, 4-1BB signaling induces a slower T cell effector response and promotes mitochondrial biogenesis, greater oxidative metabolism, and sustained T cell persistence.52, 63, 64, 65 The high effector function and self-limited expansion of CD28-based CARs may be ideal to transiently treat diseases with a rapid tumor elimination and short-term persistence of the CAR (i.e., as a bridge therapy for allogeneic hematopoietic stem cell transplantation). By contrast, 4-1BB-based CARs may be used to treat diseases in which complete responses require sustained T cell persistence.62
Similar to 4-1BB, CD27 signaling has been shown to enhance CAR T cell survival when compared with CD28.66 Incorporation of ICOS into a CAR drives CD4+ T cells toward a Th1/Th17 phenotype, enhancing T helper functions and increasing in vivo T cell persistence when compared with the widely used CD28 and 4-1BB intracellular domains.67 Recent data suggest that various lymphocyte subsets require distinct costimulation signals for optimal function and persistence. For instance, whereas the ICOS intracellular domain enhances the persistence of CD4+ CAR T cells, the 4-1BB intracellular domain provides optimal persistence in CD8+ T cells.52 One option to join the properties of different intracellular domains in one single T cell is to combine two intracellular domains in a third generation CAR. Typically, such combinations include one intracellular domain from the CD28 family and one intracellular domain from the TNFR family, resulting in the simultaneous activation of different signaling pathways.52, 60, 68, 69 Third generation CARs are consistently expressed at lower levels when compared with second generation CARs, but they usually show improved effector functions. Optimization of these more complex receptors, including the position of each intracellular domain in relation to the cell membrane, is required to attain complete synergy of the combination.52
Altogether, these findings indicate that the nature of costimulation during CAR T cell activation critically regulates the metabolism, survival, and effector function of T cells. Each costimulatory domain has unique properties, and it is unlikely that an optimal costimulatory domain will serve all purposes. Differences in the affinity of the scFv, the intensity of antigen expression, the probability of off-tumor toxicity, or the disease to be treated may influence the selection of the intracellular domain. Further understanding of the molecular signaling effects of CARs may help in choosing the right intracellular domain or combination of intracellular domains for each condition. Ultimately, carefully designed clinical trials may be required to understand the effects of costimulation on the success of CAR T cell therapies.
Avoiding Tonic Signaling
Although the main purpose of engineering T cells with CARs is to drive activation upon antigen-specific recognition in tumors, some CAR constructs can also induce antigen-independent activation, usually referred to as tonic signaling. High density of CARs at the T cell surface, usually driven by strong constitutive promoters, can result in clustering of CAR molecules that leads to chronic T cell activation. Tonic signaling can be detected during in vitro primary T cell expansions and is characterized by differences in the growth patterns, phenotype, and function of CAR T cells when compared with control T cells. The level and consequences of tonic signaling are highly dependent on the selection and configuration of CAR modules, but they usually include chronic T cell activation with accelerated differentiation and exhaustion, and impaired anti-tumor effects.52, 70 Moreover, tonic signaling in cMET-specific CARs containing the CD28 signaling domain can result in continuous in vitro proliferation, with constitutive secretion of diverse cytokines, including IL-2.71 In a GD2-specific CAR, substitution of the CD28 intracellular domain for the 4-1BB intracellular domain ameliorated the T cell exhaustion caused by tonic signaling. Although low levels of tonic signaling in 4-1BB-based CARs may be beneficial because of enhanced T cell persistence,48 recent reports indicate that in some cases, 4-1BB-driven tonic signaling may result in augmented T cell apoptosis, impaired in vitro expansion, and progressive downregulation of CAR expression.52, 72 The level of tonic signaling can also depend on the length of the hinge,45 the expression system,52, 71 and the vector used.72 In third generation CARs, tonic signaling may be mitigated by placing the 4-1BB intracellular domain distal to the cell membrane.52
Tonic signaling is not usually assessed systematically in most CAR reports. However, emerging evidence underscores the importance of avoiding the use of CARs with configurations that may induce this chronic activation. Mitigating tonic signaling may require testing different combinations of scFvs, hinges, and intracellular domains. CAR aggregation can be attenuated by using alternative promoters to reduce the levels of CAR expression; however, this can also result in impaired efficacy.52 A recent report suggests that knocking in the CAR into the TRAC locus through homology-directed recombination averts tonic CAR signaling by optimizing CAR internalization and re-expression kinetics.73
Genetic Engineering Strategies
Viral- and non-viral-based genetic engineering tools have been used to generate CAR T cells, resulting in permanent or transient expression of therapeutic genes (Table 1). However, most of the current clinical trials developing modified T cells employ the latest generations of retroviral (gamma retroviral and lentiviral) vectors.
Table 1.
A Summary of the Current Strategies Being Used to Genetically Modify T Cells Ex Vivo to Express Chimeric Antigen Receptors
| Transgene Insertion | Transgene Expression | Transgene Lifespan | Transgene Delivery | Types | Features and Observations | |
|---|---|---|---|---|---|---|
| Retroviral vectors | non-targeted integration | exogenous promoter | long | ex vivo transduction | gamma retrovirus | safe, optimized, and FDA-approved protocols; the production of the therapeutic cells is expressive |
| lentivirus | transgene size limitation | |||||
| Transposase enzymes | non-targeted integration | exogenous promoter | long | ex vivo electroporation | Sleeping Beauty | FDA-approved protocols, more economical than viral vectors, but less developed technology; transposase have to be electroporated along with the donor DNA (plasmid or minicircles) |
| piggyBac | ||||||
| mRNA | non- integrative | N/A | short | ex vivo electroporation | N/A | fast and economical method to produce CAR T cells; the transgene expression is rapidly diluted over the expansion of the T cells; ideal when first introducing a novel CAR into patients |
| Non-integrative lentivirus | non-integrative, episomal | exogenous promoter | mid-short | ex vivo transduction | NILV-S/MAR | the transient expression can be extended up to 30 days; the production of the non-integrative lentivirus is expensive and will still require constant re-dosing |
| Endonuclease enzymes | targeted integration | endogenous promoters | long | ex vivo electroporation | zinc-finger | directed transgene insertion into the host cell genome; ability to ablate specific host cell genes; endonucleases have to be electroporated along with donor DNA (AAV or linear dsDNA); further protocol optimization is required |
| TALENs | ||||||
| CRISPR/Cas9 |
NILV-S/MAR, non-integrating lentiviral vector containing a scaffold/matrix attachment region.
Retrovirus-based gene delivery is a mature, well-characterized technology, which permanently integrates the CAR into the host cell genome. Although serious adverse events were linked to retroviral gene transfer in CD34+ stem cells,74, 75 mature T cells have been safely modified with this framework now for decades with hundreds of treated patients, resulting in stable engraftments and without cell transformation.76, 77 The downsides of this approach are the cost associated with clinical-grade production and the limited amount of genetic material that can be included in the vector. A recent study using lentiviral vectors in patients with ALL reported that unintentional transduction of a single leukemic B cell during the CD19-CART manufacturing resulted in resistance to CAR T cell therapy through masking of the CD19 epitope. Although this is a rare event, this finding highlights the importance of properly isolating T cells from leukemic cells before redirection.78
Non-viral DNA transfection transposition systems have also been used for permanent expression of therapeutic payload. Sleeping Beauty (SB) transposon is the most developed system of its kind to engineer CAR T cells,79 complying with current good manufacturing practice (cGMP),80 and is currently under investigation in the clinic (ClinicalTrials.gov: NCT00968760 and NCT01653717). Multiple SB enzymes81, 82 have been used to deliver more than one transgene from a multi-cistronic single plasmid83, 84 or multiple plasmids.85 Others have developed CAR T cells using the piggyBac transposon system,86, 87, 88 which can integrate larger transgenes.89, 90 In general, transposon technology might be easier and more economical to produce than viral vectors. However, the longer expansion protocols currently employed may result in T cell differentiation, as well as impaired activity and poor persistence of the infused cells.91 In this regard, recent developments using minicircle vectors might help address these issues because of higher efficiency integrations.92
Non-integrative gene transfer, such as RNA transfection, enables transient expression of the CAR for up to 1 week. This strategy has been used to evaluate potential toxicities or to limit the side effects of the therapy.20, 93 However, the weaknesses of a transient CAR expression are that the transgene is rapidly lost, and patients will require multiple infusions for clinical effect. Alternatively, non-integrative CAR delivery, such as episomal gene transfer, may result in longer-term, but not permanent, expression.94
Finally, in order to simplify and reduce the cost of CAR T cell manufacturing, alternative approaches using in situ T cell modification are being explored. In situ T cell programming using DNA nanocarriers95 or lentiviral vectors specifically targeting human CD8+ T cells96 have been proved safe and efficacious in pre-clinical models of B cell malignancies.
Gene Editing: The New Era
Improvements to site-directed nuclease gene-editing tools are unveiling a new era for CAR T cell therapy. Most efforts at T cell gene editing thus far have been focused on developing off-the-shelf universal CAR T cells from allogeneic healthy donors. The majority of current clinical trials use autologous CAR T cells, because use of allogeneic CAR T cells could induce graft-versus-host disease (GVHD) due to TCR-mediated recognition of the recipient alloantigens. The immune system of the recipient could also attack the infused human leukocyte antigen (HLA)-disparate CAR T cells, causing rapid T cell rejection. In order to prevent GVHD, different groups have focused on removing the TCR from allogeneic CAR T cells using genome editing. Infusion of gene-edited universal CD19-CART cells in two pediatric patients with ALL induced complete responses in both patients, proving the feasibility of this approach.97 Strategies to prevent CAR T cell rejection by the recipient are also being explored and include the elimination of HLA class I expression by editing the HLA-A or β2-microglobulin genes.
Gene editing is also being explored to selectively remove the expression of T cell-suppressive genes.98, 99, 100 Checkpoint inhibitor PD-1 is probably the most prominent gene currently targeted, with PD-1 knockout anti-MUC1 CAR T cells entering clinical trials this year (ClinicalTrials.gov: NCT03525782). Recently, Ren and colleagues99 set up a versatile system for editing multiple genes simultaneously in CAR T cells through a single-vector lentiviral system.
Target-specific endonucleases have also been exploited to insert transgenes inside loci relevant for T cell biology (Table 1). Unlike random insertion strategies, this method may result in finely regulated CAR expression independent of constitutive promoters.73, 101 In a landmark study, Eyquem et al.73 demonstrated that targeting the CAR insertion into the TCR locus results in more uniform expression and increased potency over conventionally generated CAR T cells.
Different endonucleases have been used to knock out genes in T cells, including zinc-finger nucleases (ZFNs),102 TALENs,97, 103, 104 and Cas9.98, 99 For knock-in approaches, the transgene can be supplied from donor DNA using non-integrative viral vectors, such as adeno-associated viral vector type 6 (AAV6),73, 101 or non-viral approaches, such as single-stranded DNA.105 The enzymes are typically electroporated into T cells as mRNA or protein.98, 99 More recently, a study using the CRISPR/Cas9 system has demonstrated that donor DNA can be electroporated as endonuclease-complexed and long linear double-stranded DNA (dsDNA), which is otherwise toxic, yielding relatively high T cell viability and transgene insertion efficiencies.105 This system avoids the use of viral vectors, which is expensive and time-consuming to generate and test, and could therefore substantially reduce the cost and time for gene editing in T cells. Overall, this report underscores the potential to develop next generation CAR T cell therapies; however, further protocol optimization will be needed to achieve large-scale manufacturing of gene-edited CAR T cells.
Finally, a better understanding of the potential deleterious effects of genome editing is required to ensure that benefits derived from further modifications in CAR T cells are greater than risks. In this regard, whole-genome sequencing of CRISPR/Cas9 and TALEN-targeted cell lines revealed a low incidence of off-target mutations.106 Recent studies suggest that Cas9 can induce a p53-mediated toxicity in certain cell types, which can lead to a selection of p53 mutant cells more tolerant to DNA damages.107, 108 Careful monitoring of the p53 status in gene-edited cells together with efforts to enhance nuclease specificity may help reduce the potential risks of genome editing in CAR T cells.
The Next Generation of CARs
Several new approaches to enhance the therapeutic outcome of CAR T cells are being explored based on the lessons learned in recent clinical trials and fueled by rapid advances in synthetic biology. These approaches are mainly focused on improving T cell activation and persistence, overcoming tumor immunosuppression, addressing tumor escape, mitigating toxicities, and increasing access. CAR T cell therapies incorporating costimulatory molecules, inhibitory-to-costimulatory switch signals, cytokines, or safety switches have been developed to address these challenges and have been reviewed elsewhere.23, 109, 110, 111 Here, we will highlight some of the innovative strategies based on new antigen receptors able to recognize a combination of antigens in an effort to avoid antigen escape or reduce toxicity (Figure 2).
Figure 2.
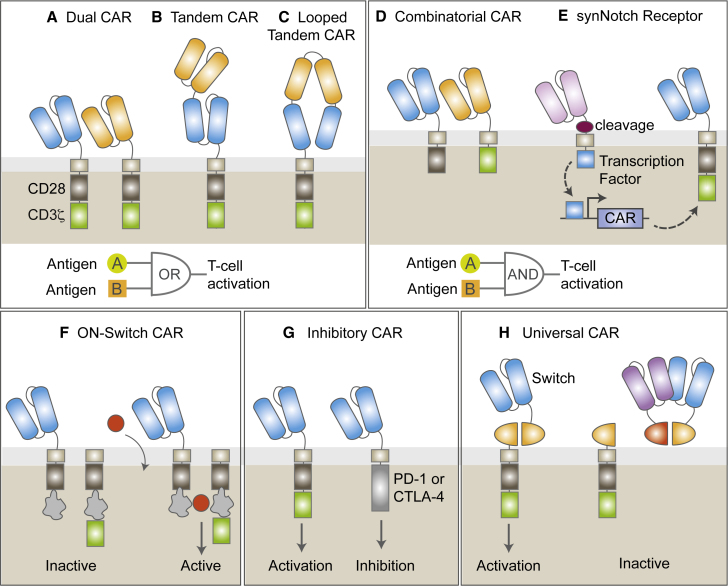
The Next Generation CARs: New Strategies to Avoid Tumor Escape and Mitigate Toxicities
Schematic representation of novel receptors. (A–C) Multitargeted CAR configurations. Dual CARs co-express two different CARs in one cell (A). Tandem CARs contain two different scFvs in a single CAR molecule that can either be stacked (B) in series or as a looped structure (C). Dual and tandem CARs function as OR-gate circuits: CAR T cells are activated following recognition of antigen A OR antigen B. (D and E) Combinatorial CARs combine two constructs: one bears the CD3z signaling motif and the other bears the costimulatory signaling domain (D). Synthetic Notch (syn-Notch) receptors induce the transcription of a CAR after antigen recognition of their cognate antigen (E). Combinatorial and syn-Notch CARs function as AND-gate circuits: CAR T cells are fully activated only when antigen A AND antigen B are recognized. (F) On-switch CAR T cells are inactive until specific activating agents are added, assembling a fully functional receptor. (G) Inhibitory CARs (iCAR) inhibit T cell activation following antigen recognition in normal cells. (H) Universal or switchable CAR T cells remain inactive until antibody-based molecules targeting a tumor antigen are supplied to reconstitute a fully active CAR construct.
Limiting Tumor Escape
A major challenge for CAR T cell therapies is tumor escape due to antigen loss in the setting of inherent tumor heterogeneity and plasticity. Loss or decreased expression of the targeted antigen after CAR T cell therapy and outgrowth of antigen-negative cells after treatment has been observed in several clinical studies,10, 17, 18, 112, 113 highlighting the importance of simultaneously targeting more than one antigen.
One approach to overcome this obstacle is to express two different CARs in one single cell in order to target two different antigens, ideally in one single bicistronic vector to ensure dual-antigen targeting in all cells, an approach known as “dual CARs.”114 Expression of two CAR molecules in one viral plasmid may require codon optimization of duplicated DNA sequences (i.e., CD3ζ) to reduce the chances of DNA recombination. Another alternative is to design a receptor fusing two scFvs with different specificities with a single intracellular module in a tandem CAR.10, 115, 116 Tandem CARs have the advantage of a smaller transgene size when compared with dual CARs, which is especially important when other transgenes (i.e., safety switches or cytokines) are also incorporated into the CAR plasmid. The main disadvantage is that in order to achieve optimal antigen recognition and full T cell activation, tandem CARs require optimization of the spacer length and linker sequence between the two scFvs, as well as the orientation of scFvs, which is more complex than with second generation CARs.117 However, computational modeling methods can help predict the structure of tandem CARs, making design and development of these receptors more feasible.115
Dual and tandem CARs are known as OR-gate CARs, as binding of either CAR to its cognate antigen is enough to drive full T cell activation. Interestingly, enhanced T cell function is observed when both targets are present, which makes them more efficient at inducing anti-tumor responses than a pooled combination of CAR T cells.114, 116, 118 One of the main limitations of OR-gate CARs is that targeting two antigens may result in increased risk for toxicity, especially when treating solid tumors. Ongoing clinical trials are currently testing dual (ClinicalTrials.gov: NCT03330691) and tandem (ClinicalTrials.gov: NCT03185494, NCT03097770, and NCT03019055) CAR T cells simultaneously targeting CD19 and CD22 or CD20 for the treatment of leukemia and lymphoma.
Minimizing Toxicities
Lack of truly tumor-specific antigens is a major limitation for the development of CAR T cells. Most of the antigens currently targeted with CAR T cells are also expressed in normal cells, where even low levels of antigen expression can potentially result in severe on-target off-tumor toxicities.12, 119, 120 Novel strategies in which engineered T cells require a specific combination of two antigens for activation are being explored to prevent toxicities. In a first approach in this direction, T cells were engineered to co-express two different CARs, one containing signal 1 (CD3z) and the other containing signal 2 (costimulation).121, 122 In this split-signal approach, recognition of a single antigen, which may happen in normal cells, leads to suboptimal T cell activation and limited killing of normal cells. In tumors, where both antigens may be expressed, full T cell activation is achieved after engagement of both scFvs to their cognate antigens.122 In a different approach, the activation of CAR T cells can be controlled in situ by the addition of a small molecule. “ON-switch” CARs are designed as fragmented CAR receptors, where the extracellular antigen-binding module is dissociated from the intracellular signaling components.123 Assembly of these two receptors in the presence of a heterodimerizing small molecule, such as tacrolimus-based analogs, leads to CAR T cell activation. The magnitude of responses depends on the dosage of the drug, which allows for titratable control. A more recent strategy involves an AND-gate recognition system in which activation of one receptor (synthetic Notch) induces the expression of a second receptor (CAR) that induces T cell activation following antigen recognition.124 Full T cell activation and tumor elimination occur only when both antigens are expressed. However, tumor escape of CAR T cells through loss of the first antigen targeted by the synthetic Notch receptor is a major limitation of this strategy.
A distinct class of strategies to limit toxicity includes the co-expression of a classic CAR with an antigen-specific inhibitory CAR (iCAR) bearing the signaling domain of an immunoinhibitory receptor (i.e., CTLA-4 or PD-1).125 Engagement of iCARs to antigens in normal cells can constrain T cell functions, which can be resumed in the absence of the iCAR-targeted antigen and following antigen recognition by the activating CAR. One of the challenges of this approach is the identification of cell surface antigens that are expressed in normal cells but absent in tumor cells, a problem that is complementary to the obstacle of identifying tumor-specific antigens absent in normal tissues.
Universal CARs: Increasing Versatility
A different strategy that could simultaneously address antigen escape while mitigating toxicities is the utilization of so-called universal CARs. Instead of engineering T cells with fixed antigen specificities, these CAR therapies are composed of two parts: (1) an antibody-based molecule that recognizes a tumor antigen and is modified to express a “switch”; and (2) a universal CAR T cell without tumor specificity by itself, which contains a construct with an extracellular portion that binds to the switch and is linked to the intracellular signaling domains. In a first approach, universal CAR T cells were designed to bind to biotinylated antigen-specific molecules.126 Other approaches incorporated the small molecule fluorescein isothiocyanate (FITC) or a short peptide in the antibody-based switch, allowing activation of the corresponding switchable CAR T cells after tumor recognition by the antibody-switch.127, 128 In a more recent approach, a split-CAR system named SUPRA CAR combines zipCAR T cell containing an extracellular leucine zipper with an scFv domain fused to a second leucine zipper (zipFv).129 These versatile systems allow researchers to easily combine universal CAR T cells with zipFvs targeting different antigens to avoid tumor escape. At the same time, modulating the dose or affinity of the antibody-based switches may control toxicities. In the case of SUPRA CAR T cells, T cell activation can be prevented when necessary by addition of a competitive zipFv that can bind with high affinity to the zipFv with tumor specificity.129
Conclusions
The clinical development of CAR T cells has undergone tremendous growth over the past decade. However, this novel therapeutic modality is still in its infancy, and much remains to be learned and improved. Clinical trials evaluating CAR T cells in patients with both hematologic malignancies and solid tumors have revealed insights into the parameters that determine clinical outcome. Based on the lessons learned, a new arsenal of novel receptors and strategies has been developed and is poised to enter clinical trials.
Preclinical studies have unveiled the importance of optimizing CAR constructs. The affinity and flexibility of the extracellular binding domain is key for improved tumor recognition, whereas the nature of costimulation during CAR T cell activation critically regulates the metabolism, survival, and effector function of T cells. A major challenge to the field is that the optimal CAR structure for each given antigen to be targeted remains empirical and involves the generation and testing of several constructs. As novel, more complex receptors emerge, optimizing each module may lead to an unrealistic number of constructs to test, both in animal models and in humans. In this regard, bridging the scientific divide between chimeric molecular engineering and protein structure identification and modeling may help predict which molecules would function optimally and reduce the number of constructs to evaluate. Identifying general rules to guide CAR design would also simplify this process. Also, a better understanding of the interactions of CAR proteins with endogenous T cell molecules and detailed mechanisms of CAR signaling would facilitate the design of more effective CAR T cell treatments.
One major limitation, especially when treating solid tumors, is the absence of truly tumor-specific antigens that are expressed in tumor cells but absent in normal tumors. Expression of the targeted antigen in normal cells can result in severe toxicity, whereas absence or decreased antigen expression in tumor cells can lead to tumor escape. Thus, it is critical to explore the tumor surfaceome, including differential post-translational modifications of tumor-associated antigens, in order to identify new targets for antibody-based therapies, such as glycan-directed CAR T cells.130 Strategies to optimize CAR specificity and reduce toxicity will be critical as we increase the number of tumor antigens to target. In this regard, recent technological advances in single-cell analysis tools and precision bioinformatics may help unveil additional differences between normal and tumor cells,131 albeit with increasing tumor heterogeneity.
Engineered T cells have shown their potential to dramatically change clinical outcomes in patients with B cell malignancies. Remarkable advances in the fields of molecular biology, immunology, gene editing, synthetic biology, and computational analysis provide new tools for CAR T cell development and are critical to achieving a greater therapeutic success when expanding this therapy to other cancer types.
Conflicts of Interest
S.G., A.D.P., and M.V.M. are listed as inventors on various patents related to chimeric antigen receptors.
References
- 1.June C.H., Sadelain M. Chimeric antigen receptor therapy. N. Engl. J. Med. 2018;379:64–73. doi: 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ribas A., Wolchok J.D. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maude S.L., Laetsch T.W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M.R., Stefanski H.E., Myers G.D. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee D.W., Kochenderfer J.N., Stetler-Stevenson M., Cui Y.K., Delbrook C., Feldman S.A., Fry T.J., Orentas R., Sabatino M., Shah N.N. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turtle C.J., Hanafi L.A., Berger C., Gooley T.A., Cherian S., Hudecek M., Sommermeyer D., Melville K., Pender B., Budiarto T.M. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Invest. 2016;126:2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park J.H., Rivière I., Gonen M., Wang X., Sénéchal B., Curran K.J., Sauter C., Wang Y., Santomasso B., Mead E. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N. Engl. J. Med. 2018;378:449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porter D.L., Hwang W.T., Frey N.V., Lacey S.F., Shaw P.A., Loren A.W., Bagg A., Marcucci K.T., Shen A., Gonzalez V. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuster S.J., Svoboda J., Chong E.A., Nasta S.D., Mato A.R., Anak Ö., Brogdon J.L., Pruteanu-Malinici I., Bhoj V., Landsburg D. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N. Engl. J. Med. 2017;377:2545–2554. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neelapu S.S., Locke F.L., Bartlett N.L., Lekakis L.J., Miklos D.B., Jacobson C.A., Braunschweig I., Oluwole O.O., Siddiqi T., Lin Y. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fry T.J., Shah N.N., Orentas R.J., Stetler-Stevenson M., Yuan C.M., Ramakrishna S., Wolters P., Martin S., Delbrook C., Yates B. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 2018;24:20–28. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolgin E. CAR-Ts move beyond B-cell cancers to myeloma. Nat. Biotechnol. 2017;35:599–601. doi: 10.1038/nbt0717-599. [DOI] [PubMed] [Google Scholar]
- 12.Morgan R.A., Yang J.C., Kitano M., Dudley M.E., Laurencot C.M., Rosenberg S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maude S.L., Barrett D., Teachey D.T., Grupp S.A. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014;20:119–122. doi: 10.1097/PPO.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gust J., Hay K.A., Hanafi L.A., Li D., Myerson D., Gonzalez-Cuyar L.F., Yeung C., Liles W.C., Wurfel M., Lopez J.A. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7:1404–1419. doi: 10.1158/2159-8290.CD-17-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newick K., O’Brien S., Moon E., Albelda S.M. CAR T cell therapy for solid tumors. Annu. Rev. Med. 2017;68:139–152. doi: 10.1146/annurev-med-062315-120245. [DOI] [PubMed] [Google Scholar]
- 16.Sotillo E., Barrett D.M., Black K.L., Bagashev A., Oldridge D., Wu G., Sussman R., Lanauze C., Ruella M., Gazzara M.R. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5:1282–1295. doi: 10.1158/2159-8290.CD-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Rourke D.M., Nasrallah M.P., Desai A., Melenhorst J.J., Mansfield K., Morrissette J.J.D., Martinez-Lage M., Brem S., Maloney E., Shen A. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017;9:eaaa0984. doi: 10.1126/scitranslmed.aaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown C.E., Badie B., Barish M.E., Weng L., Ostberg J.R., Chang W.C., Naranjo A., Starr R., Wagner J., Wright C. Bioactivity and safety of IL13Rα2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin. Cancer Res. 2015;21:4062–4072. doi: 10.1158/1078-0432.CCR-15-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed N., Brawley V.S., Hegde M., Robertson C., Ghazi A., Gerken C., Liu E., Dakhova O., Ashoori A., Corder A. Human epidermal growth factor receptor 2 (HER2)-specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J. Clin. Oncol. 2015;33:1688–1696. doi: 10.1200/JCO.2014.58.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beatty G.L., O’Hara M.H., Lacey S.F., Torigian D.A., Nazimuddin F., Chen F., Kulikovskaya I.M., Soulen M.C., McGarvey M., Nelson A.M. Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a phase 1 trial. Gastroenterology. 2018;155:29–32. doi: 10.1053/j.gastro.2018.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown C.E., Alizadeh D., Starr R., Weng L., Wagner J.R., Naranjo A., Ostberg J.R., Blanchard M.S., Kilpatrick J., Simpson J. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N. Engl. J. Med. 2016;375:2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louis C.U., Savoldo B., Dotti G., Pule M., Yvon E., Myers G.D., Rossig C., Russell H.V., Diouf O., Liu E. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knochelmann H.M., Smith A.S., Dwyer C.J., Wyatt M.M., Mehrotra S., Paulos C.M. CAR T cells in solid tumors: blueprints for building effective therapies. Front. Immunol. 2018;9:1740. doi: 10.3389/fimmu.2018.01740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrer D.C., Dörrie J., Schaft N. Chimeric antigen receptors in different cell types: new vehicles join the race. Hum. Gene Ther. 2018;29:547–558. doi: 10.1089/hum.2017.236. [DOI] [PubMed] [Google Scholar]
- 25.Mehta R.S., Rezvani K. Chimeric antigen receptor expressing natural killer cells for the immunotherapy of cancer. Front. Immunol. 2018;9:283. doi: 10.3389/fimmu.2018.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sommermeyer D., Hill T., Shamah S.M., Salter A.I., Chen Y., Mohler K.M., Riddell S.R. Fully human CD19-specific chimeric antigen receptors for T-cell therapy. Leukemia. 2017;31:2191–2199. doi: 10.1038/leu.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalziel M., Whitehouse C., McFarlane I., Brockhausen I., Gschmeissner S., Schwientek T., Clausen H., Burchell J.M., Taylor-Papadimitriou J. The relative activities of the C2GnT1 and ST3Gal-I glycosyltransferases determine O-glycan structure and expression of a tumor-associated epitope on MUC1. J. Biol. Chem. 2001;276:11007–11015. doi: 10.1074/jbc.M006523200. [DOI] [PubMed] [Google Scholar]
- 28.You F., Jiang L., Zhang B., Lu Q., Zhou Q., Liao X., Wu H., Du K., Zhu Y., Meng H. Phase 1 clinical trial demonstrated that MUC1 positive metastatic seminal vesicle cancer can be effectively eradicated by modified Anti-MUC1 chimeric antigen receptor transduced T cells. Sci. China Life Sci. 2016;59:386–397. doi: 10.1007/s11427-016-5024-7. [DOI] [PubMed] [Google Scholar]
- 29.Posey A.D., Jr., Schwab R.D., Boesteanu A.C., Steentoft C., Mandel U., Engels B., Stone J.D., Madsen T.D., Schreiber K., Haines K.M. Engineered CAR T cells targeting the cancer-associated Tn-glycoform of the membrane mucin MUC1 control adenocarcinoma. Immunity. 2016;44:1444–1454. doi: 10.1016/j.immuni.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinho S.S., Reis C.A. Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer. 2015;15:540–555. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- 31.Burns W.R., Zhao Y., Frankel T.L., Hinrichs C.S., Zheng Z., Xu H., Feldman S.A., Ferrone S., Rosenberg S.A., Morgan R.A. A high molecular weight melanoma-associated antigen-specific chimeric antigen receptor redirects lymphocytes to target human melanomas. Cancer Res. 2010;70:3027–3033. doi: 10.1158/0008-5472.CAN-09-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desplancq D., King D.J., Lawson A.D.G., Mountain A. Multimerization behaviour of single chain Fv variants for the tumour-binding antibody B72.3. Protein Eng. 1994;7:1027–1033. doi: 10.1093/protein/7.8.1027. [DOI] [PubMed] [Google Scholar]
- 33.Whitlow M., Bell B.A., Feng S.-L., Filpula D., Hardman K.D., Hubert S.L., Rollence M.L., Wood J.F., Schott M.E., Milenic D.E. An improved linker for single-chain Fv with reduced aggregation and enhanced proteolytic stability. Protein Eng. 1993;6:989–995. doi: 10.1093/protein/6.8.989. [DOI] [PubMed] [Google Scholar]
- 34.Liu X., Jiang S., Fang C., Yang S., Olalere D., Pequignot E.C., Cogdill A.P., Li N., Ramones M., Granda B. Affinity-tuned ErbB2 or EGFR chimeric antigen receptor T cells exhibit an increased therapeutic index against tumors in mice. Cancer Res. 2015;75:3596–3607. doi: 10.1158/0008-5472.CAN-15-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chmielewski M., Hombach A., Heuser C., Adams G.P., Abken H. T cell activation by antibody-like immunoreceptors: increase in affinity of the single-chain fragment domain above threshold does not increase T cell activation against antigen-positive target cells but decreases selectivity. J. Immunol. 2004;173:7647–7653. doi: 10.4049/jimmunol.173.12.7647. [DOI] [PubMed] [Google Scholar]
- 36.Sentman C.L., Meehan K.R. NKG2D CARs as cell therapy for cancer. Cancer J. 2014;20:156–159. doi: 10.1097/PPO.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guest R.D., Hawkins R.E., Kirillova N., Cheadle E.J., Arnold J., O’Neill A., Irlam J., Chester K.A., Kemshead J.T., Shaw D.M. The role of extracellular spacer regions in the optimal design of chimeric immune receptors: evaluation of four different scFvs and antigens. J. Immunother. 2005;28:203–211. doi: 10.1097/01.cji.0000161397.96582.59. [DOI] [PubMed] [Google Scholar]
- 38.James S.E., Greenberg P.D., Jensen M.C., Lin Y., Wang J., Till B.G., Raubitschek A.A., Forman S.J., Press O.W. Antigen sensitivity of CD22-specific chimeric TCR is modulated by target epitope distance from the cell membrane. J. Immunol. 2008;180:7028–7038. doi: 10.4049/jimmunol.180.10.7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilkie S., Picco G., Foster J., Davies D.M., Julien S., Cooper L., Arif S., Mather S.J., Taylor-Papadimitriou J., Burchell J.M., Maher J. Retargeting of human T cells to tumor-associated MUC1: the evolution of a chimeric antigen receptor. J. Immunol. 2008;180:4901–4909. doi: 10.4049/jimmunol.180.7.4901. [DOI] [PubMed] [Google Scholar]
- 40.Hudecek M., Lupo-Stanghellini M.T., Kosasih P.L., Sommermeyer D., Jensen M.C., Rader C., Riddell S.R. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin. Cancer Res. 2013;19:3153–3164. doi: 10.1158/1078-0432.CCR-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hudecek M., Sommermeyer D., Kosasih P.L., Silva-Benedict A., Liu L., Rader C., Jensen M.C., Riddell S.R. The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol. Res. 2015;3:125–135. doi: 10.1158/2326-6066.CIR-14-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Srivastava S., Riddell S.R. Engineering CAR-T cells: design concepts. Trends Immunol. 2015;36:494–502. doi: 10.1016/j.it.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hombach A., Hombach A.A., Abken H. Adoptive immunotherapy with genetically engineered T cells: modification of the IgG1 Fc ‘spacer’ domain in the extracellular moiety of chimeric antigen receptors avoids ‘off-target’ activation and unintended initiation of an innate immune response. Gene Ther. 2010;17:1206–1213. doi: 10.1038/gt.2010.91. [DOI] [PubMed] [Google Scholar]
- 44.Almåsbak H., Walseng E., Kristian A., Myhre M.R., Suso E.M., Munthe L.A., Andersen J.T., Wang M.Y., Kvalheim G., Gaudernack G., Kyte J.A. Inclusion of an IgG1-Fc spacer abrogates efficacy of CD19 CAR T cells in a xenograft mouse model. Gene Ther. 2015;22:391–403. doi: 10.1038/gt.2015.4. [DOI] [PubMed] [Google Scholar]
- 45.Watanabe N., Bajgain P., Sukumaran S., Ansari S., Heslop H.E., Rooney C.M., Brenner M.K., Leen A.M., Vera J.F. Fine-tuning the CAR spacer improves T-cell potency. OncoImmunology. 2016;5:e1253656. doi: 10.1080/2162402X.2016.1253656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jonnalagadda M., Mardiros A., Urak R., Wang X., Hoffman L.J., Bernanke A., Chang W.C., Bretzlaff W., Starr R., Priceman S. Chimeric antigen receptors with mutated IgG4 Fc spacer avoid fc receptor binding and improve T cell persistence and antitumor efficacy. Mol. Ther. 2015;23:757–768. doi: 10.1038/mt.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kochenderfer J.N., Feldman S.A., Zhao Y., Xu H., Black M.A., Morgan R.A., Wilson W.H., Rosenberg S.A. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J. Immunother. 2009;32:689–702. doi: 10.1097/CJI.0b013e3181ac6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Milone M.C., Fish J.D., Carpenito C., Carroll R.G., Binder G.K., Teachey D., Samanta M., Lakhal M., Gloss B., Danet-Desnoyers G. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol. Ther. 2009;17:1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bridgeman J.S., Hawkins R.E., Bagley S., Blaylock M., Holland M., Gilham D.E. The optimal antigen response of chimeric antigen receptors harboring the CD3zeta transmembrane domain is dependent upon incorporation of the receptor into the endogenous TCR/CD3 complex. J. Immunol. 2010;184:6938–6949. doi: 10.4049/jimmunol.0901766. [DOI] [PubMed] [Google Scholar]
- 50.Imai C., Mihara K., Andreansky M., Nicholson I.C., Pui C.H., Geiger T.L., Campana D. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 51.Jensen M., Tan G., Forman S., Wu A.M., Raubitschek A. CD20 is a molecular target for scFvFc:zeta receptor redirected T cells: implications for cellular immunotherapy of CD20+ malignancy. Biol. Blood Marrow Transplant. 1998;4:75–83. doi: 10.1053/bbmt.1998.v4.pm9763110. [DOI] [PubMed] [Google Scholar]
- 52.Guedan S., Posey A.D., Jr., Shaw C., Wing A., Da T., Patel P.R., McGettigan S.E., Casado-Medrano V., Kawalekar O.U., Uribe-Herranz M. Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation. JCI Insight. 2018;3:96976. doi: 10.1172/jci.insight.96976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morin S.O., Giroux V., Favre C., Bechah Y., Auphan-Anezin N., Roncagalli R., Mège J.L., Olive D., Malissen M., Nunès J.A. In the absence of its cytosolic domain, the CD28 molecule still contributes to T cell activation. Cell. Mol. Life Sci. 2015;72:2739–2748. doi: 10.1007/s00018-015-1873-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fos C., Salles A., Lang V., Carrette F., Audebert S., Pastor S., Ghiotto M., Olive D., Bismuth G., Nunès J.A. ICOS ligation recruits the p50alpha PI3K regulatory subunit to the immunological synapse. J. Immunol. 2008;181:1969–1977. doi: 10.4049/jimmunol.181.3.1969. [DOI] [PubMed] [Google Scholar]
- 55.Boomer J.S., Green J.M. An enigmatic tail of CD28 signaling. Cold Spring Harb. Perspect. Biol. 2010;2:a002436. doi: 10.1101/cshperspect.a002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watts T.H. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 57.Hendriks J., Xiao Y., Rossen J.W.A., van der Sluijs K.F., Sugamura K., Ishii N., Borst J. During viral infection of the respiratory tract, CD27, 4-1BB, and OX40 collectively determine formation of CD8+ memory T cells and their capacity for secondary expansion. J. Immunol. 2005;175:1665–1676. doi: 10.4049/jimmunol.175.3.1665. [DOI] [PubMed] [Google Scholar]
- 58.Shuford W.W., Klussman K., Tritchler D.D., Loo D.T., Chalupny J., Siadak A.W., Brown T.J., Emswiler J., Raecho H., Larsen C.P. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J. Exp. Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li G., Boucher J.C., Kotani H., Park K., Zhang Y., Shrestha B., Wang X., Guan L., Beatty N., Abate-Daga D., Davila M.L. 4-1BB enhancement of CAR T function requires NF-κB and TRAFs. JCI Insight. 2018;3 doi: 10.1172/jci.insight.121322. 121322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carpenito C., Milone M.C., Hassan R., Simonet J.C., Lakhal M., Suhoski M.M., Varela-Rohena A., Haines K.M., Heitjan D.F., Albelda S.M. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc. Natl. Acad. Sci. USA. 2009;106:3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brentjens R.J., Rivière I., Park J.H., Davila M.L., Wang X., Stefanski J., Taylor C., Yeh R., Bartido S., Borquez-Ojeda O. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fraietta J.A., Lacey S.F., Orlando E.J., Pruteanu-Malinici I., Gohil M., Lundh S., Boesteanu A.C., Wang Y., O’Connor R.S., Hwang W.T. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat. Med. 2018;24:563–571. doi: 10.1038/s41591-018-0010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao Z., Condomines M., van der Stegen S.J.C., Perna F., Kloss C.C., Gunset G., Plotkin J., Sadelain M. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell. 2015;28:415–428. doi: 10.1016/j.ccell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kawalekar O.U., O’Connor R.S., Fraietta J.A., Guo L., McGettigan S.E., Posey A.D., Jr., Patel P.R., Guedan S., Scholler J., Keith B. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity. 2016;44:380–390. doi: 10.1016/j.immuni.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 65.Salter A.I., Ivey R.G., Kennedy J.J., Voillet V., Rajan A., Alderman E.J., Voytovich U.J., Lin C., Sommermeyer D., Liu L. Phosphoproteomic analysis of chimeric antigen receptor signaling reveals kinetic and quantitative differences that affect cell function. Sci. Signal. 2018;11:eaat6753. doi: 10.1126/scisignal.aat6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Song D.G., Ye Q., Poussin M., Harms G.M., Figini M., Powell D.J., Jr. CD27 costimulation augments the survival and antitumor activity of redirected human T cells in vivo. Blood. 2012;119:696–706. doi: 10.1182/blood-2011-03-344275. [DOI] [PubMed] [Google Scholar]
- 67.Guedan S., Chen X., Madar A., Carpenito C., McGettigan S.E., Frigault M.J., Lee J., Posey A.D., Jr., Scholler J., Scholler N. ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood. 2014;124:1070–1080. doi: 10.1182/blood-2013-10-535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhong X.S., Matsushita M., Plotkin J., Riviere I., Sadelain M. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol. Ther. 2010;18:413–420. doi: 10.1038/mt.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pulè M.A., Straathof K.C., Dotti G., Heslop H.E., Rooney C.M., Brenner M.K. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol. Ther. 2005;12:933–941. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 70.Long A.H., Haso W.M., Shern J.F., Wanhainen K.M., Murgai M., Ingaramo M., Smith J.P., Walker A.J., Kohler M.E., Venkateshwara V.R. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 2015;21:581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frigault M.J., Lee J., Basil M.C., Carpenito C., Motohashi S., Scholler J., Kawalekar O.U., Guedan S., McGettigan S.E., Posey A.D., Jr. Identification of chimeric antigen receptors that mediate constitutive or inducible proliferation of T cells. Cancer Immunol. Res. 2015;3:356–367. doi: 10.1158/2326-6066.CIR-14-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gomes-Silva D., Mukherjee M., Srinivasan M., Krenciute G., Dakhova O., Zheng Y., Cabral J.M.S., Rooney C.M., Orange J.S., Brenner M.K., Mamonkin M. Tonic 4-1BB costimulation in chimeric antigen receptors impedes T cell survival and is vector-dependent. Cell Rep. 2017;21:17–26. doi: 10.1016/j.celrep.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eyquem J., Mansilla-Soto J., Giavridis T., van der Stegen S.J., Hamieh M., Cunanan K.M., Odak A., Gönen M., Sadelain M. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543:113–117. doi: 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hacein-Bey-Abina S., Von Kalle C., Schmidt M., McCormack M.P., Wulffraat N., Leboulch P., Lim A., Osborne C.S., Pawliuk R., Morillon E. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 75.Hacein-Bey-Abina S., von Kalle C., Schmidt M., Le Deist F., Wulffraat N., McIntyre E., Radford I., Villeval J.L., Fraser C.C., Cavazzana-Calvo M., Fischer A. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 76.Scholler J., Brady T.L., Binder-Scholl G., Hwang W.T., Plesa G., Hege K.M., Vogel A.N., Kalos M., Riley J.L., Deeks S.G. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci. Transl. Med. 2012;4:132ra53. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rosenberg S.A., Aebersold P., Cornetta K., Kasid A., Morgan R.A., Moen R., Karson E.M., Lotze M.T., Yang J.C., Topalian S.L. Gene transfer into humans—immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N. Engl. J. Med. 1990;323:570–578. doi: 10.1056/NEJM199008303230904. [DOI] [PubMed] [Google Scholar]
- 78.Ruella M., Xu J., Barrett D.M., Fraietta J.A., Reich T.J., Ambrose D.E., Klichinsky M., Shestova O., Patel P.R., Kulikovskaya I. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat. Med. 2018;24:1499–1503. doi: 10.1038/s41591-018-0201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Singh H., Manuri P.R., Olivares S., Dara N., Dawson M.J., Huls H., Hackett P.B., Kohn D.B., Shpall E.J., Champlin R.E., Cooper L.J. Redirecting specificity of T-cell populations for CD19 using the Sleeping Beauty system. Cancer Res. 2008;68:2961–2971. doi: 10.1158/0008-5472.CAN-07-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Singh H., Figliola M.J., Dawson M.J., Olivares S., Zhang L., Yang G., Maiti S., Manuri P., Senyukov V., Jena B. Manufacture of clinical-grade CD19-specific T cells stably expressing chimeric antigen receptor using Sleeping Beauty system and artificial antigen presenting cells. PLoS ONE. 2013;8:e64138. doi: 10.1371/journal.pone.0064138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mátés L., Chuah M.K.L., Belay E., Jerchow B., Manoj N., Acosta-Sanchez A., Grzela D.P., Schmitt A., Becker K., Matrai J. Molecular evolution of a novel hyperactive Sleeping Beauty transposase enables robust stable gene transfer in vertebrates. Nat. Genet. 2009;41:753–761. doi: 10.1038/ng.343. [DOI] [PubMed] [Google Scholar]
- 82.Jin Z., Maiti S., Huls H., Singh H., Olivares S., Mátés L., Izsvák Z., Ivics Z., Lee D.A., Champlin R.E., Cooper L.J. The hyperactive Sleeping Beauty transposase SB100X improves the genetic modification of T cells to express a chimeric antigen receptor. Gene Ther. 2011;18:849–856. doi: 10.1038/gt.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bhatnagar P., Alauddin M., Bankson J.A., Kirui D., Seifi P., Huls H., Lee D.A., Babakhani A., Ferrari M., Li K.C., Cooper L.J. Tumor lysing genetically engineered T cells loaded with multi-modal imaging agents. Sci. Rep. 2014;4:4502. doi: 10.1038/srep04502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thokala R., Olivares S., Mi T., Maiti S., Deniger D., Huls H., Torikai H., Singh H., Champlin R.E., Laskowski T. Redirecting specificity of T cells using the Sleeping Beauty system to express chimeric antigen receptors by mix-and-matching of VL and VH domains targeting CD123+ tumors. PLoS ONE. 2016;11:e0159477. doi: 10.1371/journal.pone.0159477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hurton L.V., Singh H., Najjar A.M., Switzer K.C., Mi T., Maiti S., Olivares S., Rabinovich B., Huls H., Forget M.A. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc. Natl. Acad. Sci. USA. 2016;113:E7788–E7797. doi: 10.1073/pnas.1610544113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morita D., Nishio N., Saito S., Tanaka M., Kawashima N., Okuno Y., Suzuki S., Matsuda K., Maeda Y., Wilson M.H. Enhanced expression of anti-CD19 chimeric antigen receptor in piggyBac transposon-engineered T cells. Mol. Ther. Methods Clin. Dev. 2017;8:131–140. doi: 10.1016/j.omtm.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakazawa Y., Huye L.E., Salsman V.S., Leen A.M., Ahmed N., Rollins L., Dotti G., Gottschalk S.M., Wilson M.H., Rooney C.M. PiggyBac-mediated cancer immunotherapy using EBV-specific cytotoxic T-cells expressing HER2-specific chimeric antigen receptor. Mol. Ther. 2011;19:2133–2143. doi: 10.1038/mt.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Manuri P.V.R., Wilson M.H., Maiti S.N., Mi T., Singh H., Olivares S., Dawson M.J., Huls H., Lee D.A., Rao P.H. piggyBac transposon/transposase system to generate CD19-specific T cells for the treatment of B-lineage malignancies. Hum. Gene Ther. 2010;21:427–437. doi: 10.1089/hum.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ding S., Wu X., Li G., Han M., Zhuang Y., Xu T. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell. 2005;122:473–483. doi: 10.1016/j.cell.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 90.Balciunas D., Wangensteen K.J., Wilber A., Bell J., Geurts A., Sivasubbu S., Wang X., Hackett P.B., Largaespada D.A., McIvor R.S., Ekker S.C. Harnessing a high cargo-capacity transposon for genetic applications in vertebrates. PLoS Genet. 2006;2:e169. doi: 10.1371/journal.pgen.0020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ghassemi S., Nunez-Cruz S., O’Connor R.S., Fraietta J.A., Patel P.R., Scholler J., Barrett D.M., Lundh S.M., Davis M.M., Bedoya F. Reducing ex vivo culture improves the antileukemic activity of chimeric antigen receptor (CAR) T cells. Cancer Immunol. Res. 2018;6:1100–1109. doi: 10.1158/2326-6066.CIR-17-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Monjezi R., Miskey C., Gogishvili T., Schleef M., Schmeer M., Einsele H., Ivics Z., Hudecek M. Enhanced CAR T-cell engineering using non-viral Sleeping Beauty transposition from minicircle vectors. Leukemia. 2017;31:186–194. doi: 10.1038/leu.2016.180. [DOI] [PubMed] [Google Scholar]
- 93.Beatty G.L., Haas A.R., Maus M.V., Torigian D.A., Soulen M.C., Plesa G., Chew A., Zhao Y., Levine B.L., Albelda S.M. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol. Res. 2014;2:112–120. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jin C., Fotaki G., Ramachandran M., Nilsson B., Essand M., Yu D. Safe engineering of CAR T cells for adoptive cell therapy of cancer using long-term episomal gene transfer. EMBO Mol. Med. 2016;8:702–711. doi: 10.15252/emmm.201505869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Smith T.T., Stephan S.B., Moffett H.F., McKnight L.E., Ji W., Reiman D., Bonagofski E., Wohlfahrt M.E., Pillai S.P.S., Stephan M.T. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat. Nanotechnol. 2017;12:813–820. doi: 10.1038/nnano.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pfeiffer A., Thalheimer F.B., Hartmann S., Frank A.M., Bender R.R., Danisch S., Costa C., Wels W.S., Modlich U., Stripecke R. In vivo generation of human CD19-CAR T cells results in B-cell depletion and signs of cytokine release syndrome. EMBO Mol. Med. 2018;10:e9158. doi: 10.15252/emmm.201809158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qasim W., Zhan H., Samarasinghe S., Adams S., Amrolia P., Stafford S., Butler K., Rivat C., Wright G., Somana K. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci. Transl. Med. 2017;9:eaaj2013. doi: 10.1126/scitranslmed.aaj2013. [DOI] [PubMed] [Google Scholar]
- 98.Ren J., Liu X., Fang C., Jiang S., June C.H., Zhao Y. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin. Cancer Res. 2017;23:2255–2266. doi: 10.1158/1078-0432.CCR-16-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ren J., Zhang X., Liu X., Fang C., Jiang S., June C.H., Zhao Y. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget. 2017;8:17002–17011. doi: 10.18632/oncotarget.15218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vong Q., Nye C., Hause R., Clouser C., Jones J., Burleigh S., Borges C.M., Chin M.S.Y., Marco E., Barrera L. Inhibiting TGFβ signaling in CAR T-cells may significantly enhance efficacy of tumor immunotherapy. Blood. 2017;130:1791. [Google Scholar]
- 101.MacLeod D.T., Antony J., Martin A.J., Moser R.J., Hekele A., Wetzel K.J., Brown A.E., Triggiano M.A., Hux J.A., Pham C.D. Integration of a CD19 CAR into the TCR alpha chain locus streamlines production of allogeneic gene-edited CAR T cells. Mol. Ther. 2017;25:949–961. doi: 10.1016/j.ymthe.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Torikai H., Reik A., Liu P.Q., Zhou Y., Zhang L., Maiti S., Huls H., Miller J.C., Kebriaei P., Rabinovich B. A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood. 2012;119:5697–5705. doi: 10.1182/blood-2012-01-405365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Poirot L., Philip B., Schiffer-Mannioui C., Le Clerre D., Chion-Sotinel I., Derniame S., Potrel P., Bas C., Lemaire L., Galetto R. Multiplex genome-edited T-cell manufacturing platform for "off-the-shelf" adoptive T-cell immunotherapies. Cancer Res. 2015;75:3853–3864. doi: 10.1158/0008-5472.CAN-14-3321. [DOI] [PubMed] [Google Scholar]
- 104.Berdien B., Mock U., Atanackovic D., Fehse B. TALEN-mediated editing of endogenous T-cell receptors facilitates efficient reprogramming of T lymphocytes by lentiviral gene transfer. Gene Ther. 2014;21:539–548. doi: 10.1038/gt.2014.26. [DOI] [PubMed] [Google Scholar]
- 105.Roth T.L., Puig-Saus C., Yu R., Shifrut E., Carnevale J., Li P.J., Hiatt J., Saco J., Krystofinski P., Li H. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature. 2018;559:405–409. doi: 10.1038/s41586-018-0326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Veres A., Gosis B.S., Ding Q., Collins R., Ragavendran A., Brand H., Erdin S., Cowan C.A., Talkowski M.E., Musunuru K. Low incidence of off-target mutations in individual CRISPR-Cas9 and TALEN targeted human stem cell clones detected by whole-genome sequencing. Cell Stem Cell. 2014;15:27–30. doi: 10.1016/j.stem.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Haapaniemi E., Botla S., Persson J., Schmierer B., Taipale J. CRISPR-Cas9 genome editing induces a p53-mediated DNA damage response. Nat. Med. 2018;24:927–930. doi: 10.1038/s41591-018-0049-z. [DOI] [PubMed] [Google Scholar]
- 108.Ihry R.J., Worringer K.A., Salick M.R., Frias E., Ho D., Theriault K., Kommineni S., Chen J., Sondey M., Ye C. p53 inhibits CRISPR-Cas9 engineering in human pluripotent stem cells. Nat. Med. 2018;24:939–946. doi: 10.1038/s41591-018-0050-6. [DOI] [PubMed] [Google Scholar]
- 109.Sadelain M., Rivière I., Riddell S. Therapeutic T cell engineering. Nature. 2017;545:423–431. doi: 10.1038/nature22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lim W.A., June C.H. The principles of engineering immune cells to treat cancer. Cell. 2017;168:724–740. doi: 10.1016/j.cell.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Castellarin M., Watanabe K., June C.H., Kloss C.C., Posey A.D., Jr. Driving cars to the clinic for solid tumors. Gene Ther. 2018;25:165–175. doi: 10.1038/s41434-018-0007-x. [DOI] [PubMed] [Google Scholar]
- 112.Grupp S.A., Kalos M., Barrett D., Aplenc R., Porter D.L., Rheingold S.R., Teachey D.T., Chew A., Hauck B., Wright J.F. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J., Chew A., Gonzalez V.E., Zheng Z., Lacey S.F. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ruella M., Barrett D.M., Kenderian S.S., Shestova O., Hofmann T.J., Perazzelli J., Klichinsky M., Aikawa V., Nazimuddin F., Kozlowski M. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J. Clin. Invest. 2016;126:3814–3826. doi: 10.1172/JCI87366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Grada Z., Hegde M., Byrd T., Shaffer D.R., Ghazi A., Brawley V.S., Corder A., Schönfeld K., Koch J., Dotti G. TanCAR: a novel bispecific chimeric antigen receptor for cancer immunotherapy. Mol. Ther. Nucleic Acids. 2013;2:e105. doi: 10.1038/mtna.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hegde M., Mukherjee M., Grada Z., Pignata A., Landi D., Navai S.A., Wakefield A., Fousek K., Bielamowicz K., Chow K.K. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J. Clin. Invest. 2016;126:3036–3052. doi: 10.1172/JCI83416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zah E., Lin M.Y., Silva-Benedict A., Jensen M.C., Chen Y.Y. T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol. Res. 2016;4:498–508. doi: 10.1158/2326-6066.CIR-15-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hegde M., Corder A., Chow K.K., Mukherjee M., Ashoori A., Kew Y., Zhang Y.J., Baskin D.S., Merchant F.A., Brawley V.S. Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Mol. Ther. 2013;21:2087–2101. doi: 10.1038/mt.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lamers C.H., Sleijfer S., Vulto A.G., Kruit W.H., Kliffen M., Debets R., Gratama J.W., Stoter G., Oosterwijk E. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J. Clin. Oncol. 2006;24:e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 120.Parkhurst M.R., Yang J.C., Langan R.C., Dudley M.E., Nathan D.A., Feldman S.A., Davis J.L., Morgan R.A., Merino M.J., Sherry R.M. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol. Ther. 2011;19:620–626. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lanitis E., Poussin M., Klattenhoff A.W., Song D., Sandaltzopoulos R., June C.H., Powell D.J., Jr. Chimeric antigen receptor T cells with dissociated signaling domains exhibit focused antitumor activity with reduced potential for toxicity in vivo. Cancer Immunol. Res. 2013;1:43–53. doi: 10.1158/2326-6066.CIR-13-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kloss C.C., Condomines M., Cartellieri M., Bachmann M., Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat. Biotechnol. 2013;31:71–75. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu C.Y., Roybal K.T., Puchner E.M., Onuffer J., Lim W.A. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science. 2015;350:aab4077. doi: 10.1126/science.aab4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Roybal K.T., Rupp L.J., Morsut L., Walker W.J., McNally K.A., Park J.S., Lim W.A. Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell. 2016;164:770–779. doi: 10.1016/j.cell.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fedorov V.D., Themeli M., Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci. Transl. Med. 2013;5:215ra172. doi: 10.1126/scitranslmed.3006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Urbanska K., Lanitis E., Poussin M., Lynn R.C., Gavin B.P., Kelderman S., Yu J., Scholler N., Powell D.J., Jr. A universal strategy for adoptive immunotherapy of cancer through use of a novel T-cell antigen receptor. Cancer Res. 2012;72:1844–1852. doi: 10.1158/0008-5472.CAN-11-3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rodgers D.T., Mazagova M., Hampton E.N., Cao Y., Ramadoss N.S., Hardy I.R., Schulman A., Du J., Wang F., Singer O. Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies. Proc. Natl. Acad. Sci. USA. 2016;113:E459–E468. doi: 10.1073/pnas.1524155113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ma J.S., Kim J.Y., Kazane S.A., Choi S.H., Yun H.Y., Kim M.S., Rodgers D.T., Pugh H.M., Singer O., Sun S.B. Versatile strategy for controlling the specificity and activity of engineered T cells. Proc. Natl. Acad. Sci. USA. 2016;113:E450–E458. doi: 10.1073/pnas.1524193113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cho J.H., Collins J.J., Wong W.W. Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell. 2018;173:1426–1438.e11. doi: 10.1016/j.cell.2018.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Steentoft C., Migliorini D., King T.R., Mandel U., June C.H., Posey A.D., Jr. Glycan-directed CAR-T cells. Glycobiology. 2018;28:656–669. doi: 10.1093/glycob/cwy008. [DOI] [PubMed] [Google Scholar]
- 131.Heath J.R., Ribas A., Mischel P.S. Single-cell analysis tools for drug discovery and development. Nat. Rev. Drug Discov. 2016;15:204–216. doi: 10.1038/nrd.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]