Abstract
Caulobacter flavus RHGG3T, a novel type species in the genus Caulobacter, originally isolated from rhizosphere soil of watermelon (Citrullus lanatus), has the ability to improve the growth of watermelon seedling and tolerate heavy metals. In vitro, C. flavus RHGG3T was able to solubilize phosphate (80.56 mg L−1), produce indole-3-acetic acid (IAA) (11.58 mg L−1) and was resistant to multiple heavy metals (copper, zinc, cadmium, cobalt and lead). Inoculating watermelon with this strain increased shoot and root length by 22.1% and 43.7%, respectively, and the total number of lateral roots by 55.9% compared to non-inoculated watermelon. In this study, we present the complete genome sequence of C. flavus RHGG3T, which was comprised of a single circular chromosome of 5,659,202 bp with a G + C content of 69.25%. An annotation analysis revealed that the C. flavus RHGG3T genome contained 5172 coding DNA sequences, 9 rRNA and 55 tRNA genes. Genes related to plant growth promotion (PGP), such as those associated with phosphate solubilization, nitrogen fixation, IAA, phenazine, volatile compounds, spermidine and cobalamin synthesis, were found in the C. flavus RHGG3T genome. Some genes responsible for heavy metal tolerance were also identified. The genome sequence of strain RHGG3T reported here provides new insight into the molecular mechanisms underlying the promotion of plant growth and the resistance to heavy metals in C. flavus. This study will be valuable for further exploration of the biotechnological applications of strain RHGG3T in agriculture.
Electronic supplementary material
The online version of this article (10.1007/s13205-019-1569-z) contains supplementary material, which is available to authorized users.
Keywords: Caulobacter flavus RHGG3T, Complete genome sequence, Plant growth-promoting rhizobacteria, Heavy metal resistance
Introduction
Plant growth-promoting rhizobacteria (PGPR) are well known for their abilities to promote plant growth and enhance the tolerance of plants to stressors, such as heavy metals, drought, salt, and pathogens (Bhattacharyya and Jha 2012; Xie et al. 2018; Zhang et al. 2017). PGPR are able to promote plant growth directly or indirectly through a combination of mechanisms, including nitrogen fixation, phosphate solubilization, biosynthesis of siderophores, plant growth hormones [indole-3-acetic acid (IAA)], hydrolytic enzymes, various antibiotics, as well as the induction of plant resistance (Qin et al. 2009, 2017; Wang et al. 2015). These functional properties are critical when considering the formulation of biofertilizers, which may be an advantage due to less environmental pollution than chemical applications.
In our previous study, a Gram-stain-negative, yellow-pigmented bacterium strain RHGG3T was isolated from rhizosphere soil of cultivated watermelon (Citrullus lanatus) collected from Hefei, China, and identified as a novel species Caulobacter flavus RHGG3T using a polyphasic approach (Sun et al. 2015). The genus Caulobacter, which belongs phylogenetically to the family Caulobacteraceae, contains 11 species with validly published names (Moya et al. 2017; Sun et al. 2017). Members of the genus Caulobacter have the ability to tolerate uranium, copper and chlorophenols (Ash et al. 2014; Hu et al. 2005; Yung et al. 2015). However, information on the plant growth-promoting traits of Caulobacter spp. from rhizosphere, their capacities and mechanisms in plant growth promotion and tolerance to heavy metals is relatively scarce (Pereira et al. 2016).
Notably, C. flavus RHGG3T produces IAA (11.58 mg L−1) and solubilizes phosphorus (80.56 mg L−1), suggesting its potential in plant growth promotion (Table 1). Based on IAA production and phosphate solubilization abilities, the plant root elongation promoting activity of strain RHGG3T was tested using the modified root elongation assay described by Belimov et al. (2005). Two milliliters of the bacterial suspension (5 × 107 cells mL−1) or sterile water (uninoculated control) was added to glass Petri dishes containing filter paper. The watermelon seeds were surface-sterilized with 10% (v/v) H2O2 for 20 min, washed in sterile water, and placed on wetted filter paper. The assay was performed three times with three dishes (four seeds/dish) for each treatment. Root length and the number of lateral roots of the seedlings were measured after a 7-day incubation at 28 °C in the dark. Inoculation with strain RHGG3T resulted in a significant increase in shoot length, root length and the total number of lateral roots by 22.1%, 43.7% and 55.9%, respectively (Table 1). In addition, strain RHGG3T showed resistance to multiple heavy metals (copper, zinc, cadmium, cobalt and lead) (Table 2), and tolerated Cu2+ concentrations up to 0.2 mg mL−1 on GMSB agar (Sun et al. 2015).
Table 1.
Plant beneficial traits of strain Caulobacter flavus RHGG3T and its promoting effects on watermelon seedlings
| IAA concentration (mg L−1) | Solubilized phosphate (mg L−1) | Shoot length (cm) | Root length (cm) | Number of lateral roots | |
|---|---|---|---|---|---|
| RHGG3 | 11.58 ± 0.19 | 80.56 ± 1.53 | 8.00 ± 0.66 | 11.35 ± 0.65 | 46.0 ± 7.63 |
| CK | – | – | 6.55 ± 0.47 | 7.90 ± 1.56 | 29.5 ± 4.84 |
Table 2.
Resistances of strain Caulobacter flavus RHGG3T to heavy metal ions (mg mL−1)
| Concentration | Cu2+ | Zn2+ | Cd2+ | Co2+ | Pb2+ |
|---|---|---|---|---|---|
| 0.025 | + | + | + | + | + |
| 0.05 | + | + | + | + | + |
| 0.1 | + | + | − | + | + |
| 0.2 | + | + | − | + | + |
| 0.3 | − | + | − | + | + |
| 0.4 | − | + | − | − | + |
| 0.5 | − | − | − | − | − |
+, growth; −, no growth
We performed whole-genome sequencing of strain RHGG3T to obtain detailed genetic information of C. flavus RHGG3T with plant growth-promoting and heavy metal resistance abilities. Genomic DNA was extracted using the conventional phenol/chloroform/isoamyl alcohol (25:24:1) extraction method. The efficiency of the DNA extraction was tested using 1.0% agarose gel electrophoresis, and concentration and purity were determined with a TBS-380 spectrophotometer. An 8–10-kb DNA fragment library was constructed according to the manufacturer’s instructions and sequenced on the PacBio RSII sequencing platform with a SMART cell (MajorBio Co., Shanghai, China). The filtered subreads (1,202,099,079 bp) with 214-fold genome coverage were assembled de novo using the hierarchical genome-assembly process (HGAP 3.0) (Chin et al. 2013).
Glimmer 3.0 (Delcher et al. 2007) was used to predict the protein-coding genes (open reading frames). The ribosomal RNA (rRNA) genes were predicted using Barrnap 0.4.2. The tRNA genes were predicted using tRNAscan-SEv1.3.1 (Lowe and Eddy 1997). Gene annotation was carried out by BLASTP search against the non-redundant GenBank protein database (http://www.ncbi.nlm.nih.gov/protein), Swiss-Prot database, the Clusters of Orthologous Groups of proteins (COG) database (http://www.ncbi.nlm.nih.gov/COG), and the KEGG database (http://www.genome.ad.jp/kegg). Further annotation was performed using the online RAST server online (Aziz et al. 2008). The circular genome was drawn using Circos v 0.64 (Krzywinski et al. 2009).
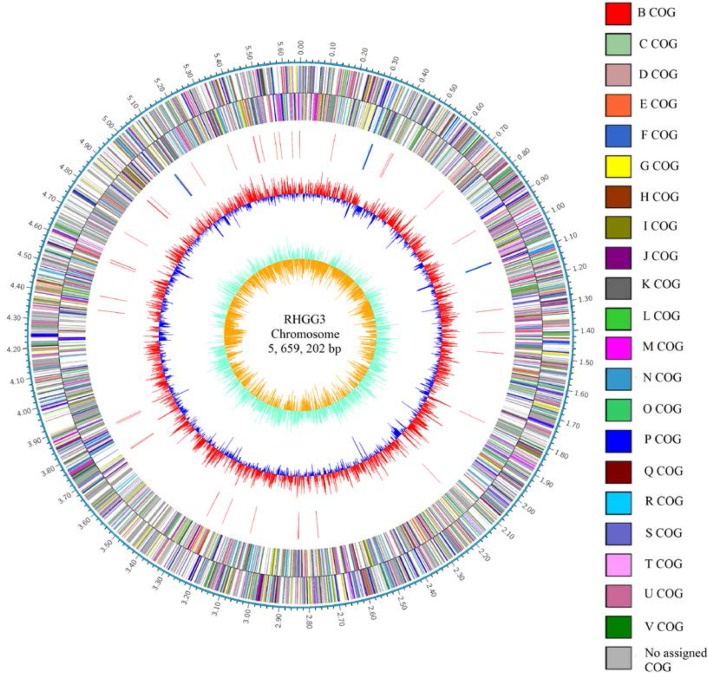
The genome of C. flavus RHGG3T contained a 5,659,202 bp circular chromosome (69.25% G + C content), including 5172 predicted protein-coding genes, 9 rRNA genes, and 55 tRNA genes (details can be seen in Table 3; Fig. 1). It is notable that C. flavus is the first completely sequenced Caulobacter genome to have three rRNA operons. A total of 2957 identified genes were classified into functional categories according to the COG designations (Tatusov et al. 2000), and the results were as follows: two genes for ‘chromatin structure and dynamics’, 164 genes for ‘translation, ribosomal structure and biogenesis’, 200 genes for ‘transcription’, 138 genes for ‘replication, recombination and repair’, 22 genes for “cell cycle control, cell division, chromosome partitioning”, 192 genes for ‘cell wall/membrane/envelope biogenesis’, 59 genes for ‘cell motility’, 136 genes for ‘posttranslational modification, protein turnover and chaperones’, 168 genes for ‘signal transduction mechanisms’, 92 genes for ‘intracellular trafficking, secretion, and vesicular transport’, 35 genes for ‘defense mechanisms, 179 genes for ‘energy production and conversion’, 226 genes for ‘amino acid transport and metabolism’, 70 genes for ‘nucleotide transport and metabolism’, 168 genes for ‘carbohydrate transport and metabolism’, 92 genes for ‘coenzyme transport and metabolism’, 168 genes for ‘lipid transport and metabolism’, 190 genes for ‘inorganic ion transport and metabolism’, 110 genes for ‘secondary metabolites biosynthesis, transport and catabolism’, 215 genes for ‘general function prediction only’ and 331 genes for ‘function unknown’ (Table S1).
Table 3.
Genome features of Caulobacter flavus RHGG3T
| Features | Value |
|---|---|
| Genome size (bp) | 5,659,202 |
| Number of contigs | 1 |
| Average GC content (%) | 69.25 |
| Total number of genes | 5172 |
| Gene total length (bp) | 5,056,212 |
| Protein-coding genes (CDSs) | 4989 |
| rRNA genes (5S, 16S, 23S) | 9 |
| tRNA genes | 55 |
Fig. 1.
Circular genome maps of Caulobacter flavus RHGG3. Rings from the outermost to the center: (1) scale marks of the genome, (2) protein-coding genes on the forward strand, (3) protein-coding genes on the reverse strand, (4) rRNA operon and tRNA genes, (5) GC content, and (6) GC skew. Circles 2 and 3 are open reading frames encoded by leading and lagging strands, respectively, with color codes for the COG functional categories: A, RNA processing and modification; B, chromatin structure and dynamics; J, translation, ribosomal structure and biogenesis; K, transcription; L, replication, recombination and repair; D, cell cycle control, cell division, chromosome partitioning; M, cell wall/membrane/envelope biogenesis; N, cell motility; O, posttranslational modification, protein turnover, chaperones; T, signal transduction mechanisms; U, intracellular trafficking, secretion, and vesicular transport; V, defense mechanisms; Z, cytoskeleton; C, energy production and conversion; E, amino acid transport and metabolism; F, nucleotide transport and metabolism; G, carbohydrate transport and metabolism; H, coenzyme transport and metabolism, I, lipid transport and metabolism; P, inorganic ion transport and metabolism; Q, secondary metabolite biosynthesis, transport and catabolism; R, general function prediction only; and S, function unknown
We identified genes involved in nutrient availability as well as IAA, phenazine, volatile compounds, spermidine, and cobalamin production in the C. flavus RHGG3T genomic sequence (Table 4). Few studies have investigated the function and mechanism of plant growth promotion in Caulobacter species. However, C. flavus RHGG3T enhanced plant growth through the production of IAA (Table 1). A sequence analysis of the C. flavus RHGG3T genome also indicated the existence of genes responsible for IAA production, such as the tryptophan biosynthesis gene cluster (trpABCDEFGRS). The C. flavus RHGG3T genome also encoded glucose dehydrogenase (gcd), polyphosphate kinase [EC 2.7.4.1] (ppk), phosphate inorganic transporter (pit), phytase (phy) and nitrogenase (fikK), which increase plant phosphorus and nitrogen uptake abilities (Table 4). Glucose dehydrogenase is critical in the production of gluconic acid which is the major mechanism for phosphate solubilization in bacteria (Achal et al. 2007). Besides IAA, volatiles (acetoin and 2,3-butanediol) produced by bacteria can promote plant growth (Ping and Boland 2004). Acetolactate synthase (alsS) and acetolactate decarboxylase (alsD) catalyze the reaction from pyruvate to acetoin, and then acetoin is converted to 2,3-butanediol, either by the bacteria or by the host plant (Ryu et al. 2003). Moreover, the C. flavus RHGG3T genome contained genes encoding acetolactate synthase (alsS), transcriptional regulator (alsR), and the acetoin utilization protein (Table 4). Antibacterial compounds, such as phenazine produced by PGP bacteria, inhibit pathogenic microbes and promote plant growth (Chen et al. 2015; He et al. 2012). The plant growth regulator spermidine has newly found roles in plant growth and the response to various abiotic stressors such as salt, drought, cold and oxidative stress (Alcazar et al. 2011). Additionally, C. flavus RHGG3T contained gene clusters responsible for spermidine/putrescine ABC transporter permease (potABCD), phenazine and cobalamin.
Table 4.
Candidate genes related to plant growth promotion in Caulobacter flavus RHGG3T genome
| Gene name | Gene ID | Gene annotation |
|---|---|---|
| Phosphate solubilization or transport genes | ||
| ppk | ORF1527 (ORF3572) | Polyphosphate kinase |
| ppnk | ORF2945 | Inorganic polyphosphate kinase |
| pit | ORF3753 | Phosphate inorganic transporter |
| pstB | ORF0866 | Phosphate ABC transporter ATP-binding protein |
| phy | ORF3225 | 3-Phytase |
| gcd | ORF3117(ORF5251, ORF1567) | Glucose dehydrogenase |
| Nitrogen fixation genes | ||
| fixK | ORF0025 | Nitrogen fixation-regulating protein FixK |
| nifU | ORF0965(ORF1242) | Nitrogen fixation protein NifU |
| nifS1 | ORF2037 | Nitrogenase metallocluster biosynthesis protein |
| nifS2 | ORF3632 | Nitrogenase metallocluster biosynthesis protein |
| glnA | ORF2439(ORF2447, ORF4112) | Glutamine synthetase genes |
| Volatile signal-related genes | ||
| acoR | ORF1750 | Acetoin catabolism regulatory protein |
| aco | ORF4739 | Acetoin utilization protein |
| ilvH | ORF2866 | Acetolactate synthase small subunit |
| ilvB | ORF2867 | Acetolactate synthase isozyme 3 large subunit |
| ilvX | ORF0594 | Putative acetolactate synthase large subunit |
| IAA-related genes | ||
| trpA | ORF1509 | Tryptophan synthase subunit alpha |
| trpB | ORF1510 | Tryptophan synthase subunit beta |
| trpF | ORF1511 | N-(5′-Phosphoribosyl)anthranilate isomerase |
| trpS | ORF1241 | Tryptophan–tRNA ligase |
| trpR | ORF3001 | TrpR-binding protein WrbA |
| trpE | ORF3811 | Anthranilate synthase subunit I |
| trpG | ORF3814 | Anthranilate synthase |
| trpD | ORF3815 | Anthranilate phosphoribosyltransferase |
| trpC | ORF3816 | Indole-3-glycerol phosphate synthase |
| Antibiotic-related genes | ||
| phzF | ORF0105 | Phenazine biosynthesis protein PhzF family |
| Others | ||
| lysR | ORF4869 | LysR transcriptional regulator |
| potD | ORF2440 | Spermidine/putrescine ABC transporter substrate-binding protein |
| potB | ORF2441 | Spermidine/putrescine ABC transporter permease |
| potC | ORF2442 | Spermidine/putrescine ABC transporter permease |
| potA | ORF2443 | Putrescine/spermidine ABC transporter ATP-binding protein |
| cobT | ORF0043 | Cobaltochelatase CobT subunit |
| cobD | ORF4262 | Cobalamin biosynthesis protein CobD |
| cobP | ORF4254 | Cobalamin biosynthesis protein |
| cobW | ORF0743 | Cobalamin biosynthesis protein CobW |
| cobS | ORF0496 | Cobalamin biosynthesis protein CobS |
| cbiG | ORF4875 | Cobalamin biosynthesis protein CbiG |
Based on the annotation, many genes involved in heavy metal resistance were identified in the C. flavus RHGG3T genome, including those encoding copper resistance proteins, multicopper oxidase, a cation transporter, and multiple heavy metal efflux pumps for cadmium, zinc and cobalt (Table 5). The C. flavus RHGG3T genome also contained czcCBA operons, including outer membrane protein genes (czcC), inner membrane protein genes (czcA), and membrane fusion protein genes (czcB) and czcD genes (Table 5). The efflux transporter protein czcCBA exports cobalt/zinc/cadmium cations from both the cytoplasm and the periplasm to outside of the cell to protect the cell from heavy metal stress (Vaccaro et al. 2016).
Table 5.
Metal resistance gene operons in the Caulobacter flavus RHGG3T genome
| Gene name | Gene ID | Gene annotation |
|---|---|---|
| Cd2+, Zn2+, Co2+ | ||
| czcD | ORF0854 | Cobalt transporter [Caulobacter cation diffusion facilitator family transporter] |
| ORF0862 | Cobalt transporter | |
| czcC | ORF1649 | Outer membrane protein |
| czcB | ORF1650 | RND family efflux transporter, MFP subunit |
| czcA | ORF1651 | Heavy metal efflux pump, cobalt–zinc–cadmium |
| zntA | ORF1654 | Cadmium-exporting ATPase |
| czcD | ORF1658 | PREDICTED: metal tolerance protein 1-like |
| czcC | ORF3713 | Metal transporter [Caulobacter metal ion efflux outer membrane factor protein family] |
| czcB | ORF3714 | Cation transporter [Caulobacter cation efflux system protein] |
| czcA | ORF3715 | Cation transporter [Caulobacter AcrB/AcrD/AcrF family protein] |
| czcD | ORF3918 | Cation diffusion facilitator family transporter |
| czcA | ORF3920 | Heavy metal efflux pump, cobalt–zinc–cadmium |
| czcB | ORF3921 | RND family efflux transporter, MFP subunit |
| czcC | ORF3922 | Outer membrane protein |
| cusB | ORF3929 | RND transporter [Caulobacter RND family efflux transporter] |
| cusA | ORF3930 | Cation transporter [Caulobacter CzcA family heavy metal efflux pump] |
| Cu2+ | ||
| copB | ORF0330 | Copper resistance protein CopB |
| copA | ORF0332 | Copper-binding protein |
| cueR | ORF1760 | Transcriptional regulator |
| cutA | ORF2296 | Cation tolerance protein CutA |
| copS | ORF2381 | Histidine kinase |
| copR | ORF2382 | Transcriptional regulator |
| cueO | ORF3812 | Multicopper oxidase type 3 |
| copA | ORF3931 | Heavy metal translocating P-type ATPase |
| copC | ORF3936 | Copper resistance protein CopC |
| copD | ORF3937 | Copper resistance D domain-containing protein |
| copB | ORF3944 | Copper resistance protein CopB |
| copA | ORF3945 | Copper-binding protein |
| ORF4873 | Copper-binding protein | |
The general features of C. flavus RHGG3T and some other Caulobacter genomes are summarized in Table 6. Genomes in the genus Caulobacter showed a high G + C content ranging from 65.8 to 69.3% (Table 6). The genome sizes of the 9 strains of Caulobacter ranged from 3.96 to 5.89 Mb, with 3630–5378 predicted genes (Table 6). Strain Caulobacter sp. K31 had the largest genome size and the maximum number of predicted genes, whereas strain C. henricii CB4T had the smallest genome size and the minimum number of predicted genes. Strains C. henricii CB4T contained one plasmid, while strain Caulobacter sp. K31 contained two plasmids. These differences in genome size suggest that the evolution of Caulobacter is coupled with different levels of horizontal gene transfer, including gene insertion and deletion.
Table 6.
Genomic features of strains in the genus Caulobacter
| Num. | Strain | Size (Mb) | Number of plasmid | Contigs | GC content (%) | CDS | rRNA | tRNA | ncRNA | Pseudogene | Type strain | GenBank accession number |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | C. flavus RHGG3T | 5.66 | 0 | 1 | 69.3 | 4989 | 9 | 55 | Yes | CP026100 | ||
| 2 | C. henricii CB4T | 3.96 | 1 | 2 | 65.8 | 3630 | 6 | 50 | 3 | 70 | Yes | CP013002; CP013003 |
| 3 | C. mirabilis FWC38 | 4.58 | 0 | 1 | 69.3 | 4246 | 6 | 46 | 3 | 44 | No | CP024201 |
| 4 | C. segnis TK0059 | 4.66 | 0 | 1 | 67.7 | 4201 | 6 | 51 | 3 | 100 | No | CP027850 |
| 5 | C. vibrioides CB2 | 4.12 | 0 | 1 | 67.2 | 3896 | 6 | 52 | 0 | 102 | Yes | P0C23313 |
| 6 | C. vibrioides CB15 | 4.02 | 0 | 1 | 67.2 | 3737 | 6 | 51 | 1 | No | AE005673 | |
| 7 | C. vibrioides CB1 | 4.14 | 0 | 1 | 67.2 | 3990 | 6 | 51 | 4 | 46 | No | CP023314 |
| 8 | C. vibrioides CB13b1a | 4.14 | 0 | 1 | 67.0 | 3775 | 6 | 51 | 4 | 0 | No | CP023315 |
| 9 | Caulobacter sp. K31 | 5.89 | 2 | 3 | 67.4 | 5378 | 6 | 49 | 3 | 74 | No | CP000927; CP000928; CP000929 |
In summary, C. flavus RHGG3T contained genes related to the promotion of plant growth and stress tolerance, such as phosphate solubilization, nitrogen fixation, production and utilization of IAA, acetoin, and spermidine, as well as the tolerance to heavy metals. Our findings provide a good explanation for the growth promotion of plants and resistance to heavy metals in plants. This is the first report on the complete genome sequence of the type strain C. flavus RHGG3T. The detailed analysis of the complete C. flavus RHGG3T genome sequence provides a molecular basis for biotechnological exploitation and applications in the field of agriculture and the environment.
Nucleotide sequence accession numbers and culture deposition
The whole-genome sequence of C. flavus RHGG3T is available in the GenBank database under accession number CP026100. The strain has been deposited into the general collection of microorganism of the Korean Collection for Type Cultures (KCTC) under accession number KCTC42581T, China General Microbiological Culture Collection Center under accession number CGMCC 1.15093T and Japan Collection of Microorganisms under accession number JCM 30763T.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (41401275, 31800057), the Anhui Provincial Major Scientific and Technological Special Project (17030701023) and National Agricultural Science and Technology Achievements Transformation Fund (2014GB2C300022).
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
References
- Achal V, Savant VV, Reddy MS. Phosphate solubilization by a wild type strain and UV-induced mutants of Aspergillus tubingensis. Soil Biol Biochem. 2007;39:695–699. doi: 10.1016/j.soilbio.2006.09.003. [DOI] [Google Scholar]
- Alcazar R, Bitrian M, Bartels D, Koncz C, Altabella T, Tiburcio AF. Polyamine metabolic canalization in response to drought stress in Arabidopsis and the resurrection plant Craterostigma plantagineum. Plant Signal Behav. 2011;6:243–250. doi: 10.4161/psb.6.2.14317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash K, Brown T, Watford T, Scott LE, Stephens C, Ely B. A comparison of the Caulobacter NA1000 and K31 genomes reveals extensive genome rearrangements and differences in metabolic potential. Open Biol. 2014;4:140128. doi: 10.1098/rsob.140128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, Meyer F, Olsen GJ, Olson R, Osterman AL, Overbeek RA, McNeil LK, Paarmann D, Paczian T, Parrello B, Pusch GD, Reich C, Stevens R, Vassieva O, Vonstein V, Wilke A, Zagnitko O. The RAST server: rapid annotations using subsystems technology. BMC Genom. 2008;9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belimov AA, Hontzeas N, Safronova VI, Demchinskaya SV, Piluzza G, Bullitta S, Glick BR. Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.) Soil Biol Biochem. 2005;37:241–250. doi: 10.1016/j.soilbio.2004.07.033. [DOI] [Google Scholar]
- Bhattacharyya PN, Jha DK. Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol. 2012;28:1327–1350. doi: 10.1007/s11274-011-0979-9. [DOI] [PubMed] [Google Scholar]
- Chen Y, Shen X, Peng H, Hu H, Wang W, Zhang X. Comparative genomic analysis and phenazine production of Pseudomonas chlororaphis, a plant growth-promoting rhizobacterium. Genom Data. 2015;4:33–42. doi: 10.1016/j.gdata.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin CS, Alexander DH, Marks P, Klammer AA, Drake J, Heiner C, Clum A, Copeland A, Huddleston J, Eichler EE, Turner SW, Korlach J. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods. 2013;10:563–569. doi: 10.1038/nmeth.2474. [DOI] [PubMed] [Google Scholar]
- Delcher AL, Bratke KA, Powers EC, Salzberg SL. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics. 2007;23:673–679. doi: 10.1093/bioinformatics/btm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, Hao K, Blom J, Ruckert C, Vater J, Mao Z, Wu Y, Hou M, He Y, Borriss R. Genome sequence of the plant growth promoting strain Bacillus amyloliquefaciens subsp. plantarum B9601-Y2 and expression of mersacidin and other secondary metabolites. J Biotechnol. 2012;164:281–291. doi: 10.1016/j.jbiotec.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Hu P, Brodie EL, Suzuki Y, McAdams HH, Andersen GL. Whole-genome transcriptional analysis of heavy metal stresses in Caulobacter crescentus. J Biotechnol. 2005;187:8437–8449. doi: 10.1128/JB.187.24.8437-8449.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya G, Yan ZF, Won KH, Yang JE, Wang QJ, Kook MC, Yi TH. Caulobacter hibisci sp. nov., isolated from rhizosphere of Hibiscus syriacus L. (Mugunghwa flower) Int J Syst Evol Microbiol. 2017;67:3167–3173. doi: 10.1099/ijsem.0.002021. [DOI] [PubMed] [Google Scholar]
- Pereira SIA, Monteiro C, Vega AL, Castro PML. Endophytic culturable bacteria colonizing Lavandula dentata L. plants: isolation, characterization and evaluation of their plant growth-promoting activities. Ecol Eng. 2016;87:91–97. doi: 10.1016/j.ecoleng.2015.11.033. [DOI] [Google Scholar]
- Ping L, Boland W. Signals from the underground: bacterial volatiles promote growth in Arabidopsis. Trends Plant Sci. 2004;9(6):263–266. doi: 10.1016/j.tplants.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Qin S, Li J, Chen HH, Zhao GZ, Zhu WY, Jiang CL, Xu LH, Li WJ. Isolation, diversity, and antimicrobial activity of rare Actinobacteria from medicinal plants of tropical rain forests in Xishuangbanna. China Appl Environ Microb. 2009;75:6176–6186. doi: 10.1128/AEM.01034-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Feng WW, Wang TT, Ding P, Xing K, Jiang JH. Plant growth-promoting effect and genomic analysis of the beneficial endophyte Streptomyces sp. KLBMP 5084 isolated from halophyte Limonium sinense. Plant Soil. 2017;416:117–132. doi: 10.1007/s11104-017-3192-2. [DOI] [Google Scholar]
- Ryu CM, Farag MA, Hu CH, Reddy MS, Wei HX, Pare PW, Kloepper JW. Bacterial volatiles promote growth in Arabidopsis. P Natl Acad Sci USA. 2003;100:4927–4932. doi: 10.1073/pnas.0730845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LN, Yang ED, Wei JC, Tang XY, Cao YY, Han GM. Caulobacter flavus sp. nov., a stalked bacterium isolated from rhizosphere soil. Int J Syst Evol Microbiol. 2015;65:4374–4380. doi: 10.1099/ijsem.0.000585. [DOI] [PubMed] [Google Scholar]
- Sun LN, Yang ED, Hou XT, Wei JC, Yuan ZX, Wang WY. Caulobacter rhizosphaerae sp. nov., a stalked bacterium isolated from rhizosphere soil. Int J Syst Evol Microbiol. 2017;67:1771–1776. doi: 10.1099/ijsem.0.001860. [DOI] [PubMed] [Google Scholar]
- Tatusov RL, Galperin MY, Natale DA, Koonin EV. The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000;28:33–36. doi: 10.1093/nar/28.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccaro BJ, Lancaster WA, Thorgersen MP, Zane GM, Younkin AD, Kazakov AE, Wetmore KM, Deutschbauer A, Arkin AP, Novichkov PS, Wall JD, Adams MW. Novel metal cation resistance systems from mutant fitness analysis of denitrifying Pseudomonas stutzeri. Appl Environ Microbiol. 2016;82:6046–6056. doi: 10.1128/AEM.01845-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JF, Zhang YQ, Li Y, Wang XM, Nan WB, Hu YF, Zhang H, Zhao CZ, Wang F, Li P, Shi HY, Bi YR. Endophytic microbes Bacillus sp. LZR216-regulated root development is dependent on polar auxin transport in Arabidopsis seedlings. Plant Cell Rep. 2015;34:1075–1087. doi: 10.1007/s00299-015-1766-0. [DOI] [PubMed] [Google Scholar]
- Xie SS, Jiang HY, Ding T, Xu QQ, Chai WB, Cheng BJ. Bacillus amyloliquefaciens FZB42 repressed plant miR846 to induce systemic resistance via jasmonic acid-dependent signaling pathway. Mol Plant Pathol. 2018;19(7):1612–1623. doi: 10.1111/mpp.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung MC, Park DM, Overton KW, Blow MJ, Hoover CA, Smit J, Murray SR, Ricci DP, Christen B, Bowman GR, Jiao Y. Transposon mutagenesis paired with deep sequencing of Caulobacter crescentus under uranium stress reveals genes essential for detoxification and stress tolerance. J Bacteriol. 2015;197:3160–3172. doi: 10.1128/JB.00382-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhong J, Liu H, Xin KY, Chen CQ, Li QQ, Wei YH, Wang Y, Chen F, Shen XH. Complete genome sequence of the drought resistance-promoting endophyte Klebsiella sp. LTGPAF-6F. J Biotechnol. 2017;246:36–39. doi: 10.1016/j.jbiotec.2017.02.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.