Abstract
A 66-year-old man with diabetes presented to the hospital with a two-month history of dyspnea, cough, rust-colored sputum, night sweats and 20 pound weight loss. He had begun smoking medical marijuana 3 months earlier. CT of the chest showed multiple bilateral large ground glass opacities with surrounding consolidation. Infectious workup was negative. BAL was non-diagnostic. He was treated with broad spectrum antibiotics without improvement. VATS was performed and cultured lung tissue grew Rhizopus species. He was started on intravenous liposomal amphotericin B and micafungin and then transitioned to oral posaconazole after two weeks. Repeat CT two months later showed stable size of the cavities. One month later he died of massive pulmonary hemorrhage. Here we document what we believe is the first known case of pulmonary mucormycosis associated with medical marijuana use.
1. Introduction
Marijuana use is widespread and increasing. In the 2015 National Survey on Drug Use and Health, 8.3% of respondents said that they had used marijuana in the past month [1]. As of April 2018, 30 states, the District of Columbia, Guam and Puerto Rico had passed medical marijuana laws permitting programs public medical marijuana [2]. The total number of medical marijuana patients is unknown but estimates place the number at greater than 2 million patients in the United States [3].
Marijuana refers to the dried leaves and flowers of the Cannabis sativa plant, which are rich in phytocannabinoids. The plant is grown either indoors or outdoors before it is harvested, cured, and dried. Molds may be present and can multiply under conditions of high moisture as with inappropriate watering, humidity or ventilation or inadequate drying and curing. Mold spores may survive the drying and curing process even under ideal conditions [4]. Marijuana obtained from medical dispensaries does not differ significantly with regards to microflora when compared to illicit marijuana. One analysis of twenty marijuana samples obtained from dispensaries in northern California showed the presence of 20 fungal genera including Aspergillus, Cryptococcus, and Mucor as well as several bacterial pathogens such as Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa [5].
Previous case reports have documented cases of pulmonary aspergillosis associated with marijuana smoking in immunocompromised patients [[6], [7], [8]]. Here we document what we believe is the first known case of pulmonary mucormycosis associated with medical marijuana use.
2. Case summary
A 66-year-old man presented to the emergency department with a two-month history of shortness of breath, cough, rust-colored sputum, night sweats and a 20 pound unintentional weight loss. He had a history of poorly controlled type 2 diabetes mellitus and chronic lower back pain. He was a 1-pack-per-day smoker for thirty years. Three months prior to presentation, he had started smoking medical marijuana for relief of his chronic back pain. The marijuana was obtained from a medical dispensary. He previously worked as an auto mechanic but had retired six months prior to presentation.
On physical examination, respiratory rate was 24 breaths per minute with an SpO2 of 98% on high flow oxygen (15 L/min). There were bilateral coarse breath sounds on pulmonary examination. White blood cell count was 17.3 × 103/μL. Blood glucose was 348 mg/dL and hemoglobin A1c was 10.0%.
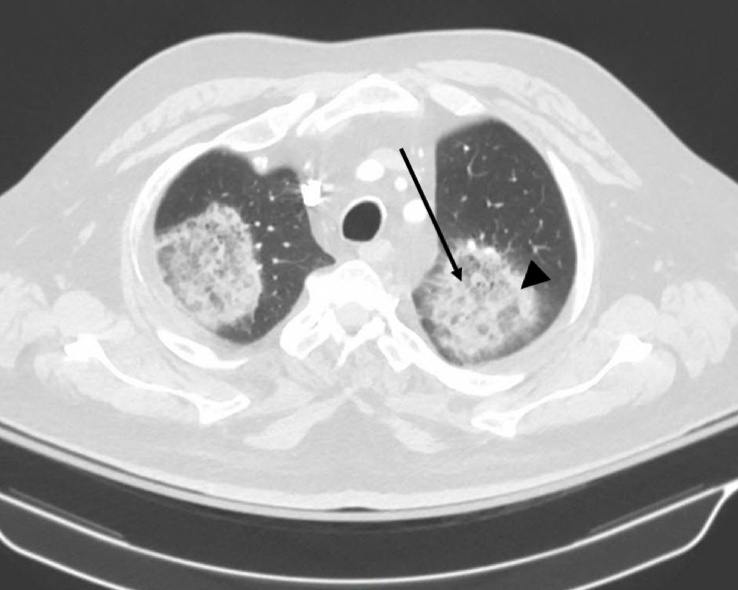
Plain film chest x-ray showed multiple large oval opacities in both lungs. Computed tomography of the chest showed multiple bilateral large ground glass opacities with surrounding areas of consolidation (reversed halo sign) (Fig. 1). Infectious workup including blood, urine and sputum cultures was negative. Vasculitis and autoimmune workups were negative. Bronchoalveolar lavage cultures grew normal oral flora. The patient was treated with a broad spectrum antibiotic regimen including vancomycin, piperacillin-tazobactam and levofloxacin without clinical improvement.
Fig. 1.
Computed tomography of the chest showing the reversed halo sign. Central ground glass opacities (arrow) are surrounded by areas of peripheral consolidation (arrowhead). The reversed halo sign may be considered pathognomonic of pulmonary mucormycosis in the right clinical context [22].
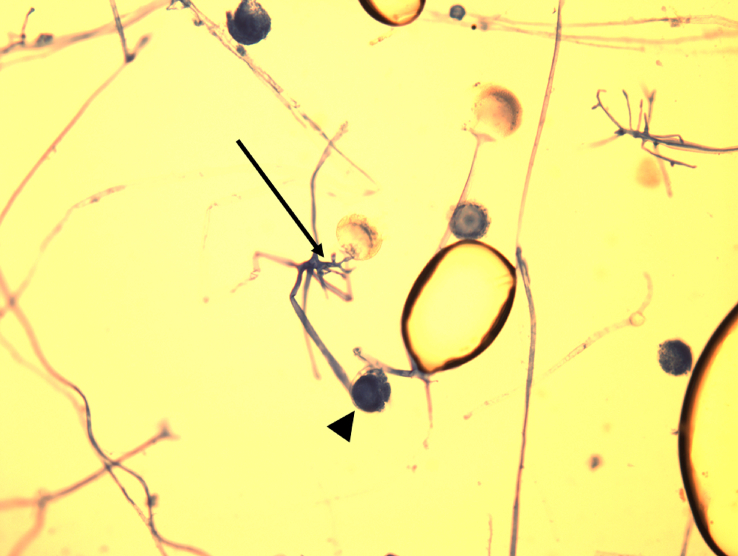
The patient was intubated and underwent video-assisted thoracoscopic surgery on hospital day 7. The lungs were noted to have areas of severely friable and inflamed lung parenchyma and pleura. Examination of Giemsa stained tissue samples revealed mycelial elements. Culture of the biopsied lung tissue grew Rhizopus species (Fig. 2).
Fig. 2.
Giemsa stain of lung biopsy showing broad, aseptate, irregularly branched hyphae characteristic of Rhizopus spp. Rhizoids (arrow) and sporangia (arrowhead) are visible.
He was started on liposomal amphotericin B and micafungin. His oxygen requirements decreased and he was extubated on post-operative day 12. He received two weeks of parenteral amphotericin and was then transitioned to oral posaconazole after clinical improvement.
Repeat CT one month later showed stable size of the cavities and continued improvement of the patient's respiratory function. He was readmitted two months later with hemoptysis which stopped spontaneously. One month after discharge from the second hospitalization, he died of massive pulmonary hemorrhage despite continued therapy with posaconazole.
3. Discussion
Mucormycosis is an angioinvasive disease caused by species of the order Mucorales, most commonly of the genera Rhizopus, Mucor, and Rhizomucor. Mucormycosis most commonly manifests as rhino-orbital-cerebral infection but may also present as pulmonary, cutaneous, gastrointestinal, central nervous system or disseminated infection. Disease is typically seen in immunocompromised patients, including those with Diabetes mellitus, particularly in the setting of diabetic ketoacidosis [9]. High plasma glucose and iron concentrations upregulate expression of glucose-regulated protein 78 (GRP78), a heat shock protein present on host endothelial cells. CotH is a fungal spore coat protein present in pathogenic Mucorales species and acts as the fungal ligand which binds to GRP78, inducing endocytosis and leading to angioinvasion [10,11].
Mucorales are widespread and are can be found where humid organic matter is exposed to heat, such as in composting vegetation or rotting fruit [12]. Marijuana, though known to contain Mucorales species, has not previously been associated with mucormycosis. Like all Zygomycetes, Mucorales produce spores that are released into the environment where they remain airborne and may eventually gain entry to the body via inhalation. Definitive diagnosis is made by histopathological, cytopathological, or direct microscopic visualization in affected organs [13]. Hyphae of mucorales species are easily damaged during biopsy or tissue preparation and thus microscopic or histopathological examination is usually more useful than culture.
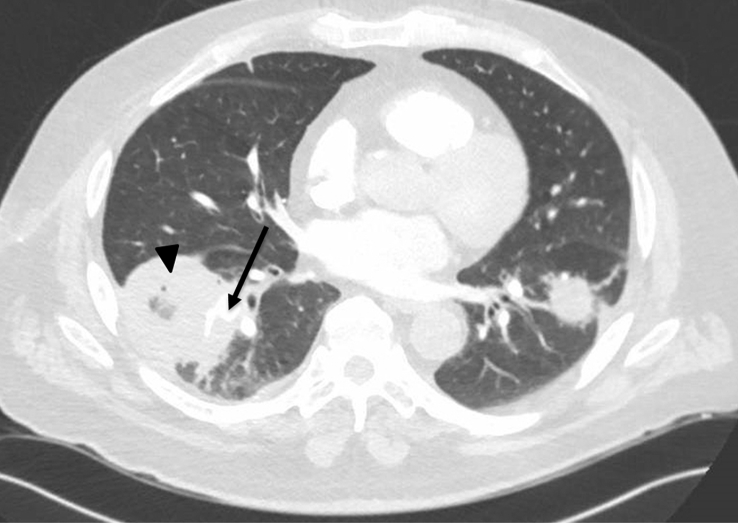
In a population studied by Roden et al., diabetics represented 36% of published mucormycosis cases [14]. Among this diabetic population, 43% presented with rhinocerebral involvement and 16% presented with pulmonary involvement. Mortality among all patients was 76% in patients with pulmonary infection. Due to the high mortality, treatment must be initiated as soon as the diagnosis is suspected rather than waiting for definitive diagnosis. Surgical debridement should be considered but is not always feasible, especially when necrotic tissue abuts important anatomic structures, as was the case in our patient (Fig. 3). First line treatment is amphotericin B lipid complex at a daily dose of >5 mg/kg or liposomal amphotericin B at a daily dose of >3 mg/kg. Patients with impaired renal function, those who fail treatment with amphotericin or develop major adverse effects should be treated with posaconazole 200 mg four times per day [15,16]. Isavuconazole is a second-generation triazole that has shown promise in treating invasive pulmonary aspergillosis and mucormycosis and may have a more favorable side effect profile than current first-line drugs [17]. Aggressive surgical resection should be considered in patients who do not show clinical improvement within 48–72 hours of starting appropriate medical therapy [18,19]. Wedge resection may be considered if disease is limited but a lobectomy is often required and pneumonectomy may be necessary for extensive disease [18]. A review of 87 cases of localized pulmonary mucormycosis without evidence of dissemination showed a 44% overall survival rate with a mortality rate of 55% in patients receiving medical therapy alone versus a mortality rate of 27% in patients who underwent surgery, most of whom also received antifungal therapy [20]. These results may be biased by the fact that patients who have extensive, multilobar disease at the time of diagnosis may not be deemed surgical candidates due to the widespread nature of their infection, as was the case with our patient.
Fig. 3.
Computed tomography demonstrating angioinvasion. A segmental artery (arrow) is obliterated by a cavitary lesion (arrowhead).
Numerous previous case reports have described cases of invasive pulmonary fungal infection associated with marijuana smoking in immunocompromised patients (Table 1). Most of these patients had hematologic malignancies, a well-known risk factor for invasive fungal disease. Our patient had no known malignancy. However, we suspect that the combination of poorly controlled diabetes and years of cigarette smoking leading to emphysema and increased susceptibility to pulmonary infection put him at increased risk of invasive fungal disease. We hypothesize that he inhaled airborne spores while smoking marijuana, leading to overwhelming pulmonary infection. Unfortunately, we were unable to perform microbiologic testing of the suspect marijuana due to the delayed presentation after onset of symptoms.
Table 1.
Selected cases of pulmonary fungal infections associated with marijuana use.
| Study | Patient age | Underlying medical condition | Amount of time using marijuana prior to presentation | Diagnosis | Treatment | Outcome |
|---|---|---|---|---|---|---|
| Stone et al. | 66 years | Diabetes | 3 months | Mucormycosis | Amphoteracin B followed by voriconazole | Death |
| Szyper-Kravitz et al. [6] | 46 years | AML on chemotherapy | Unknown | Invasive pulmonary aspergillosis | Amphoteracin B | Resolution of fever and hypoxemia within 72 hours of starting antifungal therapy |
| Cescon et al. [7] | 65 years | Stage IV colorectal cancer on chemotherapy | 6 weeks | Invasive pulmonary aspergillosis | Voriconazole | Total symptomatic and radiographic resolution of infection |
| Khwaja et al. [8] | 27 years | ALL on chemotherapy | Unknown | Unknown, presumed invasive fungal infection | Voriconazole/micafungin | Decreased size of pulmonary nodule |
| Khwaja et al. [8] | 20 years | ALL on chemotherapy | Unknown | Unknown, presumed invasive fungal infection | Voriconazole | Resolution of nodular pulmonary lesion |
| Khwaja et al. [8] | 36 years | AML on chemotherapy | Unknown | Unknown, presumed invasive fungal infection | Posaconazole | Death |
| Khwaja et al. [8] | 53 years | AML on chemotherapy | 22 years | Disseminated Fusarium infection | Liposomal amphotericin B, then combination of micafungin and voriconazole | Death |
| Gargani et al. [23] | 47 years | Rheumatoid arthritis, chronic steroid use | Unknown | Chronic pulmonary aspergillosis, aspergilloma | Posaconazole, cessation of marijuana | Cough and sputum production improved, no evidence of recurrence |
| Gargani et al. [23] | 43 years | Tetralogy of Fallot, emphysema | 34 years | Chronic pulmonary aspergillosis, aspergilloma | Voriconazole | Death |
Patients seeking pain relief by smoking marijuana may have conditions putting them at risk for opportunistic infections. In Canada and the Netherlands, where medical marijuana is dispensed under the regulation of the federal government, gamma-irradiation is used to sterilize medical marijuana before it is distributed to patients [21]. This practice is not yet commonplace in the United States, where rules and regulations vary state to state [4]. Until sterilization of medical marijuana becomes routine in the United States, physicians should counsel immunocompromised patients, including those with poorly controlled diabetes, that smoking medical marijuana puts them at risk for overwhelming pulmonary infection due to invasive fungi.
Conflicts of interest
The authors have no conflict of interest to declare.
References
- 1.Center for Behavioral Health Statistics and Quality . 2016. Key Substance Use and Mental Health Indicators in the United States: Results from the 2015 National Survey on Drug Use and Health.https://www.samhsa.gov/data/ (HHS Publication No. SMA 16-4984, NSDUH Series H-51) Available from: [Google Scholar]
- 2.State Medical Marijuana Laws. National Conference of State Legislators; Washington, DC: 2018. http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx [Internet] Available from: [Google Scholar]
- 3.Medical Marijuana Patient Numbers. Marijuana Policy Project; Washington, DC: 2017. https://www.mpp.org/issues/medical-marijuana/state-by-state-medical-marijuana-laws/medical-marijuana-patient-numbers/ [Internet] Available from: [Google Scholar]
- 4.Holmes M., Vyas J., Steinbach W. 2015 May. Microbiological Safety Testing of Cannabis.http://cannabissafetyinstitute.org/wp-content/uploads/2015/06/Microbiological-Safety-Testing-of-Cannabis.pdf Retrieved June 20, 2017, from Cannabis Safety Institute: (White paper) [Google Scholar]
- 5.Thompson G.R., Tuscano J.M., Dennis M. A microbiome assessment of medical marijuana. Clin. Microbiol. Infect. 2017;23(4):269–270. doi: 10.1016/j.cmi.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Szyper-Kravitz M., Lang R., Manor Y. Early invasive pulmonary aspergillosis in a leukemia patient linked to aspergillus contaminated marijuana smoking. Leuk. Lymphoma. 2001;42:1433–1437. doi: 10.3109/10428190109097776. [DOI] [PubMed] [Google Scholar]
- 7.Cescon D.W., Page A.V., Richardson S. Invasive pulmonary aspergillosis associated with marijuana use in a man with colorectal cancer. J. Clin. Oncol. 2008;26(13):2214–2215. doi: 10.1200/JCO.2007.15.2777. [DOI] [PubMed] [Google Scholar]
- 8.Khwaja S., Yacoub A., Cheema A. Marijuana smoking in patients with leukemia. Cancer Control. 2016 July;23(3):278–283. doi: 10.1177/107327481602300311. [DOI] [PubMed] [Google Scholar]
- 9.Spellberg B., Edwards J., Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation and management. Clin. Microbiol. Rev. 2005;18(3):566–569. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu M., Spellberg B., Phan Q. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J. Clin. Invest. 2010;120(6):1914–1924. doi: 10.1172/JCI42164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gebremariam T., Liu M., Luo G. CotH3 mediates fungal invasion of host cells during mucormycosis. J. Clin. Invest. 2014;124(1):237–250. doi: 10.1172/JCI71349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richardson M. The ecology of the Zygomycetes and its impact on environmental exposure. Clin. Microbiol. Infect. 2009;15(Suppl. 5):2–9. doi: 10.1111/j.1469-0691.2009.02972.x. [DOI] [PubMed] [Google Scholar]
- 13.Lass-Flörl C. Zygomycosis: conventional laboratory diagnosis. Clin. Microbiol. Infect. 2009;15(Suppl 5):60–65. doi: 10.1111/j.1469-0691.2009.02999.x. [DOI] [PubMed] [Google Scholar]
- 14.Roden M., Zaoutis T., Buchanana W. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin. Infect. Dis. 2005;41:634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 15.Petrikkos G. Lipid formulations of amphotericin B as the first-line treatment of zygomycosis. Clin. Microbiol. Infect. 2009;15(Suppl. 5):2–9. doi: 10.1111/j.1469-0691.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- 16.Cornely O.A., Vehreschild J.J., Rüping M. Current experience in treating invasive zygomycosis with posaconazole. Clin. Microbiol. Infect. 2009;15(Suppl. 5):2–9. doi: 10.1111/j.1469-0691.2009.02985.x. [DOI] [PubMed] [Google Scholar]
- 17.Jenks J., Salzer H., Prattes J. Spotlight on isavuconazole in the treatment of invasive aspergillosis and mucormycosis: design, development, and place in therapy. Drug Des. Dev. Ther. 2018 Apr 30;12:1033–1044. doi: 10.2147/DDDT.S145545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tedder M., Spratt J.A., Anstadt M.P. Pulmonary mucormycosis: results of medical and surgical therapy. Ann. Thorac. Surg. 1994;57(4):1044–1050. doi: 10.1016/0003-4975(94)90243-7. [DOI] [PubMed] [Google Scholar]
- 19.Cadelis G. Hemoptysis complicating bronchopulmonary mucormycosis in a diabetic patient. Rev. Pneumol. Clin. 2013 Apr;69(2):83–88. doi: 10.1016/j.pneumo.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Lee F.Y.W., Mossad S.B., Adal K.A. Pulmonary mucormycosis: the last 30 years. Arch. Intern. Med. 1999;159(12):1301–1309. doi: 10.1001/archinte.159.12.1301. [DOI] [PubMed] [Google Scholar]
- 21.Hazekamp A. Evaluating the effects of gamma-irradiation for decontamination of medicinal cannabis. Front. Pharmacol. 2016;7:108. doi: 10.3389/fphar.2016.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Legouge C., Caillot D., Chrétien M.-L. The reversed halo sign: pathognomonic pattern of pulmonary mucormycosis in leukemic patients with neutropenia? Clin. Infect. Dis. 2014;58(5):672–678. doi: 10.1093/cid/cit929. [DOI] [PubMed] [Google Scholar]
- 23.Gargani Y., Bishop P., Denning D. Too many mouldy joints – marijuana and chronic pulmonary aspergillosis. Mediterr. J. Hematol. Infect. Dis. 2011 January;3 doi: 10.4084/MJHID.2011.005. [DOI] [PMC free article] [PubMed] [Google Scholar]