Abstract
Leiomyomatosis peritonealis disseminata (LPD) is a benign disease characterized by the presence of multiple small nodules on the omentum, parietal, and visceral peritoneum. It corresponds to leiomyoma and often resembles metastases of malignant tumors; however, with favorable prognosis. Here we describe a 46-year-old woman, diagnosed with LPD, to demonstrate the etiopathogenesis of the developed leiomyomatosis following endoscopic extirpation of the uterus with the use of a power morcellator. The patient was operated for diffuse leiomyoma using a power morcellator. Six months later, during a follow-up visit, disseminated tumor nodes on the peritoneum were revealed. Histological and immunohistochemical (smooth muscle α-actin, vimentin, estrogen receptors, progesterone receptors, and Ki67) study confirmed the diagnosis of LPD. As part of the follow-up, certain regression of the tumor nodes was noted against the backdrop of the onset of menopause and the corresponding decline of estrogen levels. Currently, the prognosis is favorable and follow-up is ongoing. Such cases are rare, but the condition is particularly important due to its iatrogenic nature. It has attracted the attention of the Food and Drug Administration (FDA) because power morcellation is probably associated with the risk of spreading suspected cancerous tissue. The existing high risk of iatrogenic LPD formation indicates the need for detailed reporting of all similar clinical cases, including the established pathogenetic and pathomorphological mechanisms of this process to prevent morcellator-related complications.
Keywords: Leiomyomatosis , Peritoneum , Iatrogenic disease
What’s Known
Leiomyomatosis peritonealis disseminata (LPD) is an idiopathic benign disease characterized by the presence of multiple small nodules on the peritoneum.
It corresponds to leiomyoma and often resembles metastases of malignant tumors. Possible iatrogenic nature due to power morcellation is considered.
To date, there are only about 140 officially registered LPD cases worldwide.
What’s New
The present case report describes LPD of iatrogenic origin following a power morcellation procedure.
This case is of particular interest as it involves a woman with a normal estrogen level and definitive iatrogenic nature following laparoscopic extirpation of the uterus.
Introduction
Leiomyomatosis peritonealis disseminata (LPD) is an idiopathic benign disease characterized by the presence of multiple small nodules on the omentum, parietal, and visceral peritoneum. It corresponds to leiomyoma and often resembles metastases of malignant tumors on ultrasonography. To date, there are about 140 officially registered LPD cases worldwide.1-3 It is believed that LPD is a multifactorial disease with genetic and hormonal components leading to metaplasia of mesenchymal stem cells into smooth muscle cells, fibroblasts, myofibroblasts, and decidual cells under the influence of female sex hormones (estrogen and progesterone).2,4 There are no specific clinical symptoms. Basically, the disease manifests itself as abdominal pain and uterine bleeding. On physical (visual) examination LPD mimics peritoneal carcinomatosis, which makes verification of the diagnosis more difficult. Ultrasonography, computed tomography, and magnetic resonance imaging are among the most effective diagnostic methods. However, final verification of the pathological process is only possible with the use of morphological methods which allows identification of these tumors as low-grade leiomyomatosis nodes.4 In case of untimely treatment or the use of ineffective methods in dealing with LPD, the risk of malignization and formation of leiomyosarcoma increases significantly.
Recent scientific literature has shown that certain surgical manipulations, including power morcellation, can significantly increase the risk of dissemination of tumor formations.5 Power morcellation, or electromechanical morcellation, is an instrumental method intended to shred and easily remove tissue through a laparoscopic access port with various design variations.6 Morcellator is a surgical instrument for dividing and removal of organs and tissues directly from a cavity without excessive tissue dissection. In gynecological practice, this instrument is most often used during the majority of laparoscopic and robotic supracervical hysterectomy and myomectomy. Although the exact frequency of morcellator use is unknown, about 20,000 laparoscopic and robotic supracervical hysterectomies are performed each year in the United States.7
Morcellation procedure has attracted the attention of researchers following several reports on idiopathic leiomyosarcoma cases in patients with a history of endoscopic surgery.8 It is assumed that fragments of the uterus or myoma can be implanted into peritoneum with subsequent dissemination in women susceptible to hormonal imbalance against the backdrop of hyperestrogenism. Special attention should be paid to the risk of malignancy appearance with possible resultant upstaging of the disease and worsened prognosis. Additionally, leiomyosarcomas are the subject of considerable concern because they are often difficult to differentiate from benign leiomyomatosis preoperatively.9
The present case report describes a 46-year-old woman who was diagnosed with LPD. The case report is aimed to demonstrate etiopathogenesis of the developed peritoneal leiomyomatosis following endoscopic extirpation of the uterus with the use of a power morcellator against the backdrop of the patient’s normal hormonal status.
Case Presentation
A 46-year-old woman was admitted to the gynecological department of a hospital in Odessa (Ukraine) on 26 December 2016 with complaints of pain in the lower abdomen and bloody discharge from the genital tract. Following a complete clinical investigation, the diagnosis was confirmed as multinodular uterine myoma, adenomyosis, and cicatricial deformity of the cervix. The pelvic ultrasonography showed a 35×35 mm intramural, subserosal node at the bottom region, and a 43×40 mm subserosal node at the posterior wall of the uterus. Subsequently, diagnostic laparoscopy was performed. It was noted that the omentum was normal; dimensions of the uterus were 7×7×8 cm. The uterus was deformed due to the intramural myomatous node, subserous myomatous node, and small subserous nodes along the anterior wall of the uterus. Additionally, endometrioid heterotopias were found. Laparoscopic supracervical hysterectomy with uterine tubes using a power morcellator, posterior colpotomy, and superficial conization of the cervix was performed. Morphological study of the removed tissues confirmed diffuse leiomyoma with foci of endometriosis and chronic bilateral salpingitis.
After 6 months in June 2017, the patient visited the Genesis Clinic (Simferopol, Russian Federation) for a follow-up examination because of her relocation. She had complaints of mild to moderate abdominal pain and discomfort in the pelvic region. Laboratory tests for estrogen and progesterone levels showed no deviation from normal values. Results of routine blood test, urine test, and blood chemistry were also normal. Ultrasonography and magnetic resonance imaging were performed followed by diagnostic laparoscopy, which confirmed the presence of neoplastic disseminate nodes of whitish-gray color ranging from 0.5 to 1.5 cm in diameter diffusely located on the peritoneum. Neither exudate nor adhesive process was present. To establish the histological origin, malignancy, and probable prognosis, a biopsy sample was taken from the tumor-like formations for histological examination.
The first phase of the study included histological examination using hematoxylin and eosin (H&E) stain. Histological structure of biopsy material was represented by the fibrous and muscular bundles with the presence of spindle-shaped cells with eosinophilic cytoplasm (figure 1). Further diagnostic approach included immunohistochemical (IHC) examination which was carried out according to a standardized procedure using serial 3-5 μm paraffin sections with EnVISION™ FLEX+ imaging system (Agilent Technologies, Santa Clara, CA, USA). To determine the immune phenotype of the tumor, a monoclonal antibody to smooth muscle α-actin (SMA) and vimentin was used, as well as monoclonal antibodies to estrogen receptors (clone SP1), progesterone receptors (clone pGr636), and Ki67 (clone MIB-1). Evaluation of SMA and vimentin expression was performed on the basis of specific cytoplasmic staining of the smooth muscle cells. The color intensity was evaluated semi-quantitatively as low, moderate, or high. Evaluation of expression levels of estrogen and progesterone receptors was carried out based on the color intensity and distribution of receptors (%) according to: negative (less than 10%), low (10% to 45%), moderate (46% to 75 %), and high (76% to 100%).
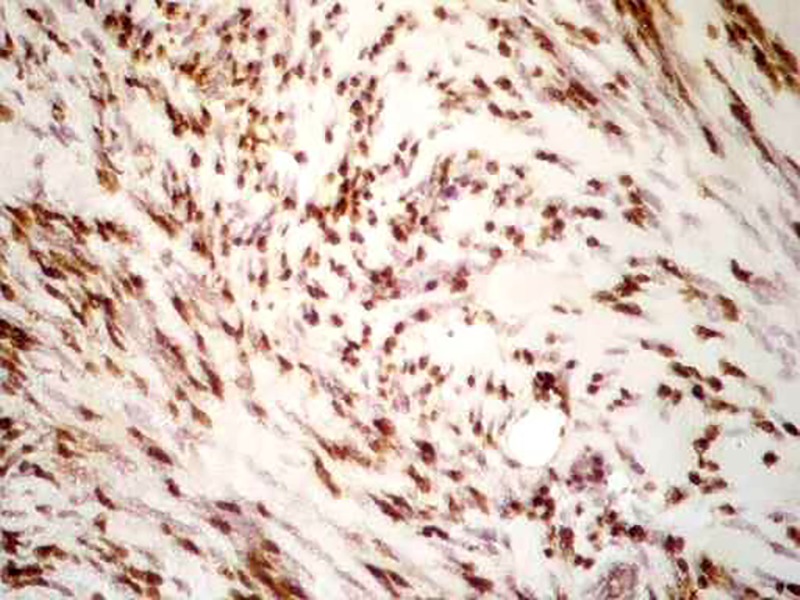
Figure1.
Proliferation of fibrous and muscular tissue showing the presence of spindle-shaped cells with eosinophilic cytoplasm (H&E stain, ×40).
IHC study revealed a high level of cytoplasmic expression of vimentin (figure 2) and SMA (figure 3), which is an indicator of tumor growth of mesenchymal origin, in particular, of smooth muscle cells. Also, there was a high expression of progesterone receptors (from 80% to 92%) (figure 4) and a low expression of estrogen receptors (from 30% to 42%) (figure 5). Finally, given that the proliferative activity of human tumor cells correlates with the degree of histological and biological malignancy, the necessary routine investigation included determination of the proliferative activity using Ki67. In this case, the level of Ki67 expression was low and accounted for only 5% to 8% (figure 6).
Figure2.
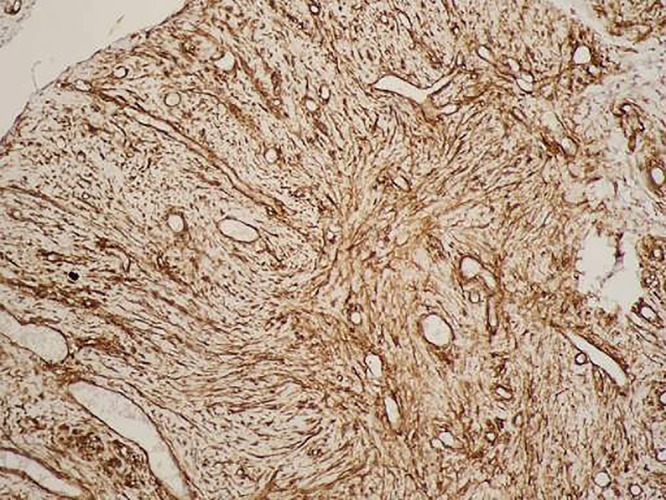
The microphotograph depicts a high level of vimentin cytoplasmic expression (vimentin, ×100).
Figure3.
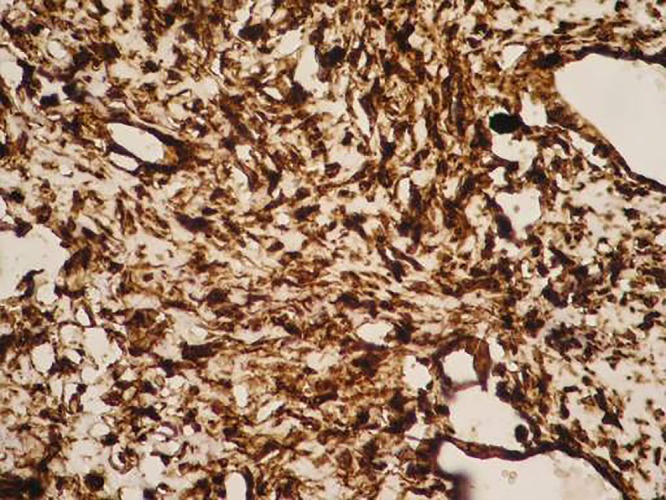
The microphotograph depicts a high level of smooth muscle actin cytoplasmic expression (smooth muscle actin, ×100).
Figure4.
The microphotograph depicts a high level of progesterone receptors intranuclear expression (progesterone receptors, ×250).
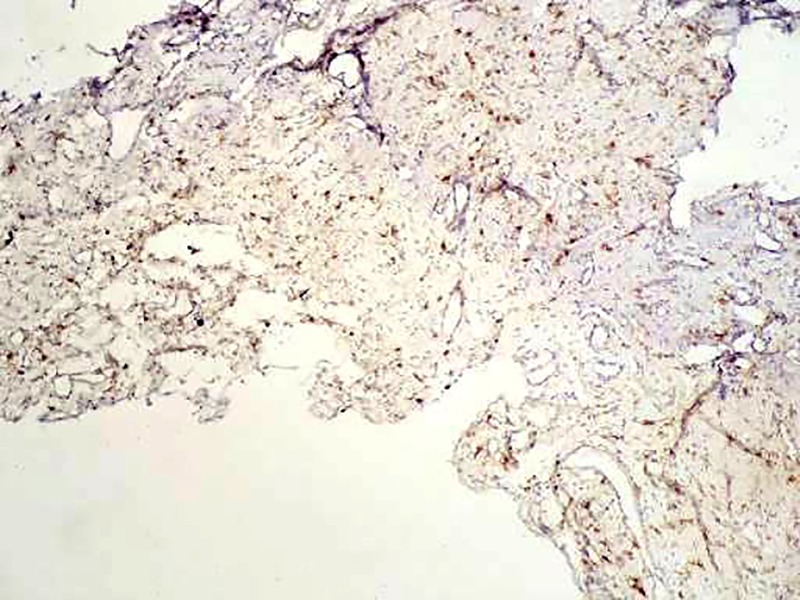
Figure5.
A low level of estrogen receptors intranuclear expression is revealed (estrogen receptors, ×250).
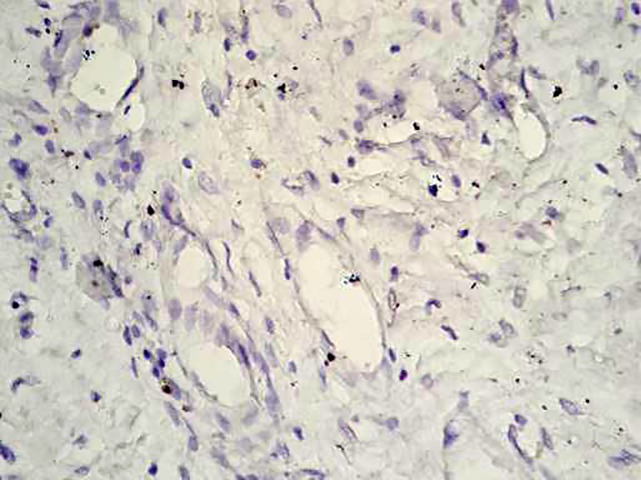
Figure6.
Low proliferative activity (Ki67) is revealed (Ki67, ×40).
Based on the results of histological and immunohistochemical studies, the diagnosis of LPD was morphologically verified. Given the age of the patient and her premenopausal state, accompanied by a natural decrease in the estrogen level, the attending gynecologist chose the strategy of an active follow-up. At the last follow-up (November 2017), according to ultrasonography investigation, there was a positive dynamic in the number and size of the leiomyomatosis nodules. Further follow-up is ongoing. Written informed consent was obtained from the patient for the publication of this case report and accompanying images.
Discussion
The present case report described a 46-year-old woman diagnosed with LPD with a probable relationship to morcellation procedure. A complex clinical-morphological study confirmed the tumor on the peritoneum as a peritoneal leiomyomatosis with a low degree of malignancy, low expression of estrogen receptors, and high expression of progesterone receptors, which allow adequate pathogenetic hormonal therapy.
This case is of particular interest as it involved a woman with a normal level of estrogen. Typically, most reported cases have involved women with an elevated estrogen level suggestive of the hormonal theory of LPD origin. This theory proposes abnormal sensitivity of submesothelial tissue to ovarian hormones. It is believed that the mechanism of the origin of LPD can be confirmed in women with unstable hormonal status since gonadectomy leads to regression of disseminated lesions, and receptors to estrogen and progesterone can be immunohistochemically verified in tumor tissue.10 Other theories support genetic factors (e.g. mutations in X, 17, 12, and 8 chromosomes) probably since they are pathogenetically linked to the uterine myomas and LPD.11
Due to previous laparoscopic hysterectomy using a power morcellator, the present case is similar to those reported in the literature and provides evidence in support of the iatrogenic LPD theory.4,8 Lu and colleagues described six patients diagnosed with peritoneal leiomyomatosis 39-132 months after using the power morcellation procedure.4 All those patients underwent secondary surgical procedures to remove disseminated nodes located mainly in the pelvic area,4 which is in contrast to our patient that underwent a conservative follow-up.
According to the iatrogenic theory of LPD, iatrogenic LPD may occur in case of a morcellation procedure. It proposes that the fragments of myomatous tissue can be implanted in the abdominal cavity that leads to metaplasia of the mesenchymal cells of the peritoneum and, in turn, promote dissemination. This may also appear under favorable conditions, particularly at high levels of estrogen. Huang and colleagues confirmed the crucial role of estrogen in the development of iatrogenic parasitic myomas in an experimental model.10 In their investigation, the estrogen levels were normal at the time of LPD diagnosis and continued to decline due to menopause. Therefore, despite the proven role of estrogen, one cannot rule out the sole iatrogenic origin. Note that the declining levels of estrogen due to natural menopause allowed avoiding repeated surgical intervention and manage our patient in a conservative manner with positive dynamics. Accordingly, the Food and Drug Administration (FDA) announced that laparoscopic power morcellation is associated with the risk of spreading suspected cancerous tissue, notably uterine sarcomas, beyond the uterus. There has also been an increasing number of reports on iatrogenic LPD following laparoscopic myomectomy and morcellation.7,12
The existing high risk of iatrogenic LPD formation indicates the need for detailed reporting of all similar clinical cases, including the established pathogenetic and pathomorphological mechanisms of this process to prevent morcellator-related complications and implementation of a personalized preventive approach due to possible malignancy. Most studies on this topic have focused on uterine morcellation; however, this device may be used during surgical interventions on other organs (e.g. the spleen and kidneys) which may also cause serious concern. In this regard, it is necessary to improve the system of monitoring surgical methods and devices to determine the risks associated with the introduction of new technologies.
Conclusion
The present case report describes LPD of iatrogenic origin following laparoscopic extirpation of the uterus using a power morcellator in a woman with a normal level of estrogen. The finding highlights the importance and awareness of such condition, especially following a morcellation procedure, despite the normal level of estrogen.
Footnotes
Conflict of Interest: None declared.
References
- 1.Al-Talib A, Tulandi T. Pathophysiology and possible iatrogenic cause of leiomyomatosis peritonealis disseminata. Gynecol Obstet Invest. 2010;69:239–44. doi: 10.1159/000274487. [DOI] [PubMed] [Google Scholar]
- 2.Fasih N, Prasad Shanbhogue AK, Macdonald DB, Fraser-Hill MA, Papadatos D, Kielar AZ, et al. Leiomyomas beyond the uterus: unusual locations, rare manifestations. Radiographics. 2008;28:1931–48. doi: 10.1148/rg.287085095. [DOI] [PubMed] [Google Scholar]
- 3.Momtahan M, Nemati M, Safaei A. Disseminated peritoneal leiomyomatosis. Iran J Med Sci. 2011;36:57–9. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu B, Xu J, Pan Z. Iatrogenic parasitic leiomyoma and leiomyomatosis peritonealis disseminata following uterine morcellation. J Obstet Gynaecol Res. 2016;42:990–9. doi: 10.1111/jog.13011. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen D, Maheshwary R, Tran C, Rudkin S, Treaster L. Diffuse peritoneal leiomyomatosis status post laparoscopic hysterectomy with power morcellation: A case report with review of literature. Gynecol Oncol Rep. 2017;19:59–61. doi: 10.1016/j.gore.2017.01.001. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylan E, Sahin C, Zeybek B, Akdemir A. Contained Morcellation: Review of Current Methods and Future Directions. Front Surg. 2017;4:15. doi: 10.3389/fsurg.2017.00015. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kho KA, Nezhat CH. Evaluating the risks of electric uterine morcellation. JAMA. 2014;311:905–6. doi: 10.1001/jama.2014.1093. [DOI] [PubMed] [Google Scholar]
- 8.Wu C, Zhang X, Tao X, Ding J, Hua K. Leiomyomatosis peritonealis disseminata: A case report and review of the literature. Mol Clin Oncol. 2016;4:957–8. doi: 10.3892/mco.2016.848. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Einstein MH, Barakat RR, Chi DS, Sonoda Y, Alektiar KM, Hensley ML, et al. Management of uterine malignancy found incidentally after supracervical hysterectomy or uterine morcellation for presumed benign disease. Int J Gynecol Cancer. 2008;18:1065–70. doi: 10.1111/j.1525-1438.2007.01126.x. [DOI] [PubMed] [Google Scholar]
- 10.Huang BS, Yang MH, Wang PH, Li HY, Chou TY, Chen YJ. Oestrogen-induced angiogenesis and implantation contribute to the development of parasitic myomas after laparoscopic morcellation. Reprod Biol Endocrinol. 2016;14:64. doi: 10.1186/s12958-016-0200-y. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyake T, Enomoto T, Ueda Y, Ikuma K, Morii E, Matsuzaki S, et al. A case of disseminated peritoneal leiomyomatosis developing after laparoscope-assisted myomectomy. Gynecol Obstet Invest. 2009;67:96–102. doi: 10.1159/000164949. [DOI] [PubMed] [Google Scholar]
- 12.Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol. 2014;21:486–91. doi: 10.1016/j.jmig.2013.12.003. [DOI] [PubMed] [Google Scholar]