Summary
Neutrophils are detected in inflamed colon in Crohn’s disease (CD). However, whether the frequency and/or activation of circulating or gut tissue neutrophils correlate with endoscopic severity remains to be investigated. A cohort of 73 CD patients was prospectively enrolled according to endoscopic severity and treatment history. Individuals with active disease were stratified using the Montreal classification. Harvey–Bradshaw Index (HBI) and Simple Endoscopic Score for Crohn’s Disease (SES‐CD) were performed at the time of ileocolonoscopy. Frequency of neutrophils and their expression of CD66b and CD64 were assessed in paired blood and colonic biopsies using flow cytometry. The percentage of neutrophils increased in inflamed colon and correlated with SES‐CD in the entire cohort of patients examined, as well as in the subgroup with inflammatory (B1) active disease. SES‐CD further correlated with neutrophil CD66b expression in mucosa but not blood and, conversely, with neutrophil CD64 expression in blood but not mucosa. However, the evaluation of neutrophil activation in mucosa when compared to blood reflected disease activity more clearly. Finally, a neutrophil activation power index (CD66b in mucosa X CD64 in blood) that correlated with SES‐CD discriminated between patients with mild and severe disease. In conclusion, the frequency and activation of colonic neutrophils correlated with SES‐CD, highlighting that mucosal neutrophils are associated with disease severity in CD.
Keywords: Crohn’s disease, CD64, CD66b, neutrophil, SES‐CD
Introduction
Crohn’s disease (CD) is a chronic inflammatory bowel disease with increasing prevalence worldwide 1. The disease is characterized by discontinuous macroscopic and microscopic inflammation that involves innate and adaptive immune cell responses 2. Neutrophil infiltration in the colonic mucosa correlates with endoscopic severity in ulcerative colitis (UC), while their implication remains to be investigated in CD 3, 4. The correlation between disease severity and accumulation and/or activation of neutrophils remains unclear in adult CD patients.
The search for biomarkers to assess disease activity and severity in CD is an area of intense investigation. CD66b is a glycosyl phosphatidylinositol (GPI) anchored glycoprotein weakly expressed on resting neutrophils, which is up‐regulated under inflammatory conditions on neutrophils as well as eosinophils by N‐formyl‐Met‐Leu‐Phe (fMLP) 5, 6. CD64 (FcγRI) is a member of the immunoglobulin (Ig)G receptor family, and cross‐linking with IgG triggers phagocytosis and respiratory burst 7, 8. CD64 expression on peripheral blood neutrophils correlated with endoscopic severity in a pediatric cohort of CD patients 9. Although CD64 and CD66b could be markers of activated neutrophils in CD, the correlation between their expression and endoscopic disease severity in adult CD remains unknown 10.
To the best of our knowledge, no single‐cell analysis study has been performed to examine the correlation between endoscopic scoring and distribution and activation of neutrophils simultaneously in blood and mucosa in a cohort of adult CD patients. In this prospective study, we found that activated neutrophils are recruited to the inflamed colon. Furthermore, their frequencies and activation correlate with endoscopic disease severity (SES‐CD).
Materials and methods
Patient population
This study was performed in accordance with the Declaration of Helsinki and was approved by the local Research Ethics Committee at Centre Hospitalier de l’Université de Montréal. Written informed consent was obtained from each participant and all samples and analyses were anonymized. We prospectively enrolled patients affected by CD (n = 73) at the time of their ileocolonoscopy at Centre Hospitalier de l’Université de Montréal (CHUM) from November 2015 to December 2017. We included patients aged 18 years and older with either active or quiescent ileocolonic or colonic Crohn’s disease. Patients were recruited at the time of diagnosis, during a flare or a follow‐up ileocolonoscopy. CD was either previously diagnosed or confirmed according to standard endoscopic and histopathological criteria 2, 11. We established our recruitment strategy according to endoscopic disease activity and treatment history. Five groups of CD patients were stratified accordingly: (1) newly diagnosed CD, (2) active CD and no treatment in the last month, (3) active CD in spite of non anti‐tumor necrosis factor (TNF) therapy [5‐aminosalicylic acid (5ASA), immunomodulators, prednisone], (4) active CD in spite of anti‐TNF therapy (anti‐TNF alone or combined with an immunomodulator) and (5) CD patients in clinical and endoscopic remission (inactive disease). Each group included approximately 15 participants. A cohort of healthy donors was created as controls (asymptomatic individuals undergoing colorectal cancer screening colonoscopy).
We excluded any patient with suspicion of bacterial or viral intestinal infection and other autoimmune conditions (primary sclerosing cholangitis, primary biliary cholangitis, celiac disease). We also excluded CD patients with exclusive ileal involvement, incomplete colonoscopy and/or treated with vedolizumab and ustekinumab.
Disease characterization
Harvey–Bradshaw Index (HBI) and Simple Endoscopic Score for Crohn’s Disease (SES‐CD) were performed at the time of blood and tissue sampling 12, 13. As currently defined in the literature, active disease corresponded to SES‐CD ≥ 3, with endoscopic remission being SES‐CD = 0–2 14. Notably, our cohort of CD patients with endoscopic inactive disease included four patients with SES‐CD = 1 or 2 (residual mucosal macroscopic inflammation) and 12 patients with SES‐CD = 0, considered to be in complete mucosal healing 15. Finally, age of onset, localization, behavior and presence of perianal disease were recorded according to the Montreal classification 16.
Sample processing and cell isolation
Blood
Three ml of heparinized peripheral venous blood were obtained from each participant at the time of the colonoscopy. Peripheral blood leukocytes were isolated through ammonium–chloride–potassium (ACK) lysis (BioWhittaker Lysing Buffer, Lonza, Walkersville, MD, USA) and centrifugation.
Tissue digestion
Eight to 10 colonic biopsies were obtained from the macroscopically most affected area in patients with SES‐CD ≥ 1. For individuals with complete mucosal healing and control group, biopsies were taken in the cecum. Briefly, we first proceeded to a chemical digestion with dithiothreitol (DTT) 1 mM (Sigma‐Aldrich, St Louis, MO, USA) and ethylenediamine tetraacetic acid (EDTA) 1 mM (Sigma Aldrich) at 37°C for 45 min, followed by enzymatic digestion with DNAse I 0·01 mg/ml (Roche, Mannheim, Germany) and collagenase D 0·25 mg/ml (Roche) at 37°C for 45 min. Mechanical dissociation before and after the enzymatic digestion was performed with gentle MACS Dissociator (Miltenyi Biotec, Bergisch Gladbach, Germany) 17.
Flow cytometry
Blood and mucosal cells were counted with Turk’s solution. Live‐dead staining was performed [LIVE/DEAD® Fixable Aqua Dead Cell Stain Kit; AmCyan (Molecular Probes®, Life Technologies Inc., ON, Canada)] to identify viable cells. Next, cells were stained for surface antigens along with human IgG to block Fc receptors for 30 min at 4°C.
Two staining panels were designed to identify the granulocytes. The first panel detected neutrophils as CD45+SSChighCD66b+CD15+CD9–FcεRI– cells 10, 18 and eosinophils as CD45+ SSChighCD66b+CD15dullCD9+/FcεRI+ cells 5, 10. The second panel detected basophils and mast cells. Basophils were CD45+SSClow Lin–human leukocyte antigen D‐related (HLA‐DR)–CRTH2+CCR3+c‐kit–CD172alowFcεRIhigh cells in peripheral blood, and as CD45+ SSClowLin–HLA‐DR–CD172alowc‐kit–FcεRI+cells in the mucosa, while mast cells were CD45+ LindullHLA‐DRdullc‐kit+FcεRI+ cells 19. See Supporting information methods for list of antibodies; 100 000 events per sample were acquired on a BD fluorescence activated cell sorter (FACS) Aria II (BD Biosciences, Franklin Lakes, NJ, USA). Data were analyzed and mean fluorescence intensity (MFI) (arithmetic mean) for CD66b and CD64 on gated neutrophils and eosinophils was calculated using FCS Express 4 (De Novo Software, Glendale, CA, USA). Data analyses were performed by two independent investigators, with one investigator blinded to the patient’s clinical status.
Cell morphology
Neutrophils and eosinophils were sorted with BD FACS Aria II, underwent cytospin and were stained with Giemsa Wright. A Leica DM4000B microscope equipped with a Leica DFC300FX camera was used for morphological identification.
Histopathology
We performed an independent retrospective histopathological analysis of biopsies taken from the same area, as the biopsies processed for flow cytometry (n = 23). This assessment was made by a gastrointestinal pathologist blinded to the clinical, endoscopic and flow cytometry results. An adaptation of the previously published Colonic Global Histological Activity Score (CGHAS) was performed using one biopsy per patient 20, 21.
Statistical analysis
We first tested our variables for normality using the D’Agostino–Pearson test. SES‐CD in the active endoscopic disease population followed a normal distribution. Pearson’s correlation test was performed when evaluating the entire cohort and Spearman’s rank correlation test with smaller subgroups. Kruskal–Wallis test followed by Dunn’s test was used when more than two groups were compared. Non‐paired data groups were compared using the Mann–Whitney U‐test, while paired data groups were compared with Wilcoxon’s matched pairs signed‐rank test. Data are shown as median with interquartile range. Receiver operating characteristic (ROC) curves with binomial exact confidence intervals were generated. The statistical significance of pairwise comparison of AUCs was evaluated with DeLong’s test using MedCalc software version 18 (Ostend, Belgium). Other statistical analyses were performed using GraphPad Prism version 7.03 (La Jolla, CA, USA). Statistical significance was defined as a P‐value < 0·05; *P <0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001.
Results
The frequency of colonic and circulating neutrophils but not eosinophils augments in active CD
In this prospective study, we quantified granulocytes in paired colonic mucosa and blood from patients with CD (n = 73) stratified according to endoscopic criteria and treatment history (Table 1). The cohort includes 57 patients with active endoscopic disease (SES‐CD ≥ 3) and 16 with endoscopic inactive disease (SES‐CD = 0–2) at the time of inclusion. Clinical remission according to HBI was observed in 46 CD participants and, in agreement with several studies 22, 23, 24, HBI and SES‐CD were not correlated in the subgroup of 23 patients with clinical activity (HBI > 4) (Supporting information, Fig. S1a,b).
Table 1.
Characteristics of participants at the time of inclusion
| Endoscopic active disease | Endoscopic inactive disease | ||
|---|---|---|---|
| SES‐CD ≥ 3 n = 57 | SES‐CD = 0 n = 12 | SES‐CD 1–2 n = 4 | |
| Female n (%) | 29 (50·9) | 7 (58·3) | 2 (50·0) |
| Age (years) median (IQR) | 38 (25.3–49.8) | 50 (46.8–52.8) | 50 (37.0–66.0) |
| Disease duration (years) median (IQR) | 5 (0–12·8) | 18 (14·3–24·4) | 23 (15·5–24·5) |
| Surgical resection history n (%) | 6 (10·5) | 2 (16·7) | 1 (25·0) |
| A1 (age at diagnosis below 16 year) n (%) | 8 (14·0) | – | – |
| A2 (age at diagnosis between 17 and 40 years) n (%) | 42 (73·7) | 11 (91·7) | 3 (75·0) |
| A3 (age at diagnosis above 40 years) n (%) | 7 (12·3) | 1 (8·3) | 1 (25·0) |
| L2 (colonic location) n (%) | 29 (50·9) | 9 (75·0) | 4 (100·0) |
| L3 (ileocolonic location) n (%) | 28 (49·1) | 3 (25·0) | – |
| B1 (non‐stricturing, non‐penetrating behavior) n (%) | 40 (70·2) | 9 (75·0) | 3 (75·0) |
| B2 (stricturing behavior) n (%) | 9 (15·8) | 1 (8·3) | – |
| B3 (penetrating behavior) n (%) | 8 (14·0) | 2 (16·7) | 1 (25·0) |
| Perianal disease n (%) | 10 (17·5) | 2 (16·7) | 1 (25·0) |
| New diagnosis n (%) | 15 (26·2) | – | – |
| No recent treatment n (%) | 14 (24·6) | 2 (16·6) | – |
| Non‐anti‐TNF treatmenta n (%) | 14 (24·6) | 5 (41·7) | 3 (75·0) |
| Anti‐TNF treatmentb n (%) | 14 (24·6) | 5 (41·7) | 1 (25·0) |
Includes 5‐aminosalicylic acid (5ASA), immunomodulators, corticosteroids [n = 4: hydrocortisone rectal foam (n = 1), prednisone (n = 1), combination of 5‐ASA and prednisone (n =1), combination of thiopurine and prednisone (n =1)].
Includes anti‐tumor necrosis factor (TNF) with or without an immunomodulator. IQR = interquartile range; SES‐CD = Simple Endoscopic Score for Crohn’s Disease.
Granulocytes were purified from inflamed mucosa and blood using selective surface markers (i.e. CD66b, CD9, CD15 and FcεRI), granularity (SSC) and cell size (FSC) to first examine their morphology. Colonic and circulating SSChighCD66b+ cells included granular CD15+CD9–FcεRI– neutrophils as well as CD15dullCD9+/FcεRI+ eosinophils (Fig. 1a, upper panels). Purified granulocytes displayed bona fide cell morphology of neutrophils and eosinophils (Fig. 1a, lower panels). The frequency of hematopoietic CD45+ cells was significantly higher in the colonic mucosa of CD patients with active disease when compared to patients with complete mucosal healing or healthy controls (Supporting information, Fig. S2a) and correlated with endoscopic severity (r = 0·38 P < 0·002). We therefore quantified each granulocyte subpopulation after gating on CD45+ cells and showed that the percentage of neutrophils but not eosinophils augmented in the inflamed colon and peripheral blood of patients with active disease (SES‐CD ≥ 3) when compared to patients with inactive disease (SES‐CD = 0–2) (Fig. 1b).
Figure 1.
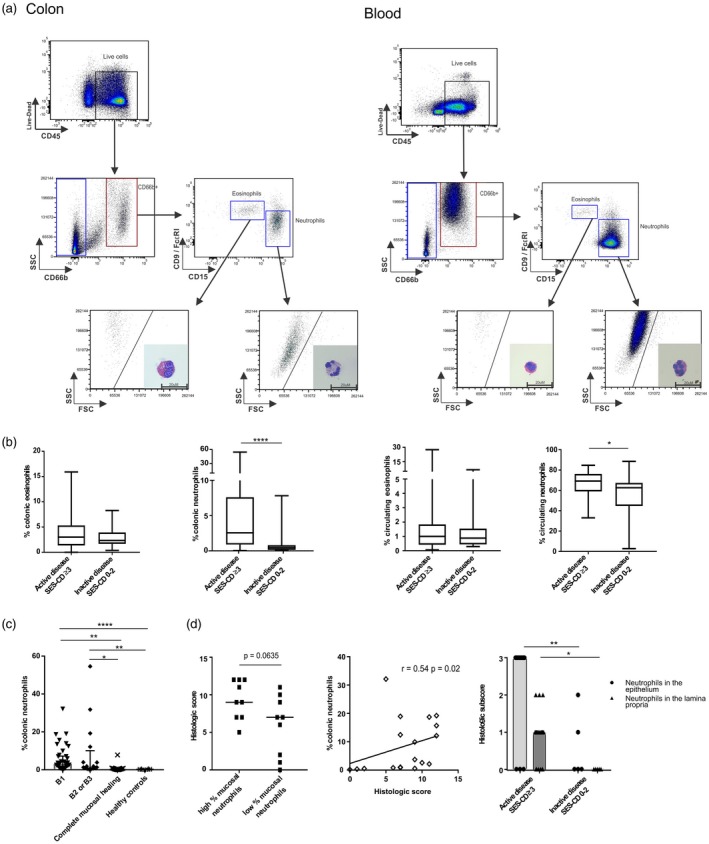
Gating strategy for identification of neutrophils and eosinophils in the mucosa and the blood of Crohn’s disease (CD) patients and cellular frequencies. (a) Representative gating strategy, cell size and morphology by Giemsa–Wright staining for identification of eosinophils and neutrophils in freshly isolated hematopoietic cells from inflamed colon mucosa and peripheral blood of CD patients. Scale bar: 20 μm. (b) Frequencies of colonic and peripheral blood eosinophils and neutrophils among patients with active (n = 57) versus inactive disease (n = 16) (Mann–Whitney U‐test). (c) Frequencies of colonic neutrophils among subgroups according to disease behaviour (Kruskal–Wallis test followed by Dunn’s test. (d) Histological score of active CD patients (n = 18) with high and low mucosal neutrophil frequencies according to the median of the distribution of neutrophils (median = 3.15%) assessed by flow cytometry left panel (Mann–Whitney U‐test), correlation between the frequencies of colonic neutrophils and histological score (middle panel) (Spearman’s rank correlation coefficient) and comparison between histological subscores relative to polymorphonuclear infiltration in active (n =18) or inactive disease (n = 5) (right panel) (Mann–Whitney U‐test); B1 *P < 0·05; **P < 0·01; ****P < 0·0001.
The cohort of patients with active endoscopic disease was further stratified according to Montreal classification 16 into non‐stricturing, non‐penetrating B1 (n = 40) and non‐B1 disease [stricturing disease (B2) or penetrating disease (B3); n = 17]. Notably, the cohort of 16 participants with inactive disease included four individuals considered in endoscopic remission with a SES‐CD of 1 or 2, but still presenting some degree of mucosal inflammation without ulceration in at least one colonic segment. As the present study aimed at evaluating the immune profile of inflamed versus strictly non‐inflamed colon, subgroups of patients with active disease were compared to the subgroup of CD patients with inactive disease in complete mucosal healing (SES‐CD = 0) or to healthy subjects (colonic samples n = 10, blood samples n = 16). Neutrophil infiltration was significantly increased in the colonic mucosa of CD patients with active B1, B2 or B3 disease when compared to CD patients with complete mucosal healing or healthy subjects (Fig. 1c). The percentage of colonic neutrophils was not influenced by treatment (Supporting information, Fig. S2b). Notably, we estimated median cell counts of neutrophils analyzed using flow cytometry at 1890 [interquartile range (IQR) = 630–5300] cells in inflamed mucosa, 130 (IQR = 30–303) cells in complete healed mucosa and 75 (IQR = 38–103) cells in healthy mucosa. Finally, no differences were observed in the frequencies of circulating neutrophils between endoscopic active disease subgroups and controls, irrespective of treatment (Supporting information, Fig. S2c).
Furthermore, histological disease activity assessment was performed in a random subgroup of 23 patients, including individuals in endoscopic remission (n = 5) or with active endoscopic disease (mild disease n = 6, moderate disease n = 6, severe disease n = 6) 21. High histological scores tended to associate with high frequencies of mucosal neutrophils and correlated positively with mucosal neutrophil frequencies in individuals with endoscopic active disease (Fig. 1d, left and middle panels). Specifically, increased neutrophilic infiltration was detected in both the epithelium and lamina propria in endoscopic active disease patients when compared to inactive disease patients (Fig. 1d, right panel).
Collectively, disease activity was associated with colonic neutrophilic infiltration, independently of disease phenotype.
The frequency of colonic neutrophils correlates with SES‐CD
We therefore asked whether frequencies of colonic neutrophils correlated with endoscopic severity. First, the percentage of colonic neutrophils correlated significantly with SES‐CD in the entire cohort of patients (r = 0·26, P < 0·03), in the subgroup with exclusive colonic disease (r = 0·38, P < 0·02), and more particularly in individuals with B1 phenotype (r = 0·45, P < 0·001) (Fig. 2a). Notably, the correlation was maintained when individuals in endoscopic remission (SES‐CD 0‐2) were excluded from the B1 subgroup (Supporting information, Fig. S2d), highlighting the association between frequencies of neutrophils and severity of mucosal inflammation.
Figure 2.
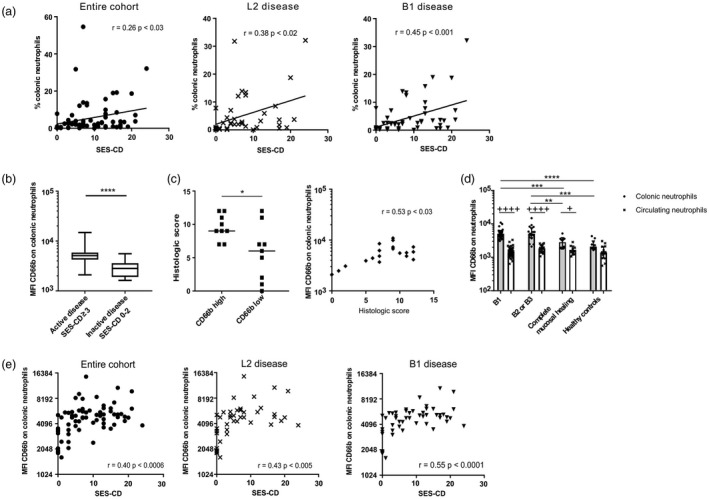
Frequency of colonic neutrophils and the expression of CD66b correlate with Simple Endoscopic Score for Crohn’s Disease (SES‐CD). (a) Correlations between mucosal neutrophil frequencies and SES‐CD in the entire cohort (n = 73), exclusive colonic disease ‘L2’ (n = 42), B1 disease (n = 52) (Pearson’s correlation coefficient). (b) CD66b mean fluorescence intensity (MFI) on neutrophils in colon of patients with active or inactive disease (Mann–Whitney U‐test). (c) Histological score of active CD patients (n = 18) with high and low CD66b MFI according to median MFI (= 5179.5) of CD66b expression on colonic neutrophils (Mann–Whitney U‐test) (left panel) and correlation between the MFI of CD66b on colonic neutrophils and histological score (n = 18, Spearman’s rank correlation coefficient). (d) CD66b MFI on neutrophils in colon and blood of individuals with B1 or non‐B1 disease, complete mucosal healing (n = 12) and healthy controls (healthy controls: colon samples n = 10, blood samples n = 16) (Kruskal–Wallis test followed by Dunn’s test (*), Wilcoxon’s matched‐pairs signed‐rank test for colonic versus circulating neutrophils (+) (healthy controls n = 10 paired samples). (e) Correlations between CD66b MFI on colonic neutrophils and SES‐CD among the entire cohort (n = 73), exclusive colonic disease (L2, n = 42), B1 disease (n = 52) (Pearson’s correlation coefficient). *+P < 0·05; **++P < 0·01; ***+++P < 0·001; ****++++P < 0·0001.
Interestingly, although eosinophils did not accumulate in inflamed CD colon (Fig. 1b), basophils but not mast cells significantly infiltrated the colon of patients with endoscopic active disease when compared to inactive disease (Supporting information, Fig. S3a,d), confirming and extending our previous study 25. More precisely, frequency of basophils augmented in the colonic mucosa in patients with active B1 when compared to B2 or B3 disease (Supporting information, Fig. S3b), while a weak correlation was observed between frequencies of mucosal basophils and SES‐CD in the entire cohort of CD patients (r = 0·25, P < 0·04) (Supporting information, Fig. S3c). In contrast, patients with active B2 or B3 disease displayed lower percentages of mast cells when compared to healthy controls (Supporting information, Fig. S3e) or the subgroup with B1 disease (P < 0·005 when the B1 and B2/B3 subgroups are compared using the Mann–Whitney U‐test).
Taken together, neutrophils and basophils but neither eosinophils nor mast cells infiltrate the inflamed colon while the percentage of neutrophils correlated with SES‐CD in patients with B1 disease.
The expression of CD66b on colonic neutrophils correlates with SES‐CD
We next investigated the activation status of neutrophils by quantifying the expression of CD66b using mean fluorescence intensity (MFI). Increased CD66b expression was detected on mucosal neutrophils in CD patients with active relative to inactive disease (Fig. 2b). High levels of CD66b expression were associated with elevated histological score (Fig. 2c, left panel) and correlated with histological score (Fig. 2c, right panel). When CD patients with endoscopic active disease were further stratified, CD66b expression was increased on mucosal neutrophils in all subgroups examined compared to control groups, irrespective of treatment (Fig. 2d and Supporting information, Fig. S4a, left panel).
We therefore asked whether CD66b up‐regulation on neutrophils occurred in the circulation or after these cells were recruited to CD colon. As further depicted in Fig. 2d, CD66b expression did not increase on circulating neutrophils in CD patients relative to healthy subjects. However, it augmented on neutrophils in inflamed or healed mucosa when compared to paired blood samples in CD, and the increase was significantly higher in patients with active relative to quiescent disease (Fig. 2d).
Finally, SES‐CD correlated significantly with CD66b expression on colonic neutrophils in the entire cohort (r = 0·40, P < 0·0006), colonic (L2) (r = 0·43, P < 0·005), ileocolonic (L3) (r = 0·38, P < 0·04) and B1 (r = 0·55, P < 0·0001) subgroups, and maintained in patients with B1 endoscopic active disease (r = 0·34, P < 0·04) (Fig. 2e and Supporting information, Fig. S4b).
Collectively, the activation of mucosal neutrophils as defined by CD66b expression correlated with SES‐CD in all but not B2/B3 subgroups of patients examined.
CD64 expression on circulating neutrophils correlates with SES‐CD
That CD66b did not increase on circulating neutrophils prompted us to examine CD64 expression, another neutrophil activation marker in blood that was previously reported to correlate with disease severity in pediatric CD patients 9. Accordingly, we observed a higher CD64 expression on circulating neutrophils in adult CD patients with active relative to inactive disease (Fig. 3a). Circulating CD64 expression augmented in all subgroups of patients including B1 and non‐B1 when compared to healthy controls (Fig. 3b). As shown for CD66b, CD64 expression was significantly up‐regulated in CD mucosa when compared to blood, independently of inflammation status (Fig. 3b). In contrast to CD66b, CD64 expression was also elevated in colon relative to blood in healthy controls and, unexpectedly, colonic neutrophils expressed significantly less CD64 in active CD patients when compared to healthy individuals (Fig. 3b). Furthermore, CD64 and CD66b were co‐expressed and up‐regulated on neutrophils when traveling from blood to inflamed colon (Fig. 3c). In active CD, circulating neutrophils were detected as CD64highCD66blow and colonic as CD64highCD66bhigh cells, while in healthy subjects and patients with complete mucosal healing, neutrophils were CD64lowCD66blow in blood and CD64highCD66blow in colon.
Figure 3.
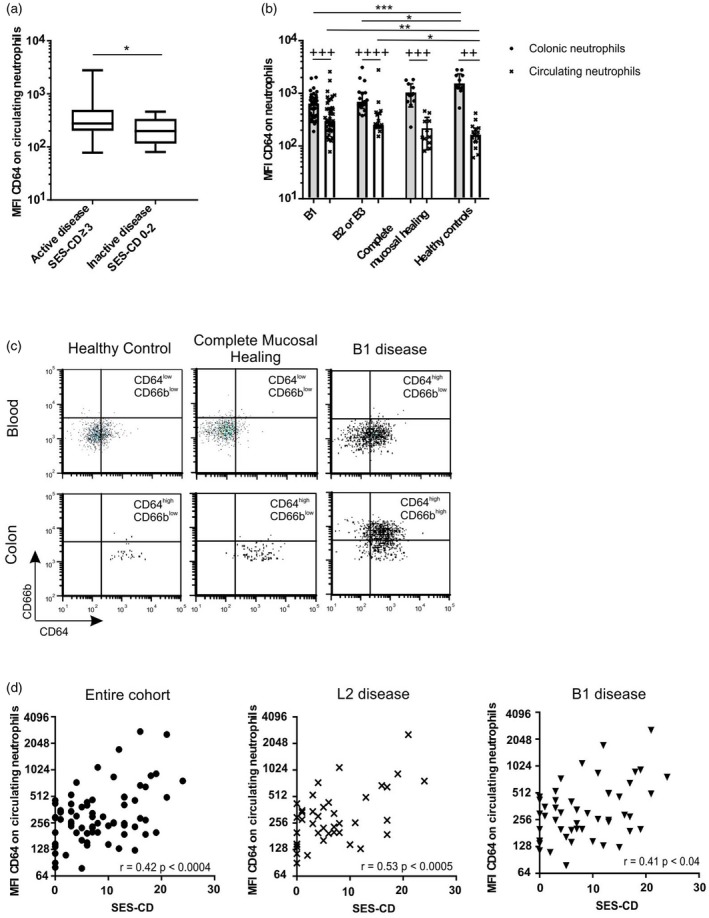
CD64 expression on circulating neutrophils correlates with Simple Endoscopic Score for Crohn’s Disease (SES‐CD). CD64 mean fluorescence intensity (MFI) on neutrophils (a) in blood of patients with active and inactive disease (Mann–Whitney U‐test), (b) in colon and blood of individuals with B1 versus non‐B1 disease, complete mucosal healing (n = 12) and healthy controls (healthy controls: colon samples: n = 10, blood samples n = 16) (Kruskal–Wallis followed by Dunn’s test between B1, B2 or B3, and healthy control groups (*), Wilcoxon matched‐pairs signed‐rank test for colonic versus circulating neutrophils (+) (healthy controls n = 10 paired samples). (c) Dot‐plot representing CD64 and CD66b co‐expression after gating on purified circulating and colonic neutrophils. (d) Correlations between CD64 MFI on circulating neutrophils and SES‐CD among the entire cohort (n = 73), exclusive colonic disease ‘L2’ (n = 42), B1 disease (n = 52) (Pearson’s correlation coefficient) *P < 0·05; **++P < 0·01; ***+++P < 0·001; ****++++P < 0·0001.
Finally, CD64 expression on circulating neutrophils correlated with SES‐CD in the entire cohort (r = 0·42, P < 0·0004), individuals with exclusive colonic involvement (L2 disease) (r = 0·53, P < 0·0005) and in patients with B1 disease (r = 0·41, P < 0·04) (Fig. 3d), a correlation which was maintained in individuals with endoscopic active B1 disease (Supporting information, Fig. S4c). However, no correlation was observed between CD64 expression and SES‐CD on circulating neutrophils in patients with ileocolonic (L3) disease (Supporting information, Fig. S4c).
Notably, as the activation of neutrophils correlated with SES‐CD, we asked whether a similar correlation could be observed for eosinophils. Both in the blood and mucosa, the expression of either CD66b or CD64 on eosinophils did not correlate with endoscopic severity (Supporting information, Table S1).
Collectively, neutrophil activation increased in inflamed colon (increased CD66b expression) and blood (increased CD64 expression) of CD patients with endoscopic active disease, and correlated with endoscopic disease severity.
Combined expression of activation markers on mucosal and circulating neutrophils discriminates disease activity and severity
Complete mucosal healing being a predictor of sustained steroid‐free remission 15, we therefore evaluated the performance of neutrophil activation status in distinguishing patients with strictly non‐inflamed mucosa (complete mucosal healing, SES‐CD = 0) from individuals with inflamed mucosa (SES‐CD ≥ 1). The latter subgroup included the four patients with SES‐CD = 1–2 who were considered in endoscopic remission but showed signs of inflammation. Examination of the subgroup with B1 disease as well as the entire cohort (Fig. 4a and Supporting information, Fig. S5a) showed that mucosal CD66b expression was superior to circulating CD64 expression on neutrophils in distinguishing complete mucosal healing from macroscopic mucosal inflammation. The performance of the activation status of mucosal neutrophils was evaluated next to discriminate between CD patients with severe disease (SES‐CD ≥ 16) or mild–moderate disease (SES‐CD = 3–15), as severe endoscopic disease could be a predictor of poor prognosis 26. As mucosal CD66b expression inconsistently discriminated disease severity in CD patients (Fig. 4b and Supporting information, Fig. S5b), we designed a ‘neutrophil activation power index’ (MFI of mucosal CD66b X circulating CD64) that reflects neutrophil activation in blood and colon. This power index correlated positively with SES‐CD in all subgroups examined (Table 2), but its performance was not superior to mucosal CD66b expression. Nonetheless, the index was significantly different between patients with severe and mild endoscopic disease, more particularly in the B1 disease subgroup (Fig. 4b right panels, Supporting information, Fig. S5b).
Figure 4.
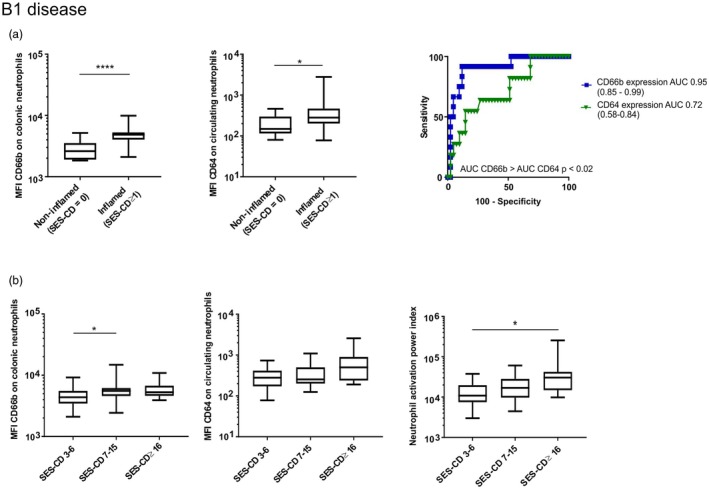
Expression of activation markers on mucosal and circulating neutrophils discriminates disease activity and severity in Crohn’s disease (CD) patients with B1 disease. (a) CD66b mean fluorescence intensity (MFI) on colonic neutrophils (left panel) and CD64 MFI on peripheral blood neutrophils (middle panel) according to the presence of complete mucosal healing [Simple Endoscopic Score for Crohn’s Disease (SES‐CD) ≥ 1: n = 43, SES‐CD = 0: n =12) (Mann–Whitney U‐test)]. Performance of each parameter to discriminate complete mucosal healing from individuals with SES‐CD ≥ 1 area under the curve (AUC) and their exact binomial confidence interval are presented as well as pairwise comparison of ROC curves (DeLong’s test) (right panel). (b) CD66b MFI on colonic neutrophils (left panel), CD64 MFI on circulating neutrophils (middle panel) and neutrophil activation power index (right panel) according to endoscopic disease severity in the subgroup with B1 disease (n = 40) (Kruskal–Wallis followed by Dunn’s test). *P < 0·05; **P < 0·01; ****P < 0·0001.
Table 2.
Correlations between neutrophils activation and SES‐CD
| % colonic neutrophils | MFI CD64 on circulating neutrophils | MFI CD66b on colonic neutrophils | Neutrophil activation power index | |
|---|---|---|---|---|
| Cohorta n = 73 | r = 0·26 P < 0·03 | r = 0·42 P < 0·0004 | r = 0·40 P < 0·0006 | r = 0·42 P < 0·0004 |
| B1a n = 52 | r = 0·45 P < 0·001 | r = 0·41 P < 0·04 | r = 0·55 P < 0·0001 | r = 0·42 P < 0·003 |
| B2B3b n = 21 | r = 0·47 P < 0·04 | r = 0·36 | r = 0·39 P = 0·08 | r = 0·54 P < 0·02 |
| Colonic (L2)a n = 42 | r = 0·38 P < 0·02 | r = 0·53 P < 0·0005 | r = 0·43 P < 0·005 | r = 0·50 P < 0·002 |
| Ileocolonic (L3)a n = 31 | r = 0·14 | r = 0·29 | r = 0·38 P < 0·04 | r = 0·32 P = 0·08 |
Pearson’s correlation coefficient.
Spearman’s rank correlation coefficient. SES‐CD = Simple Endoscopic Score for Crohn’s Disease; MFI = mean fluorescence intensity.
Collectively, these observations provide evidence that, despite the pathognomonic discontinuous mucosal lesions in CD, the activation of neutrophils correlated with endoscopic severity in inflamed mucosa.
Discussion
Neutrophils are detected in the inflamed mucosa of CD patients 20. However, how the frequency or activation of these cells correlated with disease severity remains unclear. We showed here that frequencies of neutrophils increased in inflamed colon and correlated significantly with SES‐CD. We further established that neutrophils up‐regulated CD66b expression in inflamed CD mucosa, while CD64 expression was augmented on circulating neutrophils in CD patients with endoscopic active disease relative to control groups. We next demonstrated that SES‐CD correlated with neutrophil CD66b expression in mucosa and, conversely, with neutrophil CD64 expression in blood in CD patients. We thus propose that recruitment of activated neutrophils in inflamed mucosa is associated with disease severity in Crohn’s disease.
SES‐CD was preferred to the Crohn’s disease index of severity (CDEIS) as it is validated, correlates well with CDEIS and seems to be even more reliable in evaluating changes in endoscopic severity 14, 27. HBI was selected to assess the clinical score because it is more practical to use, while correlating well with the Crohn’s disease activity index (CDAI) 12. However, in agreement with other studies 22, 23, 24, we did not observe a significant correlation between clinical and endoscopic severity. This study included a cohort of CD patients who underwent a thorough clinical evaluation to confirm diagnosis, disease activity or remission, and excluded any patient with suspicion of infection. It should be emphasized that SES‐CD was performed prospectively most of the time by the same gastroenterologist (AT), but that our cohort only included ileocolonic and colonic disease, as endoscopic scoring of exclusive ileal disease might vary in relation to the length of segment examined.
The frequencies of neutrophils were augmented in the inflamed mucosa, corroborating the neutrophil infiltration described previously in histology 20. Our study further showed an association between histological score and neutrophil activation at the single‐cell level. A correlation between frequencies of neutrophils and endoscopic severity was reported in ulcerative colitis 4 and was proposed in Crohn’s disease using quantification of fecal calprotectin or Indium‐111 scanning 28, 29. However, apparently contradictory studies suggest that CD may result from a defect in neutrophil recruitment, and that recruited neutrophils are either dysfunctional or display proinflammatory functions 18, 30, 31, 32.
Notably, among other granulocytes, basophils but not mast cells or eosinophils infiltrated the inflamed mucosa, more particularly in patients with B1 phenotype. The depletion of mast cells observed in the mucosa of patients with B2 and B3 disease could reflect the decrease in basophilic infiltrate in this subgroup of patients, acknowledging that mast cells may recruit basophils in the tissue through the release of histamine and prostaglandin D2 33.
We examined CD66b and CD64 expression, as these activation markers could be key elements in the bactericidal and cytolytic function of neutrophils. Although CD11b and CD62L are preferentially implicated in neutrophils recruitment 34, shedding of CD62L upon neutrophil activation 35 renders this marker less suitable to evaluate neutrophils’ degree of activation when compared to other surface antigens. Furthermore, CD66b is considered as a marker of activation for neutrophils in inflammatory conditions, but might also reflect neutrophil dysfunction 10, 36, 37. To our knowledge, we are the first to report a correlation between CD66b expression on mucosal neutrophils and endoscopic severity. This suggests increased interactions between neutrophils and bacterial products or, perhaps, increased local stimulation by granulocyte–macrophage colony‐stimulating factor (GM‐CSF) produced by macrophages 38, pathogenic T helper type 17 (Th17) 32, innate lymphoid cells type 3 (ILC3) 39, fibroblasts 40 and epithelial cells in inflamed CD mucosa 41. CD66b has been reported to play a role in neutrophil adhesion and superoxide production, as well as release of preformed IL‐8 in neutrophils 6, 42, 43. However, its contribution in the pathogenesis of Crohn’s disease has not been studied, except for a potential cross‐talk between CD66b expressing neutrophils and Th17 immunity 32. Thus, further investigations are warranted to assess the potential function of increased CD66b expression on mucosal neutrophils in patients with severe endoscopic disease.
CD64 is the high‐affinity Fc receptor for IgG expressed not only on neutrophils, but also on macrophages and monocytes 44. Its expression is triggered mainly by interferon (IFN)‐γ [produced by Th145, pathogenic Th17/Th146 and natural killer (NK) cells47 in inflamed CD colon], and stimulates phagocytosis and oxidative burst 7, 8. CD64 serves as a biomarker to distinguish infections from inflammatory conditions 48. Indeed, CD64 expression on circulating neutrophils is not specific to CD, as it might increase during any infectious process, including perianal or intra‐abdominal abscesses 49. Furthermore, this expression is modulated according to ethnicity and anti‐TNF response 8, 50, 51. Nonetheless, its expression level in blood, assessed by flow cytometry, correlated with clinical activity and C‐reactive protein in an adult IBD cohort 48, and more recently with SES‐CD in a pediatric cohort of CD patients 9, 52.
The present study further showed a correlation between CD64 expression on circulating neutrophils and SES‐CD in adult CD patients with endoscopic active disease only, illustrating a specific relation between levels of CD64 expression on circulating neutrophils and the extent of mucosal lesions in the small and large intestine. As CD64 may be expressed on immune cell types other than neutrophils 53, morphological studies of highly purified cells from inflamed colon were valuable to ascertain that our gating strategy was restricted to the detection of neutrophils and not eosinophils. Notably, CD64 expression was elevated in mucosa relative to blood neutrophils in both CD and healthy individuals and, unexpectedly, higher expression was detected in mucosa of healthy subjects when compared to CD patients. To our knowledge, no previous studies have quantified baseline CD64 expression on colonic neutrophils at homeostasis. We propose two non‐mutually exclusive explanations for this intriguing observation: (1) CD64 expression may reflect baseline neutrophil activation in healthy mucosa, while in CD colon neutrophils display a relative resistance towards mediators inducing CD64 expression, such as IFN‐γ and G‐CSF to the benefit of CD66b expression, corroborating previous observations of dysfunctional neutrophils 30, 54, 55; and (2) high levels of CD64 expression are not associated with increased numbers of receptor per cell but with increased avidity of the receptor for the antibody used for its detection in healthy mucosa (inside‐out signaling) 56. Notably, the frequency of CD45+ cells and accordingly the number of colonic neutrophils analyzed in the healthy control group, was low, which could lead to some technical issues for the detection of CD64 expression on neutrophils. However, that only CD64 and not CD66b expression significantly increased on neutrophils in the mucosa of healthy controls when compared to active CD patients weakens this hypothesis.
Most importantly, our data established a correlation between disease severity and CD64 expression on circulating but not mucosal neutrophils in patients with endoscopic active disease, and vice versa for CD66b expression. These observations provide further evidence that analysis of mucosal neutrophils did not include some blood neutrophils that might have contaminated the biopsy at the time of collection. Moreover, as reported in previous pediatric studies 9, 52, here the inflammatory burden was exclusively assessed through ileocolonoscopy. Consequently, the presence of upper GI tract or extensive small bowel involvement was not taken into account in quantifying overall disease severity, and might underscore the weak correlation observed between CD64 expression on circulating neutrophils and SES‐CD.
When compared to the performance of CD64 on circulating neutrophils, mucosal CD66b expression on colonic neutrophils was superior in discriminating strict absence from signs of mucosal inflammation, indicating that mucosal when compared to blood evaluation appeared to reflect disease activity more clearly. The combination of circulating and colonic neutrophil activation status using a power index discriminated between severe and mild disease. Thus, this index might offer a sensitive and specific approach to evaluate CD disease severity, which needs to be examined in large cohorts of CD patients for further validation. In this context, correlating this power index with tools assessing the entire gastrointestinal tract, such as the Lemann Index 57 or multiple aspects of disease severity, such as the recent ‘Crohn’s disease overall disease severity index’, would be of great interest 58.
Collectively, we showed that the frequency and activation of colonic neutrophils correlated with SES‐CD. Increased expression of activation markers on neutrophils, CD66b in colon and CD64 in blood correlated with endoscopic severity and more particularly in patients with B1 disease. A neutrophilic activation power index was designed to reflect mucosal inflammation and endoscopic severity, thus allowing distinction between complete mucosal healing and macroscopic inflammation, and severe from mild to moderate endoscopic disease. However, the present report exclusively examined colonic granulocyte profile in patients with L2 and L3 disease. Future studies are warranted to evaluate frequency and activation of granulocytes in relation to disease severity in adult CD patients with exclusive ileal (L1) disease. Taken together, we propose that recruitment of activated neutrophils in the inflamed colon is associated with disease severity in Crohn’s disease. However, their precise role in disease pathogenesis requires further investigation.
Disclosures
The authors disclose no conflicts of interest.
Supporting information
Fig. S1. Assessment of disease severity. (a) Patient distribution according to clinical and endoscopic activity scores (n = 73). HBI remission: < 5, mild disease: 5‐7, moderate disease: 8‐16, severe disease: > 16. SES‐CD: remission 0‐2, mild disease: 3‐6 moderate disease: 7‐15 severe disease: ≥ 16. (b) Correlation between HBI and SES‐CD among patients with active disease according to HBI (n = 23) (Spearman’s rank correlation coefficient).
Fig. S2. Frequencies of colonic and circulating neutrophils. (a) Frequencies of colonic CD45+ cells according to disease activity (active disease n = 57, complete mucosal healing n = 12 and healthy controls n = 10). Kruskal‐Wallis test followed by Dunn’s test. (b) Frequencies of colonic neutrophils according to treatment among patients with endoscopic active disease. (c) Frequencies of circulating neutrophils according to disease behaviour left panel and according to treatment (among patients with endoscopic active disease) right panel. Kruskal‐Wallis test was used to determine any statistical difference between groups. (d) Correlations between mucosal neutrophil frequencies and SES‐CD, in endoscopic active non stricturing non penetrating disease (B1) (n = 39), (Pearson correlation coefficient). **P < 0·01.
Fig. S3. Mucosal basophils infiltration correlated with SES‐CD. Frequencies of mucosal basophils among (a) patients with active or inactive disease (Mann Whitney U test) and (b) subgroups according to disease behaviour (Kruskal‐Wallis test followed by Dunn’s test). (c) Correlation between the frequency of mucosal basophils and SES‐CD (n = 73, Pearson correlation coefficient). Frequencies of colonic mast cells among (d) patients with active or inactive disease (Mann Whitney U test), and (e) subgroups according to disease behaviour (Kruskal‐Wallis test followed by Dunn’s test) *P < 0·05 ***P < 0·001.
Fig. S4. CD66b and CD64 expression on colonic and circulating neutrophils. (a) CD66b MFI on colonic neutrophils and CD64 MFI on circulating neutrophils according to treatment groups among patients with endoscopic active disease (Kruskal‐Wallis test). (b) Correlation between CD66b on colonic neutrophils and SES‐CD among individuals with ileocolonic disease “L3” (n = 31) and patients with endoscopic active B1 disease (n = 39), (Pearson correlation coefficient) (c) Correlations between CD64 MFI on circulating neutrophils and SES‐CD among patients with ileocolonic disease “L3” (n = 31) and endoscopic active B1 disease (n = 39), (Pearson correlation coefficient).
Fig. S5. Expression of activation markers on mucosal and circulating neutrophils discriminates disease activity and severity in the entire cohort of CD patients. (a) CD66b MFI on colonic mucosa neutrophils (left panel) and CD64 MFI on peripheral blood neutrophils (middle panel) according to the presence of complete mucosal healing (SES‐CD ≥ 1: n = 61, SES‐CD = 0: n = 12) (Mann‐Whitney U test). Performance of each parameter to discriminate complete mucosal healing from individuals with SES‐CD ≥ 1 AUC and their exact binomial confidence interval are presented as well as pairwise comparison of ROC curves (DeLong’s test) (right panel). (b) CD66b MFI on colonic neutrophils (left panel), CD64 MFI on circulating neutrophils (middle panel) and neutrophil activation power index (right panel) according to endoscopic disease severity (Kruskal‐Wallis followed by Dunn’s test) *P < 0·05 **P < 0·01 ****P < 0·0001.
Acknowledgments
The authors would like to thank all CD and healthy patients who kindly gave samples, as well as the nurses and gastroenterology medical staff from the Division of Gastroenterology at Centre Hospitalier de l’Université de Montréal. Special thanks to Heena Mehta for proofreading the manuscript and insightful discussions. This work was supported by Canadian Institutes of Health Research (CIHR) MOP – 130533 awarded to M. S. A. T. received a CIHR Canada Graduate Scholarship Banting and Best Master’s degree award and Phase 1 award from Fonds de Recherche Santé Québec (FRQS) Programme FRQS/MSSS de formation pour médecins résidents en médecine spécialisée visant une carrière en recherche. L. C. and M. Bs. received Doctoral training awards from FRQS.
References
- 1. Ng SC, Shi HY, Hamidi N et al Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population‐based studies. Lancet 2018;390:2769–78. [DOI] [PubMed] [Google Scholar]
- 2. Magro F, Langner C, Driessen A et al European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis 2013;7:827–51. [DOI] [PubMed] [Google Scholar]
- 3. Wera O, Lancellotti P, Oury C. The dual role of neutrophils in inflammatory bowel diseases. J Clin Med 2016;5:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lemmens B, Arijs I, Van Assche G et al Correlation between the endoscopic and histologic score in assessing the activity of ulcerative colitis. Inflamm Bowel Dis 2013;19:1194–201. [DOI] [PubMed] [Google Scholar]
- 5. Yoon J, Terada A, Kita H. CD66b regulates adhesion and activation of human eosinophils. J Immunol 2007;179:8454–62. [DOI] [PubMed] [Google Scholar]
- 6. Skubitz KM, Campbell KD, Skubitz AP. CD66a, CD66b, CD66c, and CD66d each independently stimulate neutrophils. J Leukoc Biol 1996;60:106–17. [DOI] [PubMed] [Google Scholar]
- 7. Hoffmann JJ. Neutrophil CD64: a diagnostic marker for infection and sepsis. Clin Chem Lab Med 2009;47:903–16. [DOI] [PubMed] [Google Scholar]
- 8. Hoffmeyer F, Witte K, Schmidt RE. The high‐affinity Fc gamma RI on PMN: regulation of expression and signal transduction. Immunology 1997;92:544–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Minar P, Haberman Y, Jurickova I et al Utility of neutrophil Fcgamma receptor I (CD64) index as a biomarker for mucosal inflammation in pediatric Crohn’s disease. Inflamm Bowel Dis 2014;20:1037–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lampinen M, Backman M, Winqvist O et al Different regulation of eosinophil activity in Crohn’s disease compared with ulcerative colitis. J Leukoc Biol 2008;84:1392–9. [DOI] [PubMed] [Google Scholar]
- 11. Annese V, Daperno M, Rutter MD et al European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis 2013;7:982–1018. [DOI] [PubMed] [Google Scholar]
- 12. Vermeire S, Schreiber S, Sandborn WJ, Dubois C, Rutgeerts P. Correlation between the Crohn’s disease activity and Harvey–Bradshaw indices in assessing Crohn’s disease severity. Clin Gastroenterol Hepatol 2010;8:357–63. [DOI] [PubMed] [Google Scholar]
- 13. Daperno M, D’Haens G, Van Assche G et al Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES‐CD. Gastrointest Endosc 2004;60:505–12. [DOI] [PubMed] [Google Scholar]
- 14. Vuitton L, Marteau P, Sandborn WJ et al IOIBD technical review on endoscopic indices for Crohn’s disease clinical trials. Gut 2016;65:1447–55. [DOI] [PubMed] [Google Scholar]
- 15. Baert F, Moortgat L, Van Assche G et al Mucosal healing predicts sustained clinical remission in patients with early‐stage Crohn’s disease. Gastroenterology 2010;138:463–8; quiz e10–1. [DOI] [PubMed] [Google Scholar]
- 16. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006;55:749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baba N, Van VQ, Wakahara K et al CD47 fusion protein targets CD172a+ cells in Crohn’s disease and dampens the production of IL‐1beta and TNF. J Exp Med 2013;210:1251–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kvedaraite E, Lourda M, Idestrom M et al Tissue‐infiltrating neutrophils represent the main source of IL‐23 in the colon of patients with IBD. Gut 2016;65:1632–41. [DOI] [PubMed] [Google Scholar]
- 19. Wakahara K, Baba N, Van VQ et al Human basophils interact with memory T cells to augment Th17 responses. Blood 2012;120:4761–71. [DOI] [PubMed] [Google Scholar]
- 20. D’Haens GR, Geboes K, Peeters M et al. Early lesions of recurrent Crohn’s disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology 1998;114:262–7. [DOI] [PubMed] [Google Scholar]
- 21. Laharie D, Reffet A, Belleannee G et al Mucosal healing with methotrexate in Crohn’s disease: a prospective comparative study with azathioprine and infliximab. Aliment Pharmacol Ther 2011;33:714–21. [DOI] [PubMed] [Google Scholar]
- 22. Jones J, Loftus EV Jr, Panaccione R et al Relationships between disease activity and serum and fecal biomarkers in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2008;6:1218–24. [DOI] [PubMed] [Google Scholar]
- 23. Khanna R, Nelson SA, Feagan BG et al Endoscopic scoring indices for evaluation of disease activity in Crohn’s disease. Cochrane Database Syst Rev 2016;8:CD010642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peyrin‐Biroulet L, Reinisch W, Colombel JF et al Clinical disease activity, C‐reactive protein normalisation and mucosal healing in Crohn’s disease in the SONIC trial. Gut 2014;63:88–95. [DOI] [PubMed] [Google Scholar]
- 25. Chapuy L, Bsat M, Mehta H et al Basophils increase in Crohn disease and ulcerative colitis and favor mesenteric lymph node memory TH17/TH1 response. J Allergy Clin Immunol 2014;134:978–81 e1. [DOI] [PubMed] [Google Scholar]
- 26. Ordas I, Feagan BG, Sandborn WJ. Early use of immunosuppressives or TNF antagonists for the treatment of Crohn's disease: time for a change. Gut 2011;60:1754–63. [DOI] [PubMed] [Google Scholar]
- 27. Khanna R, Zou G, Stitt L et al Responsiveness of endoscopic indices of disease activity for Crohn’s disease. Am J Gastroenterol 2017;112:1584–92. [DOI] [PubMed] [Google Scholar]
- 28. Sipponen T, Karkkainen P, Savilahti E et al Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn’s disease and histological findings. Aliment Pharmacol Ther 2008;28:1221–9. [DOI] [PubMed] [Google Scholar]
- 29. Saverymuttu SH, Camilleri M, Rees H, Lavender JP, Hodgson HJ, Chadwick VS. Indium 111‐granulocyte scanning in the assessment of disease extent and disease activity in inflammatory bowel disease. A comparison with colonoscopy, histology, and fecal indium 111‐granulocyte excretion. Gastroenterology 1986;90(5 Pt 1):1121–8. [DOI] [PubMed] [Google Scholar]
- 30. Marks DJ, Harbord MW, MacAllister R et al Defective acute inflammation in Crohn’s disease: a clinical investigation. Lancet 2006;367:668–78. [DOI] [PubMed] [Google Scholar]
- 31. Smith AM, Rahman FZ, Hayee B et al Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn’s disease. J Exp Med 2009;206:1883–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pelletier M, Maggi L, Micheletti A et al Evidence for a cross‐talk between human neutrophils and Th17 cells. Blood 2010;115:335–43. [DOI] [PubMed] [Google Scholar]
- 33. Sarfati M, Wakahara K, Chapuy L, Delespesse G. Mutual interaction of basophils and T cells in chronic inflammatory diseases. Front Immunol 2015;6:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013;13:159–75. [DOI] [PubMed] [Google Scholar]
- 35. Ivetic A. A head‐to‐tail view of L‐selectin and its impact on neutrophil behaviour. Cell Tissue Res 2018;371:437–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Torsteinsdottir I, Arvidson NG, Hallgren R, Hakansson L. Enhanced expression of integrins and CD66b on peripheral blood neutrophils and eosinophils in patients with rheumatoid arthritis, and the effect of glucocorticoids. Scand J Immunol 1999;50:433–9. [DOI] [PubMed] [Google Scholar]
- 37. Schmidt T, Brodesser A, Schnitzler N et al CD66b overexpression and loss of C5a receptors as surface markers for Staphylococcus aureus‐induced neutrophil dysfunction. PLOS ONE 2015;10:e0132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hamilton JA. Colony‐stimulating factors in inflammation and autoimmunity. Nat Rev Immunol 2008;8:533–44. [DOI] [PubMed] [Google Scholar]
- 39. Geremia A, Arancibia‐Carcamo CV. Innate lymphoid cells in intestinal inflammation. Front Immunol 2017;8:1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Egea L, Hirata Y, Kagnoff MF. GM‐CSF: a role in immune and inflammatory reactions in the intestine. Exp Rev Gastroenterol Hepatol 2010;4:723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Noguchi M, Hiwatashi N, Liu ZX, Toyota T. Increased secretion of granulocyte–macrophage colony‐stimulating factor in mucosal lesions of inflammatory bowel disease. Digestion 2001;63(Suppl 1):32–6. [DOI] [PubMed] [Google Scholar]
- 42. Stocks SC, Ruchaud‐Sparagano MH, Kerr MA, Grunert F, Haslett C, Dransfield I. CD66: role in the regulation of neutrophil effector function. Eur J Immunol 1996;26:2924–32. [DOI] [PubMed] [Google Scholar]
- 43. Schroder AK, Uciechowski P, Fleischer D, Rink L. Crosslinking of CD66B on peripheral blood neutrophils mediates the release of interleukin‐8 from intracellular storage. Hum Immunol 2006;67:676–82. [DOI] [PubMed] [Google Scholar]
- 44. Buckle AM, Jayaram Y, Hogg N. Colony‐stimulating factors and interferon‐gamma differentially affect cell surface molecules shared by monocytes and neutrophils. Clin Exp Immunol 1990;81:339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol 2014;14:329–42. [DOI] [PubMed] [Google Scholar]
- 46. Brand S. Crohn’s disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s disease. Gut 2009;58:1152–67. [DOI] [PubMed] [Google Scholar]
- 47. Takayama T, Kamada N, Chinen H et al Imbalance of NKp44(+)NKp46(‐) and NKp44(‐)NKp46(+) natural killer cells in the intestinal mucosa of patients with Crohn’s disease. Gastroenterology 2010;139:882–92, 92 e1–3. [DOI] [PubMed] [Google Scholar]
- 48. Tillinger W, Jilch R, Jilma B et al Expression of the high‐affinity IgG receptor FcRI (CD64) in patients with inflammatory bowel disease: a new biomarker for gastroenterologic diagnostics. Am J Gastroenterol 2009;104:102–9. [DOI] [PubMed] [Google Scholar]
- 49. Davis BH, Olsen SH, Ahmad E, Bigelow NC. Neutrophil CD64 is an improved indicator of infection or sepsis in emergency department patients. Arch Pathol Lab Med 2006;130:654–61. [DOI] [PubMed] [Google Scholar]
- 50. Minar P, Jackson K, Tsai YT et al A low neutrophil CD64 Index is associated with sustained remission during infliximab maintenance therapy. Inflamm Bowel Dis 2016;22:2641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wojtal KA, Rogler G, Scharl M et al Fc gamma receptor CD64 modulates the inhibitory activity of infliximab. PLOS ONE 2012;7:e43361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Minar P, Jackson K, Tsai YT, Sucharew H, Rosen MJ, Denson LA. Validation of neutrophil cd64 blood biomarkers to detect mucosal inflammation in pediatric Crohn’s disease. Inflamm Bowel Dis 2017;24:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bournazos S, Wang TT, Ravetch JV. The role and function of fcgamma receptors on myeloid cells. Microbiol Spectr 2016;4 10.1128/microbiolspec.MCHD-0045-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hayee B, Rahman FZ, Tempero J et al The neutrophil respiratory burst and bacterial digestion in Crohn’s disease. Dig Dis Sci 2011;56:1482–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jurickova I, Collins MH, Chalk C et al Paediatric Crohn disease patients with stricturing behaviour exhibit ileal granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) autoantibody production and reduced neutrophil bacterial killing and GM‐CSF bioactivity. Clin Exp Immunol 2013;172:455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brandsma AM, Jacobino SR, Meyer S, ten Broeke T, Leusen JH. Fc receptor inside‐out signaling and possible impact on antibody therapy. Immunol Rev 2015;268:74–87. [DOI] [PubMed] [Google Scholar]
- 57. Pariente B, Mary JY, Danese S et al Development of the Lemann index to assess digestive tract damage in patients with Crohn’s disease. Gastroenterology 2015;148:52–63 e3. [DOI] [PubMed] [Google Scholar]
- 58. Siegel CA, Whitman CB, Spiegel BMR et al Development of an index to define overall disease severity in IBD. Gut 2018;67:244–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Assessment of disease severity. (a) Patient distribution according to clinical and endoscopic activity scores (n = 73). HBI remission: < 5, mild disease: 5‐7, moderate disease: 8‐16, severe disease: > 16. SES‐CD: remission 0‐2, mild disease: 3‐6 moderate disease: 7‐15 severe disease: ≥ 16. (b) Correlation between HBI and SES‐CD among patients with active disease according to HBI (n = 23) (Spearman’s rank correlation coefficient).
Fig. S2. Frequencies of colonic and circulating neutrophils. (a) Frequencies of colonic CD45+ cells according to disease activity (active disease n = 57, complete mucosal healing n = 12 and healthy controls n = 10). Kruskal‐Wallis test followed by Dunn’s test. (b) Frequencies of colonic neutrophils according to treatment among patients with endoscopic active disease. (c) Frequencies of circulating neutrophils according to disease behaviour left panel and according to treatment (among patients with endoscopic active disease) right panel. Kruskal‐Wallis test was used to determine any statistical difference between groups. (d) Correlations between mucosal neutrophil frequencies and SES‐CD, in endoscopic active non stricturing non penetrating disease (B1) (n = 39), (Pearson correlation coefficient). **P < 0·01.
Fig. S3. Mucosal basophils infiltration correlated with SES‐CD. Frequencies of mucosal basophils among (a) patients with active or inactive disease (Mann Whitney U test) and (b) subgroups according to disease behaviour (Kruskal‐Wallis test followed by Dunn’s test). (c) Correlation between the frequency of mucosal basophils and SES‐CD (n = 73, Pearson correlation coefficient). Frequencies of colonic mast cells among (d) patients with active or inactive disease (Mann Whitney U test), and (e) subgroups according to disease behaviour (Kruskal‐Wallis test followed by Dunn’s test) *P < 0·05 ***P < 0·001.
Fig. S4. CD66b and CD64 expression on colonic and circulating neutrophils. (a) CD66b MFI on colonic neutrophils and CD64 MFI on circulating neutrophils according to treatment groups among patients with endoscopic active disease (Kruskal‐Wallis test). (b) Correlation between CD66b on colonic neutrophils and SES‐CD among individuals with ileocolonic disease “L3” (n = 31) and patients with endoscopic active B1 disease (n = 39), (Pearson correlation coefficient) (c) Correlations between CD64 MFI on circulating neutrophils and SES‐CD among patients with ileocolonic disease “L3” (n = 31) and endoscopic active B1 disease (n = 39), (Pearson correlation coefficient).
Fig. S5. Expression of activation markers on mucosal and circulating neutrophils discriminates disease activity and severity in the entire cohort of CD patients. (a) CD66b MFI on colonic mucosa neutrophils (left panel) and CD64 MFI on peripheral blood neutrophils (middle panel) according to the presence of complete mucosal healing (SES‐CD ≥ 1: n = 61, SES‐CD = 0: n = 12) (Mann‐Whitney U test). Performance of each parameter to discriminate complete mucosal healing from individuals with SES‐CD ≥ 1 AUC and their exact binomial confidence interval are presented as well as pairwise comparison of ROC curves (DeLong’s test) (right panel). (b) CD66b MFI on colonic neutrophils (left panel), CD64 MFI on circulating neutrophils (middle panel) and neutrophil activation power index (right panel) according to endoscopic disease severity (Kruskal‐Wallis followed by Dunn’s test) *P < 0·05 **P < 0·01 ****P < 0·0001.


