Abstract
Cancer chemotherapy dose schedules are conventionally applied intermittently, with dose duration of the order of hours, intervals between doses of days or weeks, and cycles repeated for weeks. The large number of possible combinations of values of duration, interval, and lethality has been an impediment to empirically determine the optimal set of treatment conditions. The purpose of this project was to determine the set of parameters for duration, interval, and lethality that would be most effective for treating early colon cancer. An agent-based computer model that simulated cell proliferation kinetics in normal human colon crypts was calibrated with measurements of human biopsy specimens. Mutant cells were simulated as proliferating and forming an adenoma, or dying if treated with cytotoxic chemotherapy. Using a high-performance computer, a total of 28 800 different parameter sets of duration, interval, and lethality were simulated. The effect of each parameter set on the stability of colon crypts, the time to cure a crypt of mutant cells, and the accumulated dose was determined. Of the 28 800 parameter sets, 434 parameter sets were effective in curing the crypts of mutant cells before they could form an adenoma and allowed the crypt normal cell dynamics to recover to pretreatment levels. A group of 14 similar parameter sets produced a minimal time to cure mutant cells. A different group of nine similar parameter sets produced the least accumulated dose. These parameter sets may be considered as candidate dose schedules to guide clinical trials for early colon cancer.
Keywords: colon cancer, chemotherapy, dose schedules, mutants, agent-based model, computer simulation, high-performance computer
Introduction
Colorectal cancer starts with the abnormal proliferation of mutant cells in colonic crypts. The population of mutant cells may continue to increase and form an adenoma, an early stage of colon cancer.1 The purpose of this project is to determine optimal chemotherapy dose schedules that eliminate these mutant cells before they can form an adenoma, and also the system retains crypt function.
There were an estimated 135 430 new cases of colon cancer in the United States in 2017 and an estimated 50 260 deaths.2 These deaths indicate that in spite of stool-based screening3 and colonoscopic screening for adenomas, both of which do reduce the risk of fatal colorectal cancer,4 there is still a need for other methods of early detection. As new methods for detecting pre-malignant abnormalities come to fruition, possibly by blood tests,5 the next need will be to determine the optimal therapies for such early lesions.
To determine optimal chemotherapy dose schedules for early colon cancer, it is necessary to take into account the many possible values of three parameters, the duration of the dose, the interval between doses, and the lethal effect of the dose on mutant cells and on normal cells in the crypt. Values of these three parameters may vary over a wide range. For instance, the value of duration may be from hours to days, the interval between doses may be from days to weeks, and the lethality may kill 5% to 95% of mutant cells at each treatment cycle. Because of the large possible range of values of each of the three parameters, it has not been possible to instantiate a clinical trial that would compare all of the very many possible different conditions. However, computer simulations can be used to explore many dose schedules.
For the results of computer simulations to be reliable for finding optimal conditions, two criteria need to be satisfied. The model must be realistic, and the computer system must be able to explore a large number of different parameter sets.
We previously described a realistic computer model of cell dynamics in human colon crypts.6,7 The model uses the concept of agent-based modeling, where each biological cell is represented as an agent that has specified properties and interacts with other agents and with its environment. It has the advantage of producing emergent behaviors derived from each type of agent, such as stem cells, proliferating cells, differentiated cells, and rapidly dividing mutant cells. Then the emergent properties of the system of agents (biological cells in a crypt) in different environments (cytotoxic chemotherapy) could be generated in computer simulations. The model was calibrated with measurements of cell types in human biopsy specimens. The emergent behavior of the model reproduced several properties observed in biological crypts, including the ability of normal cell dynamics to recover from perturbations such as cytotoxic drug treatments that will eliminate mutant cells.
The second criterion for the results of computer simulations to be reliable is that the computer system needs to be able to explore a large number of different parameter sets. The original colon crypt model was written in the NetLogo application. The code for this model in this application was sufficient to simulate a few parameter sets but not adequate to explore a large number of parameter sets necessary to determine which are optimum.8 To explore a large number of different chemotherapy dose schedules (duration, interval, and lethality), the NetLogo code was ported to C++, and simulations were run on high-performance computing platforms (Edison at NERSC and Beagle at the Computation Institute—University of Chicago). Such simulations were previously successful in exploring the large behavioral landscape of a different agent-based model.9,10
The goal of this project was to explore the effect of many different dose schedules for their ability to eliminate mutant cells in an adenomatous colon crypt, and to determine a subset of dose schedules that are optimum. Each dose schedule had different set of parameter values for duration, interval, and lethality. The criterion of an optimum dose schedule is either one that eliminates mutant cells in the shortest time or one that has the least accumulated dose, and that retains normal crypt function.
We report on the results of 28 800 different simulated dose schedules. We found 14 dose schedules with the shortest time to eliminate mutants from an adenomatous crypt and nine dose schedules with the least accumulated dose.
Methods
Cytotoxic chemotherapy can kill dividing mutant cells but may also kill dividing normal cells. Intermittent chemotherapy dose schedules were sought that would kill all mutant cells but allow normal cell dynamics to recover between doses and retain crypt function.
The logic for intermittent dose schedules is the following. Normal cell types in the crypt include quiescent stem cells at the bottom of the crypt, proliferating cells near the bottom third, and differentiated cells in the top two-thirds. Cells move from the region of proliferating cells up to the region of differentiated cells at the top and are removed from the crypt. A dose of a cytotoxic drug will kill proliferating cells but spare quiescent stem cells. Quiescent stem cells are resistant to cytotoxic drugs because they have a very low probability of dividing. As the normal proliferating cells are killed, some quiescent stem cells become activated and produce additional proliferating cells. If the cytotoxic dose is of short duration, the number of proliferating cells, and their differentiated progeny, will increase to the pretreatment levels. Normal cell homeostasis with quasi-stationary cell dynamics will be restored, and the crypt will recover.
An agent-based colon crypt model, written in the application NetLogo, was previously described in detail.6 The model assumes that each cell’s probability to proliferate or to die is determined by its position in two gradients along the crypt axis: a divide gradient and a die gradient. A cell’s type, stem cell, proliferating cell, or differentiated cell is determined by its position in the divide gradient. A cell born near the bottom of the crypt moves up and is removed at the top, where it undergoes apoptosis. Mutant cells are those that divide and die with a higher (though distinct) probability, than normal cells at the same spatial position in the colonic crypt.
The NetLogo model was calibrated using measurements of the number and variation of each cell type in histological sections of normal human colon biopsies. The behavior of the model was verified by its ability to reproduce the number and variation of each cell type, to undergo crypt fission by neutral drift, to have mutants proliferate to fill the crypt and form an adenoma, and to be robust and recover from perturbation from cytotoxic agents.6,7
To efficiently explore the chemotherapeutic parameter space, the NetLogo crypt model6 (https://doi.org/doi:10.7282/T3KH0QKV) was ported to C++ (https://doi.org/doi:10.7282/T33X8B0H) using established methods.9,11 For cytotoxic chemotherapy, the duration of therapy was varied from 1 to 24 time-steps with increments of one step; the interval of therapy was varied from 2 to 48 steps with increments of two steps; and the lethality factor was varied from 1 to 50 in increments of one step. To account for the effects of randomness in the model, fifty stochastic replicates were performed for each of the 28 800 cytotoxic chemotherapeutic dose schedules.
In this context, the terms duration, interval, and lethality are defined as follows: duration of the dose is the time during which cells in a crypt are exposed to the cytotoxic agent. The interval between doses is the time from the beginning of one dose to the beginning of the next dose. Lethality is the factor that increases the probability that a cell will die above that determined by its position in the crypt. The Lethality factor affects both normal cells and mutant cells. Mutant cells, in initial simulations, had a 1.16 X probability of dividing than normal cells at the same position in the crypt and a 1.1 X probability of dying than normal cells at the same position in the crypt. Each specific treatment parameterization consisted of a dose schedule described by a parameter set of one value of duration, one value of interval, and one value of lethality.
Results
Effective dose schedules
The goal of this project is to determine an optimal set of dose schedules (parameter sets; consisting of dose duration, interval between successive doses, and dose lethality) that will kill mutant cells in the crypt before they can proliferate and form an adenoma and will allow restoration of the number of normal cells and recovery of crypt function.
Dose schedules were simulated that included combinations of 24 different values of duration, 24 different values of interval, and 50 different values of lethality, for a total of 28 800 parameter sets. Each set of parameters was simulated in 50 independent runs. The output of each parameter set included whether the crypt recovered and survived a time period of 1200 steps, and if so, the time to cure the crypt of all mutants. Of the 28 800 different parameter sets, 434 (1.5 %) were effective, that is, all mutant cells were eliminated, and crypts recovered in each of the 50 runs. Each of the 434 effective parameter sets and the outputs of average time to cure, coefficient of variation of average time to cure, and accumulated dose sum are given in the table available at https://doi.org/doi:10.7282/T3D79FX4.
Dose schedules with shortest time to cure
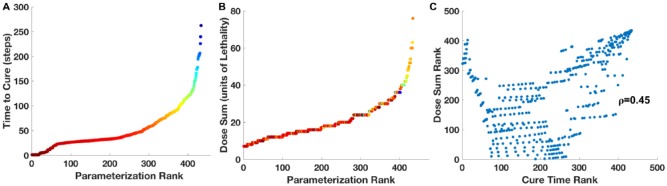
The average time to cure crypts of mutant cells was calculated over 50 stochastic replicates of each of the 434 effective dose schedules. There is a broad range of average cure times, from 2 time-steps to 263 time-steps (Figure 1A). One step equals approximately 4.5 human hours.6
Figure 1.
Distributions of average time to cure and sum dose of chemotherapy. (A): time, in simulation steps, to cure mutants versus the rank of each of 434 dose schedule parameter sets. (B): sum of lethal doses to cure mutants versus the rank of the each of the 434 dose schedule parameter sets. Points are color-coded according to the average time of 50 simulations that it takes to cure mutants, see Figure 3. (C): comparison of the ranks of parameter sets for the sum of doses and the ranks of the parameter sets for the time to cure. The ranks are not highly correlated (R2 = 0.45).
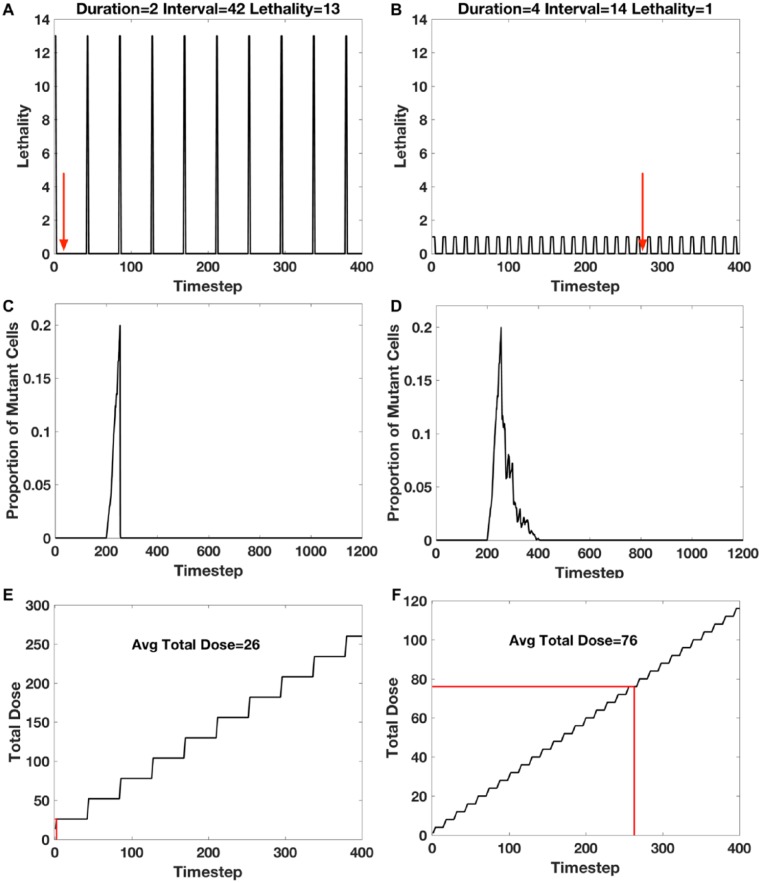
A total of 14 dose schedules had the shortest time to cure (two steps). One of these is shown in Figure 2A. These dose schedules each had a duration of two steps, had intervals of 42 to 48 steps, and lethality of 13 to 19. For comparison, the dose schedule that had the longest average cure time (263 steps) had a duration of 4, interval of 14, and lethality of 1 (Figure 2B). This indicates a range of interval/lethality combinations that have lethality sufficient to quickly kill all mutant cells but an interval that is sufficiently long for the crypt to recover before the subsequent dose, thus establishing a dynamic equilibrium between the proliferative effects of the cells comprising the crypt with the cytotoxic effects of chemotherapy.
Figure 2.
Time to cure and accumulated dose for different dose schedules. (A) and (B): dose schedules for the shortest time to cure (two time-steps), and the slowest time to cure (263 time-steps), with the indicated parameter set of duration, interval, and lethality. The red arrows indicate the average times to cure for 50 simulations. (C) and (D): example of one of 50 simulations of the kinetics of the proportion of mutants per crypt for the dose schedules shown in (A) and (B), respectively. (E) and (F): total dose accumulation as a function of time-steps for the dose schedules shown in (A) and (B), respectively. The average total dose at the time of cure is indicated by the red line.
The kinetics of the proportion of mutant cells per crypt as a function of time is shown for two example simulation runs, the shortest time to cure and the longest time to cure (Figure 2C and D, respectively). Therapy was started after the proportion of mutant cells per total cells in the crypt was greater than 0.2, and the time to cure the crypt of all mutant cells was determined. The dose schedule with the shortest time to cure could eliminate all mutant cells within two steps with only one dose, whereas the dose schedule with the longest time to cure required 20 successive intermittent doses to eliminate all mutants.
The decrease of the number of mutant cells as a function of time, and the recovery of the quasi-stationary number of each normal cell type in the crypt, can be seen in movies for the shortest time to cure (https://doi.org/doi:10.7282/T3RN3C79), and for the longest time to cure (https://doi.org/doi:10.7282/T3MW2MHK).
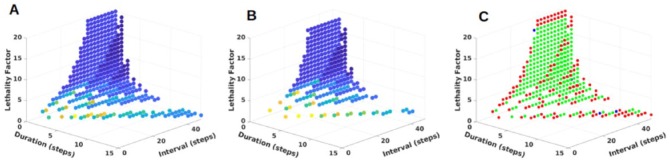
The 434 effective dose schedules can be visualized as a point in a three-dimensional (3D) space with axes of duration, interval, and lethality (Figure 3A). The time to cure for each dose schedule is represented by a point of a different color. The group of dose schedules with shortest average cure time appears at a cusp on the surface of the 3D distribution. The arrangement of the different dose schedules in the 3D space can also be visualized as the figure is rotated in a movie (https://doi.org/doi:10.7282/T3CC141S).
Figure 3.
Dose schedule parameter sets that cure mutants when chemotherapy is started at different times. Each point represents a dose schedule parameter set that yield a 100% mutant cure rate and 0% crypt mortality rate when tested over 50 stochastic replicates. The duration of chemotherapy (in simulation time-steps) is represented on the x-axis; the interval between doses (in simulation time-steps) is represented on the y-axis; cytotoxic lethality factor is represented on the z-axis. Points are color-coded based on the average time to cure a crypt of mutant cells, with red representing a treatment schedule that quickly eliminates the mutant cells and dark blue representing a treatment which takes longer to eliminate mutant cells. (A): successful parameter sets for chemotherapy that is initiated when mutant cells make up 20% of the total crypt population; (B): successful parameter sets for chemotherapy that is initiated when mutant cells make up 40% of the total crypt population. (C): comparison of parameter sets in A and B. Points shaded in red are those which are unique to the set of simulations that initiate chemotherapy when mutant cells compose 20% of the crypt; points shaded in blue are those which are unique to the set of simulations that initiate chemotherapy when mutant cells compose 40% of the crypt; points shaded in green are those which are shared between the set of simulations that initiate chemotherapy when mutant cells compose 20% of the crypt and the set of simulations that initiate chemotherapy when mutant cells compose 40% of the crypt.
Mutants can be cured in simulations with different initial conditions
The effective cure times afore mentioned were determined for therapy that was started when the number of mutant cells was 20% of the total number of cells in the crypt. The mutants were a homogeneous population of cells that had a 1.16 X probability of dividing and a 1.1 X probability of dying compared with non-mutant cells at the same position in the crypt. These values are not unique; cures can be achieved with other values. As examples, we give results with three different sets of values.
First, the treatments were started when the proportion of mutant cells per crypt was twice, that is, 40% rather than the 20% afore mentioned. Cures were also obtained when treatments were started at 40%. The distribution of cure times for doses started at 20% and 40% are shown in Figure 3A and B, respectively. The similarities and differences in the cure times are shown in Figure 3C.
Second, mutant cells were considered that grew half as fast, having half the probability of dividing, that is, 1.08 X rather than 1.16 X of that of normal cells. Cures were also obtained with the slower-growing mutant cells. Slower-growing mutants would be expected to be more resistant to cytotoxic chemotherapy than the faster-growing mutants. The average cure time of the slower-growing mutants was 17 steps rather than 2 steps of the faster-growing mutants.
Third, a heterogeneous population of mutant cells, rather than a homogeneous population, was considered. Biological crypts may be heterogeneous, containing different cells with different mutations and different drug responses.12 Cures were also obtained with a heterogeneous population of mutant cells. A heterogeneous combination of fast- and slow-growing mutants had an average cure time of 12 steps, rather than a cure time of 2 steps for the homogeneous population of faster-growing mutants.
Therefore, mutant cells can be cured under different initial conditions, for example, when treatment is started when there are different proportions of mutant cells, when the mutant cells are relatively fast or slow growing and when the population of mutant cells is homogeneous or heterogeneous.
Dose schedules with least treatment to cure
Repeated cycles of treatments (Figure 2A and B) result in the accumulation of doses over time (Figure 2E and F). Where each dose = lethality × duration, and the accumulated dose is the sum of doses up to the time that all mutants are cured. The least amount of accumulated dose needed to eliminate mutant cells may be considered as an alternative criterion for optimal therapy to the shortest time to eliminate mutant cells.
The distribution of accumulated doses to cure for each of the 434 effective treatment schedules is shown in Figure 1B. This distribution appears to be similar to the distribution of least time to cure (Figure 1A). However, the ranks of the parameter sets for accumulate doses to cure and ranks of the parameter sets for the least times to cure are not highly correlated (R2 = 0.45; Figure 1C).
It should be noted that the parameter set of the dose schedule with the least accumulated dose that cures mutants is not the same as the parameter set of the dose schedule with the sum of doses with the shortest time to cure (Figure 1C and Table 1). Therefore, the least accumulated dose to cure and the shortest time to cure are two independent criteria for optimal chemotherapy.
Table 1.
Comparison of schedules with shortest cure time and least treatment.
| Criterion | Average cure time | Dose sum | Duration | Interval | Lethality |
|---|---|---|---|---|---|
| Shortest time | 2 | 26 | 2 | 42 | 13 |
| Least treatment | 32 | 7 | 1 | 32 | 7 |
Note: where 1 time-step = 4.5 hours and dose = lethality × duration.
Discussion
In this project, many different intermittent dose schedules of a cytotoxic chemotherapeutic drug have been evaluated for their ability to eliminate mutant cells from a colon crypt before the mutants can fill the crypt and form adenoma. Eliminating mutant cells, while retaining crypt function, could intercept the progression of adenomas to adenocarcinomas.13,14
Each different dose schedule consisted of a different set of values of duration of the dose, interval between doses, and lethality of the dose. A high-performance computer was used to simulate the effect of 28 800 different sets of dose schedules. Of these, a subset of 434 dose schedules was effective, they eliminated all mutant cells and allowed crypt normal cell dynamics to recover from the lethal effects of the chemotherapy. The other dose schedules were not effective; although they eliminated mutant cells, they also killed most normal cells, resulting in total collapse of the crypt. Effective dose schedules were identified that produced the shortest time to cure all mutants from the crypt, and different effective dose schedules were identified that produced the least accumulated dose.
The ability of a subset of intermittent dose schedules to both eliminate mutant cells and retain normal cell crypt function was shown to be effective for a range of initial conditions, including the following: (1) drug treatment could be initiated when the mutants are 20% or 40% of the total number of cells in the crypt, (2) mutants could be eliminated that proliferated much faster than normal cells and are relatively sensitive to cytotoxic chemotherapy, and mutants could be eliminated that proliferate only slightly faster than normal cells and are relatively resistant to chemotherapy, and (3) the mutant populations may be homogeneous, or may be heterogeneous consisting of both fast and slow proliferating mutant cells.
Cell dynamics in the human normal and neoplastic crypts has been the subject of other modeling studies.15,16 This is partially due to the fact that cell dynamics have been well characterized experimentally.17 In a normal unperturbed crypt, homeostasis is maintained with a quasi-stationary number of total cells, and of each cell type. The average total number of cells has been measured in human colon crypts is 2427. The average number of quiescent stem cells, proliferating cells, and differentiated cells is 36,624, and 1768, respectively.6
Stem cells in the normal crypt may be quiescent or may become active18 and divide stochastically.19,20 Actively dividing stem cells yield proliferating cells, which in turn move up the crypt, differentiate, and are removed at the top.21 The rate of cell loss at the top of the crypt is balanced by the rate of stem cell division at the bottom of the crypt. This balance maintains homeostasis.22 The rate of stem cell divisions is controlled by the number of stem cells, by the number of proliferating cells, and indirectly by the number of differentiated cells.23
If a crypt is exposed to a brief low dose of a cytotoxic chemotherapeutic drug, some proliferating cells are killed, and the stem cells will respond by producing more proliferating cells. This results in restoration of homeostatic cell dynamics and recovery of crypt function. If mutant cells are also in the crypt, a longer or a more lethal dose may be required to kill the mutant cells before they can proliferate and fill the crypt to form an adenoma. However, such a longer or more lethal cytotoxic dose may overwhelm the ability of the stem cells to repopulate the crypt, and the crypt will collapse.
Cell dynamics in the colon crypt without therapy have been modeled mathematically24 and computationally6,25 and reviewed.26–28 Crypt dynamics with therapy has been modeled to determine a therapy that could eliminate mutant cancer cells but still allow recovery of normal crypt cell homeostasis. Optimal control theory for cancer therapy has been reviewed,29,30 and computer models for cancer therapy have been reviewed.31
Many of the published modeling reports provide a different perspective than the modeling results that we report. We focused our attention on an early stage of colon cancer initiated by abnormally proliferating mutant cells in a colon crypt that also had normal cells. Our agent-based computer model was calibrated with measurements of human biopsy specimens. We simulated the response to cytotoxic chemotherapy of both mutant cells and normal cells. By comparison, Panetta32 described a competition model with periodically pulsed chemotherapy and parameter values needed to prevent relapse. Gaffney33 mathematically modeled schedules with rest phases between chemotherapy and emphasized the importance of choosing the correct intervals between doses. Marcu and Bezak34 used Monte Carlo computer modeling to determine the conditions under which intermittent therapy may fail because some tumor cells repopulate the tumor between doses. Murano et al35 mathematically modeled normal crypt cell dynamics and concluded that stem cells and proliferating cells should react differently to therapy-induced apoptotic killing. Leder et al36 mathematically modeled radiation dose schedules and predicted that hyper-fractionated dosing schedules would be superior to hypo-fractionated dosing schedules. These results were confirmed for survival of irradiated mice with glioblastoma.
We have considered two kinds of optimal dose schedules, in each case allowing recovery of normal cell dynamics and crypt function. One kind of optimal schedule eliminates all mutant cells in the shortest time, and a second kind of optimal schedule eliminates all mutants with the least accumulated dose. A choice may be made between these two optimal schedules to reduce the collateral damage due to chemotherapy, such as neuropathy, cardio-toxicity, and neutropenia. Alternatively, rather than choosing one or the other, there are several parameter sets that have both intermediate times to cure and intermediate accumulated doses (Figure 2C). These parameter sets provide an opportunity to select a dose schedule treatment that is good, but not perfect, by each criterion.
Human clinical trials for chemotherapy of colon cancer have also used intermittent chemotherapy dose schedules. Many of these were designed to compare the efficacy and toxicity of intermittent dose schedules with constant treatment.37 These trials contrast with our simulations studies that were designed to compare the efficacy and toxicity of different intermittent dose schedules with each other, rather than with constant treatment. The human clinical trials varied in the intermittent strategy, including the duration of the treatment, and the interval when the treatment was restarted. Another difference between the clinical trials and our simulations was the evaluation of the outcome. The human clinical trials used tumor response and patient survival (overall or progression free), as well as toxicity, for metrics of outcome, whereas our simulations used removal of mutants from crypts (instead of tumor response) and recovery of crypts (as an indication of non-toxicity). Most intermittent trials used disease progression of individual patients to determine the interval between treatments, unlike our simulations that used fixed values for each set of dose schedules.
Two trials did use fixed values of treatment duration and interval time between treatments. Their treatment durations and intervals can be compared with those in our simulations. Van Cutsem et al38 used a treatment duration of 2 weeks and an interval between treatments of 1 week. Labianca et al39 used a treatment duration of 8 weeks and an interval between treatments of 8 weeks. In each trial, the outcome of the intermittent treatment was comparable to the outcome of constant treatment based on tumor response, survival, and toxicity. Our predicted optimum duration and interval to achieve the shortest time to eliminate all mutants was 9 hours (duration 2) and 8 days (interval 42), respectively; and the predicted optimum duration and interval to achieve the least accumulated dose to eliminate all mutants was 4.5 hours (duration 1) and 6 days (interval 32), respectively, as shown in Table 1. It is possible that improvements in intermittent therapy over constant therapy could be achieved using the treatment duration, interval between treatments, and lethality that we have predicted.
We acknowledge several limitations of this project. For instance, we have not taken into account that cytotoxic drugs may induce new mutant cells, including drug resistant mutants, as well as kill existing mutant cells. However, we have already modeled the induction of new mutant cells and shown that intermittent dose schedules can be effective in eliminating mutants that arise spontaneously or are induced by a cytotoxic drug.7 In addition, this project has not modeled chemotherapy dose schedules by a combination of two, or more, drugs. Such an extension will require considering duration, interval, and lethality of at least two drugs, and different orders of the different drugs. Simulating and evaluating the output of such a large number of sets of parameters, and large range of values of each parameter, will require machine-learning strategies for multi-object parameterization9,40 rather than the parameter sweeping strategy that was sufficient for simulating a single drug.
In conclusion, 28 800 sets of intermittent chemotherapeutic dose schedules for early colon cancer were evaluated for their ability to remove all mutant cells while retaining normal crypt function. Each dose schedule had a different duration of chemotherapy dose, different intervals between doses, and different lethality. A subset of similar dose schedules was determined that had the shortest time to cure and another subset of similar dose schedules was determined that had with the least accumulated dose. These subsets of effective dose schedules suggest candidate dose schedules for clinical trials.
Acknowledgments
C.C. thanks Gary An for discussions on agent-based modeling techniques. D.E.A. thanks the members of the Division of Life Sciences IT Support Group for computer services, the RUCore staff for file archive services, and Albertas Janulevicius for suggestions on how to increase NetLogo executions speed, which lead to the collaboration with C.C.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: C.C. used high-performance computing resources of the National Energy Research Scientific Computing Center, a DOE Office of Science User Facility supported by the Office of Science of the U.S. Department of Energy under contract no. DE-AC02-05CH11231. In addition, this research was supported by the NIH through resources provided by the University of Chicago Computation Institute (Beagle 2) and the Biological Sciences Division of the University of Chicago and Argonne National Laboratory (grant no.: 1S100D018495-01). D.E.A. was supported by the Human Genetics Institute of New Jersey, the New Jersey Breast Cancer Research Fund, and the Rutgers Cancer Institute of New Jersey (grant no.: PA30CA072720).
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: C.C. ported D.E.A.’s model NetLogo code to C++ and ran the simulations. D.E.A. wrote a first draft. Both authors produced graphs, interpreted data, and approved the final draft.
ORCID iDs: Chase Cockrell  https://orcid.org/0000-0003-3224-7617
https://orcid.org/0000-0003-3224-7617
David E Axelrod  https://orcid.org/0000-0002-0912-3870
https://orcid.org/0000-0002-0912-3870
References
- 1. Strum WB. Colorectal adenomas. N Engl J Med. 2016;374:1065–1075. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics. CA Cancer J Clin. 2017;67:177–193. [DOI] [PubMed] [Google Scholar]
- 3. Lauby-Scretan B, Vilahru N, Biachini F, Guha N, Straif K. The IARC perspective on colorectal cancer screening. N Engl J Med. 2018;378:1734–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:2541. [DOI] [PubMed] [Google Scholar]
- 5. Cohen JD, Li L, Wang Y, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science. 2018;359:926–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bravo R, Axelrod DE. A calibrated agent-based computer model of stochastic cell dynamics in normal human colon crypts useful for in silico experiments. Theor Biol Med Model. 2013;10:66–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Axelrod DE, Vedula S, Obanyi J. Effective chemotherapy of heterogeneous and drug-resistant early colon cancers by intermittent dose schedules: a computer simulation study. Cancer Chemother Pharmacol. 2017;79:889–898. [DOI] [PubMed] [Google Scholar]
- 8. Railsback S, Ayllon D, Berger U, et al. Improving execution speed of models implemented in NetLogo. J Artif Soc Soc Simul. 2017;20:3–23. [Google Scholar]
- 9. Cockrell C, An G. Sepsis reconsidered: identifying novel metrics for behavioral landscape characterization with a high-performance computing implementation of an agent-based model. J Theor Biol. 2017;430:157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cockrell C, An G. Examining the controllability of sepsis using genetic algorithms on an agent-based model of systemic inflammation. PLoS Comp Biol. 2017;14:e1005876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. An G. In silico experiments of existing and hypothetical cytokine-directed clinical trials using agent-based modeling. Crit Care Med. 2004;32:2050–2060. [DOI] [PubMed] [Google Scholar]
- 12. Borras E, San Lucas FA, Chang K, et al. Genomic landscape of colorectal mucosa and adenomas. Cancer Prev Res. 2016;9:417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leslie A, Carey FS, Pratt NR, Steel RJC. The colorectal adenoma-carcinoma sequence. Br J Surg. 2002;89:845–860. [DOI] [PubMed] [Google Scholar]
- 14. Blackurn EH. Cancer interception. Cancer Prev Res. 2011;4:787–792. [DOI] [PubMed] [Google Scholar]
- 15. Cockrell C, Christley S, An G. Investigation of inflammation and tissue patterning in the gut using a spatially explicit general-purpose model of enteric tissue (SEGMEnT). PLoS Comp Biol. 2014;10:e1003507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cockrell RC, Christley S, Chang E, An G. Towards anatomic scale agent-based modeling with a massively parallel spatially explicit general-purpose model of enteric tissue (SEGMEnT_HPC). PLoS ONE. 2015;10:e0122192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baker A-M, Cereser B, Metlon S, et al. Quantification of crypt and stem cell evolution in the normal and neoplastic human colon. Cell Rep. 2014;8:940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Snippert HJ, van der Flier LG, Sato T, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. [DOI] [PubMed] [Google Scholar]
- 20. Lopez-Garcia C, Klein AM, Simons BD, Winton DJ. Intestinal stem cell replacement follows a pattern of neutral drift. Science. 2010;330:822–825. [DOI] [PubMed] [Google Scholar]
- 21. Humphries A, Wright NA. Colonic crypt organization and tumorigenesis. Nat Rev Cancer. 2008;8:415–424. [DOI] [PubMed] [Google Scholar]
- 22. Simons BD, Clevers H. Strategies for homeostatic stem cell self-renewal in adult tissues. Cell. 2011;145:851–862. [DOI] [PubMed] [Google Scholar]
- 23. Yang J, Axelrod DE, Komarova NL. Determining the control networks regulating stem cell lineages in colonic crypts. J Theor Biol. 2017;429:190–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smallbone K, Corfe BM. A mathematical model of the colon crypt capturing compositional dynamic interactions between cell types. Int J Exp Pathol. 2014;95:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kagawa Y, Horita N, Taniguchi H, Tsuneda S. Modeling of stem cell dynamics in human colonic crypts in silico. J Gastroenterol. 2014;49:263–269. [DOI] [PubMed] [Google Scholar]
- 26. van Leeuwen IMM, Byrne HM, Jensen OE, King JR. Crypt dynamics and colorectal cancer: advances in mathematical modelling. Cell Prolif. 2006;39:157–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kershaw SK, Byrne H, Gavaghan DJ, Osborne JM. Colorectal cancer through simulation and experiment. IET Syst Biol. 2013;7:57–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. De Matteis G, Gradenzi A, Antoniotti M. A review of spatial computational models for multi-cellular systems, with regard to intestinal crypts and colorectal cancer development. J Math Biol. 2013;66:1409–1462. [DOI] [PubMed] [Google Scholar]
- 29. Swan GW. Role of optimal control theory in cancer chemotherapy. Math Biosci. 1990;101:237–284. [DOI] [PubMed] [Google Scholar]
- 30. Shi J, Alagoz O, Safa Erenay F, Su Q. A survey of optimization models on cancer chemotherapy treatment planning. Ann Oper Res. 2014;221:331–356. [Google Scholar]
- 31. Marcu LG, Harriss-Phillips WM. In silico modelling of treatment-induced tumour cell kill: developments and advances. Comput Math Methods Med. 2012;2012:960256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Panetta JC. A mathematical model of periodically pulsed chemotherapy: tumor recurrence and metastasis in a competitive environment. Bull Math Biol. 1996;58:425–447. [DOI] [PubMed] [Google Scholar]
- 33. Gaffney EA. The mathematical modelling of adjuvant chemotherapy scheduling: incorporating the effects of protocol rest phases and pharmacokinetics. Bull Math Biol. 2005;67:563–611. [DOI] [PubMed] [Google Scholar]
- 34. Marcu L, Bezak E. Modelling of tumour repopulation after chemotherapy. Australas Phys Eng Sci Med. 2010;33:265–270. [DOI] [PubMed] [Google Scholar]
- 35. Murano T, Kagawa Y, Tsuneda S. Mathematical modelling of spatio-temporal cell dynamics in colonic crypts following irradiation. Cell Prolif. 2014;47:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leder K, Pitter K, LaPlant Q, et al. Mathematical modeling of PDGF-driven glioblastoma reveals optimized radiation dosing schedules. Cell. 2014;156:603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Berry SR, Cosby R, Asmis T, Chan K, Hammad N, Krzyzanowska MK. Continuous versus intermittent chemotherapy strategies in metastatic colorectal cancer: a systematic review and meta-analysis. Ann Oncol. 2015;26:477–485. [DOI] [PubMed] [Google Scholar]
- 38. Van Cutsem E, Findlay M, Osterwalder B, et al. Capecitabine, an oral fluoropyrimidine carbamate with substantial activity in advanced colorectal cancer: results of a randomized phase II study. J Clin Oncol. 2000;18:1337–1345. [DOI] [PubMed] [Google Scholar]
- 39. Labianca R, Sobrero A, Isa L, et al. Intermittent versus continuous chemotherapy in advanced colorectal cancer: a randomised “GISCAD” trial. Ann Oncol. 2011;22:1236–1242. [DOI] [PubMed] [Google Scholar]
- 40. Cockrell C, An G. Using genetic algorithms to discover effective treatments for sepsis in an agent-based model. PLoS Compl Biol. 2018;14:e1005876. [DOI] [PMC free article] [PubMed] [Google Scholar]