Abstract
Objective:
The aim of this study was to assess the real-world effectiveness and tolerability of palbociclib combined with endocrine therapy for the treatment of hormone receptor positive (HR-positive), human epidermal growth factor receptor 2 negative (HER2-negative), advanced/metastatic breast cancer that progressed on previous endocrine therapy, and to compare these results with the outcomes of the PALOMA-3 clinical trial.
Methods:
This study was a retrospective observational cohort study including all patients who started with palbociclib in the St. Antonius Hospital between September 1, 2016 and April 1, 2018 for the treatment of HR-positive, HER2-negative advanced/metastatic breast cancer that progressed on previous endocrine therapy. Individual patient data were collected from electronic medical records. Primary study outcomes were progression-free survival (PFS) and the number of permanent treatment discontinuations before disease progression due to adverse events (AEs). Secondary outcomes were the frequency of all (serious) AEs and the frequency of and reasons for dose reductions, -interruptions and cycle delays.
Results:
A total of 46 patients were studied with a median follow-up of 13.0 months. Overall, the median PFS in real-world clinical practice was 10.0 months (95% confidence interval (CI) 4.9-15.1), compared with 9.5 months in PALOMA-3 (95% CI 9.2-11.0). Two patients discontinued treatment because of AEs. Neutropenia was the most frequent grade 3-4 AE, but with no febrile neutropenia events. Most AEs were managed with palbociclib dose modifications. Regarding these modifications, more cycle delays, less dose reductions, and less dose interruptions occurred in clinical practice compared with PALOMA-3 (59 vs 36%, 22 vs 34%, and 9 vs 54%, respectively). Patients who did not meet the PALOMA-3 study eligibility criteria (n = 16) showed a lower median PFS of 5.5 months (95% CI 4.7-6.4).
Conclusions:
The effectiveness and tolerability of palbociclib in real-world clinical practice corresponded well with the results obtained in the PALOMA-3 clinical trial. Despite the differences in dose modifications, this study suggests that there is no efficacy-effectiveness gap in this patient population.
Keywords: palbociclib, real-world, efficacy-effectiveness gap, tolerability, clinical practice, metastatic breast cancer
Introduction
In the past, studies have shown that the effects of new cancer therapies when used in daily clinical practice do not always correspond with the results from the clinical trials.1–3 For most new oncology drugs, this possible efficacy-effectiveness gap has not been evaluated nor quantified yet. Having this information available, however, is important to support clinical decision making and discussions about the high costs of many new oncology drugs.
Palbociclib, a first-in-class cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitor, is registered for the treatment of hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer.4 In the phase 3 clinical trial (PALOMA-3), the combination of fulvestrant plus palbociclib significantly improved progression-free survival (PFS) compared with fulvestrant plus placebo (median PFS 9.5 months (95% confidence interval (CI) 9.2-11.0) vs 4.6 months (95% CI 3.5-5.6), respectively) in patients who were previously treated with endocrine therapy.5 Treatment with palbociclib combined with fulvestrant was generally safe and well tolerated.6 Neutropenia was the most common adverse event (AE), which could be managed by dose reduction, dose interruption, or cycle delay.
Currently, real-world data about palbociclib are still scarce. One study provided insight into treatment and complete blood count monitoring patterns in community oncology practice, but with no outcomes data.7 One other study showed effectiveness and tolerability data of palbociclib in a selected population of patients who were previously treated with everolimus.8 Overall data about the efficacy-effectiveness relation within the marketing authorisation label have not yet been reported.
The present study assessed the real-world effectiveness and tolerability of palbociclib combined with endocrine therapy for the treatment of HR-positive, HER2-negative advanced or metastatic breast cancer that progressed on previous endocrine therapy, and compared these results with the outcomes of the PALOMA-3 clinical trial.
Methods
In this retrospective observational cohort study, all patients who received at least one dose of palbociclib in the St. Antonius Hospital (Utrecht/Nieuwegein, the Netherlands) between September 1, 2016 and April 1, 2018, and whose HR-positive, HER2-negative advanced or metastatic breast cancer had progressed on previous endocrine therapy, were identified through the Pharmacy Information System (CGM Mira, version 2.7) and included in the study. The combination of palbociclib with any endocrine therapy was accepted, which included fulvestrant and aromatase inhibitors. Individual patient data were manually collected from electronic medical records. Baseline was defined as the date of the first dose of palbociclib. The study was approved by the Institutional Review Board of the St. Antonius Hospital (R&D/Z17.092).
In our study, the primary study outcomes were PFS and the number of permanent treatment discontinuations before disease progression due to AEs. PFS was defined as the time from the start of treatment to disease progression or death, whichever occurred first. Secondary outcomes were the frequency of all (serious) AEs and the frequency of and reasons for dose reductions, -interruptions and cycle delays. AEs were graded in accordance with the maximum Common Terminology Criteria for Adverse Events (CTCAE, version 4.0).
Data were reported similarly to the data presentation in the PALOMA-3 main scientific paper.5 Standard descriptive statistics were used to report the data on patient characteristics and tolerability outcomes. The Kaplan-Meier method was used to estimate PFS, and where possible, survival curves were compared using the log-rank test. Median time of follow-up was calculated using the reversed Kaplan-Meier analysis.9 External reference data were used from Cristofanilli et al5 and Verma et al6 to compare the real-world data. Results were considered statistically significant if P < .05. Analyses were performed using IBM SPSS Statistics 24.
Results
Patient characteristics
A total of 46 patients were studied with a median follow-up of 13.0 months. Follow-up for primary outcomes ended on October 1, 2018. Follow-up for secondary outcomes ended on May 25, 2018. At baseline, median age was 67 years (range 35-85) compared with 57 years in PALOMA-3 (range 30-88) (Table 1). On average, real-world patients had had more previous lines of endocrine treatment than patients in PALOMA-3 (28% of patients in clinical practice had had ⩾3 previous lines of endocrine treatment, compared with 14% in PALOMA-3). All patients started with the recommended dose of palbociclib (125 mg daily for 21 consecutive days, followed by 7 days off treatment). Five patients received the combination of palbociclib and an aromatase inhibitor, instead of fulvestrant.
Table 1.
Baseline characteristics of patients in the real-world population and in the PALOMA-3 population.
| Real-world (N = 46) | PALOMA-3 (N = 347) | |
|---|---|---|
| Median age, years (range) | 67 (35-85) | 57 (30-88) |
| ECOG performance status | ||
| 0-1 | 44 (96%) | 347 (100%) |
| 2 | 2 (4%) | 0 |
| Menopausal status | ||
| Premenopausal or perimenopausal | 3 (7%) | 72 (21%) |
| Postmenopausal | 39 (85%) | 275 (79%) |
| Missing | 4 (9%) | 0 |
| Non-measurable disease | ||
| Bone | 13 (28%) | 75 (22%) |
| Othersa | 4 (9%) | 4 (1%) |
| Measurable disease | ||
| Any measurable disease | 29 (63%) | 268 (77%) |
| Visceral disease | 28 (61%) | 206 (59%) |
| Lung involvement | 11 (24%) | 100 (29%) |
| Liver involvement | 20 (43%) | 127 (37%) |
| Peritoneal involvementa | 3 (7%) | 2 (1%) |
| Brain or pleural involvement, or botha | 4 (9%) | 4 (1%) |
| Number of previous lines of endocrine treatment | ||
| 1 | 20 (43%) | 160 (46%) |
| 2 | 13 (28%) | 140 (40%) |
| ⩾3 | 13 (28%) | 47 (14%) |
| Purpose of most recent treatment | ||
| Adjuvant therapy | 3 (7%) | 74 (21%) |
| Treatment of advanced or metastatic breast cancer | 43 (93%) | 273 (79%) |
| Disease-free interval | ||
| Data available | 35 (76%) | 233 (67%) |
| >24 months | 33 (94%) | 192 (82%) |
| 12-24 months | 2 (6%) | 30 (13%) |
| <12 months | 0 | 11 (5%) |
| Previous endocrine therapy | ||
| Aromatase inhibitors | 18 (39%) | 137 (39%) |
| Tamoxifen | 2 (4%) | 51 (15%) |
| Aromatase inhibitors and tamoxifen | 14 (30%) | 159 (46%) |
| Aromatase inhibitors and fulvestrant | 2 (4%) | 0 |
| Aromatase inhibitors and everolimus | 1 (2%) | 0 |
| Aromatase inhibitors, fulvestrant, and tamoxifen | 2 (4%) | 0 |
| Aromatase inhibitors, everolimus, and tamoxifen | 2 (4%) | 0 |
| Aromatase inhibitors, everolimus, and fulvestrant | 1 (2%) | 0 |
| Aromatase inhibitors, everolimus, tamoxifen, and fulvestrant | 2 (4%) | 0 |
| Aromatase inhibitors, megestrol, and tamoxifen | 1 (2%) | 0 |
| Fulvestrant | 1 (2%) | 0 |
| Previous chemotherapy | ||
| Neoadjuvant or adjuvant therapy only | 16 (35%) | 139 (40%) |
| Treatment of metastatic disease (with or without adjuvant or neoadjuvant) | 14 (30%) | 113 (33%) |
| Previous sensitivity to endocrine therapy | ||
| Yes | 42 (91%) | 274 (79%) |
| No | 4 (9%) | 73 (21%) |
| Oestrogen-receptor or progesterone-receptor status | ||
| Oestrogen receptor | ||
| Positive | 46 (100%) | |
| Negative | 0 | |
| Progesterone receptor | ||
| Positive | 31 (67%) | |
| Negative | 13 (28%) | |
| Missing | 2 (4%) | |
Data are number (%), unless otherwise specified. ECOG: Eastern Cooperative Oncology Group. Some percentages may not total 100% when summed, because of rounding. PALOMA-3 data were adapted from Cristofanilli et al.5
For real-world: peritoneal and pleural involvement was considered non-measurable disease (‘Others’).
Thirty (65%) out of 46 patients treated in clinical practice met the eligibility criteria for the PALOMA-3 clinical trial. The remaining patients (n = 16) would have been excluded from participation due to Eastern Cooperative Oncology Group (ECOG) performance status 2 (n = 2), prior treatment with fulvestrant and/or everolimus (n = 11), and/or more than 1 prior line of chemotherapy for advanced disease (n = 7).
Effectiveness
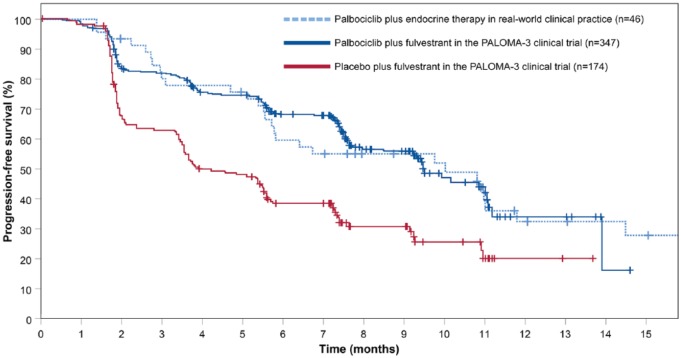
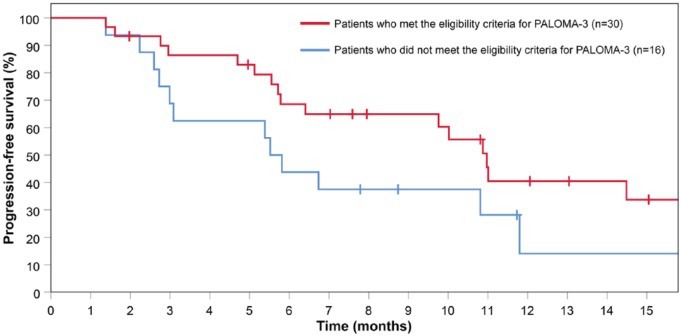
Overall, the median PFS in real-world clinical practice was 10.0 months (95% CI 4.9-15.1), compared with 9.5 months in PALOMA-3 (95% CI 9.2-11.0), with a similar trend in events over time (Figure 1). Patients who did not meet the PALOMA-3 eligibility criteria (n = 16) showed a shorter median PFS compared with the patients that did meet the PALOMA-3 eligibility criteria (median PFS 5.5 months (95% CI 4.7-6.4) vs 11.0 months (95% CI 9.7-12.3), log-rank test, P = .154) (Figure 2).
Figure 1.
Kaplan-Meier curves of progression-free survival among patients in real-world clinical practice, and patients in PALOMA-3.
PALOMA-3 data were adapted from the original PALOMA-3 publication (Cristofanilli et al5).
Figure 2.
Kaplan-Meier curves of progression-free survival among patients who did and did not meet PALOMA-3 eligibility criteria.
Tolerability
Two patients (4%) in clinical practice discontinued treatment because of AEs, which was similar to the rate of treatment discontinuation in PALOMA-3 (4%). Four (9%) of 46 patients in clinical practice had a dose interruption because of an AE, 27 (59%) had a cycle delay, and 10 (22%) had at least one dose reduction during treatment, compared with 54%, 36%, and 34%, respectively, in PALOMA-3 (Table 2). No deaths occurred in clinical practice as a result of treatment-related toxic effects, as was the case in PALOMA-3.
Table 2.
Frequency, timing, and duration of dose modifications in clinical practice and in PALOMA-3.
| Real-world (N = 46) | PALOMA-3 (N = 345) | |
|---|---|---|
| Duration of treatment (days) | 222.5 (42-693) | 232 (1-481) |
| Frequency, timing, and duration of dose reductions | ||
| No. of patients who had dose-level reduction(s), n (%) | ||
| 1 | 6 (13%) | 95 (28%) |
| 2 | 4 (9%) | 22 (6%) |
| ⩾1 | 10 (22%) | 117 (34%) |
| Patients who had dose level reduced, n (%) | ||
| To 100 mg | 10 (22%) | 108 (31%) |
| To 75 mg | 4 (9%) | 31 (9%) |
| Time course for patients who had 1 dose-level reduction (days) | ||
| Time until dose reduction from 125 to 100 mg | 166.5 (28-360) | 57 (27-293) |
| Duration receiving 100 mg | 64.5 (7-303) | 105 (13-248) |
| Time until reduction from 100 to 75 mg | 36 (29-85) | |
| Duration receiving 75 mg | 120 (17-159) | |
| Time course for patients who had 2 dose-level reductions (days) | ||
| Time until dose reduction from 125 to 100 mg | 36.5 (28-102) | 34 (27-142) |
| Duration receiving 100 mg | 49 (35-104) | 44 (10-196) |
| Time until reduction from 100 to 75 mg | 118 (63-141) | 120 (56-352) |
| Duration receiving 75 mg | 25.5 (19-44) | 81 (21-168) |
| Frequency, timing, and duration of cycle delays and dose interruptions | ||
| Patients who had cycle delay or interruptions, n (%) | ||
| Any cycle delay due to an AE | 27 (59%) | 123 (36%) |
| Any dose interruption due to an AE | 4 (9%) | 187 (54%) |
| Time to first cycle delay (days) | 28 (23-504) | 64 (31-349) |
| Time to first dose interruption (days) | 105.5 (15-315) | 18 (1-482) |
| Duration of cycle delay (days)a | 7 (3-28) | 3 (2-16) |
| Duration of dose interruption (days)a | 15.5 (13-21) | 6 (1-20) |
| Frequency of permanent treatment discontinuations | ||
| Permanent discontinuation of treatment because of AEs, n (%) | 2 (4%) | 14 (4%) |
Data are the median (range), unless otherwise specified.
PALOMA-3 data were adapted from Verma et al.6
Abbreviation: AE, adverse event.
For real-world: data are the duration of the first cycle delay/dose interruption.
Reasons for dose modifications were mostly neutropenia, and to a lesser extent other haematological AEs (eg, thrombocytopenia, leukopenia), and non-haematological AEs, such as active infection or general weakness after infection.
The most commonly reported haematological and non-haematological AEs are reported in Additional file 1. Neutropenia was the most frequently observed grade 3-4 AE (63%), which was also the case in PALOMA-3 (65%). Febrile neutropenia did not occur in clinical practice. This corresponded with the finding in PALOMA-3 that febrile neutropenia was uncommon with palbociclib treatment (0.9%). The incidence rates of any grade 3-4 AEs and any serious AEs were similar in clinical practice and in PALOMA-3 (78% vs 73%, and 13% vs 13%, respectively). A number of other AEs occurred more frequently in clinical practice than in the clinical trial, but these were mostly mild to moderate (grade 1-2) AEs, that did not seem to require additional consideration (Additional files 2 and 3).
Discussion
This study suggests that there is no efficacy-effectiveness gap for palbociclib in the treatment of HR-positive, HER2-negative advanced or metastatic breast cancer that progressed on previous endocrine therapy. The PFS in the overall population in clinical practice corresponded well with the PFS in the PALOMA-3 clinical trial. Moreover, the tolerability of palbociclib in clinical practice was generally consistent with the results in PALOMA-3. Despite the differences in dose modifications between clinical practice and clinical trial (more cycle delays, less dose reductions, and less dose interruptions in clinical practice), this did not seem to affect effectiveness.
The numerical but non-significant difference in PFS observed in the subgroup analysis of patients that did and did not meet the eligibility criteria for the clinical trial could possibly be explained by the fact that the subpopulation of non-eligible patients was a more advanced and pre-treated population. The clinical impact of this finding, however, may become less in the future, as palbociclib is now readily available as first and second line of endocrine treatment.
Besides the reduced effectiveness in PALOMA-3 non-eligible patients, we observed a slight numerical difference in median PFS for eligible patients in clinical practice vs patients in the clinical trial (median PFS 11.0 months vs 9.5 months). One possible explanation could be a closer follow-up of disease progression in the clinical trial (every 8 weeks vs every 12 weeks in clinical practice). It could also be that the choice of dose modifications of medical practitioners in clinical practice (cycle delays, instead of dose reductions) might have enhanced effectiveness. Unfortunately, this could not be explored further with the available data. At this time, the Canadian Cancer Trials Group is studying whether a continuous 100 mg every day regimen is superior to the currently recommended regimen of 125 mg every day for 3 weeks, followed by a 7-day pause.10 This study could help to better understand the correlation between dose intensity profile and effectiveness of palbociclib.
Strengths of this study include the inclusion of an unselected real-world cohort, and the access to, and use of all detailed clinical progress notes of the patients through the electronic medical records. Moreover, pharmacy dispensing data were available for all patients to confirm palbociclib dose and quantity, dates of palbociclib dispensing, and possible dose modifications.
The results of this study should be treated with caution. A major limitation of this study is the small sample size, which precludes robust conclusions. Nonetheless, a post-hoc power calculation showed that our sample has sufficient power (80%) to detect a difference in median PFS of 12% or more with the PALOMA-3 data. Other limitations of the study were inherent to the retrospective study design. This included missing data for some baseline characteristics, and in some cases, AEs were not documented in detail, which may have led to over- or underestimation of the severity. Moreover, a different interpretation of non-specific terms, such as the AE term ‘pain’, may have contributed to the differences observed between the two populations.
Conclusions
The effectiveness and tolerability of palbociclib in clinical practice corresponded well with the results obtained in PALOMA-3. Despite the differences in dose modifications, this study suggests that there is no efficacy-effectiveness gap in this population of patients with HR-positive, HER2-negative advanced or metastatic breast cancer that had progressed on previous endocrine therapy.
Supplementary Material
Acknowledgments
The St. Antonius Hospital Oncology staff (Dr Hunting, Dr de Jong, Dr Los, Dr Meerveld, Dr Melsen, Dr Verhaar) is acknowledged for their support in validating the clinical data.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: TBVB and EMWVDG conceived of and designed the study. DMTB identified eligible patients. TBVB collected and analysed the data. TBVB and EMWVDG interpreted data. TBVB drafted the manuscript. DMTB, MJA, and EMWVDG critically revised the manuscript for important intellectual content. All authors read and approved the final manuscript version.
Availability of Data and Material: The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethical Approval and Consent to Participate: The protocol was evaluated and approved by the Institutional Review Board of the St. Antonius Hospital (Utrecht/Nieuwegein, the Netherlands, protocol number R&D/z17.092). No written informed consent of the patients was requested, since only previously and routinely collected information was analysed. Individual patient data were documented anonymously.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Templeton AJ, Vera-Badillo FE, Wang L, et al. Translating clinical trials to clinical practice: outcomes of men with metastatic castration resistant prostate cancer treated with docetaxel and prednisone in and out of clinical trials. Ann Oncol. 2013;24:2972–2977. doi: 10.1093/annonc/mdt397. [DOI] [PubMed] [Google Scholar]
- 2. Karim S, Zhang-Salomans J, Biagi JJ, Asmis T, Booth CM. Uptake and effectiveness of FOLFIRINOX for advanced pancreatic cancer: a population-based study. Clin Oncol. 2018;30:e16–e21. doi: 10.1016/j.clon.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 3. Thortzen A, Thim S, Røder MA, Brasso K. A single-center experience with abiraterone as treatment for metastatic castration-resistant prostate cancer. Urol Oncol. 2016;34:291.e1–291.e7. doi: 10.1016/j.urolonc.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 4. European Medicines Agency. Ibrance, INN-palbociclib — WC500217196. Samenvatting van de productkenmerken. http://www.ema.europa.eu/docs/nl_NL/document_library/EPAR_-_Product_Information/human/003853/WC500217196.pdf. Published 2016. Accessed May 3, 2017.
- 5. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 6. Verma S, Bartlett CH, Schnell P, et al. Palbociclib in combination with fulvestrant in women with hormone receptor-positive/HER2-negative advanced metastatic breast cancer: detailed safety analysis from a multicenter, randomized, placebo-controlled, phase III study (PALOMA-3). Oncologist. 2016;21:1165–1175. doi: 10.1634/theoncologist.2016-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kish JK, Ward MA, Garofalo D, et al. Real-world evidence analysis of palbociclib prescribing patterns for patients with advanced/metastatic breast cancer treated in community oncology practice in the USA one year post approval. Breast Cancer Res. 2018;20:37. doi: 10.1186/s13058-018-0958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. du Rusquec P, Palpacuer C, Campion L, et al. Efficacy of palbociclib plus fulvestrant after everolimus in hormone receptor-positive metastatic breast cancer. Breast Cancer Res Treat. 2018;168:559–566. doi: 10.1007/s10549-017-4623-8. [DOI] [PubMed] [Google Scholar]
- 9. Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. [DOI] [PubMed] [Google Scholar]
- 10. Clinicaltrials.gov. Study comparing two different schedules of palbociclib plus second line endocrine therapy in women with estrogen receptor positive, HER2 negative advanced/metastatic breast cancer. https://clinicaltrials.gov/ct2/show/NCT02630693. Published 2017. Accessed June 26, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.