Abstract
Aim
To assess the in vitro effect of hydrogen peroxide (H2O2) on uterine contractions in pregnant and non-pregnant rats.
Methods
The study was performed at the Department of Physiology, College of Medicine, King Saud University from December 2016 to October 2017. Intact uterine samples were obtained from non-pregnant (n = 7-8) and term-pregnant (n = 6-7) rats. Small longitudinal uterine strips were dissected and mounted in an organ bath. Isometric force measurements were used to assess the effect of 400, 800, and 1000 μM H2O2 on spontaneous uterine contractions and contractions induced by oxytocin (5 nM), high calcium (Ca+2) solution (6 mmol/L), and high potassium chloride (KCl) solution (60 mmol/L).
Results
In both term-pregnant and non-pregnant uterine strips, H2O2 elicited a biphasic response, consisting of a transient contraction followed by a persistent decrease in spontaneously generated contractions, contractions induced by oxytocin, and contractions induced by high Ca+2 (all P < 0.01, compared with controls) in a concentration-dependent manner. The effect of H2O2 was more pronounced in non-pregnant than in pregnant rats (P < 0.05). In both groups, H2O2 failed to relax uterine strips pre-contracted with high-KCl solution (P > 0.05 compared with controls).
Conclusion
H2O2 was shown to be a potent uterine relaxant in pregnant and non-pregnant states. The pregnant uterus better withstood the inhibitory effect of H2O2 than non-pregnant uterus.
Uterine smooth muscles in pregnancy undergo extensive metabolic changes to support the physiological process of labor. At the onset of labor, the relatively quiescent myometria change suddenly to a very excitable tissue producing strong intermittent contractions. These contractions briefly compress the uterine blood vessels, resulting in repetitive ischemia and hypoxia (1,2), which generate reactive oxygen species (ROS), such as superoxide (O2-), hydrogen peroxide (H2O2), and peroxynitrite (NO3-) (3-5). At the same time, uterine smooth muscles produce antioxidant enzymes that minimize the destructive effect of ROS (6). H2O2 is an important signaling molecule with long half-life in biological systems and the ability to diffuse easily across the plasma membranes (7).
Hypoxia and ischemia have deleterious effects on pH and uterine metabolites, including adenosine 5′-triphosphate and phosphocreatine (1). Our previous study showed that hypoxia significantly decreased or inhibited the force of uterine contraction in rats from different gestation stages (8). At the molecular and cellular level, a uterine contraction is initiated by calcium (Ca2+) influx from the extracellular milieu via the voltage-gated calcium channels (VGCCs) or Ca2+ release from the sarcoplasmic reticulum (SR). The uterine contraction force during labor can be augmented by oxytocin, which further increases Ca2+ influx and release (9).
The contraction force induced by oxytocin was decreased in non-laboring pregnant women by O2- and H2O2 (10). However, different types of smooth muscles have different contractile response to H2O2. Aortic and airway smooth muscles contract (11,12), whereas smooth muscles of the mesenteric arteries and intestine relax (13,14). Because the contractile responses to H2O2 differ depending on the species, tissue type, experimental design, and contractile state (quiescent or pre-contracted), no consensus has been reached on the exact effect of H2O2 on a specific type of smooth muscle. Given that ROS generation within the uterine compartments is a part of the normal muscle contraction and labor process, we hypothesize that excessive ROS production could decrease the force of uterine contractions, which may be pronounced in non-pregnant uterus. The aim of this study was to determine the effects of H2O2 on spontaneously generated uterine contraction and contractions induced by oxytocin, high extracellular calcium (high-Ca2+) solution, and high potassium chloride (KCl) solution, and to examine if the response to H2O2 is gestationally different.
Material and methods
Experimental animals
The experiments included virgin non-pregnant (200 g, n = 7-8) and term-pregnant female Wistar rats (22 days of gestation, n = 6-7). The sample size was determined based on our experience and previous studies (8), which suggested that clear and consistent drug effects on uterine contraction are observed in sample sizes of 6-7. It was also based on the recommendations for the use of minimum number of animals by the UK Animals (Scientific Procedures) Act 1986. The experimental protocol was approved by and carried out according to the Institutional Animal Care Committee (IACC) of King Saud University recommendations (September 2016). The study was performed at the Department of Physiology, College of Medicine, King Saud University from December 2016 to October 2017. The animals were sacrificed by cervical dislocation under CO2 anesthesia in accordance with the UK Home Office guidelines (https://www.legislation.gov.uk/ukpga/1986/14/schedule/1). The uterus was removed and immediately placed into physiological Krebs saline solution. A longitudinal uterine strip (2 mm ×10 mm) was dissected from each uterus, followed by mechanical removal of the endometrial layer.
Solutions and chemicals
Krebs solution was composed of the following (in mmol/L): 115 NaCl, 4.7 KCl, 2 CaCl2, 1.16 MgSO4, 1.18 KH2PO4, 22 NaHCO3, and 7.88 dextrose, pH 7.4. High-KCl solution (60 mmol/L) was prepared by isosmotic substitution of KCl for NaCl. Oxytocin was used at a final concentration of 5 nM and added directly to Krebs solution. High-Ca2+ solution was prepared by increasing the extracellular CaCl2 concentration in Krebs solution from 2 to 6 mmol/L. H2O2 was added directly to the Krebs solution. All chemicals and drugs were of analytical grade and purchased from Sigma (St. Louis, MO, USA).
Isolated tissue bath protocols
The uterine strips for isometric force recordings were prepared as described in our previous study (8). Briefly, isolated uterine strips were tied up from both ends using surgical silk and mounted vertically in a tissue organ bath (Panlab, ADInstruments Ltd, Sydney, Australia). The bath was continuously perfused with a warmed Krebs solution at a rate of 4 mL/min and bubbled with 95% O2 and 5% CO2 at 37°C. The uterine strips were attached to an isometric force transducer (ADInstruments Ltd) under 1 g resting tension, and the force of contraction was measured in millinewtons. Cumulative concentrations of H2O2 (400, 800, and 1000 μM) were applied to the intact uterine strips as follows: 1) during spontaneous contraction; 2) during stimulation by oxytocin; 3) during stimulation by high-Ca2+ solution; and 4) during stimulation by high-KCl solution. In all experiments, H2O2 was applied for 20 minutes, after which the tissue was washed out to allow recovery. Each H2O2 dose was tested on new uterine strips as some uterine strips died or did not recover from the toxic effect of the drug.
Statistical analysis
Data are expressed as means ± standard deviation (SD), with “n” representing the number of uterine strips, one from each rat. The normality of data distribution was tested using Shapiro-Wilk test and by visual inspection of the histogram and normal Q-Q plots for each H2O2 concentration. Regular contractile activity in the last 10 minutes in the control Krebs solution (before adding any H2O2 concentration) was calculated as 100% control. The contractile activity in the last 10 minutes during H2O2 application was measured and expressed as a percentage of the preceding control period. Force amplitude, frequency (number of contractions in 10 min), and force integral (entire area under the curve, AUC) were compared between two groups using t test and between three groups using one-way ANOVA with Bonferroni correction. The level of significance was set at P < 0.05. The analysis was performed using OriginLab software (OriginLab, Northampton, MA, USA).
Results
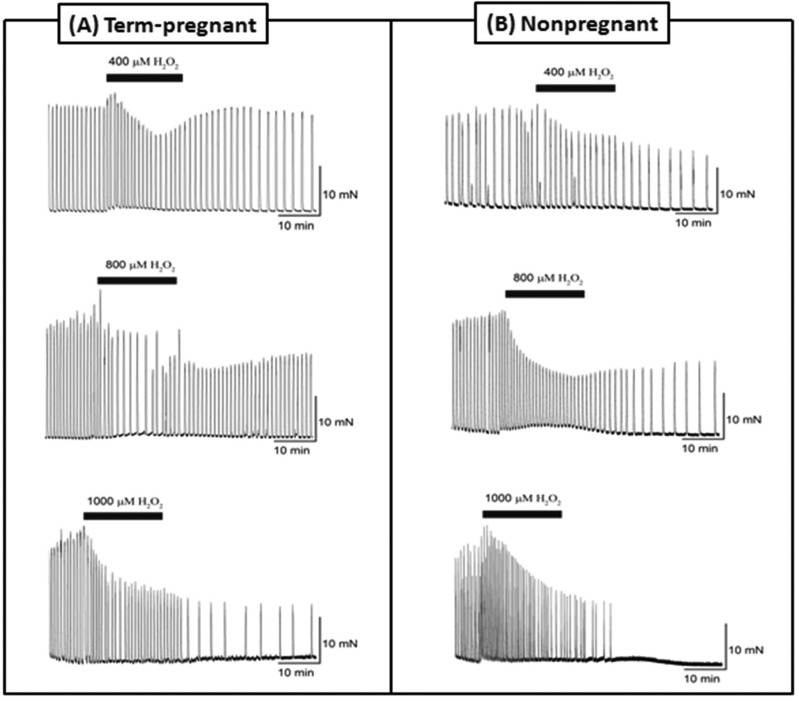
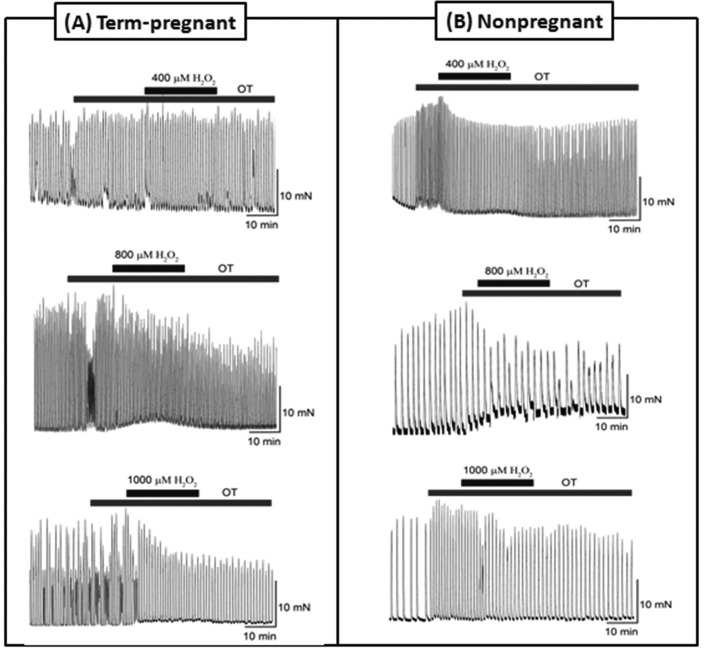
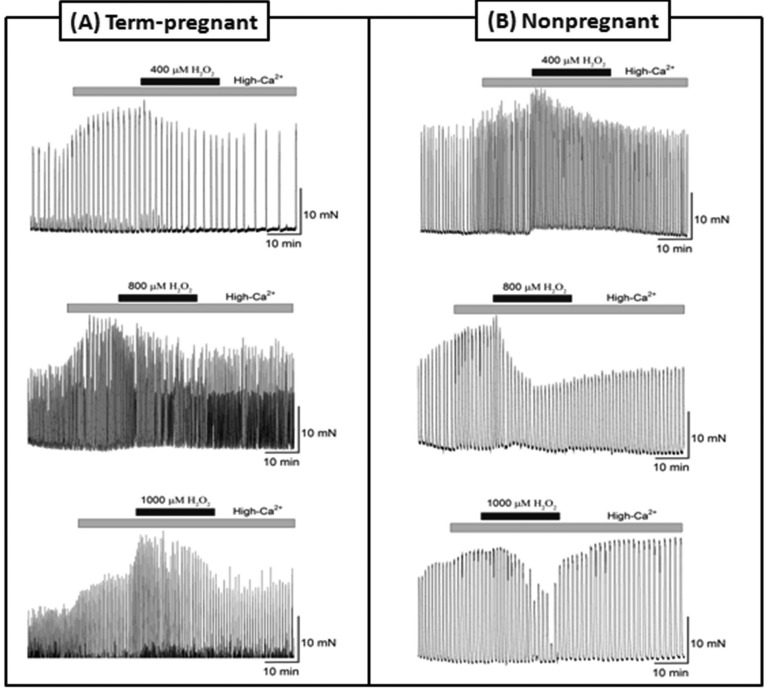
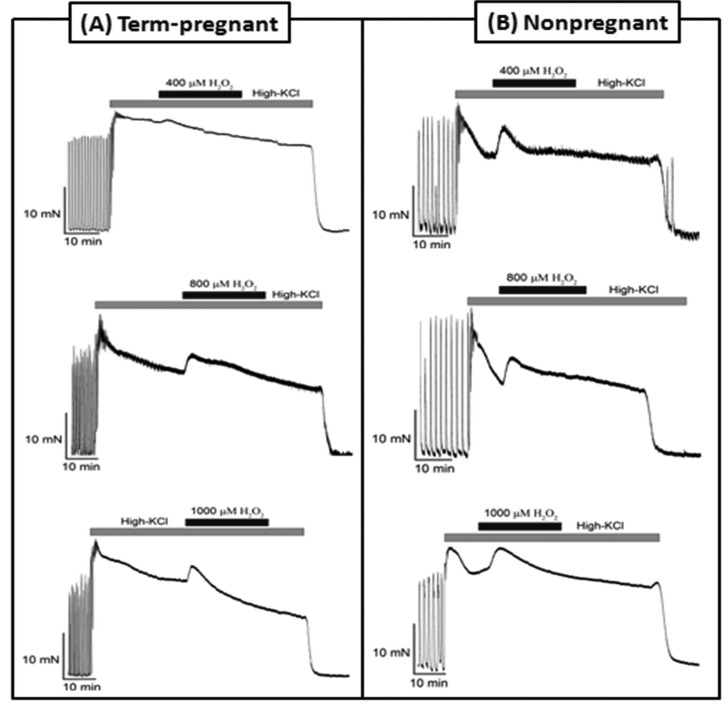
Application of 400 μM, 800 μM, and 1000 μM of H2O2 caused a transient uterine contraction followed by a marked persistent relaxation in both term-pregnant and non-pregnant rat uteri (Figure 1). Pregnant tissues tolerated the effect significantly better than non-pregnant tissues (Table 1). The same effect of all H2O2 concentrations was observed on oxytocin-induced (Figure 2, Table 2) and high calcium-induced uterine contractions (Figure 3, Table 3). In the case of high KCl-induced contractions, application of 400 μM, 800 μM, and 1000 μM of H2O2 also caused a transient contraction, but the force decrease was not significant compared with 100% control (Figure 4, Table 4).
Figure 1.
Original recordings showing the contractile responses of uterine strips to 400 μM, 800 μM, and 1000 μM of hydrogen peroxide (H2O2) during spontaneous activity in (A) term-pregnant and (B) non-pregnant rats. mN – millinewton.
Table 1.
Effects of different concentrations of hydrogen peroxide (H2O2) in vitro on spontaneous contractions of term-pregnant and non-pregnant rat uteri
| H2O2 concentrations |
|||||||
|---|---|---|---|---|---|---|---|
|
before adding H2O2 |
400 μM |
800 μM |
1000 μM |
400 μM |
800 μM |
1000 μM |
|
|
Contraction parameters (mean ± standard deviation, %) |
control |
term-pregnant (n = 7) |
non-pregnant (n = 8) |
||||
|
Amplitude |
100 |
90 ± 3* |
70 ± 6* |
56 ± 5* |
82 ± 3*† |
63 ± 3*† |
51 ± 2*† |
|
Frequency |
100 |
87 ± 8* |
75 ± 3* |
56 ± 6* |
82 ± 8* |
63 ± 8*† |
53 ± 3* |
| Area under the curve | 100 | 84 ± 5* | 73 ± 3* | 63 ± 3* | 75 ± 3*† | 65 ± 3*† | 57 ± 3*† |
*P < 0.01 compared with control (ANOVA/Bonferroni).
†P < 0.05 compared with term-pregnant (t-test).
Figure 2.
Original recordings showing the contractile responses of uterine strips in the presence of 5 nM oxytocin (OT) to 400 μM, 800 μM, and 1000 μM of hydrogen peroxide (H2O2) in (A) term-pregnant and (B) non-pregnant rats. mN – millinewton.
Table 2.
Effects of different concentrations of hydrogen peroxide (H2O2) in vitro on oxytocin-induced contractions in term-pregnant and non-pregnant rat uteri
| H2O2 concentrations |
|||||||
|---|---|---|---|---|---|---|---|
| before adding H2O2 |
400 μM |
800 μM |
1000 μM |
400 μM |
800 μM |
1000 μM |
|
| Contraction parameter (mean ± standard deviation, %) | control | term-pregnant (n = 6) |
non-pregnant (n = 7) |
||||
|
Amplitude |
100 |
82 ± 6* |
75 ± 3* |
60 ± 3* |
78 ± 3* |
67 ± 3*† |
52 ± 3*‡ |
|
Frequency |
100 |
85 ± 6* |
69 ± 3* |
64 ± 3* |
82 ± 6* |
67 ± 3* |
56 ± 3*‡ |
| Area under the curve | 100 | 73 ± 6* | 64 ± 3* | 57 ± 3* | 72 ± 3* | 64 ± 3* | 53 ± 3*† |
Figure 3.
Original recordings showing the contractile responses of uterine strips in the presence of 6 mmol/L extracellular high-calcium (Ca2+) to 400 μM, 800 μM, and 1000 μM of hydrogen peroxide (H2O2) in (A) term-pregnant and (B) non-pregnant rats. mN – millinewton.
Table 3.
Effects of different concentrations of hydrogen peroxide (H2O2) in vitro on uterine contractions induced by high-Ca2+ solution in term-pregnant and non-pregnant rat uteri
| H2O2 concentrations |
|||||||
|---|---|---|---|---|---|---|---|
| before adding H2O2 | 400 μM | 800 μM | 1000 μM | 400 μM | 800 μM | 1000 μM | |
|
Contraction parameters (mean ± standard deviation, %) |
control |
term-pregnant (n = 6) |
non-pregnant (n = 7) |
||||
|
Amplitude |
100 |
83 ± 3* |
67 ± 5* |
60 ± 3* |
82 ± 3* |
61 ± 3*† |
53 ± 3*† |
|
Frequency |
100 |
82 ± 6* |
67 ± 3* |
62 ± 3* |
80 ± 3* |
62 ± 3*† |
55 ± 3*† |
| Area under the curve | 100 | 83 ± 3* | 65 ± 3* | 64 ± 3* | 83 ± 3* | 60 ± 3*† | 58 ± 3*† |
*P < 0.01 compared with control (ANOVA/Bonferroni).
†P < 0.05 compared with term-pregnant (t-test).
Figure 4.
Original recordings showing the contractile responses of uterine strips in the presence of 60 mmol/L potassium chloride (KCl) to 400 μM, 800 μM, and 1000 μM of hydrogen peroxide (H2O2) in (A) term-pregnant and (B) non-pregnant rats. mN – millinewton.
Table 4.
Effects of different concentrations of hydrogen peroxide (H2O2) in vitro on uterine contractions induced by high potassium chloride solution in term-pregnant and non-pregnant rat uteri*
| H2O2 concentrations |
|||||||
|---|---|---|---|---|---|---|---|
| before adding H2O2 | 400 μM | 800 μM | 1000 μM | 400 μM | 800 μM | 1000 μM | |
|
Contraction parameters (mean ± standard deviation, %) |
control |
term-pregnant (n = 6) |
non-pregnant (n = 7) |
||||
| Area under the curve | 100 | 97 ± 3 | 98 ± 3 | 96 ± 6 | 96 ± 6 | 98 ± 3 | 96 ± 8 |
*There were no significant differences among the three doses of H2O2 concentrations between the two groups.
Discussion
H2O2 decreased uterine contractions induced by different mechanisms in a concentration-dependent manner in both pregnant and non-pregnant rats. However, in comparison with non-pregnant tissue, pregnant tissue tolerated the relaxant effect of H2O2 better.
H2O2 has been extensively used to induce experimental oxidative stress in isolated vascular and non-vascular smooth muscles. Our results are in agreement with previous findings on the ability of H2O2 to significantly decrease oxytocin-induced uterine contraction in pregnant women (10) and uterine contractions generated spontaneously or induced by 6 mmol/L Ca2+ in non-pregnant rats (15). H2O2 exerts its effects through cell membrane ion channels (16), potassium channels (16-19), calcium channels (20), and Ca2+-activated Cl– or Na+ currents (17).
The observed biphasic response to H2O2 consisting of an initial transient contraction followed by a persistent relaxation may be explained by Ca2+ influx or release by H2O2. These findings are supported by previous studies in other types of smooth muscles, where H2O2 application increased intracellular calcium (Ca2+)i via either calcium influx from the extracellular space (20) or calcium release from the SR (18). In other studies, blocking Ca2+ entry through VGCCs partially blocked H2O2-induced muscle contraction (11,19). In addition, blocking other Ca2+-permeable action channels, such as receptor- and store-operated channels, with a non-selective Ca2+ inhibitor markedly decreased [Ca2+]i and the contractile response to H2O2 (11).
Another proposed mechanism of H2O2-induced transient contraction is the stimulation of prostanoids biosynthesis. Transient contraction induced by H2O2 is strongly inhibited by blocking prostanoid enzymes, including cyclooxygenases and thromboxane A2 (TXA2) synthase (21,22), which are expressed by uterine smooth muscles (23,24). Therefore, we cannot exclude the possibility of prostanoids production by H2O2, which plays an essential role in the uterine activity regulation (25).
The delayed relaxation response to H2O2 may suggest other molecular mechanisms beyond the membrane channels. H2O2 could mediate myosin light chain phosphorylation, whose decrease or inhibition causes relaxation response to H2O2 (26).
High-KCl solution changes the reversal K+ potential, depolarizing the membrane, opening the VGCCs, and increasing [Ca2+]i. In addition, increasing external (K+) impairs K+ channel function by reducing the driving force for K+ efflux, thereby functionally limiting the influence of K+ channels on muscle activity (27). In our study, H2O2 failed to decrease uterine contraction induced by high-KCl, which suggests that H2O2 may not directly block VGCCs. This is consistent with the results of another study on arterial smooth muscles (28). Therefore, the relaxation response to H2O2 could be partly mediated by the activation of potassium conductance (hyperpolarization) (27), a mechanism supported by pharmacological studies on arterial smooth muscles (29,30) and electrophysiological studies on other cell types (31,32). Lucchesi et al (33) demonstrated that H2O2 elicited contraction in smooth muscle of the mesenteric arteries in compromised K+ channels (ie, in the presence of high-KCl solution), but that it elicited relaxation in uncompromised K+ channels. In the smooth muscle of blood vessels pre-contracted with high-KCl, H2O2 caused transient contraction dependent on Ca2+ influx from the extracellular space (12). We suggest that the relaxation response to H2O2 in the rat uterus may directly or indirectly involve K+ channels activation, as supported by previous reports (10,15). Although H2O2 transiently increases [Ca2+]i via Ca2+ influx pathway, high-KCl solution compromises K+ equilibrium and prevents repolarization. The existence of different types of K+ channels in the myometrium is well documented, and their stimulation is reported to cause myometrial relaxation (34). In smooth muscles of canine trachealis increased [Ca2+]i by H2O2 activated the large conductance calcium-activated potassium channels (BKCa) and promoted muscle relaxation (35). There are also several studies reporting that H2O2induces muscle relaxation by activating the voltage-gated K+ channels (15,36).
Normal uterine contractions are linked to ischemia and hypoxia within the myometrium along with the decrease in energy metabolites (37). In labor, however, uterine contractions increase in intensity, duration, and frequency, causing local hypoxic cycles and increasing the energy demand of the uterus to support the labor process. In this study, pregnant uterine tissues tolerated the effects of H2O2 better than non-pregnant tissues. This supports our pervious results, which showed that hypoxia decreased rat uterine contraction in different gestational stages, but that the term-pregnant uterus was more resistant to the deleterious effect of hypoxia than non-pregnant uterus (8) owing to pregnancy-related changes in myometrial metabolites and ion channels.
The primary limitation of our study is the death of some uterine tissues caused by the toxic effect of the high H2O2 dose (1000 μM). In addition, due to financial restrictions, we did not test whether antioxidant agents counteracted the deleterious effects of H2O2. Another limitation is the small sample size as we had to adhere to the strict IACC guidelines and use the minimum number of animals. However, the sample size was not smaller than those used in other similar studies (10,15). Post-hoc power analysis showed that comparison of AUC (1000 μM) between pregnant and non-pregnant animals during spontaneous contraction had an adequate power (0.95 at 5% significance level, G*Power 3.1.9.3, Heinrich-Heine- Universität Düsseldorf, Düsseldorf, Germany) (38), confirming that the number of animals per group was sufficient.
In conclusion, our results show that exogenous H2O2 causes transient uterine contraction followed by persistent relaxation in both pregnant and non-pregnant rats. The decrease in contraction force was observed in all uterine strips independent of the type of stimulation (spontaneous, oxytocin, high-Ca2+). However, when K+ channels were blocked by high-KCl, the relaxation response to H2O2 was inhibited. Further studies are required to unravel the cellular and molecular mechanisms of H2O2-induced relaxation before, during, and after pregnancy.
| *P < 0.01 compared with control (ANOVA/Bonferroni). |
| †P < 0.05 compared with term-pregnant (t-test). |
| ‡P < 0.01 compared with term-pregnant (t-test). |
Acknowledgments
Funding The study was funded by King Abdulaziz City for Science and Technology (KACST) (project number GSP-37-1118).
Ethical approval The experimental protocol and studies were approved and carried out according to the Institutional Animal Care Committee (IACC) of King Saud University recommendations (September 2016).
Declaration of authorship MA conceived and designed the study; RA acquired the data; RA and LD analyzed and interpreted the data; MA drafted the manuscript; MA and LD critically revised the manuscript for important intellectual content; all authors gave approval of the version to be submitted; RA and MA agree to be accountable for all aspects of the work.
Competing interests All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Larcombe-McDouall J, Buttell N, Harrison N, Wray S. In vivo pH and metabolite changes during a single contraction in rat uterine smooth muscle. . J Physiol. 1999;518:783–90. doi: 10.1111/j.1469-7793.1999.0783p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brar HS, Platt LD, DeVore GR, Horenstein J, Medearis AL. Qualitative assessment of maternal uterine and fetal umbilical artery blood flow and resistance in laboring patients by Doppler velocimetry. Am J Obstet Gynecol. 1988;158:952–6. doi: 10.1016/0002-9378(88)90100-7. [DOI] [PubMed] [Google Scholar]
- 3.Woods JR, Cavanaugh JL, Norkus EP, Plessinger MA, Miller RK. The effect of labor on maternal and fetal vitamins C and E. Am J Obstet Gynecol. 2002;187:1179–83. doi: 10.1067/mob.2002.127131. [DOI] [PubMed] [Google Scholar]
- 4.Nakai A, Oya A, Kobe H, Asakura H, Yokota A, Koshino T, et al. Changes in maternal lipid peroxidation levels and antioxidant enzymatic activities before and after delivery. J Nippon Med Sch. 2000;67:434–9. doi: 10.1272/jnms.67.434. [DOI] [PubMed] [Google Scholar]
- 5.Zyrianov V, Sumovskaya AY, Shostak A. Application of electron spin resonance for evaluation of the level of free radicals in the myometrium in full-term pregnancy with normal labour and uterine inertia. J Biosci. 2003;28:19–21. doi: 10.1007/BF02970127. [DOI] [PubMed] [Google Scholar]
- 6.Khan RN, Matharoo-Ball B, Shaw RW. Antioxidant enzyme expression, lipid peroxidation, and protein oxidation in human myometrium with parturition. Reprod Sci. 2010;17:78–84. doi: 10.1177/1933719109348027. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Zweier JL. A real-time electrochemical technique for measurement of cellular hydrogen peroxide generation and consumption: evaluation in human polymorphonuclear leukocytes. Free Radic Biol Med. 2001;31:894–901. doi: 10.1016/S0891-5849(01)00665-7. [DOI] [PubMed] [Google Scholar]
- 8.Alotaibi M, Arrowsmith S, Wray S. Hypoxia-induced force increase (HIFI) is a novel mechanism underlying the strengthening of labor contractions, produced by hypoxic stresses. Proc Natl Acad Sci U S A. 2015;112:9763–8. doi: 10.1073/pnas.1503497112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–83. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 10.Warren AY, Matharoo-Ball B, Shaw RW, Khan RN. Hydrogen peroxide and superoxide anion modulate pregnant human myometrial contractility. Reproduction. 2005;130:539–44. doi: 10.1530/rep.1.00437. [DOI] [PubMed] [Google Scholar]
- 11.Kojima K, Kume H, Ito S, Oguma T, Shiraki A, Kondo M, et al. Direct effects of hydrogen peroxide on airway smooth muscle tone: roles of Ca 2+ influx and Rho-kinase. Eur J Pharmacol. 2007;556:151–6. doi: 10.1016/j.ejphar.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Thakali K, Davenport L, Fink GD, Watts SW. Pleiotropic effects of hydrogen peroxide in arteries and veins from normotensive and hypertensive rats. Hypertension. 2006;47:482–7. doi: 10.1161/01.HYP.0000201540.91234.8f. [DOI] [PubMed] [Google Scholar]
- 13.Choi S, Yeum CH, Kim YD, Park CG, Kim MY, Park JS, et al. Receptor tyrosine and MAP kinase are involved in effects of H2O2 on interstitial cells of Cajal in murine intestine. J Cell Mol Med. 2010;14:257–66. doi: 10.1111/j.1582-4934.2008.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujimoto S, Asano T, Sakai M, Sakurai K, Takagi D, Yoshimoto N, et al. Mechanisms of hydrogen peroxide-induced relaxation in rabbit mesenteric small artery. Eur J Pharmacol. 2001;412:291–300. doi: 10.1016/S0014-2999(00)00940-7. [DOI] [PubMed] [Google Scholar]
- 15.Appiah I, Milovanovic S, Radojicic R, Nikolic-Kokic A, Orescanin-Dusic Z, Slavic M, et al. Hydrogen peroxide affects contractile activity and anti-oxidant enzymes in rat uterus. Br J Pharmacol. 2009;158:1932–41. doi: 10.1111/j.1476-5381.2009.00490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Joseph JA. Mechanisms of hydrogen peroxide-induced calcium dysregulation in PC12 cells1. Free Radic Biol Med. 2000;28:1222–31. doi: 10.1016/S0891-5849(00)00241-0. [DOI] [PubMed] [Google Scholar]
- 17.Schlief T, Heinemann SH. H2O2-induced chloride currents are indicative of an endogenous Na (+)-Ca2+ exchange mechanism in Xenopus oocytes. . J Physiol. 1995;486:123–30. doi: 10.1113/jphysiol.1995.sp020796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin M-J, Yang X-R, Cao Y-N, Sham JS. Hydrogen peroxide-induced Ca2+ mobilization in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1598–608. doi: 10.1152/ajplung.00323.2006. [DOI] [PubMed] [Google Scholar]
- 19.Santiago E, Contreras C, García-Sacristán A, Sánchez A, Rivera L, Climent B, et al. Signaling pathways involved in the H2O2-induced vasoconstriction of rat coronary arteries. Free Radic Biol Med. 2013;60:136–46. doi: 10.1016/j.freeradbiomed.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Chaplin NL, Amberg GC. Hydrogen peroxide mediates oxidant-dependent stimulation of arterial smooth muscle L-type calcium channels. Am J Physiol Cell Physiol. 2012;302:C1382–93. doi: 10.1152/ajpcell.00222.2011. [DOI] [PubMed] [Google Scholar]
- 21.Gao Y, Lee R. Hydrogen peroxide induces a greater contraction in mesenteric arteries of spontaneously hypertensive rats through thromboxane A2 production. Br J Pharmacol. 2001;134:1639–46. doi: 10.1038/sj.bjp.0704420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thakali K, Davenport L, Fink GD, Watts SW. Cyclooxygenase, p38 mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase MAPK, Rho kinase, and Src mediate hydrogen peroxide-induced contraction of rat thoracic aorta and vena cava. J Pharmacol Exp Ther. 2007;320:236–43. doi: 10.1124/jpet.106.110650. [DOI] [PubMed] [Google Scholar]
- 23.Moore F, Asboóth G, Loópez Bernal A. Thromboxane receptor signalling in human myometrial cells. Prostaglandins Other Lipid Mediat. 2002;67:31–47. doi: 10.1016/S0090-6980(01)00169-1. [DOI] [PubMed] [Google Scholar]
- 24.Swanson ML, Lei ZM, Swanson PH, Rao CV, Narumiya S, Hirata M. The expression of thromboxane A<inf>2</inf>synthase and thromboxane A<inf>2</inf>receptor gene in human uterus. Biol Reprod. 1992;47:105–17. doi: 10.1095/biolreprod47.1.105. [DOI] [PubMed] [Google Scholar]
- 25.Erkinheimo T-L, Saukkonen K, Narko K, Jalkanen J, Ylikorkala O, Ristimäki A. Expression of cyclooxygenase-2 and prostanoid receptors by human myometrium. J Clin Endocrinol Metab. 2000;85:3468–75. doi: 10.1210/jcem.85.9.6809. [DOI] [PubMed] [Google Scholar]
- 26.Fujimoto S, Mori M, Tsushima H. Mechanisms underlying the hydrogen peroxide-induced, endothelium-independent relaxation of the norepinephrine-contraction in guinea-pig aorta. Eur J Pharmacol. 2003;459:65–73. doi: 10.1016/S0014-2999(02)02825-X. [DOI] [PubMed] [Google Scholar]
- 27.Barlow RS, White RE. Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCachannel activity. Am J Physiol. 1998;275:H1283–9. doi: 10.1152/ajpheart.1998.275.4.H1283. [DOI] [PubMed] [Google Scholar]
- 28.Rogers PA, Dick GM, Knudson JD, Focardi M, Bratz IN, Swafford AN, Jr, et al. H2O2-induced redox-sensitive coronary vasodilation is mediated by 4-aminopyridine-sensitive K+ channels. Am J Physiol Heart Circ Physiol. 2006;291:H2473–82. doi: 10.1152/ajpheart.00172.2006. [DOI] [PubMed] [Google Scholar]
- 29.Ward CA, Giles WR. Ionic mechanism of the effects of hydrogen peroxide in rat ventricular myocytes. . J Physiol. 1997;500:631–42. doi: 10.1113/jphysiol.1997.sp022048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayabuchi Y, Nakaya Y, Matsuoka S, Kuroda Y. Hydrogen peroxide-induced vascular relaxation in porcine coronary arteries is mediated by Ca 2+-activated K+ channels. Heart Vessels. 1998;13:9–17. doi: 10.1007/BF02750638. [DOI] [PubMed] [Google Scholar]
- 31.Seutin V, Scuvée-Moreau J, Massotte L, Dresse A. Hydrogen peroxide hyperpolarizes rat CA1 pyramidal neurons by inducing an increase in potassium conductance. Brain Res. 1995;683:275–8. doi: 10.1016/0006-8993(95)00436-T. [DOI] [PubMed] [Google Scholar]
- 32.Filipovic DM, Reeves WB. Hydrogen peroxide activates glibenclamide-sensitive K+ channels in LLC-PK1 cells. Am J Physiol. 1997;272:C737–43. doi: 10.1152/ajpcell.1997.272.2.C737. [DOI] [PubMed] [Google Scholar]
- 33.Lucchesi PA, Belmadani S, Matrougui K. Hydrogen peroxide acts as both vasodilator and vasoconstrictor in the control of perfused mouse mesenteric resistance arteries. J Hypertens. 2005;23:571–9. doi: 10.1097/01.hjh.0000160214.40855.79. [DOI] [PubMed] [Google Scholar]
- 34.Khan RN, Matharoo-Ball B, Arulkumaran S, Ashford ML. Potassium channels in the human myometrium. Exp Physiol. 2001;86:255–64. doi: 10.1113/eph8602181. [DOI] [PubMed] [Google Scholar]
- 35.Janssen LJ, Netherton SJ, Walters DK. Ca 2+-dependent K+ channels and Na+-K+-ATPase mediate H 2 O 2-and superoxide-induced relaxations in canine trachealis. J Appl Physiol. 2000;88:745–52. doi: 10.1152/jappl.2000.88.2.745. [DOI] [PubMed] [Google Scholar]
- 36.Park SW, Noh HJ, Sung DJ, Kim JG, Kim JM, Ryu S-Y, et al. Hydrogen peroxide induces vasorelaxation by enhancing 4-aminopyridine-sensitive Kv currents through S-glutathionylation. Pflugers Arch. 2015;467:285–97. doi: 10.1007/s00424-014-1513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrison N, Wray S, Larcombe-Mcdouall JBA. 31P NMR investigation into the effects of repeated vascular occlusion on uterine metabolites, intracellular pH and force, in vivo. NMR Biomed. 1995;8:28–32. doi: 10.1002/nbm.1940080107. [DOI] [PubMed] [Google Scholar]
- 38.Faul F, Erdfelder E, Lang A-G, Buchner AG. * Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]