Abstract
Background:
Gestational diabetes mellitus (GDM) results from an imbalance between insulin resistance and insulin secretion capacity during pregnancy. Tumor necrosis factor-alpha (TNF-α) is an inflammatory cytokine that is proposed to be involved in the pathogenesis of the insulin resistance, but the findings from studies across different ethnic groups are inconsistent or even conflicting.
Aim:
The aim of this study is to determine the relationship between maternal circulating level of TNF-α and insulin resistance in pregnant Nigerian women with GDM.
Methodology:
This was a cross-sectional analytical study involving 100 women with GDM and another 100 pregnant women with normal gestation. They were evaluated between 24 and 28 weeks’ gestation. Diagnosis of GDM was based on the WHO diagnostic criteria. Fasting serum insulin and TNF-α levels were measured. Insulin resistance index was calculated as homeostasis model assessment of insulin resistance. Multivariate correlation analysis was used to determine the relationship between the maternal serum level of TNF-α and the insulin resistance.
Results:
Pregnant women with GDM had greater insulin resistance than observed in the normal controls (3.14 ± 0.19 vs. 2.89 ± 0.20, P < 0.05). There was a positive correlation between serum TNF-α level and insulin resistance among the pregnant women with GDM (r = 0.49, P < 0.05). Multiple linear regression analysis indicated that TNF-α is a predictor of insulin resistance in pregnancies complicated by GDM.
Conclusion:
It is concluded that among pregnant Nigerian women with GDM in this study, increased serum TNF-α level is associated with greater insulin resistance independent of age and body mass index.
Keywords: Body mass index, gestational diabetes mellitus, homeostasis model assessment of insulin resistance, insulin resistance, pregnancy, tumor necrosis factor-alpha, Indice de masse corporelle, diabète sucré gestationnel, évaluation du modèle d’homéostasie de la résistance à l’insuline, résistance à l’insuline, grossesse, factoralpha de nécrose tumorale
Résumé
Contexte:
Le diabète sucré gestationnel (GDM) résulte d’un déséquilibre entre la résistance à l’insuline et la capacité de sécrétion d’insuline pendant la grossesse. La factoralpha (TNFα), une nécrose tumorale, est une cytokine inflammatoire dont on pense qu’elle est impliquée dans la pathogenèse de la résistance à l’insuline, mais les résultats d’études menées dans différents groupes ethniques sont incohérents, voire contradictoires.
Objectif:
Le but de cette étude est de déterminer la relation entre le taux circulant de TNFα chez la mère et la résistance à l’insuline chez les femmes nigérianes enceintes atteintes de DG.
Méthodologie:
Il s’agissait d’une étude analytique transversale menée auprès de 100 femmes atteintes de DSG et de 100 autres femmes enceintes avec une gestation normale. Ils ont été évalués entre 24 et 28 semaines de gestation. Le diagnostic du diabète gestationnel reposait sur les critères de diagnostic de l’OMS. Les taux sériques d’insuline à jeun et de TNFα ont été mesurés. L’indice de résistance à l’insuline a été calculé en tant qu’évaluation du modèle d’homéostasie de la résistance à l’insuline. Une analyse de corrélation multivariée a été utilisée pour déterminer la relation entre le taux sérique de TNFα chez la mère et la résistance à l’insuline.
Résultats:
Les femmes enceintes atteintes de DSG présentaient une résistance à l’insuline supérieure à celle observée chez les témoins normaux (3,14 ± 0,19 vs 2,89 ± 0,20, p <0,05). Il y avait une corrélation positive entre le taux de TNFα sérique et la résistance à l’insuline chez les femmes enceintes atteintes de diabète gestationnel (r = 0,49, p <0,05). Une analyse de régression linéaire multiple a indiqué que le TNFa est un facteur prédictif de la résistance à l’insuline dans les grossesses compliquées par le diabète gestationnel.
Conclusion:
Il est conclu que chez les femmes nigérianes enceintes atteintes de DG dans cette étude, une augmentation du taux sérique de TNFα est associée à une plus grande résistance à l’insuline indépendamment de l’âge et de l’indice de masse corporelle.
INTRODUCTION
Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance with onset or first recognition during pregnancy but not meeting criteria for overt or preexisting diabetes mellitus, with or without remission after the end of pregnancy.[1] GDM has drawn scientific attention over the past decades because of its growing incidence and deleterious consequences for mothers and their offsprings.[2] Insulin resistance is the hallmark of GDM, but the exact mechanism implicated in its pathophysiology remains poorly understood. Historically, placental hormones, especially human placental lactogen, have been considered as primary mediators of insulin resistance during pregnancy leading to GDM.[3,4,5,6,7,8,9] However, more recently, an inflammatory cytokine, tumor necrosis factor-alpha (TNF-α), has been implicated in the regulation of maternal metabolism and gestational insulin resistance.[10] Several studies have investigated the relationship between serum levels of TNF-α and insulin resistance in women with GDM, but reports are inconsistent or even conflicting, especially across different ethnic groups.[11,12,13,14,15]
There is a paucity of data on the relationship between circulating levels of TNF-α and insulin resistance in women of African ancestry and living in Sub-Saharan Africa with GDM.
Thus, we investigated the association between circulating level of TNF-α and insulin resistance in Nigerian women with GDM.
METHODOLOGY
Two hundred pregnant women with singleton pregnancy at 24–28 weeks’ gestation were enrolled in the study, 100 with GDM as diagnosed by the WHO criteria (2-h 75 g oral glucose tolerance test [OGTT]: fasting serum glucose [FSG] ≥7.0 mmol/L or 2-h postload serum glucose ≥7.8 mmol/L)[16] and 100 controls (pregnant women with normal gestation). They were all recruited from pregnant women who attended antenatal clinics of Ahmadu Bello University Teaching Hospital Zaria, Sick Bay at Ahmadu Bello University Zaria, and Hajiya Gambo Sawaba General Hospital Zaria, Kaduna State, from May 2014 to June 2015. All were Nigerians of African descent. Written informed consent was obtained from each patient, before recruitment into the study. The study was approved by the Health Research Ethics Committee of Ahmadu Bello University Teaching Hospital, Zaria and Kaduna State Ministry of Health, Kaduna. Pregnant women with multiple gestations, history of pre-GDM, or any preexisting illness were excluded from the study.
Information on maternal age, gestational age, and parity were all obtained from the patients and recorded. Gestational age was based on the report of ultrasound scan.
Height and weight were measured with the patients standing without shoes or heavy outer clothing. Weight was measured using adult weighing scale (ZT-160, UK) and rounded to the nearest kilogram. Height was measured with patients standing on a hard flat surface against a wall using tape rule/stadiometer (ZT-160, UK) and rounded to the nearest centimeter. Body mass index (BMI) was calculated as the ratio of weight in kilogram to square of height in meters and expressed as kg/m2. The patients were educated regarding the procedure of OGTT and were instructed to be on their normal diet 3 days before the procedure and then fasted for 10–12 h (overnight) before the OGTT procedure. A fasting blood sample was taken for the measurement of FSG, fasting insulin, and TNF-α. The patients were then given a 75 g dose of glucose (in 300 ml of water) orally over 5–10 min. Blood sample was taken at 2 h postglucose dose for the measurement of 2 h serum glucose.
Biochemical analyses
Fasting and 2-h postload serum glucose were measured using the glucose oxidase method (LabKit France). Fasting serum insulin (FSI) was measured using human insulin ELISA kit (Perfemed Group Inc. China). Serum TNF-α was measured using human TNF-α ELISA kit (Wkea Med Supplies Corp., China). All blood samples were treated the same throughout the process of blood collection, transport, storage, and assay. All the analyses were done at the Chemical Pathology Laboratory of Ahmadu Bello University Teaching Hospital, Zaria. Insulin resistance was calculated as homeostasis model assessment of insulin resistance (HOMA-IR).[17]
HOMA-IR = FSI × FSG/22.5.[17]
FSI in μU/mL and FSG in mmol/L.
Statistical analysis
Statistical analysis was done using IBM SPSS Statistics version 20.0. Each analyte was examined for outliers and tested for normality of distribution using Kolmogorov–Smirnov test. Data presentation was done using tables and figures. Quantitative variables were summarized using measures of central tendency and dispersion. To compare mean differences between groups, t-test was used. Correlations were examined using partial correlation and linear regression analysis. A multiple linear regression analysis was performed to investigate the influence of TNF-α on insulin resistance while adjusting for confounders (age and BMI). All P values were two-sided and considered significant if <0.05.
RESULTS
Clinical and biochemical characteristics of the study patients are presented in Table 1. Both the pregnant women with GDM and the controls have similar ages of 25.6 ± 5.4 versus 26.9 ± 5.4 years, respectively (P > 0.05). Gestational age and parity also did not differ significantly between the two study groups (26.5 ± 1.7 vs. 26.7 ± 1.6 weeks, P > 0.05) and (2.3 ± 1.5 vs. 2.6 ± 1.7, P > 0.05), respectively. In comparison with the controls, pregnant women with GDM showed significantly greater pregnancy BMI (25.4 ± 4.0 vs. 23.4 ± 3.7 kg/m2, P < 0.05). Fasting insulin was also higher among the pregnant women with GDM than the controls (16.7 ± 2.5 vs. 15.5 ± 2.5 mU/ml, P < 0.05). Insulin resistance as measured by HOMA-IR indicated that the pregnant women with GDM were more insulin resistant than the controls (3.14 ± 0.19 vs. 2.89 ± 0.20, P < 0.05).
Table 1.
Clinical and biochemical characteristics of the study participants
| Mean±SD | P | ||
|---|---|---|---|
| GDM patients | Controls | ||
| Sample size (n) | 100 | 100 | |
| Age (years) | 25.6±5.4 | 26.9±5.4 | 0.074 |
| Parity | 2.3±1.5 | 2.6±1.7 | 0.166 |
| Gestational age (weeks) | 26.5±1.7 | 26.7±1.6 | 0.241 |
| Weight (kg) | 65.1±15.0 | 61.1±13.4 | 0.048 |
| Height (cm) | 159±7.5 | 161±7.0 | 0.101 |
| BMI (kg/m2) | 25.4±4.0 | 23.4±3.7 | 0.000 |
| Systolic blood pressure (mmHg) | 117.2±7.5 | 118.3±5.5 | 0.240 |
| Diastolic blood pressure (mmHg) | 78.5±4.8 | 78.8±4.1 | 0.635 |
| Fasting serum glucose (mmol/L) | 4.3±0.6 | 4.3±0.7 | 0.763 |
| 2-h serum glucose (mmol/L) | 9.0±0.9 | 6.3±0.8 | 0.000 |
| Serum fasting insulin (µU/mL) | 16.7±2.6 | 15.5±2.5 | 0.000 |
| Serum tumor necrosis factor-alpha (pg/ml) | 2.49±0.30 | 2.05±0.28 | 0.000 |
| HOMA-IR | 3.14±0.19 | 2.89±0.20 | 0.000 |
SD=Standard deviation, GDM=Gestational diabetes mellitus, HOMA-IR=Homeostasis model assessment of insulin resistance, BMI=Body mass index
The relationship between serum TNF-α level and insulin resistance appeared different between the two study groups, being only significantly associated among the pregnant women with GDM. There was a positive correlation between serum TNF-α level and HOMA-IR among the pregnant women with GDM (r = 0.49, P < 0.05) [Table 2 and Figure 1]. The observed association remained significant after adjusting for age and BMI. Multiple linear regression was used to determine the contributions of TNF-α to HOMA-IR. The adjusted R2 was 0.23, demonstrating that 23% of the variance in HOMA-IR among the pregnant women with GDM can be attributed to serum levels of TNF-α. TNF-α was found to be significant predictors of HOMA-IR in GDM [Table 3].
Table 2.
Correlation of serum tumor necrosis factor-alpha with anthropometric and biochemical factors among the study participants
| GDM patients | Controls | |||
|---|---|---|---|---|
| r | P | r | P | |
| Age (years) | 0.25 | 0.013 | 0.51 | 0.000 |
| BMI (kg/m2) | 0.19 | 0.059 | 0.83 | 0.000 |
| Fasting serum insulin (µU/ml) | 0.24 | 0.020 | 0.04 | 0.690 |
| HOMA-IR | 0.49 | 0.000 | −0.04 | 0.730 |
HOMA-IR=Homeostasis model assessment of insulin resistance, BMI=Body mass index, r=Correlation coefficient, GDM=Gestational diabetes mellitus
Figure 1.
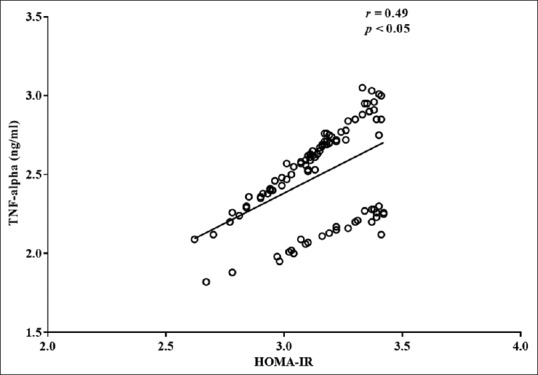
Correlation serum tumor necrosis factor-alpha levels and homeostasis model assessment of insulin resistance among the pregnant women with gestational diabetes mellitus
Table 3.
Multiple regression analysis with log homeostasis model assessment of insulin resistance as a dependent variable among the study participants
| Independent variables | GDM patients (n=100) R2=0.23 | Controls (n=100) R2=0.00 | ||||
|---|---|---|---|---|---|---|
| β | SE | P | β | SE | P | |
| Constant | 0.40 | 0.44 | ||||
| Log TNF-α | 0.25 | 0.045 | 0.000 | 0.05 | 0.049 | NS |
β=Regression coefficient, SE=Standard error, NS=Not significant, TNF-α=Tumor necrosis factor-alpha, GDM=Gestational diabetes mellitus
DISCUSSION
It was found in this study that there were no significant differences between the pregnant women with GDM and the normal controls in terms of age, weeks of gestation, and parity. It was demonstrated in this study that higher TNF-α levels among the pregnant women with GDM were strongly associated with increased insulin resistance as assessed by HOMA-IR. These associations remained significant even after adjustment for age and BMI. This is in keeping with majority of available studies, showing that higher TNF-α levels in pregnant women are associated with increased insulin resistance and therefore higher risk of developing GDM.[13,14,15]
By contrast, McLachlan et al. did not find a significant correlation between TNF-α and insulin resistance among pregnant women with GDM. Their negative findings might be explained by the limited sample size (19 GDM and 19 control participants) and the relatively older study participants (mean age of 33 years for both the GDM patients and the controls).[11] Adding to the controversy, Saucedo et al., in a study involving 60 pregnant women with GDM and 60 pregnant women with normal glucose tolerance (controls), also found no significant correlation between these parameters.[12] The discrepancy in this case may be due to the greater BMI of their study participants (mean BMI of 30 kg/m2 and 28 kg/m2 for the GDM and control groups, respectively). Differences in ethnicity of the study populations, nature of sample (serum or plasma) used, and the type of assay method used in the various studies might also be partially responsible for the discrepancies in the findings between these investigators.
TNF-α promotes serine phosphorylation of insulin receptor substrate-1, thus impairing its association with the insulin receptor.[18,19,20] Therefore, elevated levels of TNF-α in GDM could attenuate insulin signaling, thus causing the increased insulin resistance observed in GDM. It has also been demonstrated that TNF-α decreases adiponectin gene expression in human adipocytes.[20,21] Thus, the increase in TNF-α might lead to the lower level of adiponectin which is an insulin-sensitizing hormone, thereby further increasing insulin resistance.
CONCLUSION
It is concluded that among pregnant Nigerian women with GDM in this study, higher serum TNF-α level is significantly associated with increased insulin resistance. We recommend that additional investigations that will examine the effects of lifestyle factors on the serum level of TNF-α and its pattern from first to third trimester in relation to the insulin resistance are required within the population, so as to improve the understanding of mechanism underlying the alterations of this cytokine in GDM.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tracy LS, Ann JB, Mark NF. Gestational diabetes mellitus. Clin Diabetes. 2005;23:17–24. [Google Scholar]
- 2.Lacroix M, Battista MC, Doyon M, Ménard J, Ardilouze JL, Perron P, et al. Lower adiponectin levels at first trimester of pregnancy are associated with increased insulin resistance and higher risk of developing gestational diabetes mellitus. Diabetes Care. 2013;36:1577–83. doi: 10.2337/dc12-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan EA, Enns L. Role of gestational hormones in the induction of insulin resistance. J Clin Endocrinol Metab. 1988;67:341–7. doi: 10.1210/jcem-67-2-341. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed SA, Shalayel MH. Role of cortisol in the deterioration of glucose tolerance in Sudanese pregnant women. East Afr Med J. 1999;76:465–7. [PubMed] [Google Scholar]
- 5.Polderman KH, Gooren LJ, Asscheman H, Bakker A, Heine RJ. Induction of insulin resistance by androgens and estrogens. J Clin Endocrinol Metab. 1994;79:265–71. doi: 10.1210/jcem.79.1.8027240. [DOI] [PubMed] [Google Scholar]
- 6.Hornnes PJ. On the decrease of glucose tolerance in pregnancy. A review. Diabete Metab. 1985;11:310–5. [PubMed] [Google Scholar]
- 7.Barbour LA, Shao J, Qiao L, Pulawa LK, Jensen DR, Bartke A, et al. Human placental growth hormone causes severe insulin resistance in transgenic mice. Am J Obstet Gynecol. 2002;186:512–7. doi: 10.1067/mob.2002.121256. [DOI] [PubMed] [Google Scholar]
- 8.Kühl C. Glucose metabolism during and after pregnancy in normal and gestational diabetic women 1. Influence of normal pregnancy on serum glucose and insulin concentration during basal fasting conditions and after a challenge with glucose. Acta Endocrinol (Copenh) 1975;79:709–19. [PubMed] [Google Scholar]
- 9.Davis JR. Prolactin and related peptides in pregnancy. Baillieres Clin Endocrinol Metab. 1990;4:273–90. doi: 10.1016/s0950-351x(05)80051-5. [DOI] [PubMed] [Google Scholar]
- 10.Al-Noaemi MC, Shalayel MH. Radenkovic M, editor. Pathophysiology of gestational diabetes mellitus: The past, the present and the future. The past, the present and the future. 2011. [Last accessed on 2014 Jun 15]. Available from: http://www.intechopen.com/books/gestationaldiabetes/pathophysiology of gestational diabetes mellitus the past-the present and the future .
- 11.McLachlan KA, O’Neal D, Jenkins A, Alford FP. Do adiponectin, TNFalpha, leptin and CRP relate to insulin resistance in pregnancy? Studies in women with and without gestational diabetes, during and after pregnancy. Diabetes Metab Res Rev. 2006;22:131–8. doi: 10.1002/dmrr.591. [DOI] [PubMed] [Google Scholar]
- 12.Saucedo R, Zarate A, Basurto L, Hernandez M, Puello E, Galvan R, et al. Relationship between circulating adipokines and insulin resistance during pregnancy and postpartum in women with gestational diabetes. Arch Med Res. 2011;42:318–23. doi: 10.1016/j.arcmed.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Kirwan JP, Hauguel-De Mouzon S, Lepercq J, Challier JC, Huston-Presley L, Friedman JE, et al. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes. 2002;51:2207–13. doi: 10.2337/diabetes.51.7.2207. [DOI] [PubMed] [Google Scholar]
- 14.Winkler G, Cseh K, Baranyi E, Melczer Z, Speer G, Hajós P, et al. Tumor necrosis factor system in insulin resistance in gestational diabetes. Diabetes Res Clin Pract. 2002;56:93–9. doi: 10.1016/s0168-8227(01)00355-2. [DOI] [PubMed] [Google Scholar]
- 15.Laetitia G, Marilyn L, Myriam D, Marie-Claude B, Julie M, Patrice P, et al. Higher levels of tumour necrosis factor alpha are associated with elevated insulin resistance during pregnancy and increased risk of gestational diabetes mellitus. Circulation. 2013;127:AP191. [Google Scholar]
- 16.WHO/NCD/NCS/99. 2nd ed. Geneva: World Health Organization; 1999. World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and Classification of Diabetes Mellitus. [Google Scholar]
- 17.Borai A, Livingstone C, Kaddam I, Ferns G. Selection of the appropriate method for the assessment of insulin resistance. BMC Med Res Methodol. 2011;11:158. doi: 10.1186/1471-2288-11-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rui L, Aguirre V, Kim JK, Shulman GI, Lee A, Corbould A, et al. Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory ser307 via distinct pathways. J Clin Invest. 2001;107:181–9. doi: 10.1172/JCI10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kappes A, Löffler G. Influences of ionomycin, dibutyryl-cycloAMP and tumour necrosis factor-alpha on intracellular amount and secretion of apM1 in differentiating primary human preadipocytes. Horm Metab Res. 2000;32:548–54. doi: 10.1055/s-2007-978684. [DOI] [PubMed] [Google Scholar]
- 20.Friedman JE, Ishizuka T, Shao J, Huston L, Highman T, Catalano P, et al. Impaired glucose transport and insulin receptor tyrosine phosphorylation in skeletal muscle from obese women with gestational diabetes. Diabetes. 1999;48:1807–14. doi: 10.2337/diabetes.48.9.1807. [DOI] [PubMed] [Google Scholar]
- 21.Shao J, Catalano PM, Yamashita H, Ruyter I, Smith S, Youngren J, et al. Decreased insulin receptor tyrosine kinase activity and plasma cell membrane glycoprotein-1 overexpression in skeletal muscle from obese women with gestational diabetes mellitus (GDM): Evidence for increased serine/threonine phosphorylation in pregnancy and GDM. Diabetes. 2000;49:603–10. doi: 10.2337/diabetes.49.4.603. [DOI] [PubMed] [Google Scholar]


