Abstract
Background:
Lung cancer (LC) chemotherapy results in several adverse events (AEs). Data regarding supportive care medications (SCMs) offered to prevent/treat AEs in resource-limited settings are lacking. A prospective observational study was carried out to find the effectiveness of SCMs in real-life setting.
Methods:
Newly diagnosed LC patients receiving first-line chemotherapy at a tertiary referral center in North India (from July 2014 to September 2015) were enrolled. Incidence, timing of onset, duration, and grades of chemotherapy-related AEs were recorded. We assessed compliance to mandatory SCMs using a structured questionnaire. Patients also recorded various symptoms, frequency of need-based SCMs, visits to local practitioners, and hospitalization (if any) during the intercycle period.
Results:
Of the 112 patients enrolled, majority were males (83.9%, n = 94), current/ex-smokers (82.1%, n = 92), had advanced stage (Stage IIIB = 33.9% [n = 38] and Stage IV = 46.4% [n = 52]), and were non-small cell lung cancer (72.3%, n = 81). AEs were reported in 566 cycles (94%) out of a total of 602 chemotherapy cycles. Diarrhea was the most common AE (180 cycles, 29.9%) developing after a mean (standard deviation) duration of 3.6 (2.5) days and lasting for 4 (3.3) days. Vomiting (138 cycles, 22.9%) and constipation (121 cycles, 20.1%) were other common AEs. Grade 3/4 AEs occurred in 6.9% (39/566) cycles. Need-based SCMs were required in 479 of the 566 cycles (84.6%). Proportion of patients with Grade 3/4 AEs and hospitalization was highest for mucositis (16.1% Grade 3/4 and 9.7% hospitalized); followed by vomiting (10.1% Grade 3/4 and 8.7% hospitalized). Anemia was seen in 441 of 602 chemotherapy cycles (73.3%). Frequency and severity of anemia continued to increase with each chemotherapy cycle.
Conclusion:
LC chemotherapy has a high prevalence of AEs. However, the majority are low grade recovering with need-based SCMs, without any need for hospitalization.
KEY WORDS: Adenocarcinoma, anemia, chemoradiotherapy, malignancy, toxicity
INTRODUCTION
Lung cancer (LC) is a leading cause of death worldwide. Major changes in the diagnosis and management of LC have occurred over the past few decades. Advances in therapy such as targeted therapy are, however, possible only in a small subset of non-small cell lung cancer (NSCLC) patients with driver mutations.[1] Most other patients lack a driver mutation and present in an advanced stage where chemotherapy with/without radiotherapy forms the mainstay of treatment.[2] Chemotherapeutic agents target cancer cells as well as other rapidly multiplying cells of the body, hence leading to several unpleasant adverse events (AEs). The AE profile differs with age, performance status, comorbid illness, the chemotherapeutic agent used, and its dose. Randomized trials evaluating the role of various chemotherapeutic regimens provide information regarding AEs; however, these data are obtained from selected group of individuals and may not be a true representation of the real world scenario. Data on AEs and health-care resource utilization have been studied in the past for several cancers, including LC.[3,4] Most of the available literature is from developed nations, while data of AEs from developing nations are lacking.[5] The burden of LC in developing and underdeveloped nations is tremendous. Unique problems encountered in these settings include a high population burden, lack of adequate health-care facilities, lack of trained workforce, and a suboptimal doctor/nurse–patient ratio.[6] Creating awareness among patients undergoing chemotherapy, prescribing them, and educating them about need-based supportive care medicines (SCM) may be effective in resource-limited settings. The current study was, therefore, planned to evaluate the effectiveness of SCMs at a tertiary care health-care government-funded research institute in North India among LC patients undergoing first-line chemotherapy. The adverse events occurring in these individuals were studied along with the details of supportive care offered, physician visits, and hospitalization.
METHODS
This prospective observational study was conducted over a 12-month period (July 2014–July 2015) enrolling newly diagnosed LC patients attending the LC clinic of a tertiary referral center in North India. We excluded patients if they had primary tumors other than LC which had metastasized to the lungs and/or pleura, tumors of nonbronchogenic histology, and primary pleural tumors. Patients were also excluded if they refused informed consent or had received chemotherapy in the past. The study was approved by the institutional ethics committee.
Demographic data at baseline including smoking status, histology, clinicoradiological stage (TNM7), presence or absence of extrathoracic metastases, and performance statuses eastern co-operative oncology group (ECOG) and karnofsky performance status (KPS) were recorded.[7] Smoking status was quantified as the number of bidis/cigarettes smoked per day multiplied by the number of years smoked (smoking index).[8] Patients underwent complete blood count, renal, and liver function tests before each cycle of chemotherapy was administered. We also recorded the prevalence of anemia at baseline and after chemotherapy along with the nature of treatment instituted (packed red cell transfusion, erythropoietin stimulating agents, or hematinics).
Chemotherapy protocols and outcome assessments
Chemotherapy regimens used at our center and the usual management protocols were followed (published in detail previously).[6,9,10,11] We used standard platinum-based doublet regimens. The preferred non-platinum agent was pemetrexed, docetaxel, and irinotecan for adenocarcinoma, squamous, and small cell histological types, respectively. In case of unacceptable toxicity or intolerance to chemotherapy, we modified the regimen or dose in subsequent chemotherapy cycles accordingly.[6] Trained oncology nurses administered chemotherapy as per manufacturer's instruction at the day-care center of our hospital. We used the Common Toxicity Criteria for Adverse Events (version 3) to objectively assess toxicity and Response Evaluation Criteria in Solid Tumors criteria to assess the radiological tumor responses.[6,9,10,11,12,13,14] Tumor response was assessed by contrast-enhanced tomography of the thorax and upper abdomen after four cycles of chemotherapy or earlier if clinical or chest radiographic suspicion of disease progression was present. Outcomes were labeled as complete response, partial response, stable disease, or progressive disease. Two additional cycles of doublet chemotherapy (maximum six) were administered if there was an objective response to treatment.
Gastrointestinal and hematological toxicity following chemotherapy was assessed at each visit. Time of onset, duration of symptoms, and the treatment details for each of these AEs were recorded in detail. In particular, the frequency of use and effectiveness of need-based SCMs in the intercycle period were assessed using a dedicated questionnaire [Supplement 1 (188.8KB, pdf) ]. Details of AEs requiring consultation with local physician, hospitalization, and intravenous medication were also noted. We assessed compliance to mandatory and need-based SCMs using the same questionnaire [Supplement 1 (188.8KB, pdf) ]. All the study participants maintained a symptom diary to record AEs and the usage of need-based therapy in-between chemotherapy cycles. Mandatory and need-based supportive care offered between chemotherapy cycles used at our LC clinic is provided in Supplement 2 (146.9KB, pdf) .
Statistical analysis
Statistical package software (SPSS for Windows, version 22.0; IBM SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Descriptive data were presented as mean (standard deviation [SD]) or median (interquartile range) for continuous variables and as percentages for categorical variables. Differences between the means of categorical and continuous variables were compared using the Chi-square test and the Mann–Whitney U-test, respectively. P < 0.05 was considered statistically significant.
RESULTS
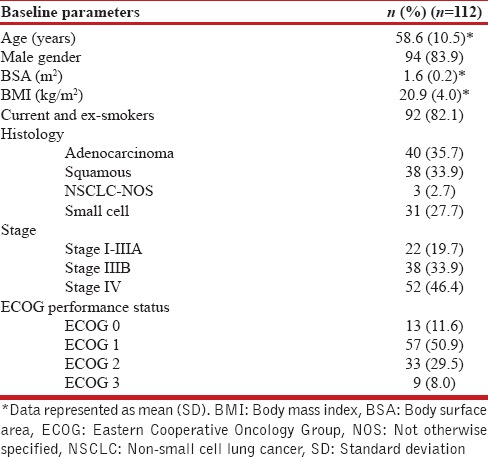
A total of 112 newly diagnosed LC patients (males, n = 94, 83.9%) with a mean (SD) age of 58.6 (10.5) years were enrolled. Baseline demographic profile, histology, anatomic staging, and performance status of these patients are shown in Table 1. Majority of the included patients were smokers (n = 92, 82.1%). The most common histologic subtype was NSCLC, of which adenocarcinoma (n = 40, 35.7%) and squamous cell carcinoma (n = 38, 33.9%) constituted the majority. Small-cell LC was seen in 31 patients (27.7%). Most of the included patients had advanced stage cancer (Stage IIIB in 38 patients [33.9%] and Stage IV in 52 patients [46.4%]), while early-stage cancer (Stage I to IIIA) was seen in 22 patients (19.7%) only.
Table 1.
Baseline characteristics of the study participants
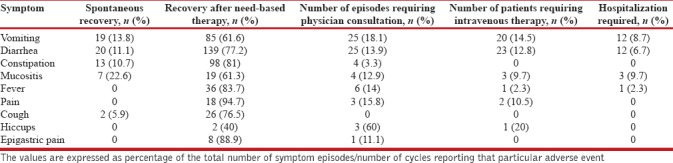
Six cycles of chemotherapy were completed in 81 patients (72.3%), and a total of 602 cycles of chemotherapy were administered in the 112 included patients. One or more of the AEs were reported in 566 cycles (94%). Diarrhea was the most commonly encountered AE seen in 69 patients (61.6%), and 180 episodes occurred (29.9% of total chemotherapy cycles) with a mean (SD) onset after 3.6 (2.5) days and lasting for a mean (SD) of 4 (3.3) days. Vomiting was seen in 59 patients (52.7%), and a total of 138 episodes (22.9% of the total cycles of chemotherapy) were reported with a mean (SD) after 3.5 (2.7) days and lasting for a mean (SD) 3.8 (3.1) days. The mean durations of other symptoms are provided in Table 2. Constipation, fever, and mucositis were the other common symptoms [Figures 1 and 2]. Only 39 episodes of AEs (6.9%) were severe (Grade 3 or Grade 4) while the remaining were low grade (Grades 1 and 2). Need-based treatment for symptoms was taken in 479 (84.6%) of the 566 cycles experiencing AEs. More than 75% patients experiencing AEs recovered with the need-based supportive management [Table 3]. Hiccup failed to improve with need-based supportive treatment and required consultation with local physician in 60% of cases (n = 3). Vomiting, diarrhea, and mucositis were the AEs which commonly required intravenous medications or hospitalization. Intravenous medications were required in 14.5% (n = 20), 12.8% (n = 23), and 9.7% (n = 3) episodes of vomiting, diarrhea, and mucositis, respectively. Twenty-eight episodes of AEs were managed after hospitalization (4.9% of 566 cycles reporting AEs). The major AEs leading to hospitalization were mucositis, vomiting, and diarrhea [Table 3].
Table 2.
Symptoms (their onset, duration, and number of episodes) reported after chemotherapy for lung cancer and compliance to need-based supportive therapy
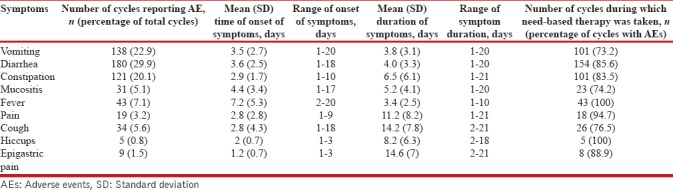
Figure 1.
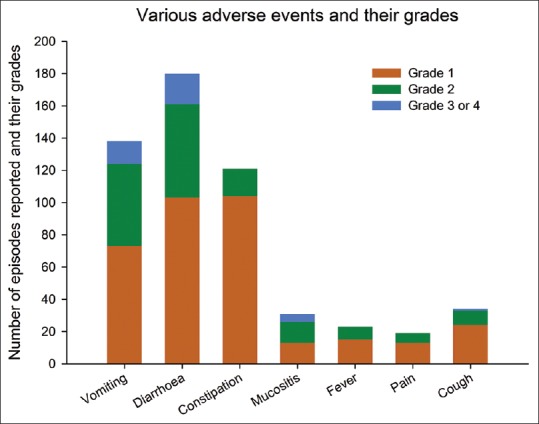
Bar chart showing the various adverse events reported and their grade
Figure 2.
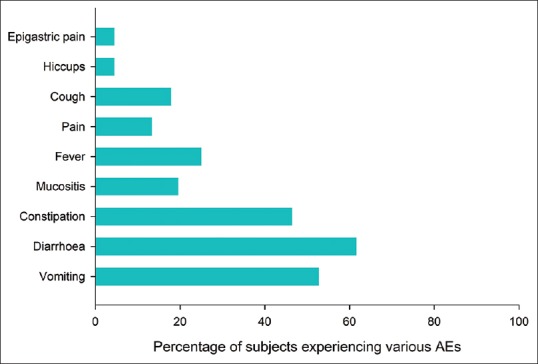
Bar chart depicting the number (%) of patients experiencing various adverse events
Table 3.
Symptoms experienced during lung cancer treatment and their recovery (either spontaneous recovery, recovery with need-based therapy, consultation with local physician, or hospitalization)
Of the 602 chemotherapy cycles, anemia was observed in 441 (73.3%). With each cycle of chemotherapy, the frequency as well as the severity of anemia continued to increase [Figure 3]. In fact, only 9.8% patients did not have anemia at the sixth cycle of chemotherapy as compared to 58% with no anemia at baseline. Hematinics were prescribed for 96.1% of the cycles with anemia, while erythropoietin and intravenous iron therapy were instituted during 30 (6.8%) and 4 (0.9%) of the 441 cycles, respectively. Transfusion to correct anemia was required in 47 (10.6%) of 441 cycles experiencing anemia.
Figure 3.
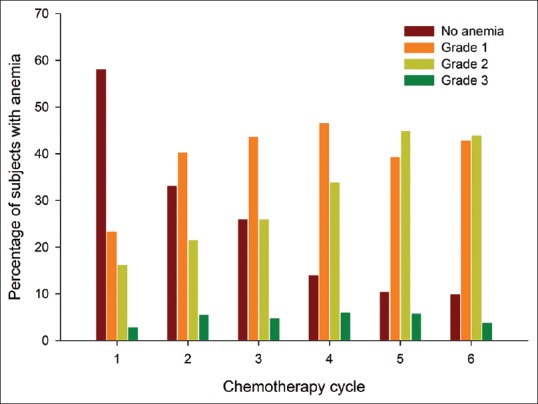
The percentage of patients experiencing various grades of anemia (Y-axis) during each chemotherapy cycle (X-axis) represented in a bar diagram
DISCUSSION
This study included 112 newly diagnosed LC patients who received first-line chemotherapy (a total of 602 cycles). Adverse events during chemotherapy were reported in 566 cycles (94%). Diarrhea was the most common AE, reported in 69 patients (61.6%) and in 29.9% of the total chemotherapy cycles. Vomiting and constipation were the other frequently reported AEs. Only 39 episodes (6.9%) of AEs were severe (Grades 3 and 4), while the remaining were low grade. Among the 566 chemotherapy cycles, need-based therapy was instituted in 479 (84.6%). Most of the AEs improved with need-based therapy or spontaneously, while hospitalization due to AEs was required in only 4.9% (28 episodes). There was a high prevalence of anemia (441 cycles [73.3%] showed anemia), and with each cycle of chemotherapy, the frequency as well as the severity of anemia continued to increase.
The toxicity profile encountered during chemotherapy varies depending on several factors. Different chemotherapy protocols, dosing regimens, and differences in genetic predisposition to various AE are certain factors that may explain such differences. For instance, in a study from Germany, Grade 3 or 4 AE occurred in 50.2% cycles. This study included 1004 chemotherapy cycles (619 for lymphoproliferative diseases and 385 for NSCLC) in 286 patients; hematologic adverse events (febrile neutropenia, infections, thrombocytopenia, and anemia) were the most frequently encountered, accounting for nearly 48% of the severe AEs. Higher proportion of hematologic AE and febrile neutropenia in this study is understandable as 156 of the 286 included patients (54.5%) had lymphoproliferative disease. Another study from Germany also observed hematologic AEs to be the most severe and bothersome.[15] The incidence and severity of hematologic AEs appear to be much higher in these European cohorts, unlike our study cohort.[4,15,16] Whether Asian patients have lesser incidence of chemotherapy-induced myelosuppression, unlike their Caucasian counterparts, needs to be explored. Gastrointestinal AEs were the most commonly encountered toxicity in our study, and diarrhea was the most frequent (29.9% cycles). The different toxicity profile encountered in our study could possibly be related to the different chemotherapy protocol used in LC as opposed to lymphoproliferative diseases.
As AEs are anticipated during chemotherapy (especially nausea and vomiting), prophylactic measures are generally administered either along with or following chemotherapy.[4] The effectiveness of prophylactic and on-demand use of supportive drugs was also noted by Ihbe-Heffinger et al. in their cohort of 120 NSCLC patients (385 chemotherapy cycles); antiemetics were the most commonly used (96.9%), and not surprisingly, the incidence of Grade 3 or 4 nausea/vomiting was low (4.6%).[15] More importantly, the dose of (cisplatin 65 mg/m2) used at our center is lesser than the conventional dose used at centers from Western countries.[6] Further, it is our practice to substitute cisplatin for carboplatin in the event of intolerance to the former. Careful dosing and dose modification in subsequent chemotherapy cycles as per patient tolerance are few factors which account for the reduced incidence of severe AEs in our study. Currently available studies are from the developed nations (the United States and Europe) and focus on the health resource utilization.[17] Studies from China describing the costs involved in managing LC patients are available; however, data on the nature and incidence of various AEs are lacking.[5] Reliable estimates of various AEs from India and other developing nations would be essential, considering the burden of LC in these parts of the world.
Anemia was another significant problem observed in our cohort and was highly prevalent, even at baseline (42%). On the other hand, in a study from Germany of 180 patients undergoing 633 chemotherapy cycles (47.2% for NSCLC and the remaining for hematological malignancies), Grade 2 anemia (as per NCI CTC version 3) was found only in 6% patients at baseline.[16] In this study, the incidence of Grade 3 or 4 anemia was only 2.5%, whereas in our cohort, the baseline prevalence of anemia (42%) and new-onset anemia or worsening of preexisting anemia following chemotherapy was much higher.[16] Further, anemia was a major cause of withholding of chemotherapy cycles in our cohort. This is consistent with our previous experiences where anemia was the most common cause of intercycle delays during chemotherapy for NSCLC.[9] Although the etiology of anemia at baseline was not evaluated in detail, nutritional deficiency is likely to play a major role. However, despite adequate compliance to hematinics, anemia continued to be a significant problem, whereas most of the other supportive care medicines used by our patients appeared to be effective. Cumulative effect of repeated cycles of chemotherapy on preexisting anemia may have contributed to such a high prevalence of anemia (only 9.8% patients were not anemic at C6). Anemia of chronic diseases is another important factor, and erythropoietin stimulating agents have been found to be useful in patients with underlying malignancy.[18] In the index study, erythropoietin was used in 6.8% of the cycles with anemia. Packed red cell transfusion was required in 10.6% of chemotherapy cycles comparable to the 14.6% requirement in NSCLC patients from Germany.[16]
Our study has a few limitations. Being a single-center study, the results cannot be generalized and we have not performed cost analysis. In high income countries, the data on various AEs are widely available; hence the literature from these developed nations focuses on estimating the cost involved in managing these AEs.[3,19] In particular, the cost involved with hospitalization is tremendous. On the other hand such data are not available for LC patients undergoing chemotherapy in India. In resource-constrained setting such as ours, hospitalization due to AEs not only increases the cost but also increases morbidity and mortality, owing to the limited availability of resources. Although we have not looked into the direct and indirect costs involved in managing these AEs, the expenditure is presumed to be high.[5] Lack of detailed evaluation for the cause of anemia is another limitation. We also have not looked into other AEs of chemotherapy such as peripheral neuropathy and fatigue. Nevertheless, our study provides an overview of real-world scenario and the profile of AEs encountered by patients from a state-run hospital of a developing nation.
In conclusion, with adequate instructions, maintenance of symptom diary, and proper use of need-based medicines, majority of the AEs can be tackled; nearly 75% either improved spontaneously or with the need-based supportive therapy. Anemia is highly prevalent in our population, and further studies to look for etiologies are essential. Our study clearly shows that the problems encountered in our setting are significantly different from those observed in the US and Europe. Larger studies reporting the chemotherapy AEs, the health expenditure involved, and measures to reduce them would be required in future.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Singh N, Bal A, Aggarwal AN, Das A, Behera D. Clinical outcomes in non-small-cell lung cancer in relation to expression of predictive and prognostic biomarkers. Future Oncol. 2010;6:741–67. doi: 10.2217/fon.10.30. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ, Jr, Wu YL. Lung cancer: Current therapies and new targeted treatments. Lancet. 2017;389:299–311. doi: 10.1016/S0140-6736(16)30958-8. [DOI] [PubMed] [Google Scholar]
- 3.Migliorino MR, Santo A, Romano G, Cortinovis D, Galetta D, Alabiso O, et al. Economic burden of patients affected by non-small cell lung cancer (NSCLC): The LIFE study. J Cancer Res Clin Oncol. 2017;143:783–91. doi: 10.1007/s00432-016-2326-x. [DOI] [PubMed] [Google Scholar]
- 4.Paessens BJ, von Schilling C, Berger K, Shlaen M, Müller-Thomas C, Bernard R, et al. Health resource consumption and costs attributable to chemotherapy-induced toxicity in German routine hospital care in lymphoproliferative disorder and NSCLC patients. Ann Oncol. 2011;22:2310–9. doi: 10.1093/annonc/mdq759. [DOI] [PubMed] [Google Scholar]
- 5.Shi J, Zhu J. Health resource utilization in patients with advanced non-small cell lung cancer receiving chemotherapy in China. Clin Drug Investig. 2016;36:77–86. doi: 10.1007/s40261-015-0356-9. [DOI] [PubMed] [Google Scholar]
- 6.Singh N, Aggarwal AN, Behera D. Management of advanced lung cancer in resource-constrained settings: A perspective from India. Expert Rev Anticancer Ther. 2012;12:1479–95. doi: 10.1586/era.12.119. [DOI] [PubMed] [Google Scholar]
- 7.Singh N, Aggarwal AN, Gupta D, Behera D, Jindal SK. Unchanging clinico-epidemiological profile of lung cancer in North India over three decades. Cancer Epidemiol. 2010;34:101–4. doi: 10.1016/j.canep.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Singh N, Aggarwal AN, Gupta D, Behera D, Jindal SK. Quantified smoking status and non-small cell lung cancer stage at presentation: Analysis of a North Indian cohort and a systematic review of literature. J Thorac Dis. 2012;4:474–84. doi: 10.3978/j.issn.2072-1439.2012.05.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh N, Aggarwal AN, Behera D, Jindal SK. Intercycle delays during chemotherapy of non-small cell lung cancer in a health care resource-constrained setting and their effect on overall survival. J Thorac Oncol. 2010;5:236–9. doi: 10.1097/JTO.0b013e3181c3f5f7. [DOI] [PubMed] [Google Scholar]
- 10.Singh N, Mootha VK, Madan K, Aggarwal AN, Behera D. Tumor cavitation among lung cancer patients receiving first-line chemotherapy at a tertiary care centre in India: Association with histology and overall survival. Med Oncol. 2013;30:602. doi: 10.1007/s12032-013-0602-z. [DOI] [PubMed] [Google Scholar]
- 11.Singh N, Singh PS, Aggarwal AN, Behera D. Comorbidity assessment using charlson comorbidity index and simplified comorbidity score and its association with clinical outcomes during first-line chemotherapy for lung cancer. Clin Lung Cancer. 2016;17:205–130. doi: 10.1016/j.cllc.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–81. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 14.Singh N, Aggarwal AN, Kaur J, Behera D. Association of graded folic acid supplementation and total plasma homocysteine levels with hematological toxicity during first-line treatment of nonsquamous NSCLC patients with pemetrexed-based chemotherapy. Am J Clin Oncol. 2017;40:75–82. doi: 10.1097/COC.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 15.Ihbe-Heffinger A, Paessens B, Berger K, Shlaen M, Bernard R, von Schilling C, et al. The impact of chemotherapy-induced side effects on medical care usage and cost in German hospital care – An observational analysis on non-small-cell lung cancer patients. Support Care Cancer. 2013;21:1665–75. doi: 10.1007/s00520-012-1711-5. [DOI] [PubMed] [Google Scholar]
- 16.Paessens B, Ihbe-Heffinger A, von Schilling C, Shlaen R, Bernard R, Peschel C, et al. Blood component use and associated costs after standard dose chemotherapy – A prospective analysis of routine hospital care in lymphoproliferative disorders and NSCLC in germany. Support Care Cancer. 2012;20:1011–21. doi: 10.1007/s00520-011-1173-1. [DOI] [PubMed] [Google Scholar]
- 17.Buck PO, Saverno KR, Miller PJ, Arondekar B, Walker MS. Treatment patterns and health resource utilization among patients diagnosed with early stage resected non-small cell lung cancer at US community oncology practices. Clin Lung Cancer. 2015;16:486–95. doi: 10.1016/j.cllc.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Vansteenkiste J, Glaspy J, Henry D, Ludwig H, Pirker R, Tomita D, et al. Benefits and risks of using erythropoiesis-stimulating agents (ESAs) in lung cancer patients: Study-level and patient-level meta-analyses. Lung Cancer. 2012;76:478–85. doi: 10.1016/j.lungcan.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 19.van der Linden N, Bongers ML, Coupé VM, Smit EF, Groen HJ, Welling A, et al. Costs of non-small cell lung cancer in the Netherlands. Lung Cancer. 2016;91:79–88. doi: 10.1016/j.lungcan.2015.10.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


